Abstract
Stimulation of the nasal mucosa by airborne irritants or water evokes a pronounced bradycardia accompanied by peripheral vasoconstriction and apnea. The dive response, which includes the trigeminocardiac reflex, is among the most powerful autonomic responses. These responses slow the heart rate and reduce myocardial oxygen consumption. Although normally cardioprotective, exaggeration of this reflex can be detrimental and has been implicated in cardiorespiratory diseases, including sudden infant death syndrome (SIDS). An essential component of the diving response and trigeminocardiac reflex is activation of the parasympathetic cardiac vagal neurons (CVNs) in the nucleus ambiguus that control heart rate. This study examined the involvement of cholinergic receptors in trigeminally evoked excitatory postsynaptic currents in CVNs in an in vitro preparation from rats. CVNs were identified using a retrograde tracer injected into the fat pads at the base of the heart. Application of the acetylcholinesterase inhibitor neostigmine significantly decreased the amplitude of glutamatergic neurotransmission to CVNs on stimulation of trigeminal fibers. Whereas nicotine did not have any effect on the glutamatergic responses, the muscarinic acetylcholine receptor (mAChR) agonist bethanechol significantly decreased the excitatory neurotransmission. Atropine, an mAChR antagonist, facilitated these responses indicating this trigeminally evoked brain stem pathway in vitro is endogenously inhibited by mAChRs. Tropicamide, an m4 mAChR antagonist, prevented the inhibitory action of the muscarinic agonist bethanechol. These results indicate that the glutamatergic synaptic neurotransmission in the trigeminally evoked pathway to CVNs is endogenously inhibited in vitro by m4 mAChRs.
INTRODUCTION
Activation of the trigeminocardiac reflex by mechanical or electrical stimulation of the trigeminal nerve evokes a pronounced decrease in heart rate, significant fall in blood pressure, and apnea and is among the most powerful autonomic reflexes (Kumada et al. 1977, 1978; Schaller 2004, 2005a,b; Schaller et al. 1999). The trigeminocardiac reflex is activated on craniofacial, orofacial, maxillofacial, and tooth extraction surgeries (Barnard and Bainton 1990; Schaller 2004; Schaller and Buchfelder 2006; Schaller et al. 1999). Similarly, the dive response, which likely includes the trigeminocardiac reflex, can be initiated by fluid or airborne irritant stimulation (Dykes 1974) of nasotrigeminal afferent nerve fibers located in the nasal mucosa (Dykes 1974; Yavari et al. 1996). This response is highly pronounced in infants, who exhibit a decrease in heart rate ranging from 5 to 51% on a single facial submersion (Goksor et al. 2002). Activation of the dive response, through stimulation of nasotrigeminal sensory fibers, evokes an increase in parasympathetic cardiac activity and pronounced bradycardia (Elsner et al. 1971; Gandevia et al. 1978).
Although the trigeminocardiac reflex and diving responses are typically protective, exaggeration of the resulting bradycardia could prove detrimental. Sudden infant death syndrome (SIDS) is the leading cause of death in infants ages 1 mo to 1 yr, occurring in 0.3 per 1,000 live births in the United States (Hunt and Hauck 2006). The most prevalent and predictive event in infants monitored for SIDS is bradycardia (Cote et al. 1998). This bradycardia precedes or is accompanied by centrally mediated life-threatening apnea (Fewell et al. 2001; Meny et al. 1994; Nachmanoff et al. 1998; Poets et al. 1999). Although the cause(s) for SIDS remains unknown, studies suggest an exaggeration of parasympathetic control of cardiac function may be involved (Divon et al. 1986; Harper and Bandler 1998; Meny et al. 1994; Schechtman et al. 1992; Spyer and Gilbey 1988). SIDS has also been linked to an abnormal or exacerbated response to trigeminal sensory nerve stimulation (Lobban 1991, 1995; Morpurgo et al. 2004). A recent case study describes an infant who succumbed to SIDS hours after having been given a bath. It was determined SIDS was triggered by temporarily plunging the infant's head underwater during bathing (Matturri et al. 2005). SIDS has also been associated with both abnormal brain stem serotonergic function and cholinergic dysfunction (Frank et al. 2001; Hunt 2005; Hunt and Hauck 2006; Kinney 2009; Kinney et al. 1995; Panigrahy et al. 2000; Paterson et al. 2006). Interestingly, deficits in cholinergic receptor density, in addition to binding dysfunction of cholinergic receptors, have been implicated as potential risk factors for SIDS (Frank et al. 2001; Kinney et al. 1995; Panigrahy et al. 2000). Infants who succumb to SIDS exhibit a decrease in choline acetyltransferase (ChAT) activity, in addition to a reduction in muscarinic acetylcholine receptor (mAChR) binding capacity (Kubo et al. 1998; Slotkin et al. 1999) and ChAT immunoreactivity (Kalaria et al. 1993; Kinney et al. 1995; Mallard et al. 1999; Sparks and Hunsaker 1991).
