Abstract
In humans, the motor system can be activated by passive observation of actions or static pictures with implied action. The origin of this facilitation is of major interest to the field of motor control. Recently it has been shown that sensorimotor learning can reconfigure the motor system during action observation. Here we tested directly the hypothesis that motor resonance arises from sensorimotor contingencies by measuring corticospinal excitability in response to abstract non-action cues previously associated with an action. Motor evoked potentials were measured from the first dorsal interosseus (FDI) while human subjects observed colored stimuli that had been visually or motorically associated with a finger movement (index or little finger abduction). Corticospinal excitability was higher during the observation of a colored cue that preceded a movement involving the recorded muscle than during the observation of a different colored cue that preceded a movement involving a different muscle. Crucially this facilitation was only observed when the cue was associated with an executed movement but not when it was associated with an observed movement. Our findings provide solid evidence in support of the sensorimotor hypothesis of action observation and further suggest that the physical nature of the observed stimulus mediating this phenomenon may in fact be irrelevant.
INTRODUCTION
It is now well established that the motor system can be activated by passive observation of actions (Fadiga et al. 1995; Gallese et al. 1996; Strafella and Paus 2000). Recent experimental evidence from human and non-human primates suggests that viewing static pictures with implied action produce a similar effect. For example, observation of pictures of hand-object interactions such as grasping activates bilateral precentral, and inferior frontal cortex, a network recruited during execution and observation of dynamic actions (Johnson-Frey et al. 2003). Greater levels of activity in dorsal premotor and primary motor cortex has also been reported before movement onset during the observation of the effector and the target involved in an action (Cisek and Kalaska 2004; Gangitano et al. 2004). Moreover the observation of static pictures of a pincer grip is sufficient to increase corticospinal excitability for the muscle that would be active during movement execution (Urgesi et al. 2006).
One possible interpretation of these findings is that the sole observation of a stimulus that has been repeatedly associated with an action through conditional motor learning (Grafton et al. 1997) is sufficient to retrieve the corresponding motor representation. Evidence in support of this hypothesis comes from a recent study showing that training individuals to perform the finger movement opposite to that observed in a video modulates corticospinal excitability in a manner consistent with the executed but not the observed movement (Catmur et al. 2007). Here we tested directly the hypothesis that it is the sensorimotor contingency between the observed stimulus and the executed action, and not the nature of the stimulus, that drives motor facilitation during action observation. For this purpose, we measured corticospinal excitability in response to abstract non-action stimuli, before and after they were visually or motorically paired to an observed finger movement. If, as we hypothesize, motor resonance is the result of sensorimotor learning, then motor facilitation in response to an abstract cue would only occur after it is motorically paired to the observed movement. If, on the contrary, sensorimotor learning is not necessary for motor resonance, then motor facilitation would be elicited by simple visual association between the abstract cue and the observed movement.
Subjects learned arbitrary visuovisual and visual motor associations between colored crosses bearing no physical resemblance with an action and abduction finger movements (index and little finger), throughout ∼12 min training sessions; corticospinal excitability for the first dorsal interosseus was measured after both types of learning. As hypothesized, observation of abstract cues modulated corticospinal excitability after visuomotor but not visuovisual learning. Our findings provide crucial evidence in support of the sensorimotor hypothesis stating that “mirror” properties develop from Hebbian associations between observed and executed actions (Keysers and Perrett 2004) and further indicate that the physical nature of the stimulus inducing motor resonance may in fact be irrelevant.
METHODS
Participants
Twenty-two right-handed volunteers [10 male, 12 female; age: 23.95 ± 3.08 (SD) yr old] participated in this study after giving written, informed consent. They did not present any neurological or psychiatric disorders and had no family history of epilepsy. The experimental procedure was approved by the local Ethics Committee and carried out according to the Declaration of Helsinki.
Participants were screened to ensure there was a specific facilitation during observation of finger movements for the first dorsal interosseus before training; particularly that the amplitude of the motor evoked potential (MEP) recorded during maximal aperture of the finger movement was significantly larger for observation of index finger movements than little finger movements. Screening was carried out based on the corticospinal excitability values obtained during the first observation block. Fifteen of 22 subjects showed a statistically significant finger-movement observation effect and were therefore included in the data analysis. The low efficiency associated with the chosen paradigm may be due to the fact that FDI contraction during this type of finger movement is modest, and/or to the use of intransitive movements, which elicit lower levels of motor facilitation than transitive actions (e.g., Enticott et al.).
