Abstract
Parasympathetic cardiac motoneurons (PCMNs) in the nucleus ambiguus (NA) play a key role in regulating cardiac functions. In this study, we examined the effects of maternal diabetes on excitability, action potential (AP) properties, and small conductance Ca2+-activated K+ (SK) currents of PCMNs. Neonatal mice from diabetic (OVE26 female, NMDM) and normal (FVB female, control) mothers that had been mated with nondiabetic fathers (FVB male) were used. Tracer XRITC was injected into the pericardial sac at P7-9 to retrogradely label PCMNs. Two days later, XRITC-labeled PCMNs were identified in brain stem slices. The responses of spike frequency, AP repolarization (half-width) and afterhyperpolarization (AHP) of PCMNs to current injections were studied using whole cell current clamp. Outward and afterhyperpolarization currents (IAHP) in response to voltage steps were measured using whole cell voltage clamp. In examining the effects of maternal diabetes on excitability and AP properties, we found that in NMDM spike frequency decreased, the half-width and AHP peak amplitude increased, and the peak amplitude of outward transient currents and IAHP increased compared with those measured in control. In examining the effects of maternal diabetes on SK channels, we found that after blockage of SK channels with a specific SK channel blocker apamin, maternal diabetes significantly increased apamin-sensitive outward transient currents and IAHP, and suppressed AHP amplitude in NMDM more than those in control. Further, apamin application increased the firing rate to current injections and completely abolished the difference of the firing rate between control and NMDM. We suggest that the augmented SK-mediated currents may contribute to the increased AHP amplitude and the attenuated excitability of PCMNs in NMDM.
INTRODUCTION
Human infants from diabetic mothers have an increased risk of developing malformations of the CNS (Ramos-Arroyo et al. 1992). Maternal diabetes decreases proliferation and increases apoptosis of neuroepithelial cells in the developing spinal cords of embryos from streptozotocin (STZ)-induced diabetic (type 1) mouse mothers (Gao and Gao 2007). Fetal hyperglycemia exposure may promote neural malformation via oxidative stress (Hockett et al. 2004). Normalization of maternal metabolism during critical perinatal development prevents neural malformation in offspring from diabetic rat mothers (Harder et al. 2003). In the autonomic nervous system, maternal diabetes impairs sympathetic and parasympathetic development in offspring (Fiorentini et al. 2005; Young and Morrison 1998). Recently Wichi et al. (2005) found that maternal diabetes impairs baroreflex control of heart rate (HR) in offspring of STZ-induced diabetic mothers. Because attenuation of baroreflex sensitivity is associated with many cardiovascular diseases including sudden death (La Rovere et al. 2008), it is critically important to study the effects of maternal diabetes on the neural components within the baroreflex circuitry.
Parasympathetic cardiac motoneurons (PCMNs) in the brain stem regulate cardiac output (Loewy and Spyer 1990; Neff et al. 2003). Previously we demonstrated that the PCMNs in the nucleus ambiguus (NA) project strongly to cardiac ganglia and that the NA has a key role in baroreflex control of HR (Cheng and Polwey 2000; Cheng et al. 2004a,b). In contrast, the dorsal motor nucleus of the vagus does not significantly contribute to baroreflex control of HR (Cheng et al. 2002). Thus far, no studies have been conducted to examine whether maternal diabetes alters excitability and action potential (AP) properties of NA PCMNs in the neonates from diabetic mothers.
In many neurons of the CNS, influx of calcium (Ca2+) during APs activates potassium (K+) channels. Activation of these channels generates currents that contribute to AP repolarization and the afterhyperpolarization (AHP) that follows them (Lancaster and Pennefather 1987; Sah and McLachlan 1992; Storm 1987). There are three major types of Ca2+-activated K+ channels: 1) large conductance (BK) voltage-sensitive channels that are potently blocked by the scorpion venom toxin charybdotoxin (ChTX) and selectively blocked by paxilline (Sanchez and McManus 1996; Strøbaek et al. 1996); 2) intermediate conductance (IK) channels that are potently blocked by ChTX and the antifungal imidazole compound clotrimazole (CTL) (Ishii et al. 1997; Logsdon et al. 1997); and 3) small conductance (SK) voltage-insensitive channels that are selectively blocked by the bee toxin apamin (Castle et al. 1989). BK currents mainly contribute to AP repolarization and the fast AHP (fAHP, a few milliseconds) (Faber and Sah 2007) that immediately follows it (Lancaster and Adams 1986; Lancaster and Nicoll 1987; Sah and McLachlan 1992; Storm 1987), whereas activation of SK currents largely generates medium AHP (mAHP; tens to several hundreds milliseconds), which significantly mediates firing frequency and patterns (Sah 1996). IK channels play a role in maintaining rapid Ca2+ entry in many cells types including myenteric neurons (Fanger et al. 2001; Tharp and Bowles 2009; Vogalis et al. 2002), but IK channels have not been reported in central neurons.
Our goal was to study the cellular mechanisms for the maternal diabetes-induced alteration of PCMN excitability. Because activation of BK and SK channels negatively regulate action potentials, we explored the contribution of BK and SK channels to the firing rate of PCMNs and the effect of maternal diabetes on BK and SK channels. According to our preliminary data, maternal diabetes attenuates excitability of PCMNs. However, blockade of BK channels with ChTX or paxilline decreased spike firing frequency in response to current injections in both control and NMDM (Lin et al. 2010a). In contrast, blockade of SK channels with apamin increased the firing rate in response to current injections and largely abolished the difference in the firing rate of PCMNs between control and NMDM (Lin et al. 2010b). Therefore we focused only on SK channels in the present study.
SK channels are expressed in many central neurons and their function in regulating neuronal excitability has been well established (Pedarzani and Stocker 2008; Stocker 2004). The activity of SK channels causes an increased interspike interval and a prolonged AHP during which the return to the resting membrane potential reflects the decay of intracellular Ca2+ levels. Blockade of SK channels with apamin increases excitability and enhancement of SK channels with enhancer 1-ethyl-2-benzimidazolinone (1-EBIO) decreases excitability (Stocker 2004). Depending on the neuronal subtype and its associated ion channels, the SK channels may contribute to regulation of burst firing (Cingolani et al. 2002; Hallworth et al. 2003; Womack and Khodakhah 2003), spike frequency adaptation (Bond et al. 1999; Vergara et al. 1998), spike patterning (Cloues and Sather 2003; Wolfart et al. 2001), and long-term potentiation (Faber et al. 2005; Ngo-Anh et al. 2005; Stackman et al. 2002) as well as the final termination of action potentials (Constanti and Sim 1987; Lancaster and Adams 1986; Schwindt et al. 1988; Storm 1990). Recently, we found that SK channels regulate the firing properties and excitability of parasympathetic cardiac motoneurons in the NA (Lin et al. 2010c).
In this study, we investigated the effects of maternal diabetes on the excitability and AP properties of PCMNs in neonatal mice. Because SK channels contribute to the intrinsic firing properties and regulation of excitability in other central neurons (Faber and Sah 2002; Sah and McLachlan 1992; Storm 1987) as well as the NA cardiac motoneurons (Lin et al. 2010c). We also examined the effect of maternal diabetes on the SK channels and the contribution of SK currents to maternal diabetes-induced changes of AP properties and excitability. We found that maternal diabetes attenuated excitability, altered AP properties, and increased SK currents that likely contribute to the increased AHP amplitude and attenuated excitability of PCMNs.
METHODS
Animal models
Neonatal mice from wild-type FVB (female) and OVE26 diabetic females (1.5–2 mo) that had been mated with wild-type FVB males (2–3 mo) were used. Female mice were separated into individual cages after they became pregnant. OVE26 mice develop type 1 diabetes due to specific overexpression of the calmodulin transgene in pancreatic beta cells (Epstein et al. 1989, 1992). OVE26 mice are a particularly valuable model of diabetes because 1) no toxic chemicals are needed to induce the disease, 2) all OVE26 mice have sustained hyperglycemia >500 mg/dl from 30 days of age and can survive well over a year without insulin administration, and 3) neonates show normal blood glucose levels before 15 days of age. Two main groups (groups 1 and 2) of neonatal mice were used. Group 1: neonates from FVB mothers that were fed by their own natural mothers (control/natural, or control for simplicity). Group 2: neonatal mice from OVE26 diabetic mothers (NMDM) were fed by their own natural diabetic mothers (NMDM/natural; or NMDM for simplicity). In this group, neonates from diabetic mothers may be either transgenic (half of the pups) or nontransgenic (half of the pups). In addition to groups 1 and 2, groups 3 and 4 were added as references to study whether the calmodulin transgene or quality of milk of diabetic mothers had any effects on body weight, blood glucose level, cell excitability, and outward currents in their offspring at the time (P9–11) of experiments. In groups 3 and 4, all neonates from OVE26 mothers were fed by normal FVB mothers (surrogate) immediately after birth. At the time of experiments (P9–11), transgenic and nontransgenic neonates could be easily distinguished by examining the size of their eyes under the light microscope [eye size is reduced by overexpression of the GR19 gene in OVE26 mice (Epstein et al. 1989)]. Transgenic and nontransgenic neonates were labeled as transgenic/surrogate (group 3) and nontransgenic/surrogate (group 4), respectively. The neonates in groups 3 and 4 were different neonates from those mice used in NMDM. Body weight, blood glucose level, and membrane properties of the neurons in these four groups are listed in Tables 1 and 2. Animals had free access to food and water, and no insulin therapy was given. All procedures were approved by the University of Central Florida Animal Care and Use Committee and followed the guidelines of National Institutes of Health. Efforts were made to reduce the number of animals used.