Previous work has shown stimulation of trigeminal sensory fibers elicits a polysynaptic excitatory glutamatergic synaptic neurotransmission to cardiac vagal neurons (CVNs) in the nucleus ambiguus (NA) (Gorini et al. 2009). Furthermore, serotonin (5-HT1A and 5-HT2A/C) receptors are endogenously active in vitro and differentially modulate this pathway in the brain stem (Gorini et al. 2009). However, little is known about the cholinergic modulation of the trigeminally evoked responses in CVNs. The goals of this study were to characterize the role of nicotinic and mAChRs in the trigeminally evoked excitatory neurotransmission to parasympathetic CVNs in the NA.
METHODS
In an initial surgery, 2- to 5-day-old Sprague–Dawley rats were anesthetized with hypothermia, which also decreased heart rate and aided in recovery. A right thoracotomy was performed and 50 μl of 1–5% of the retrograde fluorescent tracer X-rhodamine-5-(and-6)-isothiocyanate (Molecular Probes, Eugene, OR) was injected into the pericardial sac and onto the fat pads at the base of the heart. After 24–48 h of recovery, animals were anesthetized with isoflurane and killed by cervical dislocation, after which the brain tissue was placed in a 4°C physiologic saline solution [containing (in mM) 140 NaCl, 5 KCl, 2 CaCl2, 5 glucose, and 10 HEPES] bubbled with 100% O2 (pH 7.4). All animal procedures were performed with the approval of the Animal Care and Use Committee of The George Washington University in accordance with the recommendations of the panel on euthanasia of the American Veterinary Medical Association and the National Institutes of Health publication, Guide for the Care and Use of Laboratory Animals.
The medulla was removed with care to preserve the trigeminal cranial nerve rootlet, mounted on a cutting block, and placed into a vibrating blade microtome (Leica, Nussloch, Germany). A modified horizontal slice (800–900 μm) was obtained to stimulate the trigeminal nerve rootlet and record synaptic responses in CVNs. The tissue was submerged in a recording chamber that allowed perfusion (4 ml/min) above and below the slice with artificial cerebrospinal fluid [ACSF; containing (in mM) 125 NaCl, 3 KCl, 2 CaCl2, 26 NaHCO3, 5 glucose, and 5 HEPES], equilibrated with 95% O2-5% CO2 (pH 7.4).
CVNs in the NA were identified by the presence of the fluorescent tracer as described previously (Mendelowitz and Kunze 1991). Briefly, slices were viewed with infrared illumination and differential interference optics (Zeiss, Oberkochen, Germany) and under fluorescent illumination with an infrared-sensitive cooled charged-coupled device camera (Photometrics, Tucson, AZ). Neurons that contained the fluorescent tracer were identified by superimposing the fluorescent and infrared images on a video monitor (Sony, Tokyo). Our labeled preparation did not include esophageal motoneurons nor did neurons in the nucleus ambiguus pars compacta contain any significant fluorescent labeling. Patch pipettes (2.5–3.5 MOhm) were visually guided to the surface of individual CVNs using differential interference optics and infrared illumination (Zeiss). CVNs were voltage-clamped at a holding potential of −80 mV. The patch pipettes were filled with a solution that consisted of (in mM) 135 K-gluconic acid, 10 HEPES, 10 EGTA, 1 CaCl2, 1 MgCl2, and 0.005 QX-314 at a pH of 7.3.
