Abstract
In the mammalian vestibular nerve, some afferents have highly irregular interspike intervals and others have highly regular intervals. To investigate whether spike timing is determined by the afferents' ion channels, we studied spiking activity in their cell bodies, isolated from the vestibular ganglia of young rats. Whole cell recordings were made with the perforated-patch method. As previously reported, depolarizing current steps revealed distinct firing patterns. Transient neurons fired one or two onset spikes, independent of current level. Sustained neurons were more heterogeneous, firing either trains of spikes or a spike followed by large voltage oscillations. We show that the firing pattern categories are robust, occurring at different temperatures and ages, both in mice and in rats. A difference in average resting potential did not cause the difference in firing patterns, but contributed to differences in afterhyperpolarizations. A low-voltage-activated potassium current (ILV) was previously implicated in the transient firing pattern. We show that ILV grew from the first to second postnatal week and by the second week comprised Kv1 and Kv7 (KCNQ) components. Blocking ILV converted step-evoked firing patterns from transient to sustained. Separated from their normal synaptic inputs, the neurons did not spike spontaneously. To test whether the firing-pattern categories might correspond to afferent populations of different regularity, we injected simulated excitatory postsynaptic currents at pseudorandom intervals. Sustained neurons responded to a given pattern of input with more regular firing than did transient neurons. Pharmacological block of ILV made firing more regular. Thus ion channel differences that produce transient and sustained firing patterns in response to depolarizing current steps can also produce irregular and regular spike timing.
INTRODUCTION
The signature property of mammalian vestibular nerve fibers is the regularity of spike timing. The nerve comprises highly regular and highly irregular populations (Baird et al. 1988; Goldberg et al. 1990a). Spike regularity correlates with response properties to head tilt and motion (reviewed in Goldberg 2000). By average rate measures, irregular afferents are more sensitive than regular afferents to head motions at all but the lowest stimulus frequencies (Hullar et al. 2005; Sadeghi et al. 2007). Average rate, however, neglects the potential information contained in spike timing. Using mutual information methods to take spike timing into account, Sadeghi et al. (2007) found that regular afferents carry more information about the stimulus waveform, especially at frequencies <10 Hz. Thus irregular and regular afferents may use different codes to convey different aspects of the head motion stimulus, such that spike timing is not just a marker of vestibular afferent populations, but a factor in their sensory representations.
Irregular and regular afferents innervate clearly defined central and peripheral zones, respectively, of mammalian vestibular epithelia (reviewed in Goldberg 1991). Several of the numerous anatomical differences between the zones may plausibly influence spike timing. For example, the most irregular afferents of the central zones form large synaptic terminals (calyces) around the basal surfaces of one-to-three hair cells (Baird et al. 1988; Fernández et al. 1988, 1990). In these compact terminal arbors, proximity of the spike initiation zone to the postsynaptic membrane may enhance the sensitivity of the afferent to transmitter release, an inherently irregular process with Poisson-like statistics (Glowatski and Fuchs 2002). In the peripheral zones, the thin fibers of highly regular afferents form extensive dendritic arbors with dozens of small bouton terminals on multiple hair cells. Here, synaptic potentials from many synaptic terminals travel electrotonically along thin unmyelinated fibers, presumably converging and summating at a remote spike-initiating zone, a design that should filter out synaptic noise (Highstein and Politoff 1978).
Nevertheless, intracellular labeling studies suggested that morphology alone cannot account for differences in spike regularity (Baird et al. 1988; Goldberg et al. 1990b). Moreover, sharp-electrode recordings revealed that postspike afterhyperpolarizations (AHPs) are more prominent in regular afferents than in irregular afferents, indicating that the afferents' voltage-gated conductances could influence spike timing (Schessel et al. 1991). Candidate conductances have emerged from patch-clamp studies on the isolated cell bodies of vestibular afferents (reviewed in Eatock et al. 2008). Briefly, the cell bodies express diverse conductances selective for potassium (K+) (Chabbert et al. 2001a; Limón et al. 2005; Risner and Holt 2006), calcium (Ca2+) (Chambard et al. 1999; Desmadryl et al. 1997), sodium (Na+) (Chabbert et al. 1997; Schneider et al. 2006), and mixed cations (h, or HCN channels) (Chabbert et al. 2001b). There is systematic variation in the expression of certain conductances—e.g., by cells of different diameter (Limón et al. 2005)—and in firing patterns evoked by current steps (Eatock et al. 2008; Iwasaki et al. 2008) or sinusoids (Risner and Holt 2006). Iwasaki et al. (2008) found that Kv1 channels were important in setting the pattern evoked by depolarizing current steps.
The variations in step-evoked firing patterns could not be linked experimentally to spike regularity because isolated cell bodies do not fire spontaneously. Here we circumvent this difficulty by eliciting firing with excitatory postsynaptic current (EPSC)–like injections of current. These experiments show that voltage-dependent properties of vestibular afferent neurons can generate the categories of spike timing seen in vivo. We further implicate both Kv1 and Kv7 (KCNQ) ion channels in setting firing pattern (in response to current steps) and spike timing (in response to EPSC-like inputs).
METHODS
Preparations
Data were recorded from the somata of neurons isolated from the superior part of the vestibular ganglion, most from Long–Evans rats on postnatal day 0 (P0, day of birth) to P16, and some from mice (129/Sv strain), P0–P8. Animals were handled in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and all procedures were approved by the animal care committee at the Massachusetts Eye and Ear Infirmary. Chemicals were obtained from Sigma-Aldrich (St. Louis, MO) unless otherwise specified.
Temporal bones were dissected in chilled and oxygenated Liebovitz-15 (L-15) medium supplemented with 10 mM HEPES (pH 7.4, ∼315 mmol/kg); we will refer to this as the standard medium. The otic capsule was exposed and the superior part of the vestibular ganglion was detached from its distal and central nerve branches. The superior compartment supplies the utricular macula, the cristae of the lateral and anterior semicircular canals, and part of the saccular macula. The ganglion tissue was placed in the standard medium to which had been added 0.05% collagenase and 0.25% trypsin, for 25–30 min at 37°C. The ganglion tissue was then mechanically dissociated by trituration into either standard medium or a bicarbonate-buffered medium (see following text). Cells settled onto a glass-bottom culture dish precoated with poly-d-lysine.
Recordings were made after periods of 1–8 h (acute; n = 42 of 179 cells), 16–24 h (1 day in vitro; n = 123), or 40–50 h (2 days in vitro; n = 14). To compare cells at different ages, we assigned their age as the sum of the age at dissection plus the number of days spent in vitro; thus a cell recorded acutely at P9 would be compared with a cell dissociated on P7 and maintained for 2 days in vitro. Acute preparations were successful only at <P7; at older ages, satellite cells on the somata prevented sealing on the neuronal membranes with patch pipettes. Storing the cells for longer periods tended to remove the satellite cells, allowing recordings from older cells. Two storage conditions were used: 1) cells were stored overnight in standard medium (HEPES-supplemented L-15) at 4°C or 2) cells were dissociated in bicarbonate-buffered culture medium (minimal essential medium [MEM], Invitrogen, Carlsbad, CA), supplemented with 10 mM HEPES and 1% penicillin-streptomycin (Invitrogen) and incubated overnight or longer in 5% CO2-95% air at 37°C. Although neurons (and satellite cells) from older animals survived better with the second culturing method, the number of surviving cells decreased with age for both methods. For age-matched neurons from the different preparations (acutely dissociated, stored at 4°C or incubated at 37°C), we found no significant differences in the properties we measured and therefore have pooled the results.
Electrophysiology
RECORDINGS.
Isolated cells were viewed at ×630 with an inverted microscope (Olympus IMT-2; Olympus, Lake Success, NY) equipped with Nomarski optics. To avoid contamination by axonal membrane, we present data only from cells that lacked visible processes. Signals were delivered, recorded, and amplified either with an Axopatch 200A amplifier and Digidata 1200 data acquisition board controlled by pClamp 8 software or with an Axopatch 200B amplifier, Digidata 1440 board, and pClamp 10 software (MDS, Toronto, Canada). To reduce distortion of action potential shape (Magistretti et al. 1998), we recorded in the fast current-clamp mode of the amplifier.
We used filamented borosilicate glass recording pipettes with resistances of 3–5 MΩ in our standard solutions. To reduce pipette capacitance, we either wrapped electrode shanks in parafilm or coated them with a silicone elastomer (Sylgard 184; Dow Corning, Midland, MI). During ruptured-patch whole cell recordings, the loss of cellular contents can change the properties of ion channels. To minimize such changes, we used the perforated-patch method of whole cell recording in which the membrane patch contacted by the electrode is perforated by amphotericin B (Sigma-Aldrich). The amphotericin-B pores allow only small monovalent ions to pass freely (Horn and Marty 1988). The pipette solution for perforated-patch recordings contained (in mM): 75 K2SO4, 25 KCl, 5 MgCl2, 5 HEPES, 5 EGTA, 0.1 CaCl2, and 240 μg/ml amphotericin B, titrated with 13 mM KOH to a pH of 7.3 and for a final K+ concentration of about 188 mM, about 270 mmol/kg. During recordings, series resistance ranged from 12 to 27 MΩ and was compensated electronically by 80 to 50%, respectively, for final resistances of 2.4 to 12 MΩ. Activation curves (conductance–voltage [g–V] curves) were corrected off-line for residual (uncompensated) series resistance. All membrane voltages were corrected off-line for a 5.1 mV junction potential, computed with JPCalc (Barry 1994) as implemented in pClamp 10.2.
To monitor time-dependent changes in the neurons, we collected responses to standard protocols (100-ms current and voltage steps) at regular intervals. Only recordings obtained with GΩ seals and while the resting potential was stable and negative to −40 mV were included for further analysis. The bath contained fresh oxygenated standard medium, which has 5.7 mM K+ (see Preparations), for a K+ equilibrium potential of about −88 mV. Most recordings were at room temperature (22–25°C) but in some experiments the bath was heated to 35 or 37°C with a heated platform and temperature controller (TC-344B; Warner Instruments, Hamden, CT).
PHARMACOLOGY.
Stock solutions (100 μM) of α-dendrotoxin (α-DTX) were prepared in distilled water and stored at −20°C. A 10 mM stock solution of linopirdine in DMSO was prepared fresh on the day of recording. All solutions were diluted to their final concentrations in our standard L-15 solution. Drugs were locally applied to cells using a pressurized superperfusion system (Automate Scientific, Berkeley CA). It was not possible to make tail current activation curves because of interference by Na+ currents. Instead, we generated quasi-steady-state g–V curves by measuring the current at 100 ms after step onset and dividing by approximate driving force, calculated as (Vm − EK). The resulting curves were generally well fit by the Boltzmann equation
| 1 |
where gmax is the maximum conductance, V1/2 is the half-maximum activation voltage, and S is the slope factor. Pharmacology experiments were done with cultured rat neurons (P8–P17, 25–27°C). Activation curves were made only for recordings with stable series resistances.
ANALYSIS.
We analyzed data with pClamp 10 software (Clampfit; MDS Analytical Technologies, Toronto), Matlab (The MathWorks, Natick, MA), and Origin (OriginLabs, Northampton, MA). Data are given as means ± SE. Statistical significance was estimated with Student's unpaired t-test with Welsh's correction for differences in sample variance. Observed significance levels (p value) are reported as significant (p < 0.05, shown as “*” in graphs), very significant (**p < 0.01), and highly significant (***p < 0.001). Input resistance (Rin) was calculated from the voltage change produced by 10-pA hyperpolarizing current steps. We obtained membrane time constant (τm) by fitting a single exponential to the same voltage response. Membrane capacitance (Cm) is the ratio τm/Rm.
We used phase plots (Bean 2007) to estimate spike voltage threshold (Vth) as the voltage Vm, at which dVm/dt changes rapidly. We empirically determined a threshold criterion of 10 mV/ms, above the voltage noise but small enough to allow threshold detection for small spikes.
PSEUDOSYNAPTIC STIMULI.