Table 1.
Basic values in control, NMDM, transgenic, and non-transgenic mice
| Group | Body Weight, g | Blood glucose, mg/dl |
|---|---|---|
| Control | 4.1 ± 0.2 | 141.0 ± 11.0 |
| NMDM | 3.7 ± 0.2 | 131.9 ± 10.2 |
| Surrogate mother | ||
| transgenic mice | 4.3 ± 0.3# | 177.9 ± 21.6# |
| non-transgenic mice | 4.8 ± 0.3* | 197.3 ± 11.9* |
Body weights and blood glucose levels of control, neonatal mice from diabetic mothers (NMDM), transgenic/surrogate and nontransgenic/surrogate n = 7/group. There was no difference between NMDM and control groups. Values are means ± SE.
P < 0.05: compared to the control group.
P < 0.05: compared to the NMDM group.
Table 2.
Passive membrane properties and action potentials of parasympathetic cardiac neurons of control and NMDM
| Group | Vm, mV | Rin, MΩ | Cm, pF | AP Threshold, mV | AP Amplitude, mV |
|---|---|---|---|---|---|
| Control | −70.3 ± 1.1 (17) | 246.6 ± 19.0 (15) | 60.8 ± 2.8 (15) | −40.8 ± 1.2 (10) | 85.5 ± 2.0 (15) |
| NMDM | −71.4 ± 1.6 (17) | 222.1 ± 10.8 (15) | 61.6 ± 1.4 (15) | −43.2 ± 1.6 (10) | 85.5 ± 1.5 (15) |
Passive membrane properties of parasympathetic cardiac motoneurons (PCMNs) of the nucleus ambiguus (NA) in control and NMDM. No significances were found between control and NMDM. Values in parenthesis indicate the number of cells recorded. Vm, resting membrane potential; Rin, input resistance; Cm, membrane capacitance.
Fluorescent labeling of NA PCMNs and medullary slice preparation
Neonatal mice (postnatal days P7–9) were anesthetized with 3% isoflurane (Abbott Laboratories, North Chicago, IL) and cooled to ∼4°C to decrease heart rate. After a right thoracotomy was performed, the retrograde fluorescent tracer X-rhodamine-5 (and 6)-isothiocyanate (XRITC, 2%, 4–8 μl, Molecular Probes, Eugene, OR) was injected into the pericardial sac at the base of the heart. After a 48 h recovery period, neonatal mice were deeply anesthetized with 4% isoflurane. The hindbrains were rapidly removed. The brain stem including PCMNs were sliced into coronal sections (250 μm) using a vibrating blade microslicer (DTK-1000, Kyoto, Japan) and maintained in an interface chamber superfused with artificial cerebral spinal fluid (ACSF) containing (mM): 126 NaCl, 2.5 KCl, 2 CaCl2, 26 NaHCO3, 1.25 NaH2PO4, 2 MgSO4, and 10 dextrose, equilibrated with 95% O2-5% CO2, and pH adjusted to 7.4. Slices were transferred to a recording chamber maintained at room temperature (22–25°C).
Whole cell current clamp recording
Brain stem slices were viewed with infrared illumination, differential interference optics (Zeiss, Göttingen, Germany) and a near infrared-sensitive camera (AxioCom MRm, Göttingen, Germany). PCNMs in the NA were identified by the presence of the fluorescent tracer in superimposed fluorescent and infrared images (Fig. 1). Whole cell current clamp recordings were made from fluorescently labeled NA neurons. Within the recording chamber, slices were held in position using a nylon net stretched over a flattened U-shaped platinum wire and were continuously superfused at room temperature with oxygenated ACSF. When cadmium (Cd2+) was used to block voltage-gated Ca2+ channels, NaHPO4 was removed from the ACSF to prevent cadmium from precipitating out of solution. Patch electrodes used for rupture patch recordings were fabricated from borosilicate glass (1.5 mm OD; World Precision Instruments, Sarasota, FL) with a Flaming Brown horizontal puller (P-97, Sutter Instruments, Novato, CA). Electrodes were heat-polished to a final tip resistance of 3–5 MΩ and filled with a pipette solution containing (in mM) 120 potassium-gluconate, 20 KCl, 2 MgCl2, 10 HEPES, 0.1 EGTA, 2 ATP, and 0.25 GTP, with pH adjusted to 7.3 with KOH. Signals were recorded using a MultiClamp 700B patch-clamp amplifier (Axon Instruments, Foster City, CA). Responses were low-pass filtered at 3 kHz and digitized at 50 kHz with a 16 bit data acquisition system (Digidata 1322A, Axon Instruments).
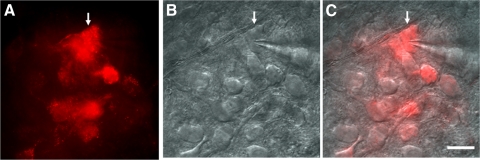
Fig. 1.
Patch-clamp recording of tracer XRITC-labeled parasympathetic cardiac motoneurons (PCMNs) in the nucleus ambiguus (NA) in brain stem slices. A: fluorescent imaging of PCMNs. ↓, the recorded PCMN in B. B: DIC imaging of single PCMNs for recording. ↓, a recorded PCMN. C: the merged image of A and B. Scale bar: 40 μm.
APs were recorded by applying a 0.5 ms depolarizing current pulse of sufficient intensity (1–2 nA) to generate a single AP. To standardize AP recordings, neurons were depolarized to a holding potential of –60 mV by DC application through the recording electrode. AP was recorded within minutes after good recording conditions were established. Spike half-width was measured as the spike width at the half-maximal voltage using Clampfit 9.2 software (Axon Instruments). AHP amplitudes were measured at 10 and 50 ms. To investigate the firing frequency of neurons, 10 current injection steps (1,000 ms each) were applied from 0 to 200 pA in 20 pA increments. The mean spike frequency was calculated as the average of the inverse of all interspike intervals in response to 1 s constant current pulse. Input resistance (Rin) was determined from a linear regression in the linear range (generally ±10 mV from resting potential) of the voltage-current relationship established by plotting the steady-state voltage changes in response to a series of de- and hyperpolarizing current injections. To determine the membrane potential threshold, AP trains were evoked by a 1.5 s, 0.2 pA /ms ramp current from a holding potential of −60 mV in control ACSF and the threshold was measured at the beginning of the first AP.
Data acquisition was performed using the ClampEx program in the pClamp 9 software package. Before seals were made on cells, offset potentials were nulled. Recordings were accepted when resting membrane potentials measured immediately after membrane rupture were more negative to −60 mV, the AP amplitude was ≥70 mV from threshold to the peak.
Whole cell voltage clamp recording
PCMNs of the NA were again identified in brain stem slices by the presence of fluorescent tracer. Whole cell voltage clamp recordings were made from neurons in the NA. To record calcium (Ca2+)-activated potassium (K+) currents, cells were superfused with an oxygenated HEPES solution containing (in mM) 140 NaCl, 3 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, 0.0005 TTX, 1 4-aminopyridine (4-AP), 0.005 glybenclamide, and 10 glucose, pH adjusted to 7.4 with NaOH. TTX, 4-AP, and glybenclamide were included routinely in the K+ recording solution unless otherwise indicated. 4-AP (1 mM) was used to reduce voltage-gated K+ currents and unmask Ca2+ dependence of K+ currents. A low concentration of 4-AP was chosen to minimize possible nonspecific blockade on other K+ currents. Experiments were conducted at room temperature (22–25°C). Electrodes were heat-polished to a final tip resistance of 2–4 MΩ and filled with pipette solution containing (in mM) 110 K-gluconate, 10 KCl, 5 NaCl, 2 MgCl2, 10 HEPES, 0.5 EGTA, 1 ATP, 0.2 GTP, and 0.1 leupeptin, with pH adjusted to 7.3 with KOH. Capacitance subtraction was used in all recordings. Membrane potential measurements were not corrected for the liquid junction potential (∼15 mV) and the series resistances were in the range of 10–20 MΩ.
Outward currents were evoked by a series of depolarizing steps from −70 to +40 mV in +10 mV increments, using 250 ms for transient current measurement or 800 ms for persistent current measurement. The peak for the transient outward current and steady-state current at 780 ms for the persistent current were measured. Current-voltage (I-V) relationships were then plotted. The mean peak values of outward currents were plotted against membrane potential and was fitted by the Boltzmann equation (Spray et al. 1981). To study the afterhyperpolarization current (IAHP), outward currents were evoked by a 100 ms pulse from a −70 mV holding potential to +10 mV followed by a 1.5 s pulse of −50 mV. The peak amplitude of IAHP was measured as the peak current after the onset of the −50 mV pulse.
Drugs and chemicals
All the channel blockers used in the present study were purchased from Sigma-Aldrich (St. Louis, MO). Blockers were applied by directly adding them to the superfusate from stock solutions.