Afferent fibers in the trigeminal nerve were stimulated, 1–2 ms duration, 2-Hz frequency, with a bipolar concentric stimulating electrode (WPI, Sarasota, FL) using a stimulus isolator (AMPI, Jerusalem, Israel). Stimulus intensity was increased in each experiment, ranging from 0.1 to 1 mA until a consistent synaptic pathway was evoked in each CVN during control conditions and then the stimulation parameters were maintained at that intensity and duration throughout the experiment. A series of 10 consecutive stimulations in each neuron was averaged and this mean value from each neuron in the population was then averaged to create a summary of results for each condition. Excitatory events were measured using pClamp 8 software (Molecular Devices, Sunnyvale, CA), which analyzes excitatory postsynaptic current (EPSC) amplitudes as peak currents subtracted from baseline currents. Baseline measurements were taken as an average over 400 ms before the stimulation and the maximum peak amplitude was detected within 20 ms, beginning from stimulation. Results are presented as mean ± SE and statistically compared with a paired Student's t-test or ANOVA with repeated measures, as appropriate (for significance of difference, P < 0.05). Only one experiment was performed per preparation. Stimulations were withheld for a period of about 5 min between stimulations 10 and 11.
The following pharmacological agents were applied by inclusion in the perfusate: the acetylcholinesterase inhibitor neostigmine (10 μM), the nicotinic acetylcholine receptor agonist nicotine (100 μM), the nicotinic acetylcholine receptor antagonist dihydro-β-erythroidine (DhβE, 100 μM), the mAChR agonist carbamyl-β-methylcholine chloride (bethanechol, 100 μM), and the mAChR antagonist atropine (10 μM). The following subtype-specific mAChR antagonists were applied: m1, pirenzepine dihydrochloride (pirenzepine, 10 μM); m2, 11-[[2-[(diethylamino)methyl]-1-piperidinyl]acetyl]-5,11-dihydro-6H-pyrido[2,3-b][1,4]benzodiazepin-6-one (AF-DX, 0.1 μM); m3, p-fluorohexahydro-sila-difenidol hydrochloride (pFHHSiD, 1 μM); and m4, tropicamide (1 μM). All drugs were purchased from Sigma-Aldrich (St. Louis, MO), with the exception of AF-DX and pirenzepine, which were purchased from Ascent Scientific (Princeton, NJ). Subtype-specific mAChR antagonist concentrations were chosen based on the literature. Specifically, in the case of m1 mAChRs, Sun et al. (2009) previously showed that 10 μM is the lowest concentration for which pirenzepine is significantly selective for m1 mAChRs in rat brain stem slices.
RESULTS
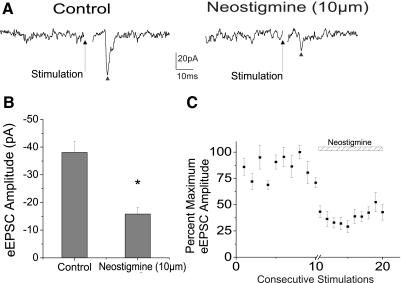
To elucidate the role of endogenous acetylcholine synaptic release in trigeminally evoked EPSCs in CVNs the acetylcholinesterase inhibitor neostigmine (10 μm) was included in the purfusate. Peak amplitudes of EPSCs, represented by a ▴, evoked in CVNs on stimulation of trigeminal afferents, were significantly reduced on application of neostigmine by 58.5 ± 3.7% (from −38.1 ± 3.9 to −15.8 ± 2.3 pA, n = 8, P < 0.05) as shown in a typical experiment (Fig. 1A). The summary data from eight experiments are represented in the bar graph (Fig. 1B) and the percentage maximum EPSC amplitude from eight experiments is illustrated in the scatterplot (Fig. 1C), indicating endogenous acetylcholine release inhibits trigeminally evoked responses in CVNs.
Fig. 1.
Application of the acetylcholinesterase inhibitor neostigmine (10 μm) significantly decreased the peak amplitude of the excitatory synaptic response to cardiac vagal neurons (CVNs). A typical experiment is shown in the top traces (A), with a ▴ indicating peak synaptic event and the summary data from 8 experiments is shown in B. C: scatterplot of percentage maximum evoked excitatory postsynaptic current (eEPSC) amplitude from 8 experiments. Neostigmine (10 μm) decreased the glutamatergic neurotransmission to cardiac vagal evoked by trigeminal afferent stimulation from −38.1 ± 3.9 to −15.8 ± 2.3 pA (by 58.5 ± 3.7%, n = 8, P < 0.05).