To drive spiking with stimuli that mimic natural synaptic input, we generated trains of simulated excitatory postsynaptic currents (pseudo-EPSCs) with pseudorandom timing. To represent the shape and size of each pseudo-EPSC, we used alpha functions
| 2 |
The parameter α, which determines the time course of the pseudo-EPSC waveform, was chosen to be 1 ms, to be consistent with voltage-clamp data on EPSCs from vestibular afferent terminals (Rennie and Streeter 2006; RA Eatock and J Xue, unpublished results) and cochlear afferent terminals (Glowatzki and Fuchs 2002). To represent the random timing of quantal synaptic input, we generated an impulse train by drawing times from a Poisson distribution (mean interval: ∼2 ms) made to be representative of synaptic arrival times in both vestibular and cochlear afferents (Glowatzki and Fuchs 2002; Holt et al. 2006, 2007). Pseudo-EPSC trains were generated by convolving the impulse trains with the EPSC-like alpha functions.
The number of pseudo-EPSC sweeps presented depended on output spike rate. Spikes were detected with the built-in spike thresholding algorithms of Clampfit 10.2. The coefficient of variation (CV) of spike times was the SD divided by the mean interspike interval. CV values are reported only for histograms with enough data points to be well formed, as assessed visually. Consequently, longer recording times were needed for trains with low rates or large SDs in the mean interval. The visual criterion translated to SE values ≤10% of the mean interval at the slowest spike rates. Ongoing recordings were monitored for consistency of resting potential, input resistance, and response to a standard current-step protocol. Recording times were as long as 1 h.
To examine the effect of pseudo-EPSC rate on firing (as shown later in Fig. 13), we generated trains of pseudo-EPSCs at uniform, predetermined, intervals (range: 10–300 ms) rather than at pseudorandom intervals.
Fig. 13.
Sustained neurons had longer integration times than did transient neurons. Uniformly spaced trains of pseudo-EPSCs were applied to a sustained neuron (A–C, P4, neuron from Fig. 10; step-evoked firing pattern shown in D) and a transient neuron (E–G, P5; step-evoked firing pattern shown in H). All spikes are truncated. Stimulus current train, shown below each response, delivered pseudo-EPSCs at intervals of 30 ms (A and E), 20 ms (B and F), or 10 ms (C and G). A–C: the sustained neuron integrated pseudo-EPSCs that were individually subthreshold (10 pA), to produce spiking for intervals <30 ms. At 30-ms intervals, the neuron did not fire (A); at 20-ms intervals, it began to fire (B); and at 10-ms intervals, it fired faster (C). E–G: the transient neuron integrated little, spiking only for pseudo-EPSCs that were individually suprathreshold; responses to 80-pA pseudo-EPSCs are shown. At 30-ms intervals (E), the neuron fired for every pseudo-EPSC. At 20-ms intervals (F), the neuron did not fire for every EPSC, but the timing of each spike was tightly coupled to the timing of a pseudo-EPSC. At 10-ms intervals (G) the neuron fired just one spike at the start of the pseudo-EPSC train.
RESULTS
Recordings were made with the perforated-patch method from vestibular ganglion somata isolated from rats (P1–P16, n = 179 neurons) and mice (P0–P8; n = 14). We first describe categories of firing patterns evoked by current steps and propose a correspondence between the step-evoked firing patterns and regularity of interspike intervals as measured in vivo. We describe experiments to test the hypothesized correspondence by measuring regularity in response to synthetic synaptic inputs. We conclude with experiments showing the involvement of two low-voltage-activated conductances in both firing patterns and spike regularity.
Firing patterns in response to current steps
Figure 1 illustrates the kinds of responses evoked by steps applied in current-clamp mode. In many neurons, the hyperpolarizing response to negative steps developed a more prominent peak as the steps grew larger (Fig. 1, A–E). The sag following the peak reflects the activation of hyperpolarization-activated currents carried by HCN channels (Ih), which are known to be expressed by vestibular ganglion somata (Chabbert et al. 2001b).
Fig. 1.
Vestibular ganglion neurons produced 2 basic firing patterns, transient and sustained, in response to depolarizing current steps. For clarity, voltage traces are offset as current steps are iterated; current step levels are shown at right. Dashed line: −65 mV for the 0-pA current step. Scale bars (top left) apply to all traces. In this and all subsequent figures, recordings were made with the perforated-patch whole cell method. Data in A–E were from rat neurons, postnatal day 13 (P13) to P16. A and B: transient neurons fired one to 2 spikes at the onset of 200-ms depolarizing current steps above a threshold value. C–E: sustained neurons were either spikers (C and D) or resonators (E). Sustained-A spikers (C) fired long trains at spike threshold. In sustained-B spikers (D), spikes built up with increasing current amplitude. Sustained resonators (E) fired one or 2 spikes followed by prominent voltage oscillations. For hyperpolarizing steps, sustained spikers showed the hyperpolarization-activated cationic current (Ih)–mediated voltage sag also seen in transient neurons, but were more likely to spike at the step offset. F: current thresholds for spiking were much lower in sustained neurons in both the first postnatal week (P1–P7, left) and at later ages (P8–P16). In this and all figures: highly significant difference, ***p < 0.001; a very significant difference, **p < 0.01; and a significant difference, *p < 0.05.
Depolarizing current steps, in contrast, differentiated the neurons by evoking two basic categories of firing pattern: transient (100/179 neurons; Fig. 1, A and B) or sustained (79/179; Fig. 1, C–E). The firing patterns of transient neurons were relatively uniform: 89 of 100 spiked only once in response to a 200-ms current step, whether at spike threshold or much larger (Fig. 1A). Some of the older transient neurons (11 of 55 neurons between P8 and P16) fired a second spike for large current steps (Fig. 1B). The firing patterns of sustained neurons (Fig. 1, C– E) were more variable. To illustrate the variability, we grouped sustained responses into three classes, but recognize that these may form a continuum. Sustained-A spiking neurons (20/79; Fig. 1C) had low current thresholds for spiking (Fig. 1F) and even at threshold fired continuously through the 200-ms step. Spike rates at threshold ranged from 10 to 50 spikes/s and increased with step size. For sustained-B spiking neurons (34/79, Fig. 1D), the duration of the spike train depended more on current intensity: the number of spikes excited by 200-ms steps of different size ranged from 1 to 10 (rates of 5–50 spikes/s) within a single neuron. A third class of sustained neurons (sustained resonators) (25/79; Fig. 1E) fired one to two small spikes followed by prominent voltage ringing. We considered these a subcategory of sustained neurons on the basis of their low current thresholds for ringing, their relatively positive resting potentials (see following text), and the tendency of sustained spikers to also ring at high current-step amplitudes (Fig. 1D, steps >100 pA).
We hypothesize that the same biophysical properties responsible for the transient and sustained firing patterns in vitro produce irregular and regular firing, respectively, in response to synaptic inputs in vivo. Before testing this hypothesis, in the following text we show that step-evoked firing patterns were robust across experimental conditions and that they correlated with other electrophysiological properties.
Firing patterns are robust and stable
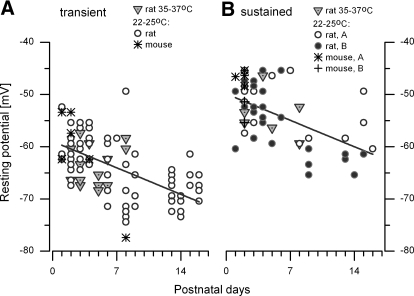
Firing pattern categories were similar at different ages (P0–P16; compare Fig. 2, A and B), at room and body temperatures (compare Fig. 2, A and C), and in rat and mouse (compare Fig. 2, A and D). Moreover, during prolonged recordings from individual neurons, firing category remained either transient or sustained as long as healthy resting potentials and input resistances were maintained. There was some movement between sustained subcategories, most frequently between sustained-A and sustained-B categories (Fig. 1, C and D). Two sustained neurons (of 79) moved between the sustained-B spiking and sustained resonant categories (not shown).
Fig. 2.
Firing pattern categories were robust and stable with changes in age, temperature, and species. Vestibular ganglion somata were from rat (A–C) and mouse (D) at ages and temperatures indicated. Responses are shown for the smallest current step to produce a spike (resolution: 10 pA), except for the mouse sustained resonator (D, right), which never spiked. Dashed line: −55 mV. Scale bars (top left) apply to all traces.
The small spike size of sustained resonators raises the question of whether they are immature. Consistent with this possibility, sustained resonators were more commonly encountered in the first postnatal week (21 of 52 sustained neurons) than in the second postnatal week (4 of 27). Because we were interested in spike timing, we focus the remainder of this study on the spiking categories (transient, sustained-A, and sustained-B).
Firing patterns correlate with other electrophysiological metrics
DIFFERENCES IN RESTING POTENTIAL DO NOT CAUSE DIFFERENCES IN FIRING PATTERN.
At all ages, resting potential was significantly more negative for transient neurons than for sustained neurons (Fig. 3). Figure 3 also shows that resting potential became more negative with age, by about 0.7 mV/day, for both transient spikers and sustained spikers. Sustained resonators (not shown) had slightly more positive resting potentials than did sustained spikers; mean values for rat data at room temperature were −48 ± 0.8 mV for 21 resonators and −51.5 ± 0.8 mV for 31 sustained spikers (A and B categories pooled; p < 0.05). In the second postnatal week, the resting potentials of sustained-B neurons became significantly more negative than those of sustained-A neurons: −62.5 ± 0.72 mV (eight neurons) versus −56.9 ± 1.65 mV (six neurons; p = 0.004), but were still more positive than transient values (−68.0 ± 0.84 mV, n = 33, P > 7; p = 0.003).
Fig. 3.
Resting potential differed between firing pattern categories and became more negative with age. A: transient spikers. B: sustained spikers, divided into sustained-A (open circles) and sustained-B (filled circles). Most data (circles) are from rat at room temperature; some are from rat at warmer temperatures (inverted triangles), or mouse at room temperature (stars and crosses). After P7, sustained-A and sustained-B spikers diverged, such that sustained-B values were more negative than sustained-A values. Lines in A and B: linear regression fits (y = mx + b) to rat data at 22–25°C. Transient neurons (68): m = 0.7 ± 0.13 mV/d, b = −58.9 ± 0.96 mV, r2 = 0.32. Sustained neurons (45); m = 0.7 ± 0.17 mV/day, b = −49.9 ± 1.20 mV, r2 = 0.28.
The differences in resting potential and firing patterns of sustained and transient neuronal somata suggest that they express different complements of voltage-gated ion channels. An alternative possibility is that the resting potential difference causes the difference in firing pattern by affecting the state of a shared set of voltage-dependent channels. To test this possibility, we looked at the effect of steady depolarization on transient neurons (Fig. 4, A and C) and of steady hyperpolarization on sustained neurons (Fig. 4, B and C). In both cases, firing pattern was unaffected by the steady change in background potential. Thus sustained and transient neurons generate different firing patterns because they have different complements of voltage-dependent channels.
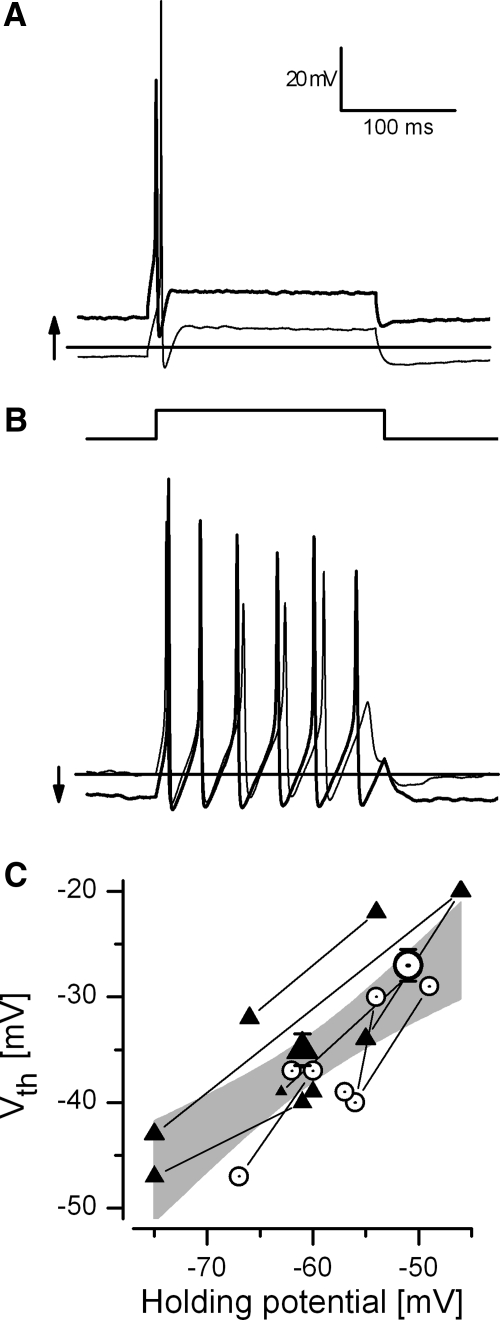
Fig. 4.
