Abstract
Activation of the medial medulla is responsible for rapid eye movement (REM) sleep atonia and cataplexy. Dysfunction can cause REM sleep behavior disorder and other motor pathologies. Here we report the behavioral effects of stimulation of the nucleus gigantocellularis (NGC) and nucleus magnocellularis (NMC) in unrestrained cats. In waking, 62% of the medial medullary stimulation sites suppressed muscle tone. In contrast, stimulation at all sites, including sites where stimulation produced no change or increased muscle tone in waking, produced decreased muscle tone during slow-wave sleep. In the decerebrate cat electrical stimulation of the NGC increased glycine and decreased norepinephrine (NE) release in the lumbar ventral horn, with no change in γ-aminobutyric acid (GABA) or serotonin (5-HT) release. Stimulation of the NMC increased both glycine and GABA release and also decreased both NE and 5-HT release in the ventral horn. Glutamate levels in the ventral horn were not changed by either NGC or NMC stimulation. We conclude that NGC and NMC play neurochemically distinct but synergistic roles in the modulation of motor activity across the sleep–wake cycle via a combination of increased release of glycine and GABA and decreased release of 5-HT and NE. Stimulation of the medial medulla that elicited muscle tone suppression also triggered rapid eye movements, but never produced the phasic twitches that characterize REM sleep, indicating that the twitching and rapid eye movement generators of REM sleep have separate brain stem substrates.
INTRODUCTION
The rostral medial medulla, including the dorsally located gigantocellularis (NGC) and the ventrally located magnocellularis (NMC) nuclei, has been implicated in the modulation of motor activity. Electrical and chemical stimulation of these regions suppresses reflex activities and muscle tone in decerebrate animals (Hajnik et al. 2000; Karlsson and Blumberg 2005; Kohyama et al. 1998; Lai and Siegel 1988; Lai et al. 1987; Magoun and Rhines 1946). Siegel et al. (1979) and Kanamori et al. (1980) reported that a group of neurons in the medial medulla increases activity during muscle tone reductions in rapid eye movement (REM) sleep and waking. Damage to this region increases tonic muscle tone during REM sleep (Holmes and Jones 1994; Schenkel and Siegel 1989). Thus the medial medulla has been hypothesized to be involved in the generation and maintenance of REM sleep atonia (Sakai et al. 1981; Siegel 1979). In contrast, Sprague and Chambers (1954) reported that electrical stimulation of the medial medulla increases motor activity in awake, chronic animals. However, rapid suppression of muscle tone with stimulation of the medial medulla in sleep in intact, unrestrained behaving animals has not been reported. The goal of the first part of our study was to address the functional role of the medial medulla in the control of motor activity across the sleep–wake cycle.
Chase and colleagues (1986) found that electrical stimulation of the NGC hyperpolarizes spinal and trigeminal motoneurons during natural REM sleep or the REM sleeplike state induced by pontine microinjection of carbachol. They also found that this hyperpolarization can be blocked by iontophoretic injection of the glycine receptor antagonist, strychnine, but not by the γ-aminobutyric acid type A (GABAA) receptor antagonists, picrotoxin or bicuculline (Chase et al. 1986; Soja et al. 1987). They hypothesized that glycinergic mechanisms play an important role in the NGC's regulation of muscle tone in REM sleep. The neurochemical mechanism underlying NMC's suppression of muscle tone remains unclear. We have demonstrated that muscle atonia induced by carbachol injection into the pontine inhibitory area (PIA) can be reversed by injection of the glutamate receptor antagonist γ-d-glutamylglycine into the NMC in the decerebrate cat (Lai and Siegel 1988). This indicates that activation of the NMC via a glutamatergic mechanism is required for muscle atonia induced by stimulation of the PIA. Indeed, electrophysiological studies demonstrated that REM-on neurons in the pons project to the NMC, which in turn projects to the spinal cord (Kanamori et al. 1980; Sakai et al. 1981). Our previous studies showed that activation of the PIA increases GABA and glycine release and decreases norepinephrine (NE) and serotonin (5-HT) release into the ventral horn (Kodama et al. 2003; Lai et al. 2001). This work therefore suggests that activation of the NMC may mediate aspects of this pattern of neurotransmitter release.
METHODS
Twenty-five adult cats of either sex, weighing 2.5–3.5 kg, were used, 12 for the chronic behavioral study and 13 for the decerebrate dialysis study. All experimental procedures were approved by the Animal Research Committee of the VA Greater Los Angeles Healthcare System.
Preparation for sleep recording and electrical stimulation in the chronic animal
Electrode implantation for standard sleep recording was performed under isoflurane (1.5%) anesthesia. Animals were first given a mixture of acepromazine, atropine, and buprenorphine (0.1/0.05/0.01 mg/kg, administered subcutaneously) to induce anesthesia, and then, a mixture of ketamine and diazepam (10/1 mg/kg, administered intravenously [iv]) for tracheal intubation. The trachea was intubated with an endotracheal tube to supply isoflurane (1.5%) and facilitate the monitoring of respiration. Stainless steel screw electrodes were implanted for electroencephalogram (EEG) and electrooculogram (EOG) recording. Stranded stainless steel wires (A-M Systems, Carlsborg, WA) were inserted into the dorsal neck muscles for electromyographic (EMG) recording and tripolar electrodes were implanted into the lateral geniculate body for ponto-geniculo-occipital (PGO) wave recording. Five pairs of 23 gauge stainless steel guide cannulae were placed straddling the midline at the medullary level. The tips of guide cannulae were placed 1.0 mm dorsal to the cerebellar surface.
One week after recovery from surgery, animals were adapted to a sound-proof, temperature- and humidity-regulated recording chamber with lights on at 7 am and off at 7 pm. A bipolar electrode (SNE-100, 2 MΩ AC impedance; Rhodes) was inserted through one of the guide cannulae into the medial medulla for electrical stimulation 1 h before the experiment. Electrical stimulation consisted of 300- to 700-ms trains of 0.2-ms rectangular cathodal pulses. The intensity of stimulation was varied from 10 μA to 150 μA using the minimal level required for eliciting change in muscle tone. The frequency of stimulation was 100–150 Hz (Lai et al. 1987). Electrical stimulations were administered during waking, slow-wave sleep (SWS), and REM sleep. Sleep–wake states, which were determined according to Ursin and Sterman (1981), were recorded through a Grass polygraph (Model 78E, Astro-Med, West Warwick, RI). EEG (1–100 Hz), EOG (1–100 Hz), EMG (100–1,000 Hz), and PGO (1–100 Hz) signals were amplified by a Grass preamplifier (Model 7P511J). EMG signals were also integrated through a Grass polygraph integrator (Model 7P10F). All recordings were displayed on a polygraph and recorded on FM magnetic tape for later analysis.
Preparation for in vivo microdialysis in the decerebrate animal
Animals were first anesthetized with thiopental (20 mg/kg, iv) for tracheal intubation. They were then given isoflurane (1.5%) for arterial cannulation and decerebration at the precollicular-postmammillary level. Laminectomy was performed on the lumbar vertebral segments, L4–L6, to expose the lumbar 7 and sacral 1 spinal cord for insertion of dialysis probes. Isoflurane was then discontinued. EMGs were recorded from the neck (occipitoscapularis and splenius) muscles using bipolar electrodes, as described in the chronic study. Blood pressure was measured through a Gould–Statham pressure transducer. Mean arterial pressure was kept at >80 mmHg. Rectal temperature was maintained at 37.5 ± 1°C by a heating pad controlled by a thermoregulator.
Delivery of 500-ms trains with 100-Hz, 0.2-ms, and 10- to 40-μA rectangular cathodal pulses was through stainless steel microelectrodes (Model 5710, AC impedance: 5 MΩ; A-M Systems) in the NGC and NMC. Train stimulations were delivered into the brain stem every 10 s over a period of 5 min. Microdialysis probes were inserted into the spinal ventral horn bilaterally 2 h before sampling. These probes (59–7005; Harvard Apparatus, South Natick, MA) had a tip length of 2 mm. Microdialysis probes were perfused with artificial cerebrospinal fluid (Harvard Apparatus) at a flow rate of 4 μl/min using an infusion pump (EPS-64; Eicom, Kyoto, Japan). Dialysates were collected for 5-min intervals during the prestimulation, stimulation, and poststimulation period. Dialysates were kept under acidic conditions (pH 3.5). The collecting polypropylene tubing was kept at 10°C and the dialysate was stored at −80°C, until analysis.