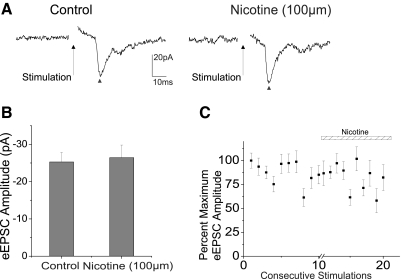
To identify whether nicotinic acetylcholine receptors (nAChRs) and/or muscarinic acetylcholine receptors (mAChRs) are involved in the cholinergic modulation of this trigeminally evoked response pathway, the effects of different nAChR and mAChR agonists and antagonists were tested. The nAChR agonist nicotine (100 μm) did not have any significant effect on the excitatory synaptic response in CVNs on trigeminal afferent stimulation (n = 8, P > 0.05). Summary data from eight experiments are shown in the bar graph in Fig. 2B, with a typical experiment illustrated in the traces (Fig. 2A), with trigeminally evoked glutamatergic neurotransmission indicated by a ▴. A scatterplot representing the percentage maximum EPSC amplitude from eight experiments is shown in Fig. 2C. Additionally, application of the nAChR antagonist DhβE (100 μm) also did not have any significant effect on trigeminal-induced EPSCs in CVNs (data not shown; n = 7 P > 0.05).
Fig. 2.
To identify specific cholinergic receptors involved in the trigeminally evoked EPSCs in CVNs, nicotine (100 μm), a nicotinic acetylcholine receptor agonist, was applied in the perfusate. Application of nicotine exhibited no significant effect on trigeminal afferent evoked glutamatergic neurotransmission, represented by a ▴, as shown in top traces (A), a typical experiment, along with summary data from 8 experiments (B and C).
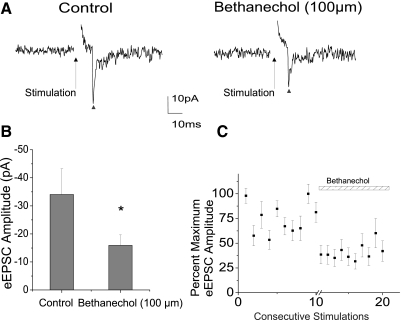
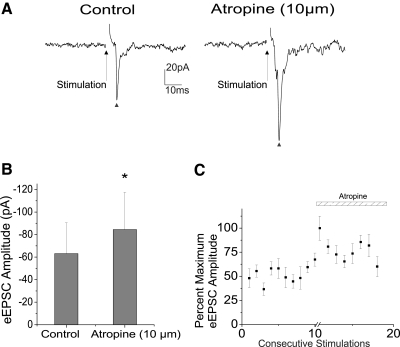
The broad mAChR agonist bethanechol (100 μm) significantly inhibited the excitatory neurotransmission to CVNs, decreasing peak amplitude from −34.1 ± 9.2 to −15.9 ± 3.7 pA (n = 8, P < 0.05). A typical experiment is shown in the top traces in Fig. 3A, with a ▴ designating the evoked synaptic response and summary data from eight experiments in Fig. 3, B and C. Conversely, the mAChR antagonist atropine (10 μm) was shown to significantly facilitate the EPSC evoked in CVNs on trigeminal afferent stimulation by 33.8 ± 20.9% (from −63.1 ± 27.4 to −84.4 ± 33.1 pA, n = 7, P < 0.05), providing additional evidence that this pathway is endogenously inhibited in vitro by acetylcholine and, in particular, mAChR activation. A ▴ indicates the peak synaptic event as seen in a typical experiment, illustrated in Fig. 4A, with summary data from seven experiments shown in the bar graph (Fig. 4B) and percentage maximum EPSC amplitude (Fig. 4C).
Fig. 3.
Application of the broad muscarinic acetylcholine receptor (mAChR) agonist bethanechol (100 μm) inhibited the responses evoked by stimulation of trigeminal afferents by an average of 53.2 ± 5.5%, from −34.1 ± 9.2 to −15.9 ± 3.7 pA (n = 8, P < 0.05). A typical experiment is illustrated in A, with ▴ indicating evoked responses and summary results from 8 experiments in the bar graph (B) and scatterplot (C).
Fig. 4.
Atropine (10 μm), a broad mAChR antagonist, induced a significant facilitation (n = 7, P < 0.05) of 33.8 ± 20.9% (from −63.1 ± 27.4 to −84.4 ± 33.1 pA) of the excitatory transmission to CVNs as indicated by ▴, and shown in a typical experiment (A) as well as in the summary data from 7 experiments (B, C).