Steady polarization did not change either the firing pattern or the voltage change required to evoke a spike. A: transient neuron (P7) driven by a 100-pA current step before (thin line) and during (thicker line) a steady approximately 12-mV depolarization. Horizontal line in A and B: −60 mV. B: sustained neuron (P8) driven by a 40-pA current step before (thin line) and during (thicker line) a steady approximately 10-mV hyperpolarization. C: dependence of the spike voltage threshold (Vth) on holding potential. Thin lines connect Vth values for single neurons held at different potentials. Triangles: transient neurons (n = 5); circles: sustained neurons (n = 4). Shaded area: 95% confidence interval (CI) for a linear regression of all data points (m = 0.7 ± 0.1 mV/mV, b = 7.5 ± 8.5 mV, r2 = 0.6). Large symbols: mean Vth ± SE (mean resting potential) for 10 transient neurons (triangle) and 10 sustained neurons (open circle); P < 8 for all neurons.
Although steady polarization did not alter the firing pattern, it did affect such spike features as action potential height, rate of action potential depolarization, and voltage threshold for spiking. At more negative background potentials (either resting or holding potentials), action potentials were larger and voltage changes (dV/dt) were faster for both transient and sustained neurons. Spike height (ΔVAP in Fig. 6A) ranged from 70 to 95 mV and did not differ significantly between transient spikers and sustained spikers at any age. Spikes of transient neurons got significantly larger by the second week, tracking the increasingly negative resting potential; spike height went from 74 ± 4.4 mV (26 neurons, P < 8) to 93 ± 4.1 mV (16 neurons, P ≥ 8) (p < 0.05).
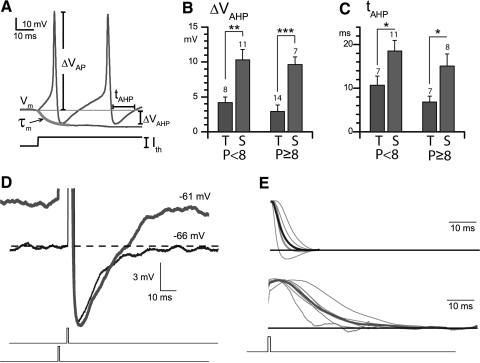
Fig. 6.
Differences between transient and sustained neurons in afterhyperpolarizations (AHPs) and subthreshold activity. A: how spike and AHP metrics were calculated. AHPs of sustained neurons were (B) larger and (C) longer than those of transient neurons at all ages tested. D: AHPs of a transient neuron (thin black line) and a sustained neuron (thicker gray line), each excited by a 1-ms current pulse of amplitude just above threshold. We aligned the AHPs on the trough to illustrate the similar recovery kinetics [alignment caused a leftward shift of the stimulus pulse (below) for the sustained neuron because of its broader spike]. E: subthreshold voltage responses of sustained neurons (n = 7, top) to 1-ms pulses were longer than those of transient neurons (n = 6, bottom). Traces were normalized to peak amplitude and averaged (thick traces).
Having a more positive resting potential, either as a sustained neuron or an artificially depolarized transient neuron, did not bring a neuron closer to voltage threshold (Vth, voltage at which spiking initiates). Instead, steady depolarization also made Vth more positive and steady hyperpolarization made Vth more negative. Figure 4C shows how Vth moved in tandem with the holding potential (by ∼0.7 mV/mV) such that depolarization by about 20 mV was required to evoke a spike for all neurons. Thus the difference in mean resting potential of transient and sustained spikers can account for the difference in average Vth between sustained neurons (−27 ± 1.5 mV, n = 10) and transient neurons (−35 ± 1.5 mV, n = 10, p < 0.001) (Fig. 4C, large symbols). Steady depolarization may fail to bring the cell closer to Vth because it inactivates more Na channels. Relief from Na channel inactivation by steady hyperpolarization may cause the more negative voltage thresholds, faster rates of depolarization, and larger spikes.
MEMBRANE TIME CONSTANT (τM).
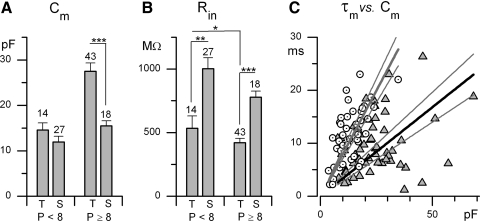
The rate of voltage change during a current injection is determined by τm: the product of input resistance (Rin) and membrane capacitance (Cm). Estimates of Cm, which is proportional to membrane surface area, indicate that in the first week, somata of sustained and transient neurons were of similar average size, but by the second week the somata of transient neurons were significantly larger (Fig. 5A). This observation supports the hypothesis that transient neurons correspond to irregular neurons because mature irregular neurons have, on average, larger somata than do regular neurons (Kevetter and Leonard 2002). We expect that cell size would further differentiate at older ages.
Fig. 5.
Passive membrane properties of transient and sustained neurons differed. A and B: membrane capacitance (Cm) and input resistance (Rin) for P1–P7 and P8–P16. Rin was significantly smaller for transient neurons over both age ranges. Cm of transient neurons increased from the first to the second week to be much larger than Cm of sustained neurons. C: membrane time constant (τm) as a function of Cm, for 45 sustained (circles) and 57 transient (triangles) neurons. Mean τm values are not significantly different but the linear regression slopes are: 340 ± 28 μs/pF (transient) vs. 800 ± 44 μs/pF (sustained). Thin lines, 95% CIs.
By itself, the increasing size of transient neurons would increase τm and slow their voltage responses; however, the increase in Cm was offset by a decrease in Rin (Fig. 5B). At all ages, Rin was significantly lower for transient neurons than for sustained spikers (Fig. 5B; p < 0.001 for the second week), contributing to their larger current thresholds (Fig. 1F) and compensating for the difference in average Cm such that mean τm values were similar: 9.4 ± 0.71 ms for 57 transient neurons and 11.4 ± 0.76 ms for 45 sustained neurons. At a given size (Cm), however, transient neurons had lower τm values (Fig. 5C, linear regressions). Both the more negative resting potentials and relatively low Rin values of transient neurons are consistent with their having additional K channels open at rest, as described in the following text. For transient neurons, Rin decreased from the first week to the second week (p < 0.05).
SPIKES AND AFTERHYPERPOLARIZATIONS.
Because spike AHPs differ between regular and irregular neurons in vivo (see introduction), we measured AHPs following the first spike evoked by a current step (just suprathreshold) (Fig. 6). AHP depth (ΔVAHP) is the difference between the resting potential and the most negative potential following a spike; AHP duration (tAHP) is the time spent below resting potential (Fig. 6A) (Bean 2007). ΔVAHP and tAHP were both much larger for sustained neurons than for transient neurons, consistent with observations for regular and irregular afferents, respectively.
These differences in AHP metrics stemmed from population differences in resting potential (which sets the endpoints of the AHP) rather than differences in the most negative (trough) potential or the time course of the repolarization. Trough potentials were similar: −70.5 ± 0.88 mV, six transient neurons versus −68.9 ± 0.87 mV, six sustained neurons. However, resting potentials differed (Fig. 3) and Fig. 6D shows how this difference can affect the appearance of AHPs. To avoid any influence of simultaneous current injection on the AHP, we triggered action potentials with pulses so brief that the action potential occurred after the pulse. As measured by tAHP and ΔVAHP, the sustained neuron's AHP was both larger and slower than the transient neuron's AHP. But there was no difference in the slope of AHP recovery, as illustrated in Fig. 6D by aligning the exemplar traces at the trough. This similarity held up when slopes were measured in six neurons of each type: 0.5 ± 0.16 mV/ms (transient) versus 0.5 ± 0.03 mV/ms (sustained). Thus it seems that the AHPs of sustained neurons took longer to recover (tAHP) and had further to go (ΔVAHP) simply because they had more positive resting potentials.
The spikes evoked by the brief pulses also differed between the two populations. Transient neurons had faster spikes with shorter onset times (time from pulse to spike peak: 4.1 ± 0.85 vs. 10.4 ± 0.35 ms, n = 6, p < 0.001) and half-widths (0.8 ± 0.09 vs. 1.6 ± 0.29 ms, p < 0.01). Subthreshold responses were also faster for transient neurons, as shown in Fig. 6E.
To summarize, vestibular ganglion somata exhibited robustly different firing patterns in response to current steps and these differences correlated with resting potential, current threshold and input resistance and the time course of spikes, AHPs, and subthreshold responses. The regular nature of the sustained firing pattern and the size and duration of the associated AHPs suggest correspondence with the in vivo regular classification. The difference in resting potentials of transient and sustained neurons did not cause the difference in firing pattern, but may be the dominant factor in AHP metrics.
Transient neurons express low-voltage-activated outward current
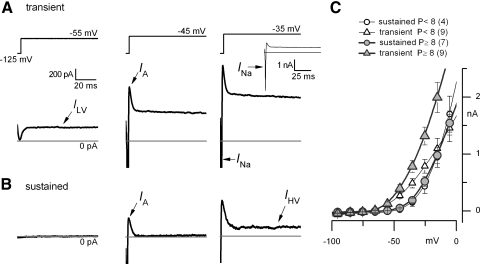
The more negative resting potentials (Fig. 3), larger current thresholds (Fig. 1F), lower input resistances (Fig. 5B), and faster subthreshold voltage changes (Fig. 6) of transient neurons might all reflect a larger resting K+ conductance. This was confirmed in voltage-clamp experiments: small depolarizing voltage steps evoked a low-voltage-activated outward current (ILV) in transient neurons, but not sustained neurons, and this current could be blocked by K-channel blockers. ILV is illustrated by the exemplar transient and sustained neurons of Fig. 7, A and B. A step to −55 mV activated a steady outward current, ILV, in the transient neuron but not in the sustained neuron. In both neurons, depolarization to −45 mV evoked a transient Na+ current, INa, and a rapidly inactivating outward current (A current, IA). In the sustained neuron, IA was the only outward current at −45 mV and it inactivated completely; in contrast, the transient neuron responded with both IA and the noninactivating ILV. Stepping to more positive voltages (−35 mV) activated a steady high-voltage-activated outward current, IHV, in both neurons. (Evidence that the current in transient neurons includes IHV was obtained by blocking ILV, as shown in the following text.)
Fig. 7.
Low-voltage-activated outward currents were larger in transient neurons than those in sustained neurons and grew with age. A and B: whole cell currents in a transient neuron (A, P13) and a sustained neuron (B, P13) in response to 3 voltage steps (top). See text. C: current–voltage (I–V) relationships taken 100 ms after the onset of the voltage step for transient (triangles) and sustained (circles) neurons, for the first (open symbols) and second (filled symbols) postnatal weeks. The outward current grew for transient neurons but not for sustained neurons (open circles are obscured by filled circles because of their similar values). By the second week, the I–V relation for transient neurons (filled triangles) was offset by close to −20 mV relative to that for sustained neurons (filled circles).
Figure 7C compares average current–voltage (I–V) relationships, taken at about 100 ms after step onset, for transient and sustained neurons in the first and second postnatal weeks. Steady current in sustained neurons did not change during this period. In both time windows, transient neurons had significantly more outward current at potentials between −55 and −25 mV; the difference increased during the second postnatal week, as ILV in transient neurons got larger. This developmental acquisition of ILV occurs in parallel with the acquisition of low-voltage-activated K channels by rat type I hair cells (Hurley et al. 2006), which in vivo provide input to irregular vestibular neurons. The developmental increase in ILV may contribute to the change in the resting potentials of transient neurons over the same period (Fig. 3).
WHAT IS ILV?