HPLC analysis
AMINO ACIDS ASSAY.
The concentration of amino acid in the perfusate was measured by high-performance liquid chromatography (HPLC; EDT-300, Eicom), with fluorescence detection (Soma S-3350; excitation at 340 nm, emission at 440 nm), and quantified with a PowerChrom (AD Instruments, Sydney, Australia) using external amino acid standards (Sigma, St. Louis, MO). Precolumn derivatization was performed with o-phthaldialdehyde/2-mercaptoethanol by an autosampler (Model 231XL; Gilson) at 10°C for 3 min. The derivatives were then separated in a liquid chromatography column (MA-5ODS; Eicom) at 30°C with 30% methanol in 0.1 M phosphate buffer (pH 6.0), being degassed by an on-line degasser (DG-100; Eicom). The detection limits for glutamate, GABA, and glycine were 20 femtomoles (fmol).
NOREPINEPHRINE AND SEROTONIN ASSAY.
The norepinephrine (NE) and serotonin (5-HT) levels in the dialysates were determined by HPLC with electrochemical detection (450 mV; DTA-300, Eicom). The mobile phase for 5-HT consisted of 80% 100 mM phosphate buffer (pH 6.0) and 20% methanol, containing 500 mg/L octanesulfonic acid and 50 mg/L EDTA-2Na. To detect NE, a mobile phase of 95% 100 mM phosphate buffer (pH 6.0) and 5% methanol, containing 400 mg/L octanesulfonic acid and 50 mg/L EDTA-2Na was used. The detection limit of our analysis system for NE and 5-HT was 0.5 fmol per 20 μl injection.
Histology
In chronic animals, an anodal DC (50 μA, 19 s) was applied through the stimulation electrode at the most ventral level. The same current was applied into the NGC and NMC in the decerebrate cat. Cats were deeply anesthetized (sodium pentobarbital, 100 mg/kg, iv) and perfused intracardially with saline, followed by 10% formalin buffer solution. Brain tissues were removed and kept in 30% sucrose in 10% formalin buffer solution. Serial sagittal sections (brains from the chronic study) and coronal sections (brains from decerebrate dialysis study) were cut at 40 μm and then stained with neutral red. Iron deposited at stimulation sites was identified with potassium ferrocyanide staining.
Data analysis
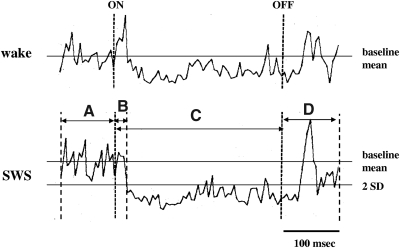
To determine the latency of stimulation-induced suppression of muscle tone in the chronic animal, trains with 300-ms duration were applied into the medulla and EMG changes were measured by stimulus-triggered averaging. Rectified and digitized EMGs were collected on a computer and averaged over a 500-ms period, from 100 ms before the stimulation to 100 ms after it, with a bin width of 100 μs. The latency of medullary stimulation-induced suppression of muscle tone was defined as the point when the EMG first fell to 2SD below the prestimulus baseline level (Fig. 1). Data from sites stimulated during waking, SWS, and REM sleep were used to analyze the relationship between sleep states, stimulation intensity, and the magnitude of muscle tone suppression. In the microdialysis experiment, baseline measures were taken from three samples prior to stimulation, during stimulation, and two samples after stimulation. The baseline and poststimulation values were averaged for each experiment, producing three values for each experiment, baseline, stimulation, and poststimulation. Statistics were performed on these data across experiments by ANOVA and follow-up Student's t-test. For graphical presentation, the changes in neurotransmitter release in the ventral horn were converted to a percentage of the baseline level. Baseline SD values were calculated from the individual animal means.
Fig. 1.
Average of electromyogram (EMG) change produced by 5 medullary stimulation trains in waking and slow-wave sleep (SWS). Trains were 300 ms in duration with 150 Hz pulse frequency and 50 μA intensity. EMG activity was averaged from 100 ms before stimulation (A), 300 ms during stimulation (C), and 100 ms after cessation of stimulation (D). B: indication of the point at which the EMG fell 2SDs below the baseline level, scored as the latency of inhibition. on and off indicate the point of stimulation onset and offset. EMGs in the figure were recorded from the left splenius.
RESULTS
Electrical stimulation across the sleep–wake cycle in the chronic cat
LOCALIZATION OF STIMULATION SITES.
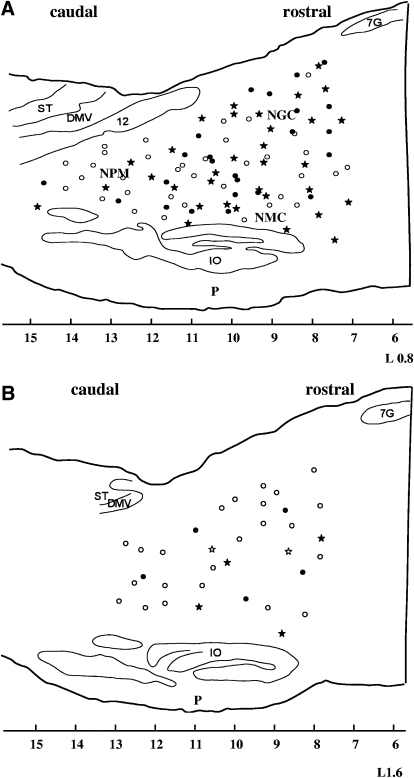
In all, 126 sites in the NGC and NMC were studied. Among them, 92 were within 1 mm (medial medulla; Fig. 2A), whereas the remaining 34 were between 1 and 2 mm from the midline (medial–lateral medulla; Fig. 2B).
Fig. 2.
Reconstruction of stimulation sites in the chronic cat. Stimulation sites located within 1 mm of the midline are presented in A (sagittal section L0.8). Sites located between 1 and 2 mm are presented in B (sagittal section L1.6). Filled circles and stars represent sites at which stimulation produced bilateral suppression of muscle tone in waking; open circles and stars represent sites at which stimulation produced no change, unilateral inhibition, or an increase in muscle tone in waking. Stars represent sites that were also tested during SWS and rapid eye movement (REM) sleep. All star sites produced suppression of muscle tone during stimulation in SWS. DMV, dorsal motor nucleus of the vagus; IO, inferior olivary nucleus; NGC, nucleus gigantocellularis; NMC, nucleus magnocellularis; NPM, nucleus paramedianus; P, pyramidal tract; ST, nucleus of the solitary tract; 7G, genu of the facial nerve; 12, hypoglossal nucleus, x-axis, mm posterior to interaural zero.
ELECTRICAL STIMULATION DURING WAKING.
Of the 92 sites in the medial medulla, electrical stimulation in 57 sites (62%) produced bilateral suppression of muscle tone followed by either an increase in muscle tone beyond baseline levels (16 cases) or a return to baseline levels (41 sites). The remaining 35 sites produced contralateral inhibition and ipsilateral facilitation (14 sites) or contralateral inhibition with no change in ipsilateral muscle tone (21 sites).
In contrast, only 9 of 34 sites (26%) located between 1 and 2 mm from the midline produced bilateral muscle tone suppression followed by either poststimulation facilitation (6 cases) or a return to baseline levels (3 cases). When stimulation was applied to the remaining 25 sites located between 1 and 2 mm from the midline, bilateral muscle tone facilitation (6 sites), no change in muscle tone bilaterally (6 sites), and contralateral muscle tone inhibition with ipsilateral facilitation on the side of stimulation (13 sites) were seen.
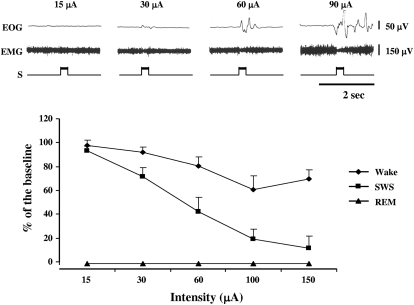
The magnitude of muscle tone suppression at medial medullary sites producing bilateral suppression was found to be stimulation intensity dependent [ANOVA, F(5,20) = 154.74, P < 0.01; Fig. 3, bottom]. A 300-ms low-intensity (<15 μA) train of 100-Hz pulses produced no detectable muscle tone change. Similarly, this stimulation failed to elicit eye movement. However, a higher intensity stimulation (60 μA) generated muscle tone suppression and eye movement at sites in both NGC and NMC (Fig. 3, top). Increased muscle tone or twitches were never generated at sites that elicited muscle tone suppression. The latency of EMG suppression was measured at 66 of the sites from which stimulation produced muscle tone suppression bilaterally. Response latency was 31.6 ± 17.8 ms and ranged from 18.3 to 46.7 ms.