Procedure and task
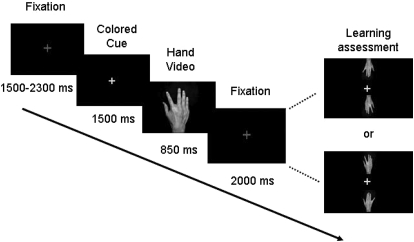
Subjects sat on a comfortable chair and viewed videos of finger movements shown at the center of a computer screen from a first person perspective. They placed their hands on both sides of a keyboard following the same posture as that shown on the screen. The experimental paradigm is illustrated in Fig. 1. All trials began with the presentation of a fixation cross. Next a colored cue appeared (the fixation cross turned red or blue) followed by a video consisting of a single abduction movement of the index or the little finger of a human right hand. Each video was composed of 32 frames, of which only the first frame was a hand at rest. Each frame was presented for 40 ms, and the total duration of the video was 1,280 ms. Time to maximal aperture was 560 ms; there were three frames of maximal aperture. The cue preceding the index finger was blue whereas the cue preceding the little finger was red.
Fig. 1.
Experimental paradigm. Shown is the sequence of stimuli presented throughout the experiment. Subjects were instructed to fixate on a gray fixation cross. After a variable interval, the cross turned into blue or red (colored cue). Blue cues preceded index finger movements, whereas red cues preceded little finger movements. Every 5 trials, learning of the visuovisual contingency was assessed through a recognition test consisting of showing 1 of the colored cues with 2 static images corresponding to each movement (learning assessment). Subjects responded by pressing 1 of 2 spatially congruent buttons.
The experiment consisted of a familiarization session, and four experimental twelve-minute blocks separated by ∼5-min rest periods: a visuovisual association block, an observation block, a visuomotor association block and a second observation block. To ensure that subjects paid attention to the stimuli, a low-contrast asterisk appeared after the finger reached maximal aperture in 20% of the trials. Subjects were told to press a pedal with their left foot whenever they detected it. All stimuli (red and blue crosses and asterisks) were presented following a pseudorandomized order and balanced within block to avoid more than three repetitions across trials.
- Familiarization session
The purpose of this session was to familiarize participants with the visuovisual learning task, the asterisk detection task, and the recognition test. Participants were instructed to learn the association between each colored cue and the corresponding observed finger movement and to press the pedal whenever they detected the asterisk. Learning was assessed through a recognition test consisting of two static images corresponding to the two finger movements, displayed above and below one of the cues (see Fig. 1). Subjects chose the finger movement corresponding to the cue by pressing one of two spatially congruent keys with their left hand as fast as they could and got immediate feedback in the form of “correct” or “incorrect”. Each recognition test was administered every five trials. To ensure that subjects learned the association between a colored cue and a finger movement and not between a colored cue and the side of the screen where action took place, images were displayed in different orientations (i.e., upright or flipped vertically; see Fig. 1 for illustration). The color of the cue was randomized to ensure it did not always match the preceding trial (1 repetition per condition took place every 4 recognition tests). The block ended when subjects reached a recognition performance criterion of 80% following ten consecutive evaluations.
- Visuovisual training block
Subjects practiced the exact same task as during the familiarization session. This overtraining was included to ensure performance reached asymptote before the first TMS session. Eighty stimuli of each cue-movie pair (160 trials in total and 32 recognition tests) were presented in a pseudorandomized order.