The association of ILV with a transient (phasic) firing pattern was previously noted by Iwasaki et al. (2008) who also studied rat vestibular ganglion somata. For neurons from animals between P5 and P7, Iwasaki et al. (2008) showed that the Kv1-channel blocker, α-DTX at 100 nM, blocked ILV and converted the transient firing pattern to a sustained (tonic) pattern. In our sample of somewhat older neurons (P8–P15), such strong sensitivity to α-DTX was not typical. In nine transient neurons studied in voltage clamp, block (by 100 nM α-DTX, measured 100 ms into a step to −45 mV from −120 mV) was almost complete (>90%) in only two and substantially less complete in the remaining seven: 55 ± 7% (range: 24–77%; Fig. 8, B–E). A standard dose for testing α-DTX sensitivity is 100 nM (Hall et al. 1994) and thus the unsuppressed current is insensitive to α-DTX (Fig. 8). In fact, in two neurons, doubling the dose did not further change the suppressed current or the firing pattern (data not shown).
Fig. 8.
Low-voltage-activated potassium current (ILV) comprised α-DTX-sensitive and linopirdine-sensitive components. A–D: whole cell currents recorded in perforated-patch mode (27°C) from a transient neuron (P10, rat) in response to a family of voltage steps. A: family of currents evoked by a standard voltage protocol (bottom): prepulse to −125 mV followed by steps between −105 and −15 mV, control (Liebovitz-15 [L-15]) external solution. The cell had INa, IA, IHV, and ILV (as in Fig. 7A). B: currents at the onset of a depolarizing step in: L-15 (black), L-15 containing 100 nM a-dendrotoxin (α-DTX, red), and L-15 containing 100 nM α-DTX + 10 μM linopirdine (dark gray). C: I–V relations taken 100 ms after the onset of voltage steps, from the cell in B. Low-voltage (LV) currents (at potentials negative to −30 mV) were partly suppressed in α-DTX (red circles) and strongly suppressed in α-DTX + linopirdine (triangles). D and E: α-DTX-sensitive and linopirdine-sensitive components of ILV activated over different timescales. D: α-DTX blocked a fast component (bottom panel; obtained by subtraction from curves above). The slow remaining component was blocked by linopirdine (E). Activation was fit with single-exponential functions (red curves) with τ values of 4.3 ms for the α-DTX-sensitive component (D, bottom), 96 ms for the α-DTX-insensitive component (E, top), and 72 ms for the linopirdine-sensitive component (E, bottom). Currents in D and E are from 2 cells, different from the cell in A–C. F: conductance–voltage (g–V) relations for the α-DTX-sensitive current (circles) and linopirdine-sensitive current (triangles). Curves: Boltzmann fits (Eq. 1; methods). G: Boltzmann fits to g–V curves, normalized by maximum conductance for each curve (thin red lines, α-DTX-sensitive conductance; thin black lines, linopirdine-sensitive conductance). Thick red and thick black lines: average g–V curves for α-DTX-sensitive current (half-maximum activation voltage [V1/2] = −44 ± 0.2 mV, slope factor [S] = 7.1 ± 0.20 mV, n = 7) and linopirdine-sensitive current (V1/2 = −41 ± 0.3 mV, S = 7.4 ± 0.41 mV, n = 5), respectively. H: activation time constants were much faster for α-DTX-sensitive currents (red circles, n = 8 neurons, some at multiple voltages) than those for α-DTX-insensitive currents (gray triangles, n = 6) and linopirdine-sensitive currents (open down triangles, n = 3). All data in this figure were taken at room temperature, P9–P15.
What channels might carry the DTX-insensitive component of ILV? KCNQ (Kv7) channels were suggested by electrophysiological recordings from calyx terminals (Hurley et al. 2006; Rennie et al. 2001) and isolated ganglion neurons (Pérez et al. 2009) and by immunolabeling in calyx terminals (Hurley et al. 2006; Kharkovets et al. 2000). Linopirdine, applied alone at a dose that is selective for KCNQ channels (10 μM; Schnee and Brown 1998), blocked ILV by 32 ± 9.6% (n = 5; range: 8–60%) and had a small effect on firing (see next section). The combination of linopirdine and α-DTX, however, was very effective, suppressing ILV by 88 ± 4% (range: 72–100%, n = 6; Fig. 8, B, C, and E). These pharmacological results indicate that Kv1 channels and KCNQ channels carried, respectively, about one half and one third of the outward current measured 100 ms into a step to −45 mV from −120 mV. Based on its voltage dependence, the remaining current could correspond to IHV in sustained neurons (Fig. 7).
Addition of new ILV channels from the first to second postnatal weeks is indicated by the data in Fig. 7C. If these channels were predominantly KCNQ channels, the age difference between our pharmacological study (P9–P15) and that of Iwasaki et al. (2008) (P5–P7) would explain the difference in the effectiveness of α-DTX at blocking ILV. Other methodological differences may also have contributed to the differences between the two studies (see discussion).
The two components of ILV differed slightly in voltage dependence (Fig. 8, F and G) and, more conspicuously, in activation time course (Fig. 8, D, E, and H). Because tail currents were contaminated with Na+ current, we took the outward current at 100 ms and divided by the driving force for each voltage to produce g–V curves. The α-DTX-sensitive (Kv1) component had a mean V1/2 value for activation of −44 ± 0.2 mV (n = 7), similar to that determined by Iwasaki et al. (2008) and a mean maximum conductance of 6.3 ± 1.55 nS. The linopirdine-sensitive (KCNQ) conductance in the same cells had a mean V1/2 value of −41 ± 0.3 mV (n = 5), 5 mV positive to the value determined for rat vestibular ganglion somata by Pérez et al. (2009), and a mean maximum conductance of 4.5 ± 1.20 nS. Activation time course was fit with single-exponential functions, as illustrated in Fig. 8, D and E. To avoid potential contamination by capacitance artifact and the onset of Na+ currents, we began the fits 8–10 ms after the onset of the voltage step. Fits were accepted only if 0.9 ≤ R2 ≤ 1 (∼80% of all fits). Experiments with both α-DTX and linopirdine indicated that most α-DTX-insensitive current was linopirdine-sensitive; this was supported by the similar activation time courses of the α-DTX-insensitive currents (obtained by subtracting currents in α-DTX from control currents; Fig. 8D) and of the linopirdine-sensitive currents (obtained by subtracting currents in α-DTX + linopirdine from currents in α-DTX alone; Fig. 8E). Mean activation time constants were 57 ± 5.7 ms (10 measurements from six neurons) for the α-DTX-insensitive current and 54 ± 8.9 ms (10 measurements from three neurons) for the linopirdine-sensitive component, over 10-fold slower than the time constants of the α-DTX-sensitive current: 3.7 ± 0.75 ms (12 measurements from eight neurons; Fig. 8, D, E, and H). The slower and faster time courses are consistent with the literature for KCNQ and Kv1 conductances, respectively (Lerche et al. 2000; Rothman and Manis 2003b; Wladyka and Kunze 2006). For example, cochlear nucleus neurons express a low-voltage-activated conductance carried by Kv1 channels (Oertel et al. 2008) and with activation time constants on the order of milliseconds (Rothman and Manis 2003b).
HOW DOES ILV SHAPE FIRING PATTERNS?
Iwasaki et al. (2008) implicated Kv1 channels in the transient firing pattern by showing that α-DTX converted firing from transient to sustained. Consistent with our voltage-clamp data, we found that the combination of α-DTX and linopirdine was usually needed for complete conversion. Figure 9 shows data from two transient neurons (Fig. 9, A and B) and one sustained-B neuron (Fig. 9C) in control, single-blocker, and combined-blocker conditions. For simplicity, the firing patterns are shown for a single current step for each neuron, but full level series (as shown in Fig. 1) were collected.
Fig. 9.
Both Kv1 and KCNQ channels contributed to the transient firing pattern. A: spiking response of a P11 transient neuron to +160-pA current steps, in control solution (left), solution containing 100 nM α-DTX (middle), and solution containing 100 nM α-DTX + 10 μM linopirdine (right). α-DTX and linopirdine converted the transient response at spike threshold to a sustained-A response. For larger steps, α-DTX by itself increased spike number (see insets in control, α-DTX panels). B: as in A, but for a P15 transient neuron and with 10 μM linopirdine as the solo drug treatment (middle). Linopirdine had no effect at spike threshold, but increased spike number for much larger current steps (inset). Linopirdine blocked ILV at −45 mV by 40% in this neuron (not shown). C: sustained-B neurons also had ILV. At spike threshold in control conditions (+80 pA), the sustained-B firing pattern of a P10 neuron was modestly affected by α-DTX but was converted to the sustained-A pattern by α-DTX + linopirdine. This neuron fired spontaneously in the dual-blocker treatment (right, inset). D–F: LV channel blockers also made transient neurons more like sustained neurons in terms of resting potential (D), current threshold for spiking (E), and input resistance (F).
In 15 of 18 transient neurons, α-DTX added no more than several spikes at the onset of the step (Fig. 9, A and C). Linopirdine alone (10 μM, n = 5) also did not effectively convert firing patterns (Fig. 9B). Coapplication of α-DTX and linopirdine, in contrast, consistently converted firing patterns from transient to sustained (9 of 11 transient neurons; Fig. 9, A and B); the remaining two neurons depolarized and converted to the sustained-C (resonant) pattern.
Coapplication of α-DTX and linopirdine to sustained-B neurons converted the firing pattern to the sustained-A pattern (Fig. 9C; n = 3), indicating that sustained-B neurons, like transient neurons, have two components of ILV. Spike accommodation (decrease in spike height during a train), typical of sustained neurons at high current amplitudes (see Fig. 1), was not strongly affected by the blockers. The blocker combination caused two of the sustained-B neurons to fire repetitively without current steps (Fig. 9C, inset), suggesting that they have a persistent inward current that is normally masked by ILV.
Coapplication of α-DTX and linopirdine also made the transient neurons more like sustained neurons in terms of resting potential (Fig. 9D), current threshold for spiking (Fig. 9E), and input resistance (Fig. 9F). The dual-blocker solution had highly significant effects on all three parameters. For resting potential and current threshold, coapplication of the blockers was much more effective than either blocker alone; however, Ith was significantly reduced by α-DTX alone and by adding linopirdine to α-DTX and Rin was significantly increased by linopirdine, whether added to the control solution or to solution containing α-DTX. Comparison of the third and fourth columns in Fig. 9, D–F shows that KCNQ channels contributed significantly to transient neurons' more negative resting potentials, larger current thresholds, and lower input resistances.
In summary, by the second postnatal week, both Kv1 and KCNQ channels contributed to ILV and shaped firing patterns in vestibular ganglion neurons.
Pseudosynaptic currents evoke spike trains of different regularity in transient and sustained neurons
To test the hypothesis that sustained neurons are regular afferents and transient neurons are irregular afferents, we used randomly timed pseudo-EPSCs (see methods) to drive spiking in 22 isolated somata (10 transient, 12 sustained). Figure 10 shows exemplar spike trains in two neurons driven by the same temporal pattern of pseudo-EPSCs. As predicted, the timing between spikes was much more regular in the sustained neuron than that in the transient neuron (Fig. 10A).
Fig. 10.
Pseudorandom trains of excitatory postsynaptic current (EPSC)–like currents evoked firing that was more regular in sustained neurons than in transient neurons. A: examples from a sustained-A, P4 neuron (top) and a transient P2 neuron (bottom), selected to have the same mean spike rate (∼13 spikes/s). Pseudo-EPSCs were delivered at the same mean rate (250/s) to each neuron but at different unitary amplitudes (40 pA for the sustained neuron vs. 200 pA for the transient neuron), as needed to drive them at similar rates. B and C: spike trains and pseudo-excitatory postsynaptic potentials (EPSPs) from the sustained and transient neurons are expanded in C for the interval in A marked by a black bar and are compared in B to intracellular, in vivo records from 2 lizard canal afferents, one regular and one irregular [adapted from Figs. 11 and 17 in Schessel et al. (1991), with permission from Elsevier and the authors]. Note similarities between the AHPs and EPSPs of the transient neuron (C, bottom) and irregular afferent (B, bottom) on the one hand and the sustained neuron (C, top) and regular afferent (B, top) on the other hand.