Fig. 3.
Top: 300-ms trains with 100-Hz pulses delivered to the medial medulla produced intensity-dependent suppression of muscle tone in waking. No detectable of change in muscle tone is seen during 15-μA stimulation. Muscle tone suppression increased as intensity increased. Higher intensity stimulations also elicited eye movement. Bottom: intensity- and SWS–wake state dependence of muscle tone suppression induced by medial medullary stimulation. Baseline was taken from the 2-min average of integrated EMG activity immediately before stimulation. The same stimulation produced a greater inhibition during SWS than that in waking. Medial medullary stimulations across the same intensity range did not change muscle activity during REM sleep, a state in which muscle tone is normally absent. EMG, electromyogram; EOG, electrooculogram; S, marker of electrical stimulation.
ELECTRICAL STIMULATION DURING SLEEP.
Among the 126 sites that were stimulated in waking, 46 sites (40 sites in the medial and 6 sites in the medial–lateral medulla; Fig. 2) were also examined during sleep. Of these 46 sites, when tested in waking, 2 sites (located in the medial–lateral medulla) produced no change in muscle tone, 28 sites (26 in the medial and 2 in the medial–lateral medulla) elicited bilateral inhibition without after-facilitation, 11 cases (9 in the medial and 2 in the medial–lateral medulla) generated bilateral inhibition followed by facilitation, and the remaining 5 cases in the medial medulla elicited contralateral inhibition with no change in ipsilateral muscle tone.
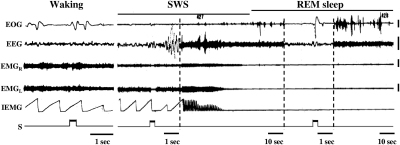
In contrast to their responses in waking, when stimulation with the same parameters was applied during SWS, bilateral inhibition without after-facilitation occurred in all cases (Fig. 4). There was a significant interaction between stimulation intensity and state (P < 0.01; Fig. 3, bottom). The magnitude of muscle tone suppression induced by medial medulla stimulation was higher in SWS than that in waking [ANOVA, F(1,5) = 445.13, P < 0.01; Fig. 3, bottom]. During REM sleep, the same stimulation did not alter signs of REM sleep, muscle atonia, or EEG desynchronization. Eye movement was induced, but we never observed twitching or increased muscle tone when medullary sites producing inhibition in SWS were stimulated during REM sleep (Fig. 4).
Fig. 4.
State-dependent response to electrical stimulation in the medial medulla; 300-ms trains with 100-Hz and 50-μA rectangular cathodal pulses failed to change muscle tone when the animal was awake. Stimulation with the same parameters applied to same site during the transition to SWS suppressed muscle tone. This stimulation did not change motor activity during the atonia of REM sleep but did elicit eye movements. Note that the time base differed across the displayed samples of SWS and REM sleep. EEG, electroencephalogram; EMGL and EMGR: left and right neck electromyogram; IEMG, integrated EMG from the left neck muscle. Calibration: 100 μV.
BEHAVIORAL RESPONSES TO STIMULATION.
With optimal stimulation parameters (500- to 700-ms train, 100 Hz, and 2.5-fold threshold intensity) applied in waking to medial medullary sites producing bilateral inhibition of tone, the animal rapidly sunk to the floor, indicating a loss of muscle tone in all limbs. Suboptimal stimulation parameters produced paresis of the forelimbs when the animal was in sitting position and dropping of the head. Behavioral changes, such as arrest of walking, grooming, and playing, were seen simultaneously with bilateral suppression of muscle tone. In addition, a variety of behavioral changes including turning of the head and pinna, swallowing, opening of the mouth, extending of the neck, and standing up were found with higher stimulation intensities (more than threefold threshold). In most cases, such movements were accompanied by ipsilateral facilitation and contralateral inhibition of neck muscle tone.
The behavioral response to electrical stimulation of the medial medulla during SWS and REM sleep was also examined. Although a reduction of muscle tone was seen in polygraphic recording, no visible response to the stimulation was observed during SWS. Similarly, no visible behavioral response was seen to medial medullary stimulation during REM sleep.
In vivo microdialysis and HPLC analysis of neurotransmitter release in the decerebrate animal
LOCALIZATION OF THE ELECTRICAL STIMULATION AND DIALYSATE COLLECTION SITES.
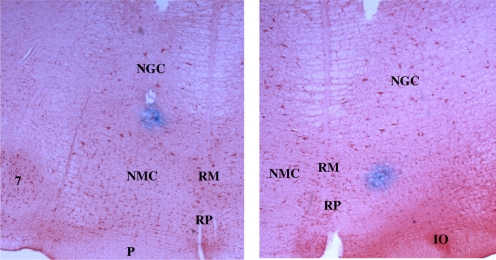
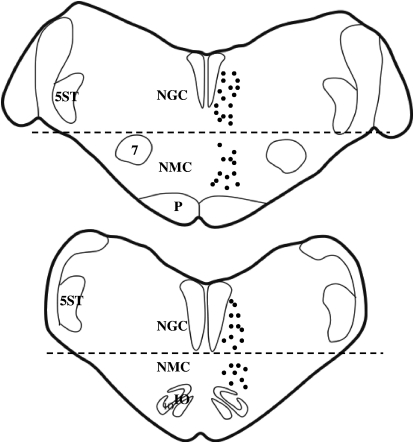
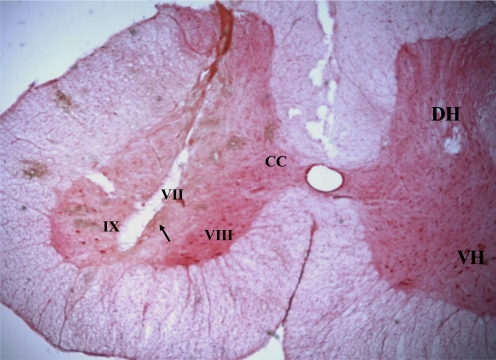
A total of 45 stimulation sites in the medial medulla producing bilateral inhibition of muscle tone were tested. Among them, 25 sites were located in the NGC and 20 in the NMC (Figs. 5 and 6). Dialysate collection sites were located in the ventral horn (Fig. 7).
Fig. 5.
Microphotographs showing examples of electrical stimulation sites in the NGC (left) and NMC (right) in the decerebrate cat. Tissue sections were processed with neutral red and counterstained with potassium ferrocyanide, producing the Prussian blue reaction at stimulation sites. IO, inferior olive; NGC, nucleus gigantocellularis; NMC, nucleus magnocellularis; P, pyramidal tract; RM, raphe magnus; RP, raphe pallidus; 7, facial nucleus.
Fig. 6.
Reconstruction of stimulation sites in the decerebrate cat. Electrical stimulations were applied to both sides of the NGC and NMC but are plotted on one side. Dashed lines represent the dorsoventral boundary between NGC and NMC. 5ST, spinal trigeminal tract.
Fig. 7.
Microphotograph showing an example of dialysate collection site in the spinal cord in the decerebrate cat. Tissue section was processed with neutral red. The tract of dialysis probe was found in the ventral horn (arrow). CC, Clarke's column; DH, dorsal horn; VH, ventral horn, lamina VII, VIII, IX.
MONOAMINE RELEASE IN THE SPINAL VENTRAL HORN DURING MEDULLARY STIMULATION-INDUCED MUSCLE ATONIA.
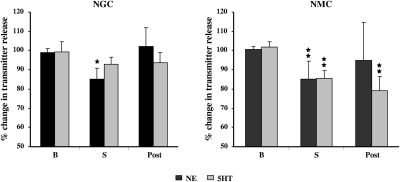
Electrical stimulation of the NGC and NMC in the decerebrate cat produced suppression of muscle tone, bilaterally. Changes in transmitter release into the ventral horn were found with both NGC and NMC stimulation. Electrical stimulation of the NGC elicited a significant decrease in NE release in the ventral horn (−13.9 ± 5.97%, P = 0.02, df = 27, t-test; Fig. 8, left), which returned to the baseline level after stimulation. On the other hand, release of 5-HT (−6.7 ± 3.77%, P = 0.18, df = 37, t-test; Fig. 8, left) into the ventral horn was not significantly changed during NGC stimulation induced suppression of muscle tone.