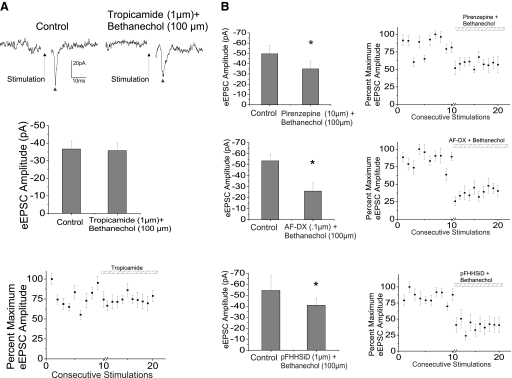
To identify the specific subtypes of mAChRs involved in the endogenous inhibition, the broad agonist bethanechol (100 μm) was applied in conjunction with subtype-specific mAChR antagonists. Tropicamide (1 μm), an m4 mAChR antagonist, prevented the inhibitory action of bethanechol (100 μm), as shown in a typical experiment (Fig. 5A), with the summary data from eight cells in the bar graph along with the percentage maximum EPSC amplitude from eight cells. The ▴ symbols are representative of trigeminally evoked glutamatergic synaptic neurotransmission to CVNs.
Fig. 5.
Identification of subtype-specific mAChRs involved in this reflex pathway was accomplished through application of specific receptor antagonists along with the broad mAChR agonist bethanechol (100 μm). m4 AChR antagonist, tropicamide (1 μm) significantly inhibited the glutamatergic neurotransmission to CVNs by 26.7 ± 3.1%, reducing peak amplitude from −36.7 ± 4.7 to −26.9 ± 3.7 pA (n = 8, P < 0.05). Tropicamide also blocked the inhibitory effect of the mAChR agonist bethanechol (100 μm) on the evoked excitatory synaptic transmission to CVNs. A typical experiment is shown in top traces and summary data from 8 experiments in the bar graph and scatterplot (A). Application of the m1, m2, and m3 AChR antagonists, pirenzepine dihydrochloride (pirenzepine, 10 μm), 11-[[2-[(diethylamino)methyl]-1-piperidinyl]acetyl]-5,11-dihydro-6H-pyrido[2,3-b][1,4]benzodiazepin-6-one (AF-DX, 0.1 μM), and p-fluorohexahydro-sila-difenidol hydrochloride (pFHHSiD, 1 μM), respectively, did not significantly inhibit the excitatory glutamatergic transmission to CVNs on trigeminal afferent stimulation. Additionally, each antagonist did not block the inhibitory effect of bethanechol (100 μm) on the evoked synaptic transmission. The summary results for pirenzepine (n = 8), AF-DX in B (n = 7), and pFHHSiD (n = 8) are shown in B. All evoked synaptic glutamatergic neurotransmission is indicated by a ▴.
The m1, m2, and m3 mAChR receptor antagonists, pirenzepine (10 μm, n = 8), AF-DX (0.1 μm, n = 7), and pFHHSiD (1 μm, n = 8), respectively, did not significantly alter the synaptic excitatory response in CVNs (P > 0.05). Additionally, the m1, m2, and m3 antagonists did not significantly alter the inhibition of the excitatory glutamatergic response in CVNs evoked by stimulation of trigeminal afferents on the addition of the mAChR agonist bethanechol (100 μm). Figure 5 shows the summary data illustrating the maintained significant decrease in peak amplitude on application of bethanechol with pirenzepine, AF-DX, and pFFHSiD (Fig. 5B). These data indicate that m4 receptors are the most likely mAChRs endogenously modulating the excitatory glutamatergic neurotransmission to CVNs on trigeminal afferent stimulation.
DISCUSSION
This study was carried out to elucidate the role of brain stem cholinergic receptors in the trigeminally evoked excitation of CVNs, providing three major conclusions: 1) neostigmine, an acetylcholinesterase inhibitor, significantly inhibited the excitatory glutamatergic trigeminally evoked responses in CVNs; 2) nicotinic acetylcholine receptors do not significantly alter this neurotransmission; and 3) this evoked response in CVNs is endogenously inhibited in vitro by m4 mAChRs.