Figure 10, B and C shows other ways in which the pseudo-EPSC-driven spiking of sustained and transient neurons resembled regular and irregular spiking, respectively, as represented by in vivo recordings from lizard canal afferents (Schessel et al. 1991). In both the regular afferent and sustained neuron, poorly resolved EPSPs are barely visible during the depolarizing ramp between spikes. (As will be shown later in Fig. 13, summation of EPSPs helps to create the depolarizing ramp and accentuates the apparent prominence of the AHPs.) In contrast, both the irregular afferent and the transient neuron had fast, clearly resolved EPSPs that, in the transient neuron, closely followed the timing of individual pseudo-EPSCs (bottom trace). The AHPs of both the transient and irregular afferents were shallow and brief compared with those of both sustained and regular afferents.
To evoke spiking in transient neurons, we had to use larger pseudo-EPSCs because of the larger current threshold. For example, for a mean rate of about 15 spikes/s (Fig. 10), unitary pseudo-EPSCs had to be fivefold larger for the transient neuron than the sustained neuron, consistent with the fivefold difference in average threshold for exciting spikes with current steps (Fig. 1F).
Because CV varies inversely with spike rate (Goldberg et al. 1990a), we compared CV values for transient and sustained neurons over a range of spike rates. To increase spike rate in a given neuron, we increased unitary pseudo-EPSC amplitude (Fig. 11, A and B); a more realistic stimulus would increase the rate by increasing unitary EPSC rate, but we needed to maintain timing patterns to allow comparison of regularity across neuronal types. Figure 11C plots CV against mean interspike interval for transient neurons (blue), sustained-A neurons (red), and sustained-B neurons (green). The range of intervals achieved by varying pseudo-EPSC amplitude was not identical for the three classes. Transient neurons could not be driven as fast as sustained neurons because the large pseudo-EPSCs that would be required caused distortion of spike and EPSP waveforms. Sustained neurons could not be driven as slowly as transient neurons because at low rates, pseudo-EPSCs did not summate sufficiently to reach spike threshold (see Fig. 13). Nevertheless, there was sufficient overlap in mean intervals to show that at similar intervals (rates), CV values were smallest for sustained-A neurons, intermediate for sustained-B neurons, and largest for transient neurons.
Fig. 11.
Spike regularity differences between transient and sustained neurons were robust over a range of mean spike intervals. A: spiking was driven at different mean intervals by varying the size of the unitary EPSC-like event from 30 to 220 pA. B: interspike interval histograms constructed from pseudo-EPSC-evoked spike trains 3.5 s in duration. Mean spike interval decreased as unitary EPSC amplitude increased. C: coefficient of variation (CV) as a function of mean interval in 22 neurons. Each symbol corresponds to one neuron, driven at multiple rates: 7 sustained-A neurons (red); 5 sustained-B neurons (green); 10 transient neurons (blue). Sustained neurons fired with shorter intervals and had lower CV values, with sustained-A neurons being the most regular. Lines: locally linear regression fits (loess algorithm, Matlab). Even at comparable rates, sustained neurons fired more regularly. D–F: step-evoked firing patterns of exemplar neurons from the set in C; the current step and the cell's symbol (C) are given at the far right.
In vestibular afferent studies, it is common to normalize CV to a value (CV*) based on empirical relationships between CV and mean rate (Goldberg et al. 1984). Those relationships are not available for the rat vestibular afferents, but if we assume that the relationships for chinchilla utricle (Goldberg et al. 1990a) and mouse canal (Lasker et al. 2008) apply to our data, then the CV values of sustained neurons and transient neurons correspond to CV* values that are in the middle of the regular and irregular CV* distributions. Consider the CV values of the exemplar neurons in Fig. 10. A CV of 0.25 at a 70-ms average interval corresponds to CV* ≅ 0.05, near the mode for regular afferents in vivo. A CV of 0.7 corresponds to CV* = 0.3, right at the mode for irregular afferents in vivo.
Generally, we could not drive spiking to the mean background rates of 40–70 spikes/s recorded in vivo in mouse canal afferents (Lasker et al. 2008; Yang and Hullar 2007) and chinchilla utricular afferents (Goldberg et al. 1990b). Possible factors include the age and temperature of our preparations. Canal afferents from early postnatal rats fire at relatively low rates (Curthoys 1979). Increasing temperature from room temperature to body temperature may more than double the maximum firing rate (see examples in Fig. 2 of step-evoked firing at body temperature and room temperature).
Blocking ILV increases regularity of responses to pseudo-EPSCs
The experiments with pseudo-EPSCs showed that step-evoked firing pattern is strongly correlated with firing regularity. We next investigated whether blocking ILV, which converted firing pattern from transient to sustained, also made pseudo-EPSC-evoked firing more regular (Fig. 12). Figure 12A shows a transient neuron driven to about 15 spikes/s in control solution and then in the blocking solution of 100 nM α-DTX plus 10 μM linopirdine. In control solution, firing was highly irregular (CV 0.8) and the unitary pseudo-EPSC amplitude needed to achieve 15 spikes/s was 340 pA. In the blocking solution, CV fell to 0.4 and the unitary pseudo-EPSC amplitude needed to achieve a similar firing rate was halved, consistent with the lower current threshold of sustained neurons (Fig. 1F). Figure 12 shows the effects of the combined blocking solution on CV in this neuron (Fig. 12B) and two other transient neurons (Fig. 12, C and D), each at multiple spiking intervals (total range: 20–200 ms). All were initially transient, with CV values and firing rates within the normal range of those established for the total transient population (thick curve). In the blocking solution, all became more regular, although not as regular as the mean for the sustained population (thin curve). These results suggest that much of the irregularity of transient neuronal somata depends on Kv1 and KCNQ subunits, but possibly not all.
Fig. 12.
Blocking ILV made the spike trains of transient neurons more regular. A, top: an irregular spike train (CV 0.8, mean interval 73 ms) from a P11 transient neuron, driven by pseudo-EPSCs with a unitary amplitude of 340 pA. Bottom: coapplication of α-DTX and linopirdine made spikes faster and more regular. (Spike rate increased in the blocker solution, so we reduced unitary amplitude to 170 pA, for a mean interval of 53 ms.) B–D: CV values as functions of mean interval are shown for 4 transient neurons before (black symbols) and after (gray symbols) ILV block: lines, average CV-interval curves (Fig. 11) for transient (thick black line) and sustained (thin gray line) neurons. Perforated-patch recordings. Data in B are from the neuron in A. E: patch rupture made a neuron more regular. Transient neuron, recorded in perforated-patch mode in control solution (black symbols; CV 0.6–0.8) and 100 μM α-DTX (black and gray symbols), which reduced CV to 0.3–0.5. The patch then ruptured and the neuron became extremely regular (gray symbols; CV 0.1–0.2).
Figure 12E shows results for a fourth transient neuron that was treated with 100 nM α-DTX alone. In perforated-patch mode, α-DTX decreased CV from 0.6–0.8 to 0.3–0.5. The patch then ruptured, after which ILV decreased further and the neuron eventually became significantly more regular, with CV as low as 0.15. Current threshold also continued to decrease, until the neuron eventually began to spike spontaneously. These results suggest that rupturing the patch may wash out part of ILV and enhance conversion to sustained, regular firing. This might help explain why α-DTX was more effective by itself in the ruptured-patch experiments of Iwasaki et al. (2008) than that in our perforated-patch recordings. The spontaneous spiking is also of interest because it indicates the existence of persistent inward currents that are normally countered by ILV (also see the example in Fig. 9C).
Sustained neurons integrate inputs more than transient neurons
One way in which ILV influences step-evoked firing patterns and irregular spike intervals is via its effect on τm and thus on integration time. To examine differences in integration by sustained and transient neurons, we applied uniformly spaced trains of pseudo-EPSCs at intervals from 10 to 300 ms and unitary amplitudes from 10 to 220 pA; amplitudes were customized for each neuron to cover the range from subthreshold to suprathreshold. Figure 13 shows results from two neurons that are representative of the 16 transient and 10 sustained neurons studied in this way.
When driven by a train of small pseudo-EPSCs at long intervals (≥30 ms; Fig. 13A), sustained neurons did not fire. Although the pseudo-EPSCs in Fig. 13, A–C were individually subthreshold, as input interval fell to <30 ms, the neuron integrated the pseudo-EPSCs to reach spike threshold (Fig. 13B). The ramp following each spike is a sum of the postspike repolarization to reach resting potential (Fig. 6D) plus the voltage change in response to fast-arriving pseudo-EPSCs. As input intervals decreased further (Fig. 13C), spike rate increased. The familiar sustained firing pattern produced by a steady depolarizing current represents the end of this continuum (Fig. 13D). All 10 sustained neurons spiked in response to 100-Hz pseudo-EPSC trains whose individual pseudo-EPSCs were subthreshold (not shown).
In contrast, transient neurons showed little temporal summation; only 2 of 16 transient neurons spiked in response to similar subthreshold EPSC trains. Usually, the neurons failed to show summation to threshold at all intervals tested (10–300 ms; not shown). Figure 13, E–G illustrates the effects of varying the rate of individually suprathreshold pseudo-EPSCs. The neuron fired one-to-one at the longest intervals (≥30 ms; Fig. 13E). As the interval decreased (Fig. 13, F and G), transient neurons became less likely to fire for each pseudo-EPSC. The reasons for this change were not investigated, but are likely to include refractoriness of Na channels induced by the average depolarization at these higher rates. This experiment shows that neuronal properties can produce irregular firing even when the input is highly regular. Further reduction of the pseudo-EPSC interval to 10 ms (Fig. 13G) or delivery of a constant step (Fig. 13H) produced only a single spike at onset.
These results illustrate the ability of sustained neurons to integrate inputs over long periods and the preference of transient neurons for transient input.
DISCUSSION
Step-evoked firing patterns of isolated vestibular somata range from sustained to transient (Eatock et al. 2008; Iwasaki et al. 2008; present study). Here we show that the ion channels responsible for the difference in firing pattern include Kv1 channels, as previously reported by Iwasaki et al. (2008), as well as KCNQ channels, as foreshadowed by immunocytochemical and electrophysiological data on afferent calyx endings (Hurley et al. 2006). We further show that these ion channels by themselves can confer irregularity in firing evoked by inputs resembling the inputs to the spike initiation zones at afferent terminals. Thus whereas differences in dendritic filtering and synaptic specializations may contribute in vivo, ion channels are likely to play a major role in setting regularity, as proposed by Smith and Goldberg (1986). Our data suggest that titration of the number of low-voltage-activated K channels active at resting potential can account for much of the variation in both step-evoked firing pattern in vitro and spike regularity in vivo. At one extreme are transient neurons, with the most numerous low-voltage-activated K channels and the most irregular spiking, and at the other extreme are the sustained-A neurons, with the fewest LV K channels and the most regular spiking.
Development of firing patterns
LV K channels are added in the first 2 wk after birth of these altricial rodents, over the same period that large numbers of LV K channels are added to the type I hair cells that are presynaptic to irregular afferents (Hurley et al. 2006). Over the same period, the activity of rat semicircular canal afferents increases (Curthoys 1979) and the animals clearly gain postural and motion control. Data from spiral ganglion suggest a mechanism for developmental regulation of firing pattern. Mouse spiral ganglion somata have step-evoked firing patterns that vary in accommodation rates along the tonotopic axis of the cochlea (Adamson et al. 2002a); the variation appears to be regulated by complementary expression of neurotrophins (NT-3 and BDNF; Adamson et al. 2002b), which are also released from vestibular epithelia (Fritzsch et al. 2005; Sugawara et al. 2007).
Neurons of central vestibular nuclei also undergo developmental increases in certain K+ currents over a similar time frame (reviewed in Straka et al. 2005). Changes can continue past the second week; spikes become briefer in medial vestibular nucleus (MVN) neurons after P15 (Dutia and Johnston 1998). Whether maturational changes in the activity of vestibular afferents influence electrophysiological maturation of brain stem neurons is not established, although this kind of effect is a tenet of developmental neurobiology (Katz and Shatz 1996). In rat MVN neurons, the acquisition of K+ currents speeds up action potentials and hyperpolarizations, allowing mature neurons to fire at high rates (>100 Hz) and to show a linear dependence of rate on injected current over a broad range (Murphy and Dulac 2001). Increasing numbers of K channels in rat MVN neurons, unlike the ganglion neurons, can lead to more sustained spiking. In maturing chick tangential neurons, in contrast, Peusner et al. (1998) observed down-regulation of LV K+ currents and more sustained spike patterns, observations that are consistent with the role of LV channels in rat primary afferent neurons, but following the opposite developmental trend.