- Observation blocks
During observation blocks, subjects continued to perform the same task but were instructed to passively observe the stimuli and press the pedal when they detected an asterisk. Motor evoked potentials of the FDI were recorded in all trials. A single TMS pulse was applied per trial during the presentation of the cue (cue condition), the observed finger movement (finger-movement observation condition) or a blank screen (baseline condition). In the first trial, pulses were delivered 200 ms before the cue ended (total cue duration = 1,500 ms). The timing of stimulation, close to the onset of the finger movement, was chosen to ensure subjects were paying attention to the cue. In the second part, pulses were applied at the maximal aperture of the finger movement. Corticospinal excitability is greater during maximal aperture when muscle contraction is maximal (e.g., Gangitano et al. 2004). Finally, in the last trial, pulses were applied 1500 ms after the end of the video and 3,300 ms before the onset of the next trial. This timing was chosen to ensure that corticospinal excitability from the previous trial had returned to baseline (Gangitano et al. 2004) and to avoid cumulative effects of TMS (≥5,700 ms elapsed between pulses). Each observation block consisted of a total of 40 trials for the cue condition (20 per cue), 40 trials for the finger-movement observation condition (20 per finger movement) and 20 trials for the baseline condition. One observation block took place after the visuovisual association block and the second one after the visuomotor association block. Only 10 recognition tests (1 every 10 trials) were administered during these blocks to monitor performance throughout the TMS sessions.
- Visuomotor training block
Participants viewed the same stimuli as in the previous block but were told to execute the finger movement corresponding to each cue as fast as possible starting at the onset of the observed finger movement. To assess the level of visuomotor association, a third “neutral” cue providing no information about the upcoming finger movement was added (green cue). The reaction time, measured as the time elapsed between the onset of the observed movement and the onset of the actual movement, was recorded using a custom made electronic device. To avoid vision of their finger movements, subjects placed their hands in their lap over the recording device. During training, cue duration was varied between 1,000 and 1,500 ms to prevent a temporal association between the cue and the movement. Trials in which subjects executed the movement before the cue ended were discarded. No recognition tests were administered during this block. Visuomotor learning was assessed by comparing reaction times (RT) to valid cues versus RTs to neutral cues.
TMS and corticospinal excitability
Single pulses of transcranial magnetic stimulation were delivered using a Magstim 200 (Magstim, Whitland, UK) through a 70 mm figure-of-eight coil positioned over the optimum scalp location corresponding to the left motor cortex with the handle pointing backward at 45° from the midline. Earplugs were provided to protect participants' hearing. Superficial cup electrodes were placed following a belly-tendon mount over the FDI of the right hand. Ground electrodes were placed over the right wrist. Electromyographical recordings were obtained using two AC amplifiers (P5 series, Grass Instruments) with a bandwidth between 10 and 1,000 Hz. The signal was amplified 1,000 times, digitized at 5,000 Hz (National Instruments, Austin, TX) and collected in a PC using a program written in LabView (LabView 8.1, National Instruments). Time interval between TMS pulses varied between 5,700 and 6,500 ms to avoid cumulative effects of the stimulation.
Before starting the experiment, the optimal scalp position to evoke motor evoked potentials from the FDI was identified. Motor threshold was determined as the intensity to produce 5 out of 10 motor evoked potentials of ≥50 μV. This location was marked on the scalp with a soft-tipped pen. During observation blocks, the TMS coil was placed over the mark and a few TMS pulses were delivered to ensure that the size and shape of MEPs were optimal. The head coil was fixed in position using an articulated arm (Manfrotto, Venice, Italy), and head movements were restrained using a bite-bar. During observation blocks, stimulation intensity was adjusted to evoke MEP amplitudes >1 mV.
Data analysis
Off-line signal processing was carried out in Matlab (The Mathworks). MEPs were quantified by measuring their peak-to-peak amplitude. Trials were discarded when baseline EMG activity exceeded 100 μV, 100 ms prior to the TMS pulse. Outliers were discarded based on Grubbs criterion (α = 0.05). Peak-to-peak values for each condition were adjusted by the baseline for each observation block.
Statistical assessment was carried out using a statistical package (SPSS software package; San Rafael, CA). Visuovisual and visuomotor learning, asterisk detection and differences in corticospinal excitability were analyzed using repeated measures ANOVA.
RESULTS
Behavioral results
Subjects learned the association between each colored cue and the observed finger movement during the familiarization session and reached asymptote during the first observation block [recognition performance: visuovisual training block, mean = 97% correct (range = 90–100%), 1st observation block, mean = 99% correct (range = 90 to 100%), 2nd observation block, mean = 97% correct (range = 60–100%)]. The fact that there were no statistical differences in recognition performance or reaction time across the two observation blocks suggests that further exposure to the visual pairs in subsequent blocks did not result in additional visuovisual learning [recognition performance, 1-way repeated measures ANOVA: F(2,14) = 0.6, P = 0.5; reaction time, 2-way repeated measures ANOVA followed by Sidak, 1st observation block vs. 2nd observation block P = 0.571].