Cholinergic modulation of brain stem cardiorespiratory network function is complex. Previous work has shown that nAChRs play a major role in brain stem cardiorespiratory function (Dwyer et al. 2008; Huang et al. 2007; Kamendi et al. 2006; Wang et al. 2003). Activation of nAChRs, specifically beta2 nAChRs, at presynaptic terminals by endogenous acetylcholine release facilitates GABAergic inhibitory inputs to CVNs and likely generates respiratory sinus arrhythmia (Evans et al. 2005; Wang et al. 2003). CVNs in the NA receive cholinergic inputs from multiple areas in the brain stem (Bieger 1993; Milner et al. 1989). Release of acetylcholine increases the frequency of glutamatergic synaptic inputs thought to originate from synaptic terminals of nucleus tractus solitarius neurons via activation of presynaptic α7-containing nicotinic receptors (Wang et al. 2001). Other sources of cholinergic inputs to CVNs could include the pedunculopontine tegmental nucleus as well as the laterodorsal tegmental nucleus (Jones and Beaudet 1987; Shiromani et al. 1988), both of which send projections to the cranial nerve nuclei, including the NA. It is also possible that cholinergic inputs to CVNs originate from surrounding cholinergic cells in the NA (Bouairi et al. 2006), lateral tegmentum area (Winzer-Serhan and Leslie 1997), and passing fibers from pontine structures (Jones 1990).
Interestingly, however, our data suggest that nicotinic receptors do not play a significant role in modulation of the trigeminally evoked responses in CVNs because the glutamatergic neurotransmission evoked on trigeminal nerve stimulation did not exhibit any significant change on application of nicotine or the nicotinic receptor antagonist DhβE. Surprisingly, however, the responses evoked by trigeminal afferent stimulation are endogenously modulated by mAChRs. Neostigmine decreased the trigeminally evoked activation of CVNs and, demonstrating a role of muscarinic receptors, the muscarinic receptor antagonist atropine increased the neurotransmission to CVNs and bethanechol, a muscarinic receptor agonist, decreased this response pathway. This modulation is mediated by m4 mAChRs.
Prenatal nicotine exposure is a known cause of change in both nAChR and mAChR function and density and is thought to be one likely cause of SIDS (Campos et al. 2009; Coddou et al. 2009; Fewell et al. 2001; Matturri et al. 2004; Thiriez et al. 2009). Prenatal exposure to nicotine has been shown to exaggerate and alter the GABAergic pathways to CVNs as well as the synaptic pathways to CVNs in response to hypoxia (Huang et al. 2007; Kamendi et al. 2006). In addition to changes in nicotinic receptor function, fetal exposure to nicotine also decreases brain stem mAChR expression and binding in rat neonates through suppression of mRNA expression (Zhu et al. 1998). Reduced cholinergic binding has been suggested to be responsible for the perturbed ventilatory response in sudden infant death (Kinney et al. 1995; Kubo et al. 1998; Matturri et al. 2004). However, this cholinergic receptor irregularity may also be linked to a decrease in choline acetyltransferase (ChAT) activity (Oda 1999). Victims of SIDS have exhibited a reduction in ChAT-immunoreactive neurons in both the hypoglossal and dorsal motor nucleus (Mallard et al. 1999; Oda 1999). A decrease in cholinergic activity in the brain stem would likely lead to a reduction in endogenously active in vitro m4 mAChR activity, causing a disinhibition and an exaggerated trigeminocardiac reflex. Future experiments are necessary to test whether fetal nicotine exposure alters the excitatory glutamatergic neurotransmission to CVNs on trigeminal afferent stimulation via changes in mAChR function and may provide a new mechanism responsible for the exaggeration of the trigeminocardiac reflex that occurs in some victims of SIDS.
In addition to respiratory modulation of CVNs, cholinergic receptors and, in particular mAChRs, also play a major role in sleep-dependent changes. Brain stem cholinergic neurotransmission is an integral component of rapid-eye-movement (REM) sleep generation (Vazquez and Baghdoyan 2004; Verret et al. 2005). Changes in cholinergic receptor activation and potentiation of ethmoidal nerve-evoked respiratory suppression (Dutschmann and Herbert 1999) have been associated with altered infant sleep–wake cycles, which is also associated with SIDS (Datta et al. 2009; Frank et al. 2001; Oda 1999). Activation of sensory trigeminal afferents during REM sleep has been shown to contribute to the REM sleep-associated respiratory failures in SIDS (Dutschmann and Herbert 1999; Gould et al. 1988).