Data analysis
Data were presented as means ± SE. Body weight, blood glucose levels, membrane properties, spike half-width, AHP, outward currents, IAHP, and decay time constants between two groups were compared using Student's t-test. Spike frequency-current intensity curves, current-voltage curves and AHP-time relationships between groups were compared using two-way ANOVA with repeated measures followed by a Tukey-Kramer post hoc test. P < 0.05 was considered as significant.
RESULTS
General conditions
The body weights and blood glucose levels of the four different groups were measured on the experimental day (Table 1). The blood was sampled from the tail of nonfasting neonates, and blood glucose levels were measured using a blood glucose monitoring system (Nova Biomedical, Waltham, MA). Compared with control group, body weights and blood glucose levels in NMDM and transgenic/surrogate were not significantly different. Body weights and blood glucose levels in surrogate mother groups were significantly higher than in those in the corresponding groups with natural mothers (control and NMDM; P < 0.05). As shown in Zheng et al. (2004), the blood glucose levels of FVB control mice were <200 mg/dl. Therefore we consider that the animals used in the present experiments were all within the normal blood glucose range. Along with the data in Figs. 2, C and D, and 5E, we conclude that the small difference of body weight (range: 3.7–4.8 g) or variation of blood glucose levels (131.9–197.3 mg/dl) among groups did not affect spike frequency and outward currents.
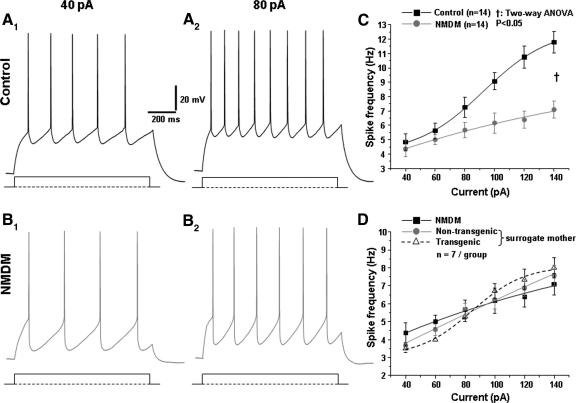
Fig. 2.
Maternal diabetes reduced the excitability of PCMNs in response to current injections. A, 1 and 2: representative PCMN spikes in response to current injections at 40 and 80 pA in a control animal. B, 1 and 2: representative PCMN spikes in response to current injections at 40 and 80 pA in a neonatal mouse with diabetic mother (NMDM). C: mean spike frequency was markedly reduced in NMDM compared with that in control animal (†P < 0.05). D: mean spike frequencies in response to current injections (40–140 pA) were comparable in NMDM (with nontransgenic and transgenic neonates from their own diabetic mothers), nontransgenic neonates fed by surrogate nondiabetic mothers, and transgenic neonates fed by surrogate nondiabetic mothers.
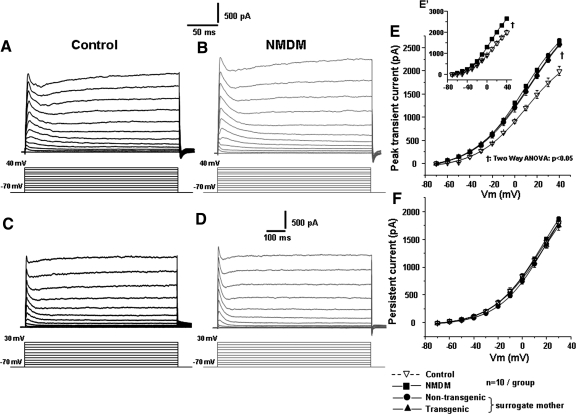
Fig. 5.
Maternal diabetes increased the transient outward current in PCMNs. A: control. The outward currents were evoked by the voltage steps from −70 to +40 mV for 250 ms in 10 mV steps every 5 s to show the transient currents. B: NMDM. The outward currents were evoked by voltage steps using the same protocol as in A. C: control. Outward currents evoked by voltage steps from −70 to +30 mV for 800 ms in 10 mV steps every 5 s to show the persistent currents. D: NMDM. Outward currents evoked by voltage steps using the same protocol as in C. E: the transient outward currents in NMDM significantly increased compared with control (as shown in inset E′, †P < 0.05), but the transient outward currents were similar in NMDM, nontransgenic neonates/surrogate mothers and transgenic neonates/surrogate mothers (as shown in E. P > 0.05). F: the persistent outward currents were not significantly different among the four different groups. Vm: membrane potential. The symbols for different mouse groups are shown at the bottom right of the figure.
Membrane properties
Table 2 lists the membrane properties of PCMNs of control and NMDM groups. The average resting membrane potentials, input resistances, thresholds, and mean amplitudes of APs did not significantly differ between these two groups.
Maternal diabetes reduced spike frequency of PCMNs in response to current injections
In the present study, 126 cells from 38 control mice and 159 cells from 28 NMDM mice were analyzed. All recorded neurons were strongly labeled by the tracer XRITC. Figure 1 shows XRITC labeled PCMNs (Fig. 1A) and one of these labeled neurons was recorded using an electrode pipette (B). Current-induced trains of APs in both groups are shown in Fig. 2, A, 1 and 2, and B, 1 and 2. Trains of APs were evoked using 1 s depolarizing current steps of varying amplitudes (40–140 pA). Spike frequency or firing rate increased as a function of current intensity but was significantly decreased by maternal diabetes (Fig. 2C, P < 0.05). Spike frequencies in response to current injections in transgenic and nontransgenic neonates from surrogate mothers were compared with that in NMDM, and no significant differences were found between these three groups (Fig. 2D, P > 0.05). Thus calmodulin transgene or milk did not significantly alter the excitability of PCMNs. Therefore we only compared the data obtained from control (group 1) and NMDM (group 2) using current clamp in the rest of our experiments.
Maternal diabetes increased the spike half-width and AHP
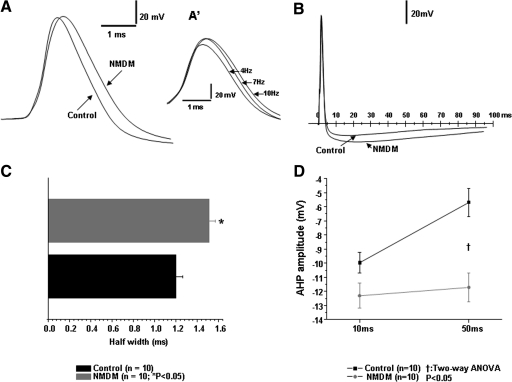
Under physiological conditions, spike broadening has been shown to be frequency dependent in a number of different neurons (Aldrich et al. 1979; Ma and Koester 1996; Quattrocki et al. 1994; Shao et al. 1999). As shown in Fig. 3, A′, inset, the spike widths of the first spike selected from the 4, 7, and 10 Hz AP trains in PCMNs induced by different current intensities of 1,000 ms were different. To standardize AP recordings, the membrane potential was held at −60 mV (below the threshold), and the AP was evoked by a 0.5-ms depolarizing current injection with an intensity that only generated a single spike (Fig. 3, A and B). Compared with control, maternal diabetes significantly increased the spike half-width from 1.20 ± 0.07 to 1.51 ± 0.06 ms (Fig. 3, A and C; P < 0.05). In addition to AP half-width, AHP amplitudes of these neurons were measured. Compared with control, AHP amplitudes at 10 ms and 50 ms were all significantly greater in NMDM, respectively (Fig. 3, B and D; P < 0.05).
Fig. 3.
Maternal diabetes broadened the spike half-width and increased the amplitude of afterhyperpolarization (AHP) of action potentials (AP) in PCMNs. A and C: maternal diabetes significantly broadened the spike half-width (*P < 0.01). Inset A′: the 1st spikes in the AP trains of 4, 7, and 10 Hz that were evoked by adjusting current intensity. The higher the frequency of spike trains, the wider the 1st spike. B and D: maternal diabetes significantly increased the amplitude of AHP at 10 and 50 ms from the onset of AP (†P < 0.05).
Transient and persistent outward currents of PCMNs
The extracellular recording solution contained a mixture of channel blockers: TTX, 0.5 μM, for Na+ current; 4-AP, 1 mM, for transient voltage-gated K+ current; and glybenclamide, 5 μM, for ATP-sensitive K+ current. These agents were added routinely to the recording perfusate unless otherwise stated. To record apamin-sensitive currents, tetraethylammonium (TEA, 1 mM) was also added to suppress voltage-dependent K+ currents.
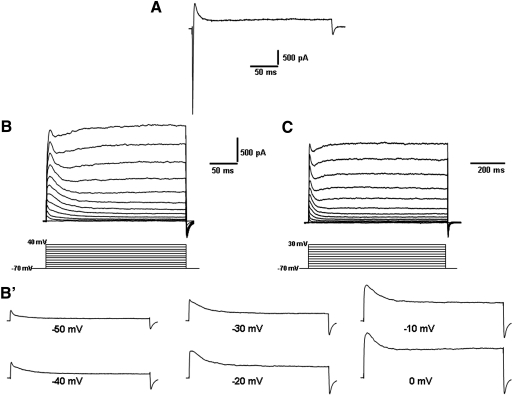
Figure 4A shows the in- and outward currents in a PCMN, evoked by a depolarizing pulse of +10 mV from a holding potential of −70 mV before application of any channel blockers. The inward currents were blocked by TTX. Figure 4B shows a family of outward currents evoked by 250 ms steps from a holding potential of −70 mV to +40 mV in 10 mV increments after application of TTX. Each outward current was composed of a rapidly activating transient component that reached the peak within several ms and then rapidly inactivated, followed by a persistent component (delayed rectifier-type K+-like current). The expanded traces in Fig. 4B′ were from corresponding current traces in B to show individual traces more clearly. Figure 4C includes a family of outward currents from a representative PCMN evoked by 800 ms steps from a holding potential of −70 to +30 mV in 10 mV increments after application of TTX at a compressed time scale to show the persistent currents.