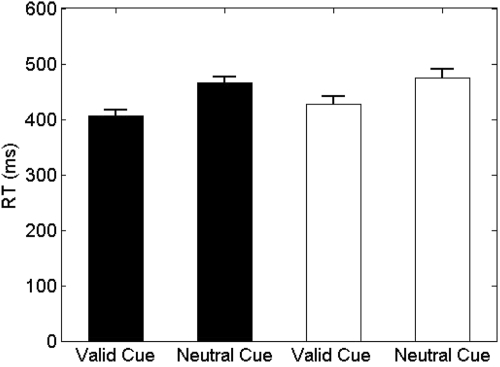
Figure 2 indicates that subjects learned the visuomotor association between each cue and the actual finger movement successfully. Visuomotor training yielded faster RTs for predictive cues than for neutral cues [2-way repeated measures ANOVA on the log10 of RTs: main effect of finger F(1,14) = 3.30, P = 0.09; main effect of cue (valid, neutral) F(1,14) = 64.55, P < 0.001; finger × cue interaction F(1,14) = 1.48, P = 0.24]. Accuracy did not differ across conditions [2-way repeated measures ANOVA; main effect of finger F(1,14) = 0.12, P = 0.73; main effect of cue F(1,14) = 3.73, P = 0.74; finger × cue interaction F(1,14) = 1.09, P = 0.31]. Finally, assessment of asterisk detection showed that subjects paid comparable amounts of attention to visual stimuli throughout the experiment [visuovisual training block = 90% correct (range = 68–100%); 1st observation block = 95% correct (range = 80–100%); 2nd observation block = 89% correct (range = 60–100%), 1-way ANOVA: F(2,14) = 1.47, P = 0.247].
Fig. 2.
Sensorimotor learning. Shown are the means ± SE corresponding to the reaction times of the little (□) and index finger (■) during the sensorimotor training block in response to valid and neutral cues.
Corticospinal excitability
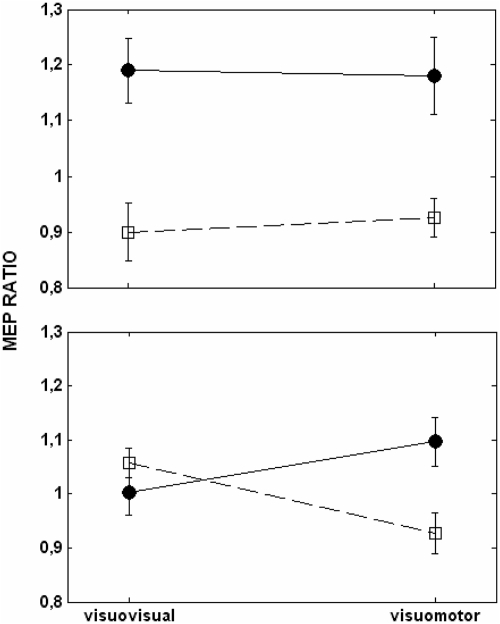
Corticospinal excitability for the first dorsal interosseus of the right hand was measured by delivering single pulses of transcranial magnetic stimulation (TMS) over the left primary motor cortex during the presentation of the cue and at the maximal aperture of the observed finger movement. Figure 3 shows the results obtained during observation of finger movements (top) and abstract stimuli (bottom) after visuovisual and visuomotor training. Corticospinal excitability measured at the maximal aperture of the finger movement was higher during the observation of index finger than little finger movements regardless of the type of training (top). Corticospinal excitability measured during the presentation of the abstract stimulus was similar for both cues after visuovisual training but increased for the cue associated with the index finger after visuomotor training (bottom). A three-way repeated measures ANOVA with training (visuovisual and visuomotor training), stimulus type (observed movement and cue), and finger (index and little finger) as within-subject factors yielded a significant training × stimulus type × finger interaction [F(1,14) = 8.76, P = 0.01]. Breaking down the triple interaction by stimulus type yielded a significant finger × training interaction during observation of the cue [F(1,14) = 7.19, P = 0.01], but this interaction was not significant during observation of finger movements [F(1,14) = 0.04, P = 0.84]. Further comparison between cue type (index and little finger) and baseline, post visuomotor training revealed a significant increment of corticospinal excitability for the cue associated with the index finger (P = 0.05) and a marginally significant suppression of corticospinal excitability for the cue associated with the little finger (P = 0.08). The results suggest that corticospinal excitability is modulated by observation of an abstract cue only after it has been motorically mapped to an action.