Significance of excitable ganglion somata
Spikes initiate in the peripheral terminal of a vestibular afferent, in or below the sensory epithelium, then propagate along the peripheral neural process to the soma, through the soma, and along the central neuronal process to their brain stem targets. Our proposal—that somatic firing patterns provide insight into the intrinsic voltage-dependent properties that set regularity at the peripheral spike initiation zones—requires that similar voltage-gated channels be expressed in both locations. Several pieces of evidence support this premise. First, as shown here, somatic ion channels succeed in reproducing spike timing and properties of AHPs and EPSPs as recorded in vivo from afferent fibers. Second, antibody labeling locates relevant ion channels in both the somata and peripheral terminals (KCNQ4 channels: Hurley et al. 2006; NaV1.5 channels: Wooltorton et al. 2007), as well as neurotransmitter receptors (Dayanithi et al. 2007; Rabejac et al. 1997) and endogenous Ca2+ buffers (Kevetter and Leonard 2002; Leonard and Kevetter 2002). Third, dorsal root ganglion somata also express ion channels believed to be important in shaping responses at the peripheral terminals (e.g., Binshtok et al. 2007; Zimmerman et al. 2007). Ion channel expression at the soma may reflect afferent terminal expression because some of the ion channels that are made for the terminal insert into the somatic membrane. Culturing may also up-regulate such insertion as neurons respond to being cut off from synaptic inputs and other aspects of in vivo existence; but we note that similar data were obtained from acute preparations recorded within 1–5 h of isolation (see methods). Alternatively, somatic excitability may be a normal way for the neuron's center of protein synthesis to monitor and respond to spiking activity (Amir and Devor 2003).
Step-evoked firing patterns
We report that firing patterns and their correlations with such electrophysiological properties as resting potential and input resistance are similar in different temperatures, ages, and species. We have also recorded transient and sustained patterns in afferents in semi-intact preparations of the ganglion and attached sensory epithelia (unpublished observations). Other groups have implicated diverse K channels in the step-evoked firing patterns of vestibular ganglion somata (Iwasaki et al. 2008; Limón et al. 2005; Pérez et al. 2009; Risner and Holt 2006), but results differ between studies. We are now in a position to account for some of the differences on the basis of cell selection procedures, recording methods, and developmental stage.
Soto and colleagues (Limón et al. 2005; Pérez et al. 2009) do not report sustained firing in control conditions. This can be understood from their emphasis on large neurons, which are expected to represent irregular afferents. Their work shows a relatively large KCNQ (“M”) current in the largest neurons in their population. For these neurons, KCNQ channel blockers alone sufficed to convert firing patterns from transient to sustained (Pérez et al. 2009). This suggests that KCNQ current is a more important component of ILV in the largest neurons than in the smaller neurons that we and others sampled. The largest somata supply the central zones with the most irregular, calyx-only afferents and immunolabeling indicates that such terminals have the most KCNQ4 subunits (Hurley et al. 2006; Lysakowski and Price 2003).
Risner and Holt (2006), working with mouse vestibular ganglion cells in the first postnatal week, found two populations with different current thresholds to spike. Based on the difference in current thresholds shown in Fig. 1F, our transient and sustained-spiker (A + B) categories correspond at least approximately to their high-threshold and low-threshold categories. Their neurons with higher thresholds included the largest neurons in the sample, consistent with our transient firing population. They found that a high dose of 4-aminopyridine (4-AP) blocked A-current and a slower, more negatively activating current that is likely to correspond to ILV. Indeed, their report of the effect of 10 mM 4-AP on step-evoked firing patterns is consistent with our results and those of Iwasaki et al. (2008) using more selective blockers of ILV.
Iwasaki et al. (2008) were the first to link step-evoked firing patterns to low-voltage-activated ion channels in rat vestibular ganglion neurons. Their phasic, tonic, and intermediate categories correspond to our transient, sustained-A, and sustained-B categories, but their results differ from ours in several significant ways. They did not report the sustained resonators (sustained-C neurons) that we found in significant numbers in the first postnatal week and in smaller numbers later. The mean capacitance values (cell sizes) were small and did not increase with age, in contrast to our results and those of Limón et al. (2005). Thus their methods selected against the increasingly large transient neurons, which may explain why their older samples had higher fractions of sustained neurons. This in turn may help explain why average resting potential did not become more negative with age: based on our data (Fig. 3), increasing the fraction of sustained neurons would offset the overall trend toward deeper resting potentials. The lack of KCNQ current in their sample may also have made resting potentials more positive. As noted earlier, KCNQ current is larger in older neurons and may also wash out in ruptured-patch recordings, which were used by Iwasaki et al. (2008). In addition, their use of papain to dissociate neurons may have altered ion channel expression (Armstrong and Roberts 2001).
Mechanisms of spike regularity
COMPARISON WITH A PUBLISHED MODEL OF SPIKE REGULARITY IN VESTIBULAR AFFERENTS.
Goldberg et al. (1984) found that regular and irregular vestibular afferents responded differently to extracellular current stimuli. These results, in addition to the different appearance of AHPs (see Fig. 10B), pointed to postsynaptic mechanisms of spike regularity. Smith and Goldberg (1986) simulated the physiological range of regularity by covarying EPSP size and the duration of a hypothetical AHP conductance. They proposed that regular afferents express an additional AHP conductance, which would cause more prominent AHPs and, by reducing input resistance, higher thresholds for spiking in response to extracellular current injection.
Our results support the general proposal that differences in afferent ion channel expression give rise to differences in spike regularity, but suggest a different specific mechanism, i.e., that vestibular afferents are regular because they lack low-voltage-activated (LV) channels, not because they have AHP channels. In our results, the more prominent AHPs of sustained (putative regular) neurons arise from their higher resting potentials (Figs. 3 and 6D) and greater integration of rapidly arriving EPSCs (Fig. 13B), both of which can be understood from the relative lack of LV channels (Fig. 7C). In addition, the relatively high-input resistance (reflecting the absence of ILV) gave sustained neurons lower thresholds to currents injected directly into the neuron through the patch electrode (Fig. 1F).
Thus the two studies put forth opposing predictions regarding the active conductances at the spike initiation zones of regular and irregular afferents. For the AHP conductance model, the interpretation relies on several assumptions about the nature of the extracellular current stimulus, most notably that the current acted only at the afferents' spike trigger zones. Our interpretation requires the selective expression of LV conductances at the afferent terminals of irregular afferents, which remains to be proven. Supporting evidence is provided by the more intense immunolabeling for KCNQ4 channels at central (highly irregular) calyces than at peripheral (more regular) calyces (Hurley et al. 2006; Lysakowski and Price 2003). KCNQ (Kv7) ion channels colocalize with Na channels at the axon initial segment in CA1 neurons and at nodes of Ranvier in peripheral and central nerve fibers; in both locations, the KCNQ channels serve to prevent repetitive firing by deepening the resting potential and increasing the spike threshold (reviewed in Brown and Passmore 2009). The strong and adjacent immunolocalization of KCNQ4 and NaV1.5 subunits in calyx inner face membranes (Hurley et al. 2006; Wooltorton et al. 2007) suggests that this location may be a site of spike initiation.
INFLUENCE OF AFFERENT ION CHANNELS ON FIRING PATTERNS.
To measure spike regularity, we drove spiking in isolated neurons with stimuli designed to mimic the essential qualities (timing, size, wave form) of true EPSCs. The results showed that step-evoked firing patterns correlated with pseudo-EPSC-driven regularity and that manipulations of firing pattern affected regularity as well. In particular, blocking ILV not only converted the step-evoked firing pattern from transient to sustained, but also converted the pseudo-EPSC-driven spike timing from irregular to regular. These results are the first experimental evidence of specific ion channel differences that shape firing pattern regularity in vestibular afferent neurons.
Low-voltage-activated currents are found in neurons of the timing pathways of the auditory brain stem, where they assist in temporal precision (Kopp-Scheinpflug et al. 2003; Oertel et al. 2000; Rothman and Manis 2003c). Like our transient neurons, cells in the cochlear nucleus with low-voltage-activated K+ currents spike only at the onset of depolarizing current steps (Bal and Oertel 2001; Rothman and Manis 2003a). Rothman and Manis (2003c) developed a neuronal model from cochlear nucleus neuron data, which included voltage-dependent currents similar to those that are clearly present in vestibular ganglion neurons: Na+, A, HV, LV, and HCN currents. The LV currents in the cochlear nucleus neurons are carried by Kv1 channels, as indicated by DTX sensitivity, immunolabeling, and study of neurons in null mutant animals. In the absence of ILV, the model neurons produced sustained responses to current steps; adding ILV changed the response to a single onset spike (transient pattern). As in the transient neurons described here, ILV also hyperpolarized the model neuron, reduced its input resistance, and sped up membrane recovery times. Thus the model captures much of what we see in vestibular ganglion neurons, where ILV may similarly serve to improve temporal precision. To convey rapid head movement signals to compensatory reflexes, irregular afferents may depend on both temporal precision, which is improved by ILV, and high conduction velocity, which is conferred by large axonal diameters.
The identification of a KCNQ (Kv7) component of ILV, together with immunolocalization of KCNQ channels to calyceal afferent terminals, suggests a way in which efferents might modulate afferent firing. The M current of brain neurons comprises KCNQ channels (sometimes with other channels) that are inhibited by neurotransmitters and hormones acting via receptors coupled to G proteins, leading to excitation (Brown and Passmore 2009). Similar modulation may occur in vestibular epithelia, where efferent terminals release acetylcholine (ACh) onto afferent terminals (Goldberg and Fernández 1980; Perachio and Kevetter 1989), which express muscarinic (as well as nicotinic) ACh receptors (Li et al. 2007). Pérez et al. (2009) showed pharmacologically that muscarinic receptors are coupled to KCNQ channels in vestibular ganglion somata. If the same is true at the distal terminals in the sensory epithelia, then ACh release could inhibit ILV, making the afferents more excitable (a known effect of shocking efferents to mammalian vestibular afferents; Goldberg and Fernández 1980) and possibly more regular at the same time (Fig. 12). A strong effect on regularity could change the nature of the information carried by the afferent, from a rate-based code to a timing code better at representing the stimulus waveform (Sadeghi et al. 2007).
The effectiveness of ILV block at converting firing patterns and the modeling by Rothman and Manis (2003c) together argue that LV channels play a dominant role in setting the step-evoked firing patterns we have observed. Our results, together with previous reports by Iwasaki et al. (2008) and Pérez et al. (2009), show that in the vestibular ganglion both Kv1 and KCNQ channels play a role. Their different kinetics may mean that in vivo, the two channel types have the greatest impact over different frequency ranges. Kv1 channels may respond more rapidly and show long-term inactivation, whereas KCNQ channels, with their slower activation and lack of inactivation, may act more like a background conductance. Several of our results, including action of ILV blockers on sustained-B neurons (Figs. 9C and 12E) and data on resting potential (Fig. 3), suggest that by the second postnatal week sustained-B neurons, like transient neurons, have ILV. Their resting potentials are intermediate between those of sustained-A and transient neurons, suggesting that they have some combination of smaller ILV and/or larger subthreshold inward currents. Thus gradation in LV channel expression might account for the heterogeneity in the sustained class.
The potency of LV-channel blockers in converting firing pattern implies that the high-voltage-activated (HV) channels and A channels have less impact on firing pattern. The HV current in vestibular ganglion neurons may include multiple Ca2+-dependent K channels, which Limón et al. (2005) showed are differentially expressed in small- and large-diameter somata. Block of BK channels or SK channels modestly affected the number of spikes in sustained-B (“intermediate”) neurons (Iwasaki et al. 2008).
HCN currents are common in vestibular ganglion neurons (Chabbert et al. 2001b; Fig. 1) and may increase with maturation (Pérez et al. 2009). Rothman and Manis (2003c) showed that HCN channels can indirectly shape firing patterns and subthreshold potentials by depolarizing resting potential and thereby increasing the resting activation of ILV. In our neurons, simply changing the resting potential by injecting current did not change firing pattern (Fig. 4, A and B), but we did not examine the effect of blocking HCN channels.