Fig. 4.
Outward currents in PCMNs. A: a current trace was evoked by a voltage step without TTX to show an inward current and an outward current. B: superimposed outward current traces in response to voltage steps (10 mV, 250 ms, every 5 s) from a holding potential of −70 to +40 mV in a bath solution containing 0.5 μM TTX, 1 mM 4-aminopyridine (4-AP), and 5 μM glybenclamide. The inward current was completely blocked. B′: 6 expanded current traces taken from B showing the transient outward current at depolarizing voltages from −50 to 0 mV. C: currents were elicited by depolarizing voltage steps (−70 to +30 mV) from a holding potential of −70 mV (10 mV, 800 ms, 5 s) at a compressed time window to show persistent currents.
Maternal diabetes increased transient outward currents of PCMNs
In Fig. 5, A and B, depolarizing voltage steps of +10 mV from the holding potential of −70 to +40 mV induced a family of outward K+ current traces, including transient currents followed by persistent current traces in both control and NMDM. Compared with control, maternal diabetes significantly increased the peaks of the transient currents in NMDM as shown in the inset (Fig. 5E′) of Fig. 5E. In Fig. 5E, the peaks of the transient currents were almost identical in NMDM, transgenic neonates and nontransgenic neonates from surrogate mothers. Thus calmodulin transgene or milk did not significantly alter the transient outward currents of PCMNs. Therefore we did not differentiate the transgenic and nontransgenic neonates in NMDM in the following experiments. Figure 5, C and D, shows the outward currents in a compressed time scale for measurement of persistent currents. There were no significant differences between the four groups (Fig. 5F). Therefore we did not further examine persistent currents.
Maternal diabetes increased SK transient outward currents
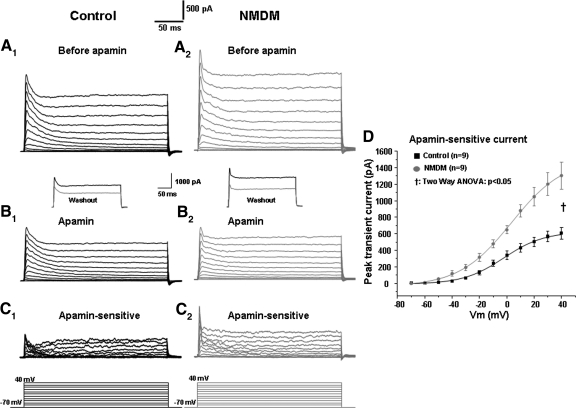
Because maternal diabetes increased transient outward K+ currents, we further examined the effect of maternal diabetes on SK transient currents and SK channel-mediated AP repolarization. To obtain the SK currents, outward currents were first evoked by voltage steps of +10 mV from −70 to +40 mV before apamin application (Fig. 6A, 1 and 2). Following administration of 100 nM apamin (a specific SK channel blocker), the outward currents were evoked again (Fig. 6B, 1 and 2). By subtracting outward currents evoked after apamin application from those before apamin application (Fig. 6, A1 and B1, and A2 and B2), apamin-sensitive currents were obtained (C, 1 and 2). The current-voltage curves are shown in Fig. 6D. Maternal diabetes significantly increased the apamin-sensitive transient currents (P < 0.05, Fig. 6D).
Fig. 6.
Maternal diabetes increased the small conductance Ca2+-activated K+ (SK) transient outward currents in PCMNs: effect of the SK channel blocker apamin. Membrane potential was held at −70 mV and stepped from −70 to +40 mV for 250 ms with 10 mV steps every 5 s as shown in the bottom of the traces. A1–C1: control. Outward currents evoked by voltage steps before (A1) and after (B1) application of apamin. C1: apamin-sensitive current (subtraction of A1 and B1). A2–C2: NMDM. Outward currents evoked by voltage steps before (A2) and after (B2) application of apamin. C2: apamin-sensitive currents (subtraction of A2 and B2). Insets in B1 and B2: the black trace (before apamin) and gray trace (after apamin washout) showing that a well-known irreversible effect after apamin blockade (Wolfart et al. 2001). D: apamin-sensitive transient outward currents were significantly increased in NMDM compared with control (†P < 0.05).
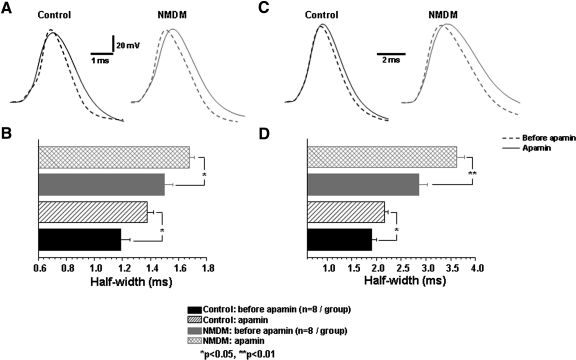
Effect of SK channels on the repolarization half-width of AP was determined by application of apamin. Using current clamp recording, a single AP was first evoked as above. As shown in Fig. 7, A and B, application of apamin significantly increased the spike half-width in both control (before: 1.19 ± 0.07 ms; after: 1.37 ± 0.05 ms; *P < 0.05) and NMDM groups (before: 1.50 ± 0.06 ms; after: 1.68 ± 0.04 ms; *P < 0.05). Under this experimental condition (i.e., 0.5 ms depolarizing current), the increase of spike half-width after application of apamin was comparable between the two groups (control: 13.6 ± 4.1% vs. NMDM: 10.6 ± 4.0%). Under a different experimental condition that used a longer depolarizing current (250 ms; Fig. 7, C and D), application of apamin also significantly increased the spike half-width in both control (before: 1.91 ± 0.1 ms and apamin: 2.16 ± 0.1 ms, *P < 0.05) and NMDM groups (before: 2.85 ± 0.2 ms, after: 3.62 ± 0.2 ms; **P < 0.01). In addition, apamin significantly broadened the spike half-width in NMDM (21.3 ± 0.2%) more than that in control (11.6 ± 0.1%) (n = 8/group, P < 0.05).
Fig. 7.
Apamin application increased spike half-width: effects of 0.5 ms (A and B) and 250 ms (C and D) current injections. A: representative AP traces evoked by a 0.5 ms depolarization current in control and NMDM before (- - -) and after apamin (—) application. B: apamin significantly increased spike half-width in both control and NMDM, (*P < 0.05). The increase of spike half-width after application of apamin was comparable in control and NMDM. C: representative AP traces evoked by a 250 ms depolarization current in control and NMDM before (- - -) and after apamin (—) application. D: apamin significantly increased spike half-width in both control and NMDM (P < 0.05). Apamin significantly broadened the spike half-width in NMDM more than that in control (P < 0.05).
Maternal diabetes increased apamin-sensitive IAHP and augmented the contribution of SK channels to AHP
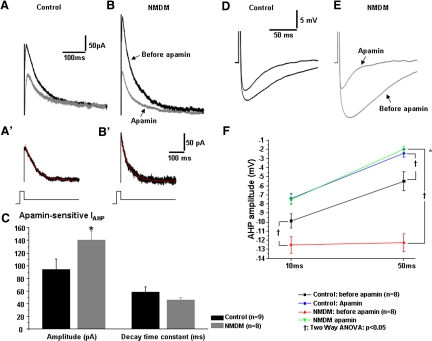
To determine whether maternal diabetes altered the contribution of SK channels to IAHP and AHP, we blocked SK channels with apamin in control and NMDM (Fig. 8, A and B). Apamin application reduced the peak amplitudes of IAHP in the control (Fig. 8A) and NMDM groups (B). The peak amplitudes of IAHP were measured and compared between control and NMDM. The top (dark) traces in Fig. 8, A and B, are representative recordings of IAHP in control and NMDM before apamin was administered. The peak amplitudes of IAHP were significantly increased in NMDM (314.3 ± 47.1 pA) compared with control (186.6 ± 47.0 pA, P < 0.05). Thus maternal diabetes significantly increased IAHP.
Fig. 8.
Maternal diabetes increased apamin afterhyperpolarization (AHP) currents (IAHP) and augmented the contribution of SK channels to AHP. IAHP current was evoked by a depolarizing voltage pulse of +10 mV (100 ms) from holding potential −70 mV followed by a returning −50 mV step. The peak of IAHP was measured. A (control) and B (NMDM). The black trace is the IAHP before apamin, and the gray trace is the IAHP after apamin. A′ (control) and B′ (NMDM). The apamin-sensitive IAHP was obtained by subtracting the current after apamin from the current before apamin. The decay of apamin IAHP was fitted by a single exponential equation (red line). C: maternal diabetes significantly increased apamin-sensitive IAHP (*P < 0.05) but did not change the decay time constant. D and E: representative AHP waveforms of control (D) and NMDM (E) PCMNs before and after apamin. F: apamin significantly reduced AHP in both groups (†P < 0.05). In addition, maternal diabetes induced a significant greater reduction of AHP compared with control (*P < 0.05).