Fig. 8.
Changes in norepinephrine (NE) and serotonin (5-HT) release into the spinal ventral horn during electrical stimulation in the NGC and NMC. Baseline (B) level was the average of 3 consecutive 5-min dialysates. A decrease in NE release in the ventral horn was seen during NGC stimulation. A decrease in both NE and 5-HT release in the ventral horn was seen with NMC stimulation. NE levels in the spinal cord returned to baseline level after the cessation of both NGC and NMC stimulation (Post). However, serotonin levels in the spinal cord remained below baseline level after NMC stimulation.
As with NGC stimulation, NMC stimulation significantly decreased NE release in the ventral horn (−15.3 ± 9.66%, P = 0.002, df = 25, t-test; Fig. 8, right). NE levels in the ventral horn returned to the baseline level after NMC stimulation. 5-HT release into the ventral horn was also significantly decreased during NMC stimulation (−16.4 ± 4.26%, P = 0.008, df = 21, t-test, Fig. 8, right). Its level remained below the baseline level (−20.8 ± 7.3%, P = 0.007, df = 21, t-test) after NMC stimulation.
AMINO ACID RELEASE IN THE VENTRAL HORN DURING MEDULLARY STIMULATION.
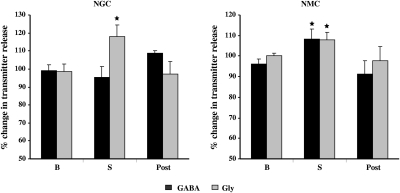
Changes in the release of the inhibitory amino acids, glycine and GABA, into the ventral horn were also found during medial medullary stimulation. Electrical stimulation of the NGC elicited an increase in glycine release in the ventral horn (19.6 ± 6.43%, P = 0.02, df = 27, t-test; Fig. 9, left). GABA release in the ventral horn was not changed during NGC stimulation (−3.9 ± 6.17%, P = 0.58, df = 45, t-test; Fig. 9, left). However, NMC stimulation significantly increased both glycine (7.8 ± 3.5%, P = 0.03, df = 35, t-test; Fig. 9, right) and GABA release (8 ± 4.75%, P = 0.04, df = 37, t-test; Fig. 9, right). Levels of both glycine and GABA release into the ventral horn returned to baseline after the cessation of stimulation of both NGC and NMC.
Fig. 9.
Changes in γ-aminobutyric acid (GABA) and glycine (Gly) release into the spinal ventral horn during electrical stimulation in the NGC and NMC. NGC stimulation increased glycine release into the ventral horn, whereas NMC stimulation increased GABA and Gly release into the spinal cord. GABA and Gly levels returned to baseline levels after both NGC and NMC stimulation.
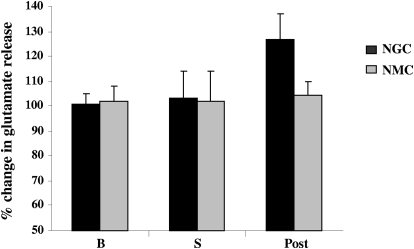
In contrast to the increased release of inhibitory amino acids, glutamate release into the spinal ventral horn was not changed from the baseline levels during NGC or NMC stimulation-elicited muscle tone suppression (NGC stimulation: 2.4 ± 6.65%, P = 0.73, df = 33, t-test; NMC stimulation: 0.43 ± 3.32%, P = 0.88, df = 31, t-test; Fig. 10).
Fig. 10.
Glutamate release into the ventral horn was not changed during NGC and NMC stimulations. After NGC stimulation, a nonsignificant trend toward increased glutamate release was seen.
DISCUSSION
Technical consideration
A head and body restrained animal has been widely used in the identification of the transmitters involved in the control of spinal neuron activity in REM sleep (Chase et al. 1986; Soja et al. 1987; Taepavarapruk et al. 2008). However, the restraint prevents head and neck responses and obscures limb responses. In the current study we have observed the effects of NGC and NMC stimulation in the unrestrained animal for the first time.
Electrical stimulation activates both cell bodies and passing fibers. Thus one may argue that the suppression of muscle tone and change in transmitter release in the ventral horn induced by the medullary stimulation may result from an activation of pontine reticulospinal fibers, involved in the generation of REM sleep atonia (Sakai 1981). However, we believe the effects induced by medial medullary stimulation result from activation of medullary neurons adjacent to the electrode based on the following evidence. First, neurons in the pons project to the medial medulla (Lai et al. 1999; Luppi et al. 1988; Sakai 1980; Shiromani et al. 1990). Second, glutamate agonist injection into the NMC and acetylcholine (ACh) injection into the nucleus paramedianus elicit muscle atonia in the decerebrate (Hajnik et al. 2000; Lai and Siegel 1991) and chronic animal (Lai and Siegel 1988). Third, the glutamate antagonist γ-d-glutamylglycine, injected into the NMC, reversed pontine carbachol injection induced atonia, indicating that pontine regulation of muscle activity is mediated through the NMC (Lai and Siegel 1988). Finally, we found an increase in glutamate and ACh levels in the NMC and nucleus paramedianus during natural REM sleep in the freely moving cat (Kodama et al. 1992, 1998).
In the present study, we found that the latency of medial medulla stimulation induced suppression of muscle tone in the freely moving animal was comparable to that in the decerebrate animal (Kohyama et al. 1998; Lai et al. 1987). We showed that electrical stimulation of the medial medulla suppresses muscle tone in the neck, forelimb, and hindlimb musculature (Hajnik et al. 2000; Kohyama et al. 1998; Lai et al. 1987). In the current study we have focused on collecting dialysates for HPLC analysis from the lumbar ventral horn to avoid the possibility of respiratory effects from damage to the cervical ventral horn. Changes in transmitter release into the cervical and lumbar ventral horns during medullary stimulation may share the pattern we have observed in lumbosacral cord.
Behavioral responses to the medullary stimulation
Activation of the medial medulla has long been known to inhibit somatic reflexes and suppress muscle tone in decerebrate animals (Hajnik et al. 2000; Karlsson and Blumberg 2005; Lai and Siegel 1988, 1991; Lai et al. 1987; Magoun and Rhines 1946; Sprague and Chambers 1954). Consistent with studies in decerebrate animals, our present study showed that stimulation of the medial medulla suppressed muscle tone during waking and SWS bilaterally in the intact, freely moving animal. Behavioral responses to medial medullary stimulation in waking included suppression of neck muscle tone bilaterally and relaxation of the fore- and hindlimb. These changes are similar to motor changes seen in cataplexy. Cataplexy has been found to be linked to a marked increase in the activity of medullary neurons that are normally active only during REM sleep atonia (Siegel et al. 1991). Lateral medullary stimulation produced unilateral or bilateral facilitation of the neck musculature accompanied by turning of the head, swallowing, or opening of the mouth, consistent with the motor activity correlates of activity in single reticular neurons recorded in the lateral medulla of unrestrained cats (Siegel and Tomaszewski 1983; Siegel et al. 1983).
We found that the magnitude and pattern of medullary inhibition of motor activity are dependent on stimulation intensity and sleep state. Consistent with studies in decerebrate animals (Hajnik et al. 2000; Lai et al. 1987; Magoun 1950), our current study also showed that sites that generated suppression of muscle activity in the freely moving cat are concentrated within 1 mm of the medullary midline.
In the present study we demonstrated that medial medullary stimulation produces a state-dependent effect on muscle tone in the freely moving animal. Using the same parameters, electrical stimulation applied to the medial medulla during SWS produced a larger suppression of muscle tone than that applied in waking. This effect may be partially due to the decreased activity of monoaminergic neurons in SWS. These neurons facilitate muscle tone in waking. We have previously shown that decreased activity of noradrenergic and serotonergic neurons is linked to muscle tone suppression in both REM sleep and cataplexy (John et al. 2004; Wu et al. 1999, 2004). We have also reported that the majority of medial reticular brain stem neurons related to motor activation have greatly reduced activity or are silent in SWS (Shouse et al. 1989; Siegel and Tomaszewski 1983; Siegel et al. 1981, 1983). Most of these neurons are glutamatergic (Lai et al. 1993, 1999). With the activity of these monoaminergic and glutamatergic facilitatory pathways already greatly reduced in SWS, the stimulus-induced activation of medial medullary inhibitory mechanisms is less likely to produce substantial depolarization of these excitatory reticulospinal neurons. Medial medullary stimulation will then produce an unopposed inhibition in SWS rather than the simultaneous inhibition and excitation likely triggered in waking.