OTHER POSSIBLE FACTORS IN SPIKE REGULARITY.
Although afferent ion channels alone can create different spike regularities similar to those measured in vivo, contributions may also come from hair cell or synaptic specializations and dendritic morphology. Frog saccular afferents show highly regular firing that is driven by the sharp electrical resonances of hair cells (Rutherford and Roberts 2009). Although mammalian vestibular hair cells do not show such sharp electrical tuning (Holt et al. 1999; Rennie et al. 1996), hair cells from central and peripheral zones differ in ion channel expression (Holt et al. 1999; Weng and Correia 1999), which may affect receptor potentials and therefore synaptic drive. Synaptic and dendritic morphology also differs with zone (reviewed in Eatock et al. 2008). Peripheral, regular afferents sampled from numerous bouton terminals on many hair cells spread over a wider area (Goldberg et al. 1990b) and thus seem optimized for spatial and temporal integration of inputs. Electrotonic filtering as EPSPs travel from postsynaptic membranes to spike initiation zones may act in concert with the high-input resistance to enhance temporal integration. These factors should combine to make extrastriolar regular afferents good at extracting low-frequency signals from noise. In contrast, striolar irregular afferents seem designed to emphasize temporal fidelity. They minimize spatial and temporal integration by sampling from few hair cells over a restricted area, have spike initiation sites close to synaptic release sites, and express ILV, which keeps membrane recovery times relatively short for the large terminal size.
REGULAR AND IRREGULAR VESTIBULAR PATHWAYS.
The large size of the central/striolar calyx terminals and afferent fibers, the large numbers of presynaptic ribbons, and their high-pass response dynamics all point to specialization of the irregular pathway for both temporal precision and speed of conduction. The presence of LV channels, which reduce the membrane time constant, also fits this view. Speed may be critical for moving the eye, head, and body rapidly enough to compensate for head motions with high-frequency components. In contrast, regular afferents may use a spike timing code that at low stimulus frequencies provides greater sensitivity and conveys more information than the rate code of irregular afferents (Sadeghi et al. 2007). Consistent with this suggestion, the angular vestibuloocular reflex engaged by low-frequency (<4 Hz) head motions is dominated in monkeys by regular afferent input (Minor and Goldberg 1991).
Whether these parallel incoming pathways are preserved centrally is not clear. Although there is a great deal of anatomical overlap of central projections, detailed study reveals significant differences in the localization and cellular targets of regular and irregular afferents (Sato and Sasaki 1993; see reviews by Goldberg 2000; Peterson 1998). In vestibular nuclei of diverse species, but especially frog, Straka and colleagues (Pfanzelt et al. 2008; Straka et al. 2005) distinguish two neuronal types expressing different complements of K channels: a more low-pass-filtered, linear, tonic type (A) and a more high-pass-filtered, nonlinear, phasic type (B). They propose that the properties of type A and type B neurons are matched to the properties of regular and irregular primary afferents, respectively, and that this subdivision is fundamental to the organization of vestibular signals.
GRANTS
The research was supported by National Research Service Award Training Grant F32-DC-93602 to R. Kalluri and National Institute on Deafness and Other Communication Disorders Grant R01-DC-02290 to R. A. Eatock.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank A. Casserly, T. Kimm, and B. Winterroth for technical help; D. Sands and J. Cunha for administrative help; Dr. Bertrand Delgutte and Dr. John Guinan for helpful discussions; and Dr. William F. Sewell, Dr. Bruce Bean, and X. Liu for helpful comments on various versions of the manuscript.
Present addresses: R. Kalluri, Communication and Auditory Neuroscience Division, House Ear Institute, 2100 W. 3rd St., Los Angeles, CA 90057 (RKalluri@hei.org); J. Xue, Department of Image Sciences, Strong Memorial Hospital, 601 Elmwood Avenue, Box 648, Rochester, NY 14642 (Jingbing_Xue@URMC.Rochester.edu).
REFERENCES
- Adamson et al., 2002b.Adamson CL, Reid MA, Davis RL. Opposite actions of brain-derived neurotrophic factor and neurotrophin-3 on firing features and ion channel composition of murine spiral ganglion neurons. J Neurosci 22: 1385–1396, 2002b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamson et al., 2002a.Adamson CL, Reid MA, Mo ZL, Bowne-English J, Davis RL. Firing features and potassium channel content of murine spiral ganglion neurons vary with cochlear location. J Comp Neurol 447: 331–350, 2002a [DOI] [PubMed] [Google Scholar]
- Amir and Devor, 2003.Amir R, Devor M. Electrical excitability of the soma of sensory neurons is required for spike invasion of the soma, but not for through-conduction. Biophys J 84: 2181–2191, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong and Roberts, 1998.Armstrong CE, Roberts WM. Electrical properties of frog saccular hair cells: distortion by enzymatic dissociation. J Neurosci 18: 2962–2973, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird et al., 1988.Baird RA, Desmadryl G, Fernández C, Goldberg JM. The vestibular nerve of the chinchilla. II. Relation between afferent response properties and peripheral innervation patterns in the semicircular canals. J Neurophysiol 60: 182–203, 1988 [DOI] [PubMed] [Google Scholar]
- Bal and Oertel, 2001.Bal R, Oertel D. Potassium currents in octopus cells of the mammalian cochlear nucleus. J Neurophysiol 86: 2299–2311, 2001 [DOI] [PubMed] [Google Scholar]
- Barry, 1994.Barry PH. JPCalc, a software package for calculating liquid junction potential corrections in patch-clamp, intracellular, epithelial and bilayer measurements and for correcting junction potential measurements. J Neurosci Methods 51: 107–116, 1994 [DOI] [PubMed] [Google Scholar]
- Bean, 2007.Bean BP. The action potential in mammalian central neurons. Nat Rev Neurosci 8: 451–465, 2007 [DOI] [PubMed] [Google Scholar]
- Binshtok et al., 2007.Binshtok AM, Bean BP, Woolf CJ. Inhibition of nociceptors by TRPV1-mediated entry of impermeant sodium channel blockers. Nature 449: 607–610, 2007 [DOI] [PubMed] [Google Scholar]
- Brown and Passmore, 2009.Brown DA, Passmore GM. Neural KCNQ (Kv7) channels. Br J Pharmacol 156: 1185–1195, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabbert et al., 2001a.Chabbert C, Chambard JM, Sans A, Desmadryl G. Three types of depolarization-activated potassium currents in acutely isolated mouse vestibular neurons. J Neurophysiol 85: 1017–1026, 2001a [DOI] [PubMed] [Google Scholar]
- Chabbert et al., 1997.Chabbert C, Chambard JM, Valmier J, Sans A, Desmadryl G. Voltage-activated sodium currents in acutely isolated mouse vestibular ganglion neurones. Neuroreport 8: 1253–1256, 1997 [DOI] [PubMed] [Google Scholar]
- Chabbert et al., 2001b.Chabbert C, Chambard JM, Valmier J, Sans A, Desmadryl G. Hyperpolarization-activated (Ih) current in mouse vestibular primary neurons. Neuroreport 12: 2701–2704, 2001b [DOI] [PubMed] [Google Scholar]
- Chambard et al., 1999.Chambard JM, Chabbert C, Sans A, Desmadryl G. Developmental changes in low and high voltage-activated calcium currents in acutely isolated mouse vestibular neurons. J Physiol 518: 141–149, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curthoys, 1979.Curthoys IS. The development of function of horizontal semicircular canal primary neurons in the rat. Brain Res 167: 41–52, 1979 [DOI] [PubMed] [Google Scholar]
- Dayanithi et al., 2007.Dayanithi G, Desmadryl G, Travo C, Chabbert C, Sans A. Trimetazidine modulates AMPA/kainate receptors in rat vestibular ganglion neurons. Eur J Pharmacol 574: 8–14, 2007 [DOI] [PubMed] [Google Scholar]
- Desmadryl et al., 1997.Desmadryl G, Chambard JM, Valmier J, Sans A. Multiple voltage-dependent calcium currents in acutely isolated mouse vestibular neurons. Neuroscience 78: 511–522, 1997 [DOI] [PubMed] [Google Scholar]
- Dutia and Johnston, 1998.Dutia MB, Johnston AR. Development of action potentials and apamin-sensitive after-potentials in mouse vestibular nucleus neurones. Exp Brain Res 118: 148–154, 1998 [DOI] [PubMed] [Google Scholar]
- Eatock et al., 2008.Eatock RA, Xue J, Kalluri R. Ion channels in mammalian vestibular afferents may set regularity of firing. J Exp Biol 211: 1764–1774, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández et al., 1988.Fernández C, Baird RA, Goldberg JM. The vestibular nerve of the chinchilla. I. Peripheral innervation patterns in the horizontal and superior semicircular canals. J Neurophysiol 60: 167–181, 1988 [DOI] [PubMed] [Google Scholar]
- Fernández et al., 1990.Fernández C, Goldberg JM, Baird RA. The vestibular nerve of the chinchilla. III. Peripheral innervation patterns in the utricular macula. J Neurophysiol 63: 767–780, 1990 [DOI] [PubMed] [Google Scholar]
- Fritzsch et al., 2005.Fritzsch B, Pauley S, Matei V, Katz DM, Xiang M, Tessarollo L. Mutant mice reveal the molecular and cellular basis for specific sensory connections to inner ear epithelia and primary nuclei of the brain. Hear Res 206: 52–63, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowatzki and Fuchs, 2002.Glowatzki E, Fuchs PA. Transmitter release at the hair cell ribbon synapse. Nat Neurosci 5: 147–154, 2002 [DOI] [PubMed] [Google Scholar]
- Goldberg, 1991.Goldberg JM. The vestibular end organs: morphological and physiological diversity of afferents. Curr Opin Neurobiol 1: 229–235, 1991 [DOI] [PubMed] [Google Scholar]
- Goldberg, 2000.Goldberg JM. Afferent diversity and the organization of central vestibular pathways. Exp Brain Res 130: 277–297, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg et al., 1990a.Goldberg JM, Desmadryl G, Baird RA, Fernández C. The vestibular nerve of the chinchilla. IV. Discharge properties of utricular afferents. J Neurophysiol 63: 781–790, 1990a [DOI] [PubMed] [Google Scholar]
- Goldberg et al., 1990b.Goldberg JM, Desmadryl G, Baird RA, Fernández C. The vestibular nerve of the chinchilla. V. Relation between afferent discharge properties and peripheral innervation patterns in the utricular macula. J Neurophysiol 63: 791–804, 1990b [DOI] [PubMed] [Google Scholar]
- Goldberg and Fernández, 1980.Goldberg JM, Fernández C. Efferent vestibular system in the squirrel monkey: anatomical location and influence on afferent activity. J Neurophysiol 43: 986–1025, 1980 [DOI] [PubMed] [Google Scholar]
- Goldberg et al., 1984.Goldberg JM, Smith CE, Fernández C. Relation between discharge regularity and responses to externally applied galvanic currents in vestibular nerve afferents of the squirrel monkey. J Neurophysiol 51: 1236–1256, 1984 [DOI] [PubMed] [Google Scholar]
- Hall et al., 1994.Hall A, Stow J, Sorensen R, Dolly JO, Owen D. Blockade by dendrotoxin homologues of voltage-dependent K+ currents in cultured sensory neurones from neonatal rats. Br J Pharmacol 113: 959–967, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highstein and Politoff, 1978.Highstein SM, Politoff AL. Relation of interspike baseline activity to the spontaneous discharges of primary afferents from the labyrinth of the toadfish, Opsanus tau. Brain Res 150: 182–187, 1978 [DOI] [PubMed] [Google Scholar]