Fig. 3.
Corticospinal excitability of 1st dorsal interoseus (FDI). Motor evoked potentials were measured for the FDI during the maximal aperture of finger movements (top) and during the presentation of the cue (bottom) after visuovisual and visuomotor training. Shown are the means ± SE of the ratio obtained from adjusting the MEPs for the index (●) and the little finger (□) by the baseline.
DISCUSSION
In this study, we have addressed the hypothesis that sensorimotor contingencies mediate motor facilitation during observation of simple finger movements. For this purpose, we trained subjects to learn arbitrary associations between two non-action, abstract stimuli and two different finger movements and compared corticospinal excitability elicited by passive observation of the abstract stimuli once they had been visually or motorically associated with the corresponding movement. Our results show evidence in support of our hypothesis. Specifically, corticospinal excitability was higher during the observation of a colored cue that preceded a movement involving the recorded muscle than during the observation of a different colored cue that preceded a movement involving a different muscle of the same hand. Crucially, this modulation was only attained when cues were motorically associated with the finger movement but not when they were visually associated with it.
The pattern of results depicted in Fig. 3 further suggests that visuomotor training was accompanied by an increment in corticospinal excitability relative to the baseline for the cue associated with movement of the index finger and a relative decrease for the cue associated with movement of the little finger (however, note that the latter was marginally significant P = 0.08). This pattern of facilitation for the involved muscle and suppression for the uninvolved muscle mimics that found for observation of finger movements at a smaller scale, and has previously been reported by Catmur et al. (2007) using a similar experimental paradigm. It is possible that this opposite modulation of corticospinal excitability reflects cortico-cortical inhibition through horizontal connections between neighboring finger representations of the FDI and ADM (Sanes and Donoghue 1995).
Our findings are of significant relevance to the field of motor control. First, they corroborate that neither the dynamic component of an action nor the physical implication of action is necessary to activate the motor system; what we show to be crucial is the sensorimotor contingency. Second, and further to the previous point, they suggest that the nature of the visual stimuli mediating this phenomenon is fairly unrestrained. Third, they provide experimental support for the hypothesis posited elsewhere (Keysers and Perrett 2004) that neurons with “mirror” properties develop as a result of sensorimotor experience. In the following paragraphs, we discuss these strengths and possible caveats of our study.
Previous studies have shown that the dynamic component of an action is not necessary to elicit motor resonance. For example, the presentation of a static picture with impending movement increases corticospinal excitability (Urgesi et al. 2006), whereas the presentation of the target and effector involved in an action can also induce activation of the AO network (Johnson-Frey et al. 2003). Similarly, viewing the target and the cursor representing the hand, is sufficient to activate neurons of the dorsal premotor cortex previous to the initiation of a movement performed by the experimenter (Cisek and Kalaska 2004). Our demonstration that an abstract stimulus bearing no physical resemblance with an action can elicit motor resonance after sensorimotor learning, suggests that the physical nature of the observed stimulus may be in fact irrelevant to the induced response. These results corroborate those by Wonfensteller and collaborators, showing that nonstandard, sensorimotor mapping can change the specificity of the ventral and dorsal premotor cortex for processing object and spatial properties of attended stimuli, respectively (Wolfensteller et al. 2004).