It is likely that muscarinic receptors alter the sleep–wake cycles via alterations in protein kinase A (PKA) activity (Caulfield and Birdsall 1998; Gillin and Sitaram 1984; Wess et al. 2007). mAChRs are G-protein coupled receptors (GPCRs) (Caulfield and Birdsall 1998; Wess et al. 2007), with m4 mAChRs primarily coupling to Gi/Go type G proteins (Sawaguchi et al. 2002; Wess et al. 2007). Once activated, m4 mAChRs activate a series of second-messenger systems that inhibit PKA and, subsequently, down-regulate cyclic adenosine monophosphate (cAMP) (Caulfield and Birdsall 1998; Datta et al. 2009; Wess et al. 2007). PKA is critical for the generation of REM sleep and regulation of sleep and wakefulness (Datta et al. 2009; Frank et al. 2001). Abnormalities in REM sleep, specifically longer latencies to REM sleep and lower REM sleep amounts (Coons and Guilleminault 1985; Frank et al. 2001; Harper et al. 1983), evoke changes in cardiovascular system activity, including highly variable heart rate (del Bo et al. 1982; George and Kryger 1985; Koehler et al. 1998; Verrier et al. 1998) and apnea-associated bradyarrhythmias (Gabriel and Albani 1976; Koehler et al. 1998). Additionally, mAChRs mediate REM sleep as well as parasympathetic cardiovascular regulation via vasoactive neuropeptides (VNs) and cAMP production, respectively (Staines 2005, 2006). Changes in cAMP levels have been shown to cause both irregular VN production and VN dysfunction (Staines 2005, 2006). It has also been suggested that VN abnormalities increase expression of N-methyl-d-aspartate receptor mRNA on postganglionic parasympathetic neurons (Robertson et al. 1998). The changes in cAMP levels by endogenous activation of m4 mAChRs by the trigeminocardiac reflex are a possible cellular target for alterations in sleep–wake states that may also increase the risk of SIDS.
In summary, results from this study demonstrate that the trigeminally evoked synaptic responses to CVNs are endogenously inhibited in vitro by mAChRs, specifically m4 AChRs, but are unaffected by nicotinic receptor activation. These results provide a cellular mechanism by which cholinergic dysfunction may play a role in victims of SIDS and describe a novel muscarinic modulation of trigeminally evoked responses in CVNs.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-59895 and HL-72006 to D. Mendelowitz.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- Barnard and Bainton, 1990.Barnard NA, Bainton R. Bradycardia and the trigeminal nerve. J Craniomaxillofac Surg 18: 359–360, 1990 [DOI] [PubMed] [Google Scholar]
- Bieger, 1993.Bieger D. The brainstem esophagomotor network pattern generator: a rodent model. Dysphagia 8: 203–208, 1993 [DOI] [PubMed] [Google Scholar]
- Bouairi et al., 2006.Bouairi E, Kamendi H, Wang X, Gorini C, Mendelowitz D. Multiple types of GABAA receptors mediate inhibition in brain stem parasympathetic cardiac neurons in the nucleus ambiguus. J Neurophysiol 96: 3266–3272, 2006 [DOI] [PubMed] [Google Scholar]
- Campos et al., 2009.Campos M, Bravo E, Eugenin J. Respiratory dysfunctions induced by prenatal nicotine exposure. Clin Exp Pharmacol Physiol 36: 1205–1217, 2009 [DOI] [PubMed] [Google Scholar]
- Caulfield and Birdsall, 1998.Caulfield MP, Birdsall NJ. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev 50: 279–290, 1998 [PubMed] [Google Scholar]
- Coddou et al., 2009.Coddou C, Bravo E, Eugenin J. Alterations in cholinergic sensitivity of respiratory neurons induced by pre-natal nicotine: a mechanism for respiratory dysfunction in neonatal mice. Philos Trans R Soc Lond B Biol Sci 364: 2527–2535, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coons and Guilleminault, 1985.Coons S, Guilleminault C. Motility and arousal in near miss sudden infant death syndrome. J Pediatr 107: 728–732, 1985 [DOI] [PubMed] [Google Scholar]
- Cote et al., 1998.Cote A, Hum C, Brouillette RT, Themens M. Frequency and timing of recurrent events in infants using home cardiorespiratory monitors. J Pediatr 132: 783–789, 1998 [DOI] [PubMed] [Google Scholar]