Apamin-sensitive IAHP was obtained by subtracting IAHP after apamin application from the IAHP before apamin (Fig. 8, A′ and B′). Compared with control, the peak amplitude of apamin-sensitive IAHP in NMDM was significantly increased (Fig. 8C, *P < 0.05). The decay time constant of the apamin-sensitive IAHP, as fitted by a single exponential equation, was comparable in control and NMDM (Fig. 8C).
Using current clamp recording, an AP was first evoked by a 0.5-ms depolarizing current of sufficient intensity to generate a single spike. As shown in Fig. 8, D–F, application of apamin significantly decreased the spike AHP in both control and NMDM groups (†P < 0.05). Noticeably, application of apamin induced a significantly greater reduction of spike AHP in NMDM (from −12.5 ± 0.9 to −7.5 ± 0.5 mV at 10 ms and from −12.3 ± 1.0 to −2.0 ± 0.4 mV at 50 ms) than that in control (from −9.9 ± 0.8 to −7.4 ± 0.6 mV at 10 ms and from −5.5 ± 1.1 to −2.4 ± 0.4 mV at 50 ms; †P < 0.05). After blockade of SK channels with apamin, the AHP amplitudes at 10 ms (NMDM: −7.5 ± 0.5 mV; control: −7.4 ± 0.6 mV) and at 50 ms (NMDM: −2.0 ± 0.4 mV; control: −2.4 ± 0.4 mV) were not significantly different.
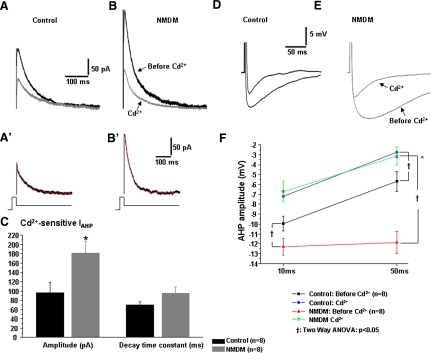
Because SK channels are Ca2+-dependent, we examined whether blockade of Ca2+ may yield a similar effect on IAHP and AHP as seen in apamin application. Therefore we blocked Ca2+ influx using the general Ca2+ channel blocker Cd2+ in control and NMDM. To evoke IAHP, a 100 ms pulse of +10 mV from a holding potential of −70 mV was given and immediately followed by a 1.5 s −50 mV pulse. The peak amplitudes of IAHP were measured and compared between control and NMDM. The top (dark) traces in Figs. 9, A and B, are representative recordings of IAHP in control and NMDM before Cd2+ was administered. As shown in Fig. 8, A and B, maternal diabetes again significantly increased IAHP. Cd2+ bath application reduced the amplitude of IAHP in both control (Fig. 9A) and NMDM groups (B). Cd2+–sensitive IAHP was obtained by the difference of the IAHP before and after application of Cd2+ (Fig. 9A', 9B'). Similar to apamin application, the peak amplitude of Cd2+-sensitive IAHP was significantly increased in NMDM (Fig. 9C, *P < 0.05). The decay time constant of Cd2+-sensitive IAHP, as fitted by a single exponential equation, was comparable in control and NMDM (Fig. 9C).
Fig. 9.
Dependency of maternal diabetes-induced increase of AHP currents (IAHP) and AHP on Ca2+influx in PCMNs. The IAHP current and AHP were evoked and measured as in Fig. 8. In addition, AHP was evoked by a current injection of sufficient intensity to generate a single AP. The peak of AHP was measured at 10 and 50 ms. A: (control) and B (NMDM). The black trace is the IAHP before Cd2+, and the gray trace is the IAHP after Cd2+ application. A′ (control) and B′ (NMDM). The Cd2+–sensitive IAHP was obtained by subtracting the current after Cd2+ from the current before Cd2+ application. The decay of Cd2+–sensitive IAHP was fitted by a single exponential equation (red line). C: maternal diabetes significantly increased Cd2+–sensitive IAHP (*P < 0.05) but did not increase decay time constant. D and E: representative AHP waveforms of control (D) and NMDM (E) PCMNs before and after Cd2+ application. F: Cd2+ significantly reduced AHP in both groups (†P < 0.05). After application of Cd2+, the AHP amplitude at 10 and 50 ms in NMDM was comparable to control, indicating that blockade of Ca2+influx completely abolished maternal diabetes-induced increase of AHP. In addition, maternal diabetes induced a significant greater reduction of AHP compared with control (*P < 0.05).
Using current clamp recording, an AP was first evoked by a 0.5-ms depolarizing current of an intensity which was sufficient to generate a single spike. As shown in Fig. 9, D–F, maternal diabetes significantly increased the spike AHP in NMDM before Cd2+ application at 10 and 50 ms (†P < 0.05; also see Fig. 3, B–D). Cd2+ significantly decreased the spike AHP in both control and NMDM groups (†P < 0.05). Noticeably, consistent with apamin examination, application of Cd2+ induced a significantly greater reduction of spike AHP in NMDM (from −12.3 ± 0.9 to −6.7 ± 1.1 mV at 10 ms and from −11.9 ± 1.1 to −3.1 ± 0.9 mV at 50 ms) than that in control (from −10.0 ± 0.7 to −7.2 ± 0.5 mV at 10 ms and from −5.7 ± 1.0 to −2.7 ± 0.5 mV at 50 ms; P < 0.05; Fig. 9F). After blockade of Ca2+ influx, the AHP amplitude was not significantly different between the two groups.
Apamin increased spike frequency and abolished the difference of spike frequency of PCMNs in response to current injections between control and NMDM
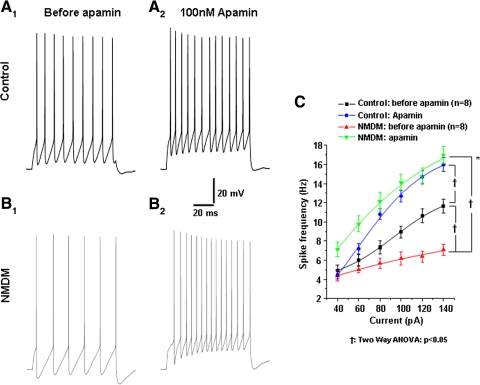
AP trains were evoked by 1 s depolarizing current steps of varying intensities (40–140 pA). Current (100 pA)-induced AP trains before and after apamin application are shown in Fig. 10, A, 1 and 2 (control group) and B, 1 and 2 (NMDM group). Consistent with Fig. 2C, maternal diabetes significantly decreased spike frequency in NMDM compared with control (Fig. 10C, black and red lines). As shown in Fig. 10C, spike frequency increased as a function of current intensity in both control and NMDM. To test the effect of SK channels on excitability, we first blocked SK channels with apamin. Apamin significantly increased spike frequency (†P < 0.05) and largely abolished the spike frequency difference between the two groups (i.e., spike frequency after apamin was not significantly different between control and NMDM).
Fig. 10.
Apamin application abolished the difference in spike frequencies of PCMNs in response to current injections between control and NMDM. A, 1 and 2: control; B, 1 and 2: NMDM. Representative traces of AP trains evoked by 1 s-depolarizing current (100 pA) injection during control aCSF and after apamin application in control and NMDM. C. Before apamin, spike frequency was significantly different in control and NMDM (†P < 0.05), which is consistent with Fig. 2C. After apamin application, spike frequency significantly increased in both groups (†P < 0.05), with a significant greater increase in NMDM compared with control (*P < 0.05). There was no difference of spike frequencies between control and NMDM, indicating the difference of spike frequencies between control and NMDM completely diminished.
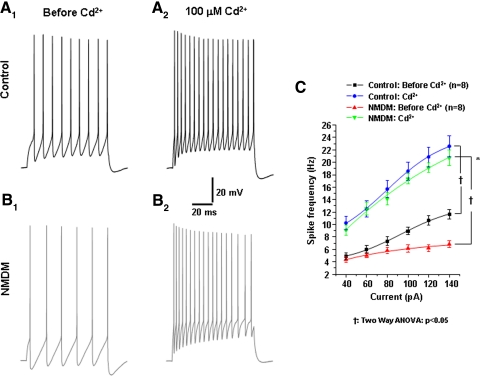
Again because SK channels are Ca2+-dependent, we examined whether blockade of Ca2+ may yield a similar effect on spike frequency to that seen in apamin application. Therefore we blocked Ca2+ influx using Cd2+ in control and NMDM. Similar to apamin, Cd2+ application significantly increased spike frequency in both control (Fig. 11, A, 1 and 2, and C) and NMDM (B, 1 and 2, and C). Cd2+ application diminished the difference of spike frequency between the two groups (i.e., spike frequency after Cd2+ was not significantly different between control and NMDM; Fig. 11C).
Fig. 11.
Effect of blockade of Ca2+ influx on spike frequency of PCMNs in control and NMDM. Cd2+ application abolished the difference in spike frequency of PCMNs in response to current injections between control and NMDM. A, 1 and 2: control; B, 1 and 2: NMDM. Representative traces of AP trains evoked by 1 s depolarizing current (100 pA) injection during control Artificial cerebrospinal fluid and after Cd2+ application in control and NMDM. C. Before Cd2+, spike frequencies were significantly different between control and NMDM (†P < 0.05) which is consistent with Fig. 10C. After Cd2+ application, spike frequency significantly increased in both groups (†P < 0.05), with a significant greater increase in NMDM compared with control (*P < 0.05). There was no difference of spike frequencies between control and NMDM, confirming that the difference of spike frequencies between control and NMDM was Ca2+-dependent.