Furthermore, the poststimulation facilitation of muscle tone that we see in some medullary areas with stimulation during waking was not found when stimulations were administered during SWS. The lack of poststimulation facilitation of muscle tone may have resulted from the suppression of sensory feedback during sleep. Neurons in the medial medulla respond to somatic noxious (pinching) and innocuous cutaneous stimuli (Drew et al. 1996; Nagata et al. 2003; Oliveras et al. 1989). Somatosensory transmission has been reported to be decreased during drowsy and SWS states (Coenen 1995). Thus muscle tone suppression followed by motor activation induced by medial medullary stimulation in waking may be a forebrain (Brown 1913) and/or peripheral sensory feedback-mediated behavioral response that controls posture.
Inhibitory postsynaptic potentials can be induced by NGC stimulation during REM sleep (Chase et al. 1986). Since muscle tone is already absent during REM sleep, no further reduction occurs, as seen in the present study. However, we found that medial medullary stimulation during REM sleep, as well as during waking, that produced suppression of muscle activity, induces rapid eye movement, a phenomenon that has not previously been reported. Many laboratories (Aserinsky and Kleitman 1953; Chase and Morales 2005; Siegel 2005) have reported that phasic muscle excitation frequently accompanies rapid eye movement during REM sleep. The lack of such activity after stimulation of the medial medulla, as seen in the current study, suggests that medullary activity is not the source of these muscle twitches. Unit recording revealed that neuronal activity in the dorsolateral tegmental nucleus increases during myoclonic twitch with atonic muscle activity in the decerebrate infant rat (Karlsson et al. 2005), indicating that the dorsolateral tegmental nucleus may be the source in the generation of phasic motor activity during REM sleep.
Transmitter release into the ventral horn during medullary stimulation-induced muscle atonia
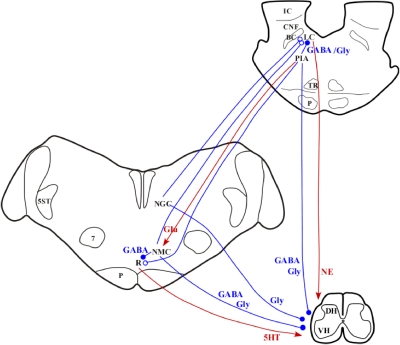
We showed in our previous studies that medullary release of glutamate and corticotrophin-releasing factor are responsible for the NMC-induced suppression of muscle tone. However, NGC-induced suppression of muscle tone is not mediated by medullary neurons responding to these two transmitters (Lai and Siegel 1988, 1991, 1992). These results suggest that NMC and NGC control of muscle activity is mediated by different pathways (Fig. 11).
Fig. 11.
Hypothetical neural circuitry in the pontomedullary reticular formation and transmitters involved in the control of muscle tone. The red lines represent excitatory effects and blue lines represent inhibitory effects on target sites (arrows and circles). The filled and open circles represent known and unknown inhibitory transmitters. Activation of the locus ceruleus (LC) and medullary raphe nuclei (R) produced NE and 5-HT release into the spinal cord and increased motor activity. Activation of the pontine inhibitory area (PIA) either by electrical stimulation or by injection of cholinergic and glutamatergic agonists activates glutamatergic neurons, which project to the NMC and activate GABAergic and glycinergic spinal projecting neurons. Activation of both the PIA and NMC also inhibits LC and raphe neuronal activity. Thus an increase in inhibitory amino acids and a decrease in NE and 5-HT into the spinal cord are caused by stimulation of the PIA and the NMC. However, GABAergic and glycinergic projections from the PIA to the spinal cord cannot be ruled out. In contrast, stimulation of the NGC activates NGC glycinergic neuronal activity and inhibits LC noradrenergic neuronal activity, and thus increases glycine release and decreases NE release into the spinal cord. BC, brachium conjunctivum; CNF, cuneiform nucleus; Glu, glutamate; IC, inferior colliculus; TR, tegmental reticular nucleus.
We hypothesize that the PIA control of muscle activity is mediated by the NMC. Consistent with this hypothesis, in the present study we found that stimulation of the NMC increases GABA and glycine release and decreases NE and 5-HT release into the spinal ventral horn, a pattern that resembles that seen with PIA stimulation (Kodama et al. 2003; Lai et al. 2001). On the other hand, an increase in glycine release and a decrease in NE release were found during NGC stimulation, without an increase in GABA or decrease in 5-HT release.
It has been hypothesized that a glycinergic mechanism is involved in REM sleep atonia (Chase et al. 1986). Glycinergic neurons in the medullary reticular formation are activated during REM sleeplike activity induced by pontine carbachol injection in the cat (Morales et al. 2006) and pontine bicuculline and kainate injection in the rat (Boissard et al. 2002). However, systemic injection of strychnine, a glycine antagonist, failed to block the medullary stimulation-induced suppression of muscle rigidity in the decerebrate cat (Llinás 1964), indicating that nonglycinergic mechanisms triggered by medullary stimulation are sufficient to suppress muscle tone. Report of a recent study concluded that infusion of strychnine, bicuculline, and strychnine mixed with bicuculline into the trigeminal motor and hypoglossal nuclei increases phasic but not tonic masseter and genioglossus muscle activity in REM sleep in the rat (Brooks and Peever 2008; Morrison et al. 2003). This is consistent with our finding of a small but significant increase in glycine and GABA release during medullary stimulation in the present study. Phasic changes in glycine and GABA release during medullary stimulation would be expected to make a relatively small contribution to a dialysate collected continuously over a 5-min period.
Our current observations in the decerebrate cat showed that NGC stimulation increased glycine release and NMC stimulation increases both glycine and GABA release into the ventral horn with suppression of muscle tone. Glycine and GABA release in the ventral horn may come from either spinal interneurons, which contain glycine and GABA (Todd and Sullivan 1990) and are activated by medullary stimulation (Takakusaki et al. 2003), or from reticulospinal glycinergic and GABAergic neurons. Glycinergic and GABAergic neurons in the NGC and NMC have been shown to project to the spinal cord (Holstege 1991; Holstege and Bongers 1991; Jones et al. 1991; Reichling and Basbaum 1990). Medullary GABAergic neuron activity has been reported to be negatively correlated with muscle activity (Maloney et al. 2000). Activation of medullary glycinergic and GABAergic neurons has been found in the rat recovering from REM sleep deprivation (Maloney et al. 2000; Sapin et al. 2009). However, the number of GABAergic neurons projecting from the NGC to the spinal cord is small (Jones et al. 1991). On the other hand, the NMC sends a substantial GABAergic projection to the spinal ventral horn (Holstege 1991) and inhibits motoneuronal activity (Holstege and Calkoen 1990). This may explain why we see that GABA release into the ventral horn is increased during NMC stimulation, but is not altered during NGC stimulation.
Our present study also showed a decrease in NE release into the ventral horn during NGC and NMC stimulations. Noradrenergic mechanisms have been shown to be involved in the modulation of motor activity. Prazosin, a noradrenergic α1 receptor antagonist, injected into the hypoglossal nucleus decreases hypoglossal nerve activity (Fenik et al. 2004). White and Neuman (1983) showed that iontophoretic application of NE into the spinal cord increases spinal motoneuron excitability. It has not been determined whether noradrenergic effects are mediated directly at the motoneuronal membrane or by adjacent interneurons. Schwartz et al. (2008), working in anesthetized rats, found that NE had little effect on masseter motoneurons in the absence of glutamate. However, they concluded that in the unanesthetized animal, it is likely that NE in combination with waking levels of glutamate produces a potent facilitation of muscle tone. Thus NE appears to interact with glutamate in regulating tone.