- Holt et al., 2007.Holt JC, Chatlani S, Lysakowski A, Goldberg JM. Quantal and nonquantal transmission in calyx-bearing fibers of the turtle posterior crista. J Neurophysiol 98: 1083–1101, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt et al., 2006.Holt JC, Xue JT, Brichta AM, Goldberg JM. Transmission between type II hair cells and bouton afferents in the turtle posterior crista. J Neurophysiol 95: 428–452, 2006 [DOI] [PubMed] [Google Scholar]
- Holt et al., 1999.Holt JR, Vollrath MA, Eatock RA. Stimulus processing by type II hair cells in the mouse utricle. Ann NY Acad Sci 871: 15–26, 1999 [DOI] [PubMed] [Google Scholar]
- Horn and Marty, 1988.Horn R, Marty A. Muscarinic activation of ionic currents measured by A new whole-cell recording method. J Gen Physiol 92: 145–159, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hullar et al., 2005.Hullar TE, Della Santina CC, Hirvonen T, Lasker DM, Carey JP, Minor LB. Responses of irregularly discharging chinchilla semicircular canal vestibular-nerve afferents during high-frequency head rotations. J Neurophysiol 93: 2777–2786, 2005 [DOI] [PubMed] [Google Scholar]
- Hurley et al., 2006.Hurley KM, Gaboyard S, Zhong M, Price SD, Wooltorton JR, Lysakowski A, Eatock RA. M-like K+ currents in type I hair cells and calyx afferent endings of the developing rat utricle. J Neurosci 26: 10253–10269, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki et al., 2008.Iwasaki S, Chihara Y, Komuta Y, Ito K, Sahara Y. Low-voltage-activated potassium channels underlie the regulation of intrinsic firing properties of rat vestibular ganglion cells. J Neurophysiol 100: 2192–2204, 2008 [DOI] [PubMed] [Google Scholar]
- Katz and Shatz, 1996.Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science 274: 1133–1138, 1996 [DOI] [PubMed] [Google Scholar]
- Kevetter and Leonard, 2002.Kevetter GA, Leonard RB. Molecular probes of the vestibular nerve. II. Characterization of neurons in Scarpa's ganglion to determine separate populations within the nerve. Brain Res 928: 18–29, 2002 [DOI] [PubMed] [Google Scholar]
- Kharkovets et al., 2000.Kharkovets T, Hardelin JP, Safieddine S, Schweizer M, El-Amraoui A, Petit C, Jentsch TJ. KCNQ4, A K channel mutated in A form of dominant deafness, is expressed in the inner ear and the central auditory pathway. Proc Natl Acad Sci USA 97: 4333–4338, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp-Scheinpflug et al., 2003.Kopp-Scheinpflug C, Fuchs K, Lippe WR, Tempel BL, Rübsamen R. Decreased temporal precision of auditory signaling in Kcna1-null mice: an electrophysiological study in vivo. J Neurosci 23: 9199–9207, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasker et al., 2008.Lasker DM, Han GC, Park HJ, Minor LB. Rotational responses of vestibular-nerve afferents innervating the semicircular canals in the C57BL/6 mouse. J Assoc Res Otolaryngol 9: 334–348, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard and Kevetter, 2002.Leonard RB, Kevetter GA. Molecular probes of the vestibular nerve. I. Peripheral termination patterns of calretinin, calbindin and peripherin containing fibers. Brain Res 928: 8–17, 2002 [DOI] [PubMed] [Google Scholar]
- Lerche et al., 2000.Lerche C, Scherer CR, Seebohm G, Derst C, Wei AD, Busch AE, Steinmeyer K. Molecular cloning and functional expression of KCNQ5, a potassium channel subunit that may contribute to neuronal M-current diversity. J Biol Chem 275: 22395–22400, 2000 [DOI] [PubMed] [Google Scholar]
- Li et al., 2007.Li G, Kevetter GA, Leonard RB, Prusak DJ, Wood TG, Correia MJ. Muscarinic acetylcholine receptor subtype expression in avian vestibular hair cells, nerve terminals and ganglion cells. Neurosci 146: 384–402, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limón et al., 2005.Limón A, Pérez C, Vega R, Soto E. Ca2+-activated K+-current density is correlated with soma size in rat vestibular-afferent neurons in culture. J Neurophysiol 94: 3751–3761, 2005 [DOI] [PubMed] [Google Scholar]
- Lysakowski and Price, 2003.Lysakowski A, Price SD. Potassium channel localization in sensory epithelia of the rat inner ear. Abstr Assoc Res Otolaryngol 26: 1534, 2003 [Google Scholar]
- Magistretti et al., 1998.Magistretti J, Mantegazza M, de Curtis M, Wanke E. Modalities of distortion of physiological voltage signals by patch-clamp amplifiers: a modeling study. Biophys J 74: 831–842, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor and Goldberg, 1991.Minor L, Goldberg JM. Vestibular-nerve inputs to the vestibulo-ocular reflex: a functional-ablation study in the squirrel monkey. J Neurosci 11: 1636–1648, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy and Du Lac, 2001.Murphy GJ, Du Lac S. Postnatal development of spike generation in rat medial vestibular nucleus neurons. J Neurophysiol 85: 1899–1906, 2001 [DOI] [PubMed] [Google Scholar]
- Oertel et al., 2000.Oertel D, Bal R, Gardner SM, Smith PH, Joris PX. Detection of synchrony in the activity of auditory nerve fibers by octopus cells of the mammalian cochlear nucleus. Proc Natl Acad Sci USA 97: 11773–11779, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel et al., 2008.Oertel D, Shatadal S, Cao XJ. In the ventral cochlear nucleus Kv1.1 and subunits of HCN1 are colocalized at surfaces of neurons that have low-voltage-activated and hyperpolarization-activated conductances. Neuroscience 154: 77–86, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perachio and Kevetter, 1989.Perachio AA, Kevetter GA. Identification of vestibular efferent neurons in the gerbil: histochemical and retrograde labeling. Exp Brain Res 78: 315–326, 1989 [DOI] [PubMed] [Google Scholar]
- Pérez et al., 2009.Pérez C, Limón A, Vega R, Soto E. The muscarinic inhibition of the potassium M-current modulates the action-potential discharge in the vestibular primary-afferent neurons of the rat. Neuroscience 158: 1662–1674, 2009 [DOI] [PubMed] [Google Scholar]
- Peterson, 1998.Peterson EH. Are there parallel channels in the vestibular nerve? News Physiol Sci 13: 194–201, 1998 [DOI] [PubMed] [Google Scholar]
- Peusner et al., 1998.Peusner KD, Gamkrelidze G, Giaume C. Potassium currents and excitability in second-order auditory and vestibular neurons. J Neurosci Res 53: 511–520, 1998 [DOI] [PubMed] [Google Scholar]
- Pfanzelt et al., 2008.Pfanzelt S, Rössert C, Rohregger M, Glasauer S, Moore LE, Straka H. Differential dynamic processing of afferent signals in frog tonic and phasic second-order vestibular neurons. J Neurosci 28: 10349–10362, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabejac et al., 1997.Rabejac D, Devau G, Raymond J. AMPA receptors in cultured vestibular ganglion neurons: detection and activation. Eur J Neurosci 9: 221–228, 1997 [DOI] [PubMed] [Google Scholar]
- Rennie et al., 1996.Rennie KJ, Ricci AJ, Correia MJ. Electrical filtering in gerbil isolated type I semicircular canal hair cells. J Neurophysiol 75: 2117–2123, 1996 [DOI] [PubMed] [Google Scholar]
- Rennie and Streeter, 2006.Rennie KJ, Streeter MA. Voltage-dependent currents in isolated vestibular afferent calyx terminals. J Neurophysiol 95: 26–32, 2006 [DOI] [PubMed] [Google Scholar]
- Rennie et al., 2001.Rennie KJ, Weng T, Correia MJ. Effects of KCNQ channel blockers on K(+) currents in vestibular hair cells. Am J Physiol Cell Physiol 280: C473–C480, 2001 [DOI] [PubMed] [Google Scholar]
- Risner and Holt, 2006.Risner JR, Holt JR. Heterogeneous potassium conductances contribute to the diverse firing properties of postnatal mouse vestibular ganglion neurons. J Neurophysiol 96: 2364–2376, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman and Manis, 2003a.Rothman JS, Manis PB. Differential expression of three distinct potassium conductances in ventral cochlear nucleus neurons. J Neurophysiol 89: 3070–3082, 2003a [DOI] [PubMed] [Google Scholar]
- Rothman and Manis, 2003b.Rothman JS, Manis PB. Kinetic analyses of three distinct potassium conductances in ventral cochlear nucleus neurons. J Neurophysiol 89: 3083–3096, 2003b [DOI] [PubMed] [Google Scholar]
- Rothman and Manis, 2003c.Rothman JS, Manis PB. The roles potassium currents play in regulating the electrical activity of ventral cochlear nucleus neurons. J Neurophysiol 89: 3097–3113, 2003c [DOI] [PubMed] [Google Scholar]
- Rutherford and Roberts, 2009.Rutherford MA, Roberts WM. Spikes and membrane potential oscillations in hair cells generate periodic afferent activity in the frog sacculus. J Neurosci 29: 10025–10037, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi et al., 2007.Sadeghi SG, Chacron MJ, Taylor MC, Cullen KE. Neural variability, detection thresholds, and information transmission in the vestibular system. J Neurosci 27: 771–781, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato and Sasaki, 1993.Sato F, Sasaki H. Morphological correlations between spontaneously discharging primary vestibular afferents and vestibular nucleus neurons in the cat. J Comp Neurol 333: 554–566, 1993 [DOI] [PubMed] [Google Scholar]
- Schessel et al., 1991.Schessel DA, Ginzberg R, Highstein SM. Morphophysiology of synaptic transmission between type I hair cells and vestibular primary afferents. An intracellular study employing horseradish peroxidase in the lizard, Calotes versicolor. Brain Res 544: 1–16, 1991 [DOI] [PubMed] [Google Scholar]
- Schnee and Brown, 1998.Schnee ME, Brown BS. Selectivity of linopirdine (DuP 996), a neurotransmitter release enhancer, in blocking voltage-dependent and calcium-activated potassium currents in hippocampal neurons. J Pharmacol Exp Ther 286: 709–717, 1998 [PubMed] [Google Scholar]
- Schneider et al., 2006.Schneider D, Hurley KM, Wooltorton JR, Eatock RA. Voltage-gated Na+ and Ca2+ currents in rodent vestibular ganglion cells. Abstr Assoc Res Otolaryngol 29: 956, 2006 [Google Scholar]
- Smith and Goldberg, 1986.Smith CE, Goldberg JM. A stochastic afterhyperpolarization model of repetitive activity in vestibular afferents. Biol Cybern 54: 41–51, 1986 [DOI] [PubMed] [Google Scholar]
- Straka et al., 2005.Straka H, Vibert N, Vidal PP, Moore LE, Dutia MB. Intrinsic membrane properties of vertebrate vestibular neurons: function, development and plasticity. Prog Neurobiol 76: 349–392, 2005 [DOI] [PubMed] [Google Scholar]
- Sugawara et al., 2007.Sugawara M, Murtie JC, Stankovic KM, Liberman MC, Corfas G. Dynamic patterns of neurotrophin 3 expression in the postnatal mouse inner ear. J Comp Neurol 501: 30–37, 2007 [DOI] [PubMed] [Google Scholar]
- Weng and Correia, 1999.Weng T, Correia MJ. Regional distribution of ionic currents and membrane voltage responses of type II hair cells in the vestibular neuroepithelium. J Neurophysiol 82: 2451–2461, 1999 [DOI] [PubMed] [Google Scholar]
- Wladyka and Kunze, 2006.Wladyka CL, Kunze DL. KCNQ/M-currents contribute to the resting membrane potential in rat visceral sensory neurons. J Physiol 575: 175–189, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooltorton et al., 2007.Wooltorton JR, Gaboyard S, Hurley KM, Price SD, Garcia JL, Zhong M, Lysakowski A, Eatock RA. Developmental changes in two voltage-dependent sodium currents in utricular hair cells. J Neurophysiol 97: 1684–1704, 2007 [DOI] [PubMed] [Google Scholar]
- Yang and Hullar, 2007.Yang A, Hullar TE. Relationship of semicircular canal size to vestibular-nerve afferent sensitivity in mammals. J Neurophysiol 98: 3197–3205, 2007 [DOI] [PubMed] [Google Scholar]
- Zimmermann et al., 2007.Zimmermann K, Leffler A, Babes A, Cruz MC, Carr RW, Kobayashi J-I, Nau C, Wood JN, Reeh PW. Sensory neuron sodium channels NaV1.8 is essential for pain at low temperatures. Nature 447: 855–859, 2007 [DOI] [PubMed] [Google Scholar]