DISCUSSION
We have demonstrated that maternal diabetes significantly reduced the spike frequency, broadened AP repolarization (increased AP repolarization half-width), and increased AHP amplitude of NA PCMNs in NMDM. These results indicate that maternal diabetes decreases PCMN excitability, possibly due to increased AP broadening and increased AHP. Because SK channels mediate spike AHP and excitability (Stocker 2004), we hypothesized that maternal diabetes alters SK currents. Indeed our data indicated that maternal diabetes increased SK-mediated currents that may contribute to the increased AHP in NMDM. Blockade of SK channels with apamin substantially increased excitability and largely abolished maternal diabetes-induced reduction of spike frequency in NMDM. Therefore we suggest that the increased SK currents contribute to the reduced excitability of PCMNs in NMDM.
Identity of NA cardiac motoneurons
In the present study, we injected tracer XRITC into the pericardial sac at the base of atria at P7–9. After 2 days, cardiac motoneurons in the NA were brightly labeled by XRITC as shown in Fig. 1. One concern is that the NA is a large nucleus that projects to parasympathetic ganglia in the heart as well as other organs near the heart (Bieger and Hopkins 1987). The external formation of NA is the major source projecting to the heart in adult animals (Bieger and Hopkins 1987). However, the external formation could not be clearly identified at the age of P9–11. Therefore whether tracer injection only labeled cardiac motoneurons in the NA was a concern. To minimize the leakage of tracer to other organs, we injected a small amount of tracer (4–8 μl) into the pericardial sac. Using this injection volume at the base of the atria but outside the pericardial sac in preliminary studies, we occasionally found some weakly labeled neurons randomly distributed in the NA region. Thus we only recorded brightly labeled neurons in the NA in the experiment. In such a way, we could optimally avoid noncardiac neurons in the NA that might have been weakly labeled due to leakage of tracer to other organs.
Fast, medium, and slow AHP of PCMNs
In many neurons, AHP has multiple components, including fast, medium, and slow components (fAHP, mAHP, and sAHP). The fAHP that follows a single AP lasts a few to ten ms (Sah 1996; Storm 1989). The mAHP and sAHP have much longer durations (mAHP: from tens to several hundred ms; sAHP: from several hundred ms to seconds) (Faber and Sah 2002; Sah and Faber 2002). The mAHP largely contributes to spike frequency and the sAHP mainly contributes to spike train adaption (Faber and Sah 2002). Unlike other neurons (Saar and Barkai 2003; Saar et al. 2001), we did not differentiate the different types of AHPs because there were not clear boundaries between fAHP, mAHP, and sAHP traces in PCMNs as seen in some other central neurons (Li and Bennett 2007; Stocker et al. 1999). Instead, we measured AHP at 10 and 50 ms in neurons in a solution which did not contain any channel blockers. Approximately 10 ms is in the fast or early medium duration range of AHP, and 50 ms is in the range of medium AHP. Because AHP almost returned to the resting membrane potentials at 100 ms for single spikes in control (Fig. 3B), PCMNs may not have a significant sAHP.
Maternal diabetes reduced excitability, broadened AP repolarization and increased AHP
Because the NA is one of the critical components in regulating cardiac function, we examined excitability and AP properties of PCMNs. Maternal diabetes did not change the passive membrane properties and threshold but significantly reduced the spike frequency in response to current injections, broadened spike, and increased AHP amplitude. Because broadened spike and increased AHP slow the AP recovery, they together may contribute to reduced excitability of PCMNs in NMDM.
Maternal diabetes increased the contribution of SK channels to AHP
In the brain, SK channels are widely expressed in many regions including hippocampal neurons, lateral amygdala neurons, the dorsal motor nucleus and nucleus ambiguus (Faber and Sah 2002; Pedarzani et al. 2000; Sailer et al. 2004; Stocker 2004; Stocker and Pedarzani 2000). Consistent with these studies, we have shown that NA PCMNs do have SK channels in this study and Lin et al. (2010c). Previously, it has been reported that SK channels contribute more to mAHP without significant contribution to AP repolarization in other central neurons (Faber and Sah 2002; Stocker 2004). In our study, we demonstrated that SK channels contribute significantly to AP repolarization because apamin blockade of SK channels induces a significant increase in spike half-width of PCMNs in both control and NMDM. Further, we found maternal diabetes increased SK transient outward currents, which may contribute to augmented transient outward currents in NMDM.
One concern may be that apamin may alter the AP depolarizing input resistance (Rinput). We have calculated the Rinput, which was defined as the ratio of membrane potential change (ΔVm) relative to membrane potential baseline and current injection increase (ΔI) at the beginning of the first AP in a ramp test. During apamin application, Rinput decreased in NMDM, whereas it increased in control as compared with that before apamin application (data not shown here). According to Ohm's law (outward currents = ΔVm /Rinput), for the same ΔVm, the outward currents increased during apamin application because apamin decreased Rinput in NMDM compared with that without apamin. Thus the actual value of apamin-sensitive currents obtained from subtraction of the currents before and after apamin application should be smaller than what we have shown here in NMDM. In contrast, because apamin application increased Rinput in control compared with that without apamin, the actual value of the apamin-sensitive currents obtained from subtraction of the currents before and after apamin application should be larger than what we have shown here in control. Therefore the conclusion that maternal diabetes increases the apamin-sensitive currents as shown in Fig. 6 should be a conservative estimate and correct. At this time, we cannot explain why apamin application increases Rinput in control but decreases it in NMDM.
Because transient outward currents may contribute to repolarization (Giese et al. 1998; Ma and Koester 1996; Wilson et al. 2002), we had initially postulated that maternal diabetes might have decreased SK transient outward currents as an explanation for why maternal diabetes induced an increase of spike half-width. Opposite to our assumption, our data showed that maternal diabetes actually increased the SK transient outward currents. If transient outward currents were indeed to contribute to repolarization, then we would expect that the increased SK transient outward currents were associated with a larger percentage increase of spike half-width in NMDM than in control. However, our data in Fig. 7, A and B, show that the percentage increase of spike half-width in NMDM and control were comparable under the experimental conditions to evoke single APs using the depolarizing current of 0.5 ms. However, using a longer depolarizing current (250 ms) to evoke single APs, we found that maternal diabetes significantly increased the percentage of spike broadening in NMDM more than that in control (Fig. 7, C and D). Therefore the increased SK outward currents in NMDM might have a significant effect on AP repolarization. Nevertheless the increased SK channel outward currents could not explain the overall AP broadening in NMDM. Although the increased SK outward currents could not explain the overall AP broadening, they may contribute to the increased AHP in NMDM because transient outward currents were reported to contribute to AHP (Giese et al. 1998; Wilson et al. 2002).
Because SK channel-mediated IAHP contributes to AHP in many central neurons (Lancaster and Adams 1986; Lancaster and Nicoll 1987; Sah 1996; Sah and McLachlan 1992; Stocker et al. 1999; Stocker 2004), we then examined the effect of maternal diabetes on SK channel-mediated IAHP and the effect of blockade of SK channels on AHP in control and NMDM. We found that maternal diabetes increased SK channel-mediated IAHP and augmented AHP at 10 ms (fAHP) and 50 ms (mAHP). Thus we suggest that maternal diabetes increases SK channel IAHP, and this may in turn contribute to the increased AHP in NMDM. Very importantly, blockade of SK channels with apamin completely abolished the maternal diabetes-induced increase of AHP at 10 ms (fAHP) and 50 ms (mAHP), strongly suggesting that SK channels play a major role in maternal diabetes-induced increase of AHP. In our study, we also used the Ca2+-channel antagonist Cd2+ to block Ca2+ influx. Similar to apamin application, Cd2+ application increased Cd2+-sensitive IAHP and completely diminished the difference of AHP at 10 ms (fAHP) and 50 ms (mAHP) to current injections between control and NMDM, This confirms that Ca2+-activated K+ channels (most likely SK channels) play a major role in maternal diabetes-induced increase in AHP of PCMNs. As to whether other Ca2+-activated K+ channels (BK) contribute to maternal diabetes-induced changes in AHP should be investigated (Lin et al. 2010a).
In this study we used two different methods to examine the effect of maternal diabetes on SK currents (transient currents and IAHP). We found that both SK transient currents and IAHP increased in NMDM, which may contribute to maternal diabetes-increased AHP.
Maternal diabetes-induced reduction of excitability in PCMNs attributes to increased SK currents
It has been well established that blockade of SK channels reduces AHP and increases firing frequency in other central neurons and that increasing SK currents with channel enhancers increases AHP and reduces firing frequency (Stocker 2004). Consistent with this report, we found that apamin application substantially increased spike firing frequency in both control and NMDM in the present study. Very importantly, apamin application completely abolished the difference of spike frequency responses to current injections between control and NMDM, strongly suggesting that SK channels play a major role in maternal diabetes-induced decrease in excitability of PCMNs. In our study, we also used the Ca2+-channel antagonist Cd2+ to block the Ca2+ influx. Similar to apamin application, Cd2+ application also completely eliminated the difference of spike frequency response to current injections between control and NMDM, confirming that Ca2+-activated K+ channels (most likely SK channels) play a major role in maternal diabetes-induced decrease in excitability of PCMNs. As to whether other Ca2+-activated K+ channels contribute to maternal diabetes-induced changes in excitability should be investigated (Lin et al. 2010a).