The rostral medial medulla contains very few noradrenergic neurons (Jones and Beaudet 1987). These neurons do not project to the spinal cord (Westlund et al. 1983, 1984). Thus the decrease in NE release during NGC and NMC stimulation seen in the present study most likely resulted from an inhibition of the pontine noradrenergic groups (Fig. 11). Neurons in the medial medulla have been shown to project to the A5, A6, and A7 groups (Clark and Proudfit 1991; Jones and Yang 1985; Verret et al. 2006). Stimulation of sites in the medial medulla that suppress muscle tone consistently produces a profound inhibition of the activity of A6 LC neurons (Mileykovskiy et al. 2000), which project to the spinal cord (Clark and Proudfit 1991; Fung et al. 1994; Lai and Barnes 1985). Activation of locus ceruleus facilitates spinal motoneuron activity (Fung and Barnes 1987; Lai et al. 1989), whereas cessation of locus ceruleus noradrenergic neuronal activity elicits cataplexy (Wu et al. 1999).
Serotonin has been shown to increase motoneuron excitability (Fung and Barnes 1989; White and Fung 1989) and increase muscle tone in SWS and REM sleep (Jelev et al. 2001). Serotonergic spinal projecting neurons have been found in the NMC and medullary raphe nuclei (Bowker et al. 1983). However, spinally projecting serotonergic neurons were not found in the NGC. We found that 5-HT release into the ventral horn is decreased during NMC but not NGC stimulation. The decrease in 5-HT release into the ventral horn with NMC stimulation continued for 10 min after the cessation of stimulation. The long-lasting decrease in 5-HT release may be mediated by a local GABAergic inhibition of 5-HT neurons. Approximately 45% of synapses in the NMC and raphe nuclei are GABAergic (Cho and Basbaum 1991). The majority of GABAergic neurons in the NMC are interneurons and innervate the medullary and raphe descending neurons (Holmes et al. 1994; Reichling and Basbaum 1990). Thus stimulation of the NMC may activate GABAergic interneuron activity, which in turn inhibits serotonergic neuronal activity and decreases 5-HT release into the spinal cord.
It has been hypothesized that a glutamatergic mechanism in the spinal cord is involved in the control of motor activity in REM sleep. Specific lesions of glutamatergic neurons in the supraolivary medulla, corresponding to the caudal medulla of the nucleus paramedianus in the cat, increase both tonic and phasic motor activity in REM sleep in the mouse (Vetrivelan et al. 2008). In the present study, changes in glutamate release into the ventral horn were not found during NGC and NMC stimulation. Thus the mechanisms that underlie rostral and caudal medial medulla roles in the control of muscle tone may differ, with GABAergic and glycinergic reticulospinal projections from the rostral medulla and glutamatergic projections from the caudal medulla contributing to REM sleep atonia.
Physiological implications and conclusions
Lesions in the medial medulla produced REM sleep without atonia and an increase in phasic motor activity in REM sleep in the cat (Holmes and Jones 1994; Schenkel and Siegel 1989), symptoms seen in REM sleep behavior disorder patients (Montplaisir et al. 1997; Schenck and Mahowald 1990). Our present study demonstrated that activation of the medial medulla produces a sleep state-dependent suppression of muscle tone. The sleep state-dependent effect on muscle activity may result from a combination of an activation of neurons that normally increase firing rate during REM sleep and of a suppression of sensory inputs during sleep. Our previous (Kodama et al. 2003; Lai et al. 2001) and present studies show that pontine and NMC stimulations produce a similar pattern of change in neurotransmitter release into the ventral horn. Injection of glutamate antagonists into the NMC reverses pontine stimulation-induced muscle atonia (Lai and Siegel 1988). Therefore we hypothesize that the pontine control of muscle tone is mediated through glutamatergic activation of the NMC. The components of the muscle tone suppression mechanism activated by NGC and NMC activation differ from each other and from those triggered by pontine stimulation.
GRANTS
This work was supported by National Institutes of Health Grants NS-14610 and MH-64109 to J. M. Siegel and NS-042566 to Y.-Y. Lai and the Medical Research Service of the Department of Veterans Affairs, Veterans Affairs Greater Los Angeles Healthcare System Sepulveda.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- Aserinsky and Kleitman, 1953.Aserinsky E, Kleitman N. Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science 118: 273–274, 1953 [DOI] [PubMed] [Google Scholar]
- Boissard et al., 2002.Boissard R, Gervasoni D, Schmidt MH, Barbagli B, Fort P, Luppi PH. The rat ponto-medullary network responsible for paradoxical sleep onset and maintenance: a combined microinjection and functional neuroanatomical study. Eur J Neurosci 16: 1959–1973, 2002 [DOI] [PubMed] [Google Scholar]
- Bowker et al., 1983.Bowker RM, Westlund KN, Sullivan MC, Wilber JF, Coulter JD. Descending serotonergic, peptidergic and cholinergic pathways from the raphe nuclei: a multiple transmitter complex. Brain Res 288: 33–48, 1983 [DOI] [PubMed] [Google Scholar]
- Brooks and Peever, 2008.Brooks PL, Peever JH. Glycinergic and GABAA-mediated inhibition of somatic motoneurons does not mediate rapid eye movement sleep motor atonia. J Neurosci 28: 3535–3545, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, 1913.Brown TG. On postural and non-postural activities of the mid-brain. Proc R Soc Lond B Biol Sci 87: 145–163, 1913 [Google Scholar]
- Chase and Morales, 2005.Chase MH, Morales FR. Control of motor neurons during sleep. In: Principles and Practice of Sleep Medicine (4th ed.), edited by Kryger MH, Roth T, Dement WC. Philadelphia, pa: Elsevier/Saunders, 2005, p. 254–268 [Google Scholar]
- Chase et al., 1986.Chase MH, Morales FR, Boxer PA, Fung SJ, Soja P. Effect of stimulation of the nucleus reticularis gigantocellularis on the membrane potential of cat lumbar motoneurons during sleep and wakefulness. Brain Res 386: 237–244, 1986 [DOI] [PubMed] [Google Scholar]
- Cho and Basbaum, 1991.Cho HJ, Basbaum AI. GABAergic circuitry in the rostral ventral medulla of the rat and its relationship to descending antinociceptive controls. J Comp Neurol 303: 316–328, 1991 [DOI] [PubMed] [Google Scholar]
- Clark and Proudfit, 1991.Clark FM, Proudfit HK. Projections of neurons in the ventromedial medulla to pontine catecholamine cell groups involved in the modulation of nociception. Brain Res 540: 105–115, 1991 [DOI] [PubMed] [Google Scholar]
- Coenen, 1995.Coenen AM. Neuronal activities underlying the electroencephalogram and evoked potentials of sleeping and waking: implications for information processing. Neurosci Biobehav Rev 19: 447–463, 1995 [DOI] [PubMed] [Google Scholar]
- Drew et al., 1996.Drew T, Cabana T, Rossignol S. Responses of medullary reticulospinal neurons to stimulation of cutaneous limb nerves during locomotion in intact cats. Exp Brain Res 111: 153–168, 1996 [DOI] [PubMed] [Google Scholar]
- Fenik et al., 2004.Fenik VB, Davies RO, Kubin L. Combined antagonism of aminergic excitatory and amino acid inhibitory receptors in the XII nucleus abolishes REM sleep-like depression of hypoglossal motoneuronal activity. Arch Ital Biol 142: 237–249, 2004 [PubMed] [Google Scholar]
- Fung and Barnes, 1987.Fung SJ, Barnes CD. Membrane excitability changes in hindlimb motoneurons induced by stimulation of the locus coeruleus in cats. Brain Res 402: 230–242, 1987 [DOI] [PubMed] [Google Scholar]
- Fung and Barnes, 1989.Fung SJ, Barnes CD. Raphe-produced excitation of spinal cord motoneurons in the cat. Neurosci Lett 103: 185–190, 1989 [DOI] [PubMed] [Google Scholar]
- Fung et al., 1994.Fung SJ, Reddy VK, Zhuo H, Liu RH, Wang Z, Barnes CD. Anatomical evidence for the presence of glutamate or enkephalin in noradrenergic projection neurons of the locus coeruleus. Microscopy Res Tech 29: 219–225, 1994 [DOI] [PubMed] [Google Scholar]