Our findings are consistent with the physiological model developed by Keysers and Perrett (2004) to explain the origin of action understanding based on Hebbian learning. According to this view, “mirror” properties may emerge from Hebbian associative learning at the neural connections involved in processing the visual and motor aspects of manipulative actions, which include the superior temporal sulcus (STS), the area PF of the inferior, posterior parietal cortex (PF), and the ventral premotor cortex (F5). The connectivity among the visual STS, F5, and PF is weak and unselective in infants; frontal regions may be active when an infant monkey grasps an object, whereas STS may fire when he sees another individual performing that action. Yet over time, as the monkey executes the action and simultaneously observes his own or someone else's action, STS neurons responding to the sight of the action would fire in synchrony with those in PF and F5 generating the motor commands, thereby leading to Hebbian plasticity at these interconnections. After the association is established, the mere observation of the grasping behavior would activate F5 and PF. Such physiological mechanism may mediate the association between an object and the movements necessary to operate it, which would explain why the mere observation of a familiar tool elicits activation of the premotor cortex (Chao and Martin 2000; Grafton et al. 1997). A similar mechanism may also be at the basis of motor facilitation during observation of static pictures with impending action mentioned in the preceding text.
An associative mechanism of this kind would also explain the occurrence of motor facilitation in response to an abstract stimulus after it has been repeatedly paired with a finger movement. We hypothesize that this new sensorimotor mapping is likely to emerge from strengthening the connections of a different network including the dorsal premotor cortex and the posterior parietal cortex, both of which have been linked to preparation and execution of these kind of intransitive movements (Passingham 1985; Petrides 1985; Thoenissen et al. 2002). Our study provides three relevant pieces of information that may enrich the existing model: that sensorimotor contingencies do not need to match kinematically, that motor resonance can emerge after only 12 min of sensorimotor training, and that sensorimotor learning may shape motor resonance throughout adulthood.
One could claim that motor facilitation elicited by the abstract cue does not reflect motor resonance in response to the associated movement but simply its anticipation. We find this possibility unlikely. Solid experimental evidence indicates that assessing the expected outcome of a sequence of abstract stimuli is associated with activation of a sensorimotor circuit including the ventral premotor, middle frontal, presupplementary motor, and intraparietal cortex (Schubotz 2007; Schubotz and von Cramon 2004). Yet if motor facilitation reported in our study was due to anticipation of the upcoming finger movement, it should have occurred after visuovisual learning, which was not the case. On the other hand, the fact that neither performance nor reaction time differed for recognition across the two TMS sessions, rules out the possibility that motor facilitation in response to the cue was due to the cumulative summation of all the preceding visuovisual associations.
Although visuomotor training significantly modulated corticospinal excitability in response to the cue, it had no impact on the motor response to the observed finger movement. At first sight, this may appear contradictory because one would expect that practicing a movement would lead to greater MEPs. Experimental evidence gathered from training on a very similar finger movement paradigm shows a significant effect of motor training after 12 blocks of 72 trials for each finger movement and overnight consolidation (Catmur et al. 2007). In our study, however, subjects learned the visuomotor association for only 160 trials. Although this interval appears sufficient to associate a visual stimulus with a motor command, it may not be enough to enhance corticospinal excitability over its initial level. We predict that prolonging the period of visuomotor training and/or overnight consolidation would enhance motor facilitation in response to the observation of both stimuli. Finally, it is important to consider that to keep the context of the task constant, the visuomotor association between the cue and the executed movement took place while subjects were simultaneously observing the videos of finger movements. Therefore we cannot rule out that imitation may be necessary to achieve motor facilitation in response to abstract cues.
In sum, we have shown that motor resonance can be triggered by nonbiological, abstract stimuli arbitrarily associated with an action. Our results provide evidence suggesting that it is the sensorimotor contingency between the observed stimuli and the associated action what drives activity in the motor system of the observer. Our findings are consistent with the hypothesis posited elsewhere (Keysers and Perrett 2004) that “mirror” properties develop as a result of sensorimotor associations. Further investigation is necessary to address the nature and the reversibility of this phenomenon.
GRANTS
The study was supported by the National Agency for the Promotion of Science and Technology, the International Brain Research Organization, and the Fogarty International Research Collaboration Award (NIH).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank K. Watkins, S. Grafton, and N. Jenkinson for thoughtful comments.