Summary
In this study, we found that maternal diabetes significantly reduced spike frequency, broadened the AP, and increased the AHP amplitude of NA PCMNs in NMDM. In addition, we demonstrated that maternal diabetes increased SK channel-mediated currents, which may likely contribute to the increased AHP in NMDM. Blockade of SK channels with apamin almost completely diminished the maternal diabetes-induced difference in AHP and excitability between control and NMDM. Therefore we conclude that augmented SK currents may play an important role in maternal diabetes-induced reduction of excitability of PCMNs in NMDM.
GRANTS
This work was supported by NIH Grants HL-79636 and AG-021020.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- Aldrich et al., 1979.Aldrich RW, Getting PA, Thompson SH. Mechanism of frequency-dependent broadening of molluscan neuron soma spikes. J Physiol 291: 531–544, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieger and Hopkins, 1987.Bieger D, Hopkins DA. Viscerotopic representation of the upper alimentary tract in the medulla oblongata in the rat: the nucleus ambiguus. J Comp Neurol 262: 546–562, 1987 [DOI] [PubMed] [Google Scholar]
- Bond et al., 1999.Bond CT, Maylie J, Adelman JP. Small-conductance calcium-activated potassium channels. Ann NY Acad Sci 868: 370–378, 1999 [DOI] [PubMed] [Google Scholar]
- Castle et al., 1989.Castle NA, Haylett DG, Jenkinson DH. Toxins in the characterization of potassium channels. Trends Neurosci 12: 59–65, 1989 [DOI] [PubMed] [Google Scholar]
- Cheng et al., 2002.Cheng Z, Guo SZ, Lipton AJ, Gozal D. Domoic acid lesions in nucleus of the solitary tract: time-dependent recovery of hypoxic ventilatory response and peripheral afferent axonal plasticity. J Neurosci 22: 3215–3226, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng and Powley, 2000.Cheng Z, Powley TL. Nucleus Ambiguus projections to cardiac ganglia of rat atria: an anterograde tracing study. J Comp Neurol 424: 588–606, 2000 [PubMed] [Google Scholar]
- Cheng et al., 2004a.Cheng Z, Zhang H, Guo SZ, Wurster R, Gozal D. Differential control over vagal efferent postganglionic neurons in rat intrinsic cardiac ganglia by neurons in the nucleus ambiguus and the dorsal motor nucleus of the vagus: anatomical evidence. Am J Physiol Regulatory Integrative Comp Physiol 286: R625–R633, 2004a [DOI] [PubMed] [Google Scholar]
- Cheng et al., 2004b.Cheng Z, Zhang H, Yu J, Wurster R, Gozal D. Attenuation of baroreflex sensitivity following domoic acid lesion of the nucleus ambiguus of rats. J Appl Physiol 96: 1137–1145, 2004b [DOI] [PubMed] [Google Scholar]
- Cingolani et al., 2002.Cingolani LA, Gymnopoulos M, Boccaccio A, Stocker M, Pedarzani P. Developmental regulation of small-conductance Ca2+-activated K+ channel expression and function in rat Purkinje neurons. J Neurosci 22: 4456–4467, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloues and Sather, 2003.Cloues RK, Sather WA. Afterhyperpolarization regulates firing rate in neurons of the suprachiasmatic nucleus. J Neurosci 23: 1593–1604, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constanti and Sim, 1987.Constanti A, Sim JA. Calcium-dependent potassium conductance in guinea pig olfactory cortex neurons in vitro. J Physiol 387: 173–194, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein et al., 1989.Epstein PN, Overbeek PA, Means AR. Calmodulin-induced early onset diabetes in transgenic mice. Cell 58: 1067–1073, 1989 [DOI] [PubMed] [Google Scholar]
- Epstein et al., 1992.Epstein PN, Ribar TJ, Decker GL, Yaney G, Means AR. Elevated B-cell calmodulin produces a unique insulin secretory defect in transgenic mice. Endocrinology 130: 1387–1393, 1992 [DOI] [PubMed] [Google Scholar]
- Faber et al., 2005.Faber ES, Delaney AJ, Sah P. SK channels regulate excitatory synaptic transmission and plasticity in the lateral amygdala. Nat Neurosci 8: 635–641, 2005 [DOI] [PubMed] [Google Scholar]
- Faber and Sah, 2002.Faber ESL, Sah P. Physiological role of calcium-activated potassium currents in the rat lateral amygdala. J Neurosci 22: 1618–1628, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber and Sah, 2007.Faber ES, Sah P. Functions of SK channels in central neurons. Clin Exp Pharmacol Physiol 34: 1077–1083, 2007 [DOI] [PubMed] [Google Scholar]
- Fiorentini et al., 2005.Fiorentini A, Perciaccante A, Paris A, Serra P, Tubani L. Circadian rhythm of autonomic activity in non diabetic offsprings of type 2 diabetic patients. Cardiovasc Diabetol 4: 15–22, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanger et al., 2001.Fanger CM, Rauer H, Neben AL, Miller MJ, Rauer H, Wulff H, Rosa JC, Ganellin CR, Chandy KG, Cahalan MD. Calcium-activated potassium channels sustain calcium signaling in T lymphocytes. Selective blockers and manipulated channel expression levels. J Biol Chem 276: 12249–12256, 2001 [DOI] [PubMed] [Google Scholar]
- Gao and Gao, 2007.Gao Q, Gao YM. Hyperglycemic condition disturbs the proliferation and cell death of neural progenitors in mouse embryonic spinal cord. Int J Dev Neurosci 25: 349–357, 2007 [DOI] [PubMed] [Google Scholar]
- Giese et al., 1998.Giese KP, Storm JF, Reuter D, Fedorov NB, Shao LR, Leicher T, Pongs O, Silva AJ. Reduced K+ channel inactivation, spike broadening, and after-hyperpolarization in Kvbeta1. 1-deficient mice with impaired learning. Learn Mem. 5: 257–273, 1998 [PMC free article] [PubMed] [Google Scholar]
- Hallworth et al., 2003.Hallworth NE, Wilson CJ, Bevan MD. Apamin-sensitive small conductance calcium-activated potassium channels, through their selective coupling to voltage-gated calcium channels, are critical determinants of the precision, pace, and pattern of action potential generation in rat subthalamic nucleus neurons in vitro. J Neurosci 23: 7525–42, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder et al., 2003.Harder T, Franke K, Fahrenkrog S, Aerts L, Van Bree R, Van Assche FA, Plagemann A. Prevention by maternal pancreatic islet transplantation of hypothalamic malformation in offspring of diabetic mother rats is already detectable at weaning. Neurosci Lett 352: 163–166, 2003 [DOI] [PubMed] [Google Scholar]
- Hockett et al., 2004.Hockett PK, Emery SC, Hansen L, Masliah E. Evidence of oxidative stress in the brains of fetuses with CNS anomalies and islet cell hyperplasia. Pediatr Dev Pathol 7: 370–9, 2004 [DOI] [PubMed] [Google Scholar]
- Ishii et al., 1997.Ishii TM, Silvia C, Hirschberg B, Bond CT, Adelman JP, Maylie J. A human intermediate conductance calcium-activated potassium channel. Proc Natl Acad Sci USA 94: 11651–11656, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rovere et al., 2008.La Rovere MT, Pinna GD, Raczak G. Baroreflex sensitivity: measurement and clinical implications. Ann Noninvasive Electrocardiol 13: 191–207, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster and Adams, 1986.Lancaster B, Adams PR. Calcium-dependent current generating the afterhyperpolarization of hippocampal neurons. J Neurophysiol 55: 1268–1282, 1986 [DOI] [PubMed] [Google Scholar]
- Lancaster and Pennefather, 1987.Lancaster B, Pennefather P. Potassium currents evoked by brief depolarizations in bull-frog sympathetic ganglion cells. J Physiol 387: 519–548, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster and Nicoll, 1987.Lancaster B, Nicoll RA. Properties of two calcium-activated hyperpolarizations in rat hippocampal neurones. J Physiol 389: 187–204, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li and Bennett, 2007.Li X, Bennett DJ. Apamin-sensitive calcium-activated potassium currents (SK) are activated by persistent calcium currents in rat motoneurons. J Neurophysiol 97: 3314–3330, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin et al., 2010a.Lin M, Chen QH, Li L, Wurster RD, Liu YQ, Cheng Z. Maternal diabetes (MD) increases large conductance Ca2+-activated K+ (BK) currents which alter action potential (AP) properties but do not affect excitability of parasympathetic cardiac motoneurons (PCMNs) in the nucleus ambiguus (NA) of neonatal mice (Abstract). Exp Biol 7205, 2010a [Google Scholar]
- Lin et al., 2010b.Lin M, Chen QH, Wurster RD, Li L, Harden SW, Liu YQ, Cheng Z. Maternal diabetes increases small conductance Ca2+-activated K+ (SK) currents which alter action potential properties and excitability of parasympathetic cardiac motoneurons (PCMNs) in the nucleus ambiguus (NA) of neonatal mice (Abstract). Expl Biol 4560, 92802, 2010b [Google Scholar]