- Hajnik et al., 2000.Hajnik T, Lai Y-Y, Siegel JM. Atonia-related regions in the rodent pons and medulla. J Neurophysiol 84: 1942–948, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes and Jones, 1994.Holmes CJ, Jones BE. Importance of cholinergic, GABAergic, serotonergic and other neurons in the medial medullary reticular formation for sleep-wake states studied by cytotoxic lesions in the cat. Neuroscience 62: 1179–1200, 1994 [DOI] [PubMed] [Google Scholar]
- Holstege, 1991.Holstege JC. Ultrastructural evidence for GABAergic brain stem projections to spinal motoneurons in the rat. J Neurosci 11: 159–167, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstege and Bongers, 1991.Holstege JC, Bongers CMH. A glycinergic projection from the ventromedial lower brainstem to spinal motoneurons. An ultrastructural double labeling study in rat. Brain Res 566: 308–315, 1991 [DOI] [PubMed] [Google Scholar]
- Holstege and Calkoen, 1990.Holstege JC, Calkoen F. The distribution of GABA in lumbar motoneuronal cell groups. A quantitative ultrastructural study in rat. Brain Res 530: 130–137, 1990 [DOI] [PubMed] [Google Scholar]
- Jelev et al., 2001.Jelev A, Sood S, Liu H, Nolan P, Horner RL. Microdialysis perfusion of 5-HT into hypoglossal motor nucleus differentially modulates genioglossus activity across natural sleep-wake states in rats. J Physiol 532: 467–481, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John et al., 2004.John J, Wu M-F, Boehmer LN, Siegel JM. Cataplexy-active neurons in the posterior hypothalamus: implications for the role of histamine in sleep and waking behavior. Neuron 42: 619–634, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones and Beaudet, 1987.Jones BE, Beaudet A. Distribution of acetylcholine and catecholamine neurons in the cat brainstem: A choline acetyltransferase and tyrosine hydroxylase immunohistochemical study. J Comp Neurol 261: 15–32, 1987 [DOI] [PubMed] [Google Scholar]
- Jones et al., 1991.Jones BE, Holmes CJ, Rodriguez-Veiga E, Mainville L. GABA-synthesizing neurons in the medulla: their relationship to serotonin-containing and spinally projecting neurons in the rat. J Comp Neurol 313: 349–367, 1991 [DOI] [PubMed] [Google Scholar]
- Jones and Yang, 1985.Jones BE, Yang TZ. The efferent projections from the reticular formation and the locus coeruleus studied by anterograde and retrograde axonal transport in the rat. J Comp Neurol 242: 56–92, 1985 [DOI] [PubMed] [Google Scholar]
- Kanamori et al., 1980.Kanamori N, Sakai K, Vouvet M. Neuronal activity specific to paradoxical sleep in the ventromedial medullary reticular formation of unrestrained cats. Brain Res 189: 251–255, 1980 [DOI] [PubMed] [Google Scholar]
- Karrlsson and Blumberg, 2005.Karrlsson KE, Blumberg MS. Active medullary control of atonia in week-old rats. Neuroscience 130: 275–283, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karrlsson et al., 2005.Karrlsson KE, Gall AJ, Mohns EJ, Seelke AMH, Blumberg MS. The neural substrates of infant sleep in rats. PLoS Biol 3: e143, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama et al., 1992.Kodama T, Lai Y-Y, Siegel JM. Enhancement of acetylcholine release during REM sleep in the caudomedial medulla as measured by in vivo microdialysis. Brain Res 580: 348–350, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama et al., 1998.Kodama T, Lai Y-Y, Siegel JM. Enhanced glutamate release during REM sleep in the rostromedial medulla as measured by in vivo microdialysis. Brain Res 780: 178–181, 1998 [PMC free article] [PubMed] [Google Scholar]
- Kodama et al., 2003.Kodama T, Lai Y-Y, Siegel JM. Changes in inhibitory amino acid release linked to pontine-induced atonia: an in vivo microdialysis study. J Neurosci 23: 1548–1554, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohyama et al., 1998.Kohyama J, Lai Y-Y, Siegel JM. Reticulospinal systems mediate atonia with short and long latencies. J Neurophysiol 80: 1839–1851, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai and Barnes, 1985.Lai Y-Y, Barnes CD. A spinal projection of serotonergic neurons of the locus coeruleus in the cat. Neurosci Lett 58: 159–164, 1985 [DOI] [PubMed] [Google Scholar]
- Lai et al., 1993.Lai Y-Y, Clements JR, Siegel JM. Glutamatergic and cholinergic projections to the pontine inhibitory area identified with horseradish peroxidase retrograde transport and immunohistochemistry. J Comp Neurol 336: 321–330, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai et al., 1999.Lai Y-Y, Clements JR, Wu XY, Shalita T, Wu JP, Kuo JS, Siegel JM. Brainstem projections to the ventromedial medulla in cat: retrograde transport horseradish peroxidase and immunohistochemical studies. J Comp Neurol 408: 419–436, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai et al., 2001.Lai Y-Y, Kodama T, Siegel JM. Changes in monoamine release in the ventral horn and hypoglossal nucleus linked to pontine inhibition of muscle tone: an in vivo microdialysis study. J Neurosci 21: 7384–7391, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai and Siegel, 1988.Lai Y-Y, Siegel JM. Medullary regions mediating atonia. J Neurosci 8: 4790–4796, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai and Siegel, 1991.Lai Y-Y, Siegel JM. Pontomedullary glutamate receptors mediating locomotion and muscle tone suppression. J Neurosci 11: 2931–2937, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai and Siegel, 1992.Lai Y-Y, Siegel JM. Corticotropin-releasing factor mediated muscle atonia in pons and medulla. Brain Res 575: 63–68, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai et al., 1987.Lai Y-Y, Siegel JM, Wilson WJ. Effect of blood pressure on medial medulla-induced muscle atonia. Am J Physiol Heart Circ Physiol 252: H1249–H1257, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai et al., 1989.Lai Y-Y, Strahlendorf HK, Fung SJ, Barnes CD. The actions of two monoamines on spinal motoneurons from stimulation of the locus coeruleus in the cat. Brain Res 484: 268–272, 1989 [DOI] [PubMed] [Google Scholar]
- Llinás, 1964.Llinás R. Mechanisms of supraspinal actions upon spinal cord activities. Pharmacological studies on reticular inhibition of alpha extensor motoneurons. J Neurophysiol 27: 1127–1137, 1964 [DOI] [PubMed] [Google Scholar]
- Luppi et al., 1988.Luppi PH, Sakai K, Fort P, Salvert D, Jouvet M. The nuclei of origin of monoaminergic, peptidergic, and cholinergic afferents to the cat nucleus reticularis magnocellularis: a double-labeling study with cholera toxin as a retrograde tracer. J Comp Neurol 277: 1–20, 1988 [DOI] [PubMed] [Google Scholar]
- Magoun and Rhines, 1946.Magoun HW, Rhines R. An inhibitory mechanism in the bulbar reticular formation. J Neurophysiol 9: 165–171, 1946 [DOI] [PubMed] [Google Scholar]
- Maloney et al., 2000.Maloney K, Mainville L, Jones BE. c-fos expression in GABAergic, serotonergic, and other neurons of the pontomedullary reticular formation and raphe after paradoxical sleep deprivation and recovery. J Neurosci 20: 4669–4679, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mileykovskiy et al., 2000.Mileykovskiy BY, Kiyashchenko LI, Kodama T, Lai Y-Y, Siegel JM. Activation of pontine and medullary motor inhibitory regions reduces discharge in neurons located in the locus coeruleus and the anatomical equivalent of the midbrain locomotor region. J Neurosci 20: 8551–8558, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montplaisir et al., 1997.Montplaisir J, Boucher S, Poirier G, Lavigne G, Lapierre O, Lesperance P. Clinical, polysomnographic, and genetic characteristics of restless legs syndrome: a study of 133 patients diagnosed with new standard criteria. Mov Disord 12: 61–65, 1997 [DOI] [PubMed] [Google Scholar]
- Morales et al., 2006.Morales FR, Sampogna S, Rampon C, Luppi PH, Chase MH. Brainstem glycinergic neurons and their activation during active (rapid eye movement) sleep in the cat. Neuroscience 142: 37–47, 2006 [DOI] [PubMed] [Google Scholar]