REFERENCES
- Catmur et al., 2007.Catmur C, Walsh V, Heyes C. Sensorimotor learning configures the human mirror system. Curr Biol 17: 1527–1531, 2007 [DOI] [PubMed] [Google Scholar]
- Chao and Martin, 2000.Chao LL, Martin A. Representation of manipulable man-made objects in the dorsal stream. Neuroimage 12: 478–484, 2000 [DOI] [PubMed] [Google Scholar]
- Cisek and Kalaska, 2004.Cisek P, Kalaska JF. Neural correlates of mental rehearsal in dorsal premotor cortex. Nature 431: 993–996, 2004 [DOI] [PubMed] [Google Scholar]
- Enticott et al., 2010.Enticott PG, Kennedy HA, Bradshaw JL, Rinehart NJ, Fitzgerald PB.Understanding mirror neurons: evidence for enhanced corticospinal excitability during the observation of transitive but not intransitive hand gestures. Neuropsychologia 48: 2675–2680, 2010 [DOI] [PubMed] [Google Scholar]
- Fadiga et al., 1995.Fadiga L, Fogassi L, Pavesi G, Rizzolatti G. Motor facilitation during action observation: a magnetic stimulation study. J Neurophysiol 73: 2608–2611, 1995 [DOI] [PubMed] [Google Scholar]
- Gallese et al., 1996.Gallese V, Fadiga L, Fogassi L, Rizzolatti G.Action recognition in the premotor cortex. Brain 119: 593–609, 1996 [DOI] [PubMed] [Google Scholar]
- Gangitano et al., 2004.Gangitano M, Mottaghy FM, Pascual-Leone A. Modulation of premotor mirror neuron activity during observation of unpredictable grasping movements. Eur J Neurosci 20: 2193–2202, 2004 [DOI] [PubMed] [Google Scholar]
- Grafton et al., 1997.Grafton ST, Fadiga L, Arbib MA, Rizzolatti G. Premotor cortex activation during observation and naming of familiar tools. Neuroimage 6: 231–236, 1997 [DOI] [PubMed] [Google Scholar]
- Johnson-Frey et al., 2003.Johnson-Frey SH, Maloof FR, Newman-Norlund R, Farrer C, Inati S, Grafton ST. Actions or hand-object interactions? Human inferior frontal cortex and action observation. Neuron 39: 1053–1058, 2003 [DOI] [PubMed] [Google Scholar]
- Keysers and Perrett, 2004.Keysers C, Perrett DI. Demystifying social cognition: a Hebbian perspective. Trends Cogn Sci 8: 501–507, 2004 [DOI] [PubMed] [Google Scholar]
- Passingham, 1985.Passingham RE. Premotor cortex: sensory cues and movement. Behav Brain Res 18: 175–185, 1985 [DOI] [PubMed] [Google Scholar]
- Petrides, 1985.Petrides M. Deficits in non-spatial conditional associative learning after periarcuate lesions in the monkey. Behav Brain Res 16: 95–101, 1985 [DOI] [PubMed] [Google Scholar]
- Sanes et al., 1995.Sanes JN, Donoghue JP, Thangaraj V, Edelman RR, Warach S. Shared neural substrates controlling hand movements in human motor cortex. Science 268: 1775–1777, 1995 [DOI] [PubMed] [Google Scholar]
- Schubotz, 2007.Schubotz RI. Prediction of external events with our motor system: towards a new framework. Trends Cogn Sci 11: 211–218, 2007 [DOI] [PubMed] [Google Scholar]
- Schubotz and von Cramon, 2004.Schubotz RI, von Cramon DY. Sequences of abstract nonbiological stimuli share ventral premotor cortex with action observation and imagery. J Neurosci 24: 5467–5474, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strafella and Paus, 2000.Strafella AP, Paus T. Modulation of cortical excitability during action observation: a transcranial magnetic stimulation study. Neuroreport 11: 2289–2292, 2000 [DOI] [PubMed] [Google Scholar]
- Thoenissen et al., 2002.Thoenissen D, Zilles K, Toni I. Differential involvement of parietal and precentral regions in movement preparation and motor intention. J Neurosci 22: 9024–9034, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urgesi et al., 2006.Urgesi C, Moro V, Candidi M, Aglioti SM. Mapping implied body actions in the human motor system. J Neurosci 26: 7942–7949, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfensteller et al., 2004.Wolfensteller U, Schubotz RI, von Cramon DY. “What” becoming “where”: functional magnetic resonance imaging evidence for pragmatic relevance driving premotor cortex. J Neurosci 24: 10431–10439, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]