- Loewy and Spyer, 1990.Loewy AD, Spyer KM. Central Regulation of Autonomic Functions. New York: Oxford, 1990 [Google Scholar]
- Logsdon et al., 1997.Logsdon NJ, Kang J, Togo JA, Christian EP, Aiyar J. A novel gene, hKCa4, encodes the calcium-activated potassium channel in human T lymphocytes. J Biol Chem 272: 32723–6, 1997 [DOI] [PubMed] [Google Scholar]
- Ma and Koester, 1996.Ma M, Koester J. The role of K+ currents in frequency-dependent spike broadening in Aplysia R20 neurons: a dynamic-clamp analysis. J Neurosci 16: 4089–4101, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff et al., 2003.Neff RA, Wang J, Baxi S, Evans C, Mendelowitz D. Respiratory sinus arrhythmia: endogenous activation of nicotinic receptors mediates respiratory modulation of brain stem cardioinhibitory parasympathetic neurons. Circ Res 93: 565–572, 2003 [DOI] [PubMed] [Google Scholar]
- Ngo-Anh et al., 2005.Ngo-Anh TJ, Bloodgood BL, Lin M, Sabatini BL, Maylie J, Adelman JP. SK channels and NMDA receptors form a Ca2+-mediated feedback loop in dendritic spines. Nat Neurosci 8: 642–649, 2005 [DOI] [PubMed] [Google Scholar]
- Pedarzani et al., 2000.Pedarzani P, Kulik A, Muller M, Ballanyi K, Stocker M. Molecular determinants of Ca2+-dependent K+ channel function in rat dorsal vagal neurones. J Physiol 527: 283–290, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedarzani and Stocker, 2008.Pedarzani P, Stocker M. Molecular and cellular basis of small—and intermediate-conductance, calcium-activated potassium channel function in the brain. Cell Mol Life Sci 65: 3196–3217, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quattrocki et al., 1994.Quattrocki EA, Marshall J, Kaczmarek LK. A Shab potassium channel contributes to action potential broadening in peptidergic neurons. Neuron 12: 73–86, 1994 [DOI] [PubMed] [Google Scholar]
- Ramos-Arroyo et al., 1992.Ramos-Arroyo MA, Rodriguez-Pinilla E, Cordero JF. Maternal diabetes: the risk for specific birth defects. Eur J Epidemiol 8: 503–508, 1992 [DOI] [PubMed] [Google Scholar]
- Saar and Barkai, 2003.Saar D, Barkai E. Long-term modifications in intrinsic neuronal properties and rule learning in rats. Eur J Neurosci 17: 2727–2734, 2003 [DOI] [PubMed] [Google Scholar]
- Saar et al., 2001.Saar D, Grossman Y, Barkai E. Long lasting cholinergic modulation underlies rule learning in rats. J Neuorsci 21: 1385–1392, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah, 1996.Sah P. Ca2+-activated K+ currents in neurons: types, physiological roles and modulation. Trends Neurosci 19: 150–154, 1996 [DOI] [PubMed] [Google Scholar]
- Sah and Faber, 2002.Sah P, Faber ES. Channels underlying neuronal calcium-activated potassium currents. Prog Neurobiol 66: 345–53, 2002 [DOI] [PubMed] [Google Scholar]
- Sah and McLachlan, 1992.Sah P, McLachlan EM. Potassium currents contributing to action potential repolarization and the afterhyperpolarization in rat vagal motoneurons. J Neurophysiol 68: 1834–1841, 1992 [DOI] [PubMed] [Google Scholar]
- Sailer et al., 2004.Sailer CA, Kaufmann WA, Marksteiner J, Knaus HG. Comparative immunohistochemical distribution of three small-conductance Ca2+-activated potassium channel subunits, SK1, SK2, and SK3 in mouse brain. Mol Cell Neurosci 26: 458–69, 2004 [DOI] [PubMed] [Google Scholar]
- Sanchez and McManus, 1996.Sanchez M, McManus OB. Paxilline inhibition of the alpha-subunit of the high-conductance calcium-activated potassium channel. Neuropharmacology 35: 963–968, 1996 [DOI] [PubMed] [Google Scholar]
- Schwindt et al., 1988.Schwindt PC, Spain WJ, Foehring RC, Stafstrom CE, Chubb MC, Crill WE. Multiple potassium conductances and their functions in neurons from cat sensorimotor cortex in vitro. J Neurophysiol 59: 424–49, 1988 [DOI] [PubMed] [Google Scholar]
- Shao et al., 1999.Shao LR, Halvorsrud R, Borg-Graham L, Storm JF. The role of BK-type Ca2+-dependent K+ channels in spike broadening during repetitive firing in rat hippocampal pyramidal cells. J Physiol) 521: 135–146, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spray et al., 1981.Spray DC, Harris AL, Bennett MV. Equilibrium properties of a voltage-dependent junctional conductance. J Gen Physiol 77: 77–93, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackman et al., 2002.Stackman RW, Hammond RS, Linardatos E, Gerlach A, Maylie J, Adelman JP, Tzounopoulos T. Small conductance Ca2+-activated K+ channels modulate synaptic plasticity and memory encoding. J Neurosci 22: 10163–10171, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker, 2004.Stocker M. Ca2+-activated K+ channels: molecular determinants and function of the SK family. Nat Rev Neurosci 5: 758–770, 2004 [DOI] [PubMed] [Google Scholar]
- Stocker et al., 1999.Stocker M, Krause M, Pedarzani P. An apamin-sensitive Ca2+-activated K+ current in hippocampal pyramidal neurons. Proc Natl Acad Sci USA 96: 4662–4667, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker and Pedarzani, 2000.Stocker M, Pedarzani P. Differential distribution of three Ca2+-activated K+ channel subunits, SK1, SK2, and SK3, in the adult rat central nervous system. Mol Cell Neurosci 15: 476–93, 2000 [DOI] [PubMed] [Google Scholar]
- Storm, 1987.Storm JF. Action potential repolarization and a fast after-hyperpolarization in rat hippocampal pyramidal cells. J Physiol) 385: 733–759, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm, 1989.Storm JF. An after-hyperpolarization of medium duration in rat hippocampal pyramidal cells. J Physiol 409: 171–190, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm, 1990.Storm JF. Potassium currents in hippocampal pyramidal cells. Prog Brain Res 83: 161–187, 1990 [DOI] [PubMed] [Google Scholar]
- Strøbaek et al., 1996.Strøbaek D, Christophersen P, Holm NR, Moldt P, Ahring PK, Johansen TE, Olesen SP. Modulation of the Ca2+-dependent K+ channel, hslo, by the substituted diphenylurea NS 1608, paxilline and internal Ca2+. Neuropharmacology 35: 903–914, 1996 [DOI] [PubMed] [Google Scholar]
- Tharp and Bowles, 2009.Tharp DL, Bowles DK. The intermediate-conductance Ca2+ -activated K+ channel (KCa3.1) in vascular disease. Cardiovasc Hematol Agents Med Chem 7: 1–11, 2009 [DOI] [PubMed] [Google Scholar]
- Vergara et al., 1998.Vergara C, Latorre R, Marrion NV, Adelman JP. Calcium-activated potassium channels. Curr Opin Neurobiol 8: 321–329, 1998 [DOI] [PubMed] [Google Scholar]
- Vogalis et al., 2002.Vogalis F, Harvey JR, Neylon CB, Furness JB. Regulation of K+ channels underlying the slow afterhyperpolarization in enteric afterhyperpolarization-generating myenteric neurons: role of calcium and phosphorylation. Clin Exp Pharmacol Physiol 29: 935–43, 2002 [DOI] [PubMed] [Google Scholar]
- Wichi et al., 2005.Wichi RB, Souza SB, Casarini DE, Morris M, Barreto-Chaves ML, Irigoyen MC. Increased blood pressure in the offspring of diabetic mothers. Am J Physiol Regulatory Integrative Comp Physiol 288: R1129–1133, 2005 [DOI] [PubMed] [Google Scholar]
- Wilson et al., 2002.Wilson JM, Coderre E, Renaud LP, Spanswick D. Active and passive membrane properties of rat sympathetic preganglionic neurones innervating the adrenal medulla. J Physiol 545: 945–60, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfart et al., 2001.Wolfart J, Neuhoff H, Franz O, Roeper J. Differential expression of the small-conductance, calcium-activated potassium channel SK3 Is critical for pacemaker control in dopaminergic midbrain neurons. J Neurosci 21: 3443–3456, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack and Khodakhah, 2003.Womack MD, Khodakhah K. Somatic and dendritic small-conductance calcium-activated potassium channels regulate the output of cerebellar purkinje neurons. J Neurosci 23: 2600–2607, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young and Morrison, 1998.Young JB, Morrison SF. Effects of fetal and neonatal environment on sympathetic nervous system development. Diabetes Care 21, Suppl 2: B156–160, 1998 [PubMed] [Google Scholar]
- Zheng et al., 2004.Zheng S, Noonan WT, Metreveli NS, Coventry S, Kralik PM, Carlson EC, Epstein PN. Development of late-stage diabetic nephropathy in OVE26 diabetic mice. Diabetes 53: 3248–3257, 2004 [DOI] [PubMed] [Google Scholar]