- Morrison et al., 2003.Morrison JL, Sood S, Liu H, Park E, Liu X, Nolan P, Horner RL. Role of inhibitory amino acids in control of hypoglossal motor outflow to genioglossus muscle in naturally sleeping rats. J Physiol 552: 975–991, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata et al., 2003.Nagata T, Suzuki H, Zhang R, Ozaki M, Kawakami Y. Mechanical stimulation activates small fiber mediated nociceptive responses in the nucleus gigantocellularis. Exp Brain Res 149: 505–511, 2003 [DOI] [PubMed] [Google Scholar]
- Oliveras et al., 1989.Oliveras JL, Vos B, Martin G, Montagne J. Electrophysiological properties of ventromedial medulla neurons in response to noxious and non-noxious stimuli in the awake, freely moving rat: a single-unit study. Brain Res 486: 1–14, 1989 [DOI] [PubMed] [Google Scholar]
- Reichling and Basbaum, 1990.Reichling DB, Basbaum AI. Contribution of brainstem GABAergic circuitry to descending antinociceptive controls I. GABA-immunoreactive projection neurons in the periaqueductal gray and nucleus raphe magnus. J Comp Neurol 302: 370–377, 1990 [DOI] [PubMed] [Google Scholar]
- Sakai, 1980.Sakai K. Some anatomical and physiological properties of ponto-mesencephalic tegmental neurons with specific reference to the PGO waves and postural atonia during paradoxical sleep in the cat. In: The Reticular Formation Revisited, edited by Hobson JA, Brazier MAB. New York: Raven Press, 1980, p 427–447 [Google Scholar]
- Sakai et al., 1981.Sakai K, Sastre JP, Kanamori N, Jouvet M. State-specific neurons in the ponto-medullary reticular formation with special reference to the postural atonia during paradoxical sleep in the cat. In: Brain Mechanisms and Perceptual Awarencess, edited by Pompeiano O, Marsan CA. New York: Raven Press, 1981, p. 405–429 [Google Scholar]
- Sapin et al., 2009.Sapin E, Lapray D, Berod A, Goutagny R, Leger L, Ravassard P, Clement O, Hanriot L, Fort P, Luppi PH. Localization of the brainstem GABAergic neurons controlling paradoxical (REM) sleep. PLoS One 4: e4272, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenck and Mahowald, 1990.Schenck CH, Mahowald MW. Polysomnographic, neurologic, psychiatric, and clinical outcome report on 70 consecutive cases with REM sleep behavior disorder (RBD): sustained clonazepam efficacy in 89.5% of 57 treated patients. Cleveland Clin J Med Suppl 57: S9–S23, 1990 [Google Scholar]
- Schenkel and Siegel, 1989.Schenkel E, Siegel JM. REM sleep without atonia after lesions of the medial medulla. Neurosci Lett 98: 159–165, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz et al., 2008.Schwarz PB, Yee N, Mir S, Peever JH. Noradrenaline triggers muscle tone by amplifying glutamate-driven excitation of somatic motoneurons in anaesthetized rats. J Physiol 586: 5787–5802, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiromani et al., 1990.Shiromani PJ, Lai Y-Y, Siegel JM. Descending projections from the dorsolateral pontine tegmentum to the paramedian reticular nucleus of the caudal medulla in the cat. Brain Res 517: 224–228, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shouse et al., 1989.Shouse MN, Siegel JM, Wu MF, Szymusiak R, Morrison AR. Mechanisms of seizure suppression during rapid eye movement sleep in cats. Brain Res 505: 271–282, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel, 1979.Siegel JM. Behavioral relations of medullary reticular formation cells. Exp Neurol 65: 691–698, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel, 2005.Siegel JM. Functional implication of sleep development. PLoS Biol 3: e178, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel et al., 1991.Siegel JM, Nienhuis R, Fahringer HM, Paul R, Shiromani P, Dement WC, Mignot E, Chiu C. Neuronal activity in narcolepsy: identification of cataplexy related cells in the medial medulla. Science 262: 1315–1318, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel et al., 1981.Siegel JM, Nienhuis R, Wheeler RL, McGinty DJ, Harper RM. Discharge pattern of reticular formation unit pairs in waking and REM sleep. Exp Neurol 74: 875–891, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel and Tomaszewski, 1983.Siegel JM, Tomaszewski KS. Behavioral organization of reticular formation: studies in the unrestrained cat. I. Cells related to axial, limb, eye, and other movements. J Neurophysiol 50: 696–716, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel et al., 1983.Siegel JM, Tomaszewski KS, Wheeler RL. Behavioral organization of reticular formation: studies in the unrestrained cat. II. Cells related to facial movements. J Neurophysiol 50: 717–723, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel et al., 1979.Siegel JM, Wheeler RL, McGinty DJ. Activity of medullary reticular formation neurons in the unrestrained cat during waking and sleep. Brain Res 179: 49–60, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soja et al., 1987.Soja PJ, Morales FR, Baranyi A, Chase MH. Effect of inhibitory amino acid antagonists on IPSPs induced in lumbar motoneurons upon stimulation of the nucleus reticularis gigantocellularis during active sleep. Brain Res 423: 353–358, 1987 [DOI] [PubMed] [Google Scholar]
- Sprague and Chambers, 1954.Sprague JM, Chambers WW. Control of posture by reticular formation and cerebellum in the intact, anesthetized and unanesthetized and in decerebrated cat. Am J Physiol 176: 52–64, 1954 [DOI] [PubMed] [Google Scholar]
- Taepavarapruk et al., 2008.Taepavarapruk N, Taepavarapruk P, John J, Lai Y-Y, Siegel JM, Phillips AG, McErlane SA, Soja PJ. State-dependent changes in glutamate, glycine, GABA, and dopamine levels in cat lumbar spinal cord. J Neurophysiol 100: 598–608, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakusaki et al., 2003.Takakusaki K, Kohyama J, Matsuyama K. Medullary reticulospinal tract mediating a generalized motor inhibition in cats III. Functional organization of spinal interneurons in the lower lumbar segments. Neuroscience 121: 731–746, 2003 [DOI] [PubMed] [Google Scholar]
- Todd and Sullivan, 1990.Todd AJ, Sullivan AC. Light microscope study of the coexistence of GABA-like and glycine-like immunoreactivities in the spinal cord of the rat. J Comp Neurol 296: 496–505, 1990 [DOI] [PubMed] [Google Scholar]
- Ursin and Sterman, 1981.Ursin R, Sterman MB. A Manual for Standardized Scoring of Sleep and Waking States in the Adult Cat. Los Angeles, CA: Univ. of California Los Angeles/Brain Research Institute, 1981 [Google Scholar]
- Verret et al., 2006.Verret L, Fort P, Gervasoni D, Leger L, Luppi PH. Localization of the neurons active during paradoxical (REM) sleep and projecting to the locus coeruleus noradrenergic neurons in the rat. J Comp Neurol 495: 573–586, 2006 [DOI] [PubMed] [Google Scholar]
- Vetrivelan et al., 2008.Vetrivelan R, Fuller PM, Tong Q, Lu J. Medullary circuitry regulating rapid eye movement sleep and motor atonia. J Neurosci 29: 9361–9369, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlund et al., 1983.Westlund KN, Bowker RM, Ziegler MG, Coulter JD. Noradrenergic projections to the spinal cord of the rat. Brain Res 263: 15–31, 1983 [DOI] [PubMed] [Google Scholar]
- Westlund et al., 1984.Westlund KN, Bowker RM, Ziegler MG, Coulter JD. Origins and terminations of descending noradrenergic projections to the spinal cord of monkey. Brain Res 292: 1–16, 1984 [DOI] [PubMed] [Google Scholar]
- White and Fung, 1989.White SR, Fung SJ. Serotonin depolarizes cat spinal motoneurons in situ and decreases motoneuron afterhyprpolarizing potentials. Brain Res 502: 205–213, 1989 [DOI] [PubMed] [Google Scholar]
- White and Neuman, 1983.White SR, Neuman RS. Pharmacological antagonism of facilitatory but not inhibitory effects of serotonin and norepinephrine on excitability of spinal motoneurons. Neuropharmacology 22: 489–494, 1983 [DOI] [PubMed] [Google Scholar]
- Wu et al., 1999.Wu M-F, Gulyani SA, Yau E, Mignot E, Phan B, Siegel JM. Locus coeruleus neurons: cessation of activity during cataplexy. Neuroscience 91: 1389–1399, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu et al., 2004.Wu M-F, John J, Boehmer LN, Yau D, Nguyen GB, Siegel JM. Activity of dorsal raphe cells across the sleep-waking cycle and during cataplexy in narcoleptic dogs. J Physiol 554: 202–215, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]