- Datta et al., 2009.Datta S, Siwek DF, Stack EC. Identification of cholinergic and non-cholinergic neurons in the pons expressing phosphorylated cyclic adenosine monophosphate response element-binding protein as a function of rapid eye movement sleep. Neuroscience 163: 397–414, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Bo et al., 1982.del Bo A, Ledoux JE, Tucker LW, Harshkfield GA, Reis DJ. Arterial pressure and heart rate changes during natural sleep in rat. Physiol Behav 28: 425–429, 1982 [DOI] [PubMed] [Google Scholar]
- Divon et al., 1986.Divon MY, Winkler H, Yeh SY, Platt LD, Langer O, Merkatz IR. Diminished respiratory sinus arrhythmia in asphyxiated term infants. Am J Obstet Gynecol 155: 1263–1266, 1986 [DOI] [PubMed] [Google Scholar]
- Dutschmann and Herbert, 1999.Dutschmann M, Herbert H. Pontine cholinergic mechanisms enhance trigeminally evoked respiratory suppression in the anesthetized rat. J Appl Physiol 87: 1059–1065, 1999 [DOI] [PubMed] [Google Scholar]
- Dwyer et al., 2008.Dwyer JB, Broide RS, Leslie FM. Nicotine and brain development. Birth Defects Res C Embryo Today 84: 30–44, 2008 [DOI] [PubMed] [Google Scholar]
- Dykes, 1974.Dykes RW. Factors related to the dive reflex in harbor seals: sensory contributions from the trigeminal region. Can J Physiol Pharmacol 52: 259–265, 1974 [DOI] [PubMed] [Google Scholar]
- Elsner et al., 1971.Elsner R, Gooden BA, Robinson SM. Arterial blood gas changes and the diving response in man. Aust J Exp Biol Med Sci 49: 435–444, 1971 [DOI] [PubMed] [Google Scholar]
- Evans et al., 2005.Evans C, Wang J, Neff R, Mendelowitz D. Hypoxia recruits a respiratory-related excitatory pathway to brainstem premotor cardiac vagal neurons in animals exposed to prenatal nicotine. Neuroscience 133: 1073–1079, 2005 [DOI] [PubMed] [Google Scholar]
- Fewell et al., 2001.Fewell JE, Eliason HL, Crisanti KC. Prenatal exposure to nicotine attenuates stress-induced hyperthermia in 7- to 8-week-old rats upon exposure to a novel environment. Physiol Behav 74: 595–601, 2001 [DOI] [PubMed] [Google Scholar]
- Frank et al., 2001.Frank MG, Srere H, Ledezma C, O'Hara B, Heller HC. Prenatal nicotine alters vigilance states and AChR gene expression in the neonatal rat: implications for SIDS. Am J Physiol Regul Integr Comp Physiol 280: R1134–R1140, 2001 [DOI] [PubMed] [Google Scholar]
- Gabriel and Albani, 1976.Gabriel M, Albani M. Cardiac slowing and respiratory arrest in preterm infants. Eur J Pediatr 122: 257–261, 1976 [DOI] [PubMed] [Google Scholar]
- Gandevia et al., 1978.Gandevia SC, McCloskey DI, Potter EK. Reflex bradycardia occurring in response to diving, nasopharyngeal stimulation and ocular pressure, and its modification by respiration and swallowing. J Physiol 276: 383–394, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George and Kryger, 1985.George CF, Kryger MH. Sleep and control of heart rate. Clin Chest Med 6: 595–601, 1985 [PubMed] [Google Scholar]
- Gillin and Sitaram, 1984.Gillin JC, Sitaram N. Rapid eye movement (REM) sleep: cholinergic mechanisms. Psychol Med 14: 501–506, 1984 [DOI] [PubMed] [Google Scholar]
- Goksor et al., 2002.Goksor E, Rosengren L, Wennergren G. Bradycardic response during submersion in infant swimming. Acta Paediatr 91: 307–312, 2002 [DOI] [PubMed] [Google Scholar]
- Gorini et al., 2009.Gorini C, Jameson HS, Mendelowitz D. Serotonergic modulation of the trigeminocardiac reflex neurotransmission to cardiac vagal neurons in the nucleus ambiguus. J Neurophysiol 102: 1443–1450, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould et al., 1988.Gould JB, Lee AF, Morelock S. The relationship between sleep and sudden infant death. Ann NY Acad Sci 533: 62–77, 1988 [DOI] [PubMed] [Google Scholar]
- Harper and Bandler, 1998.Harper RM, Bandler R. Finding the failure mechanism in sudden infant death syndrome. Nat Med 4: 157–158, 1998 [DOI] [PubMed] [Google Scholar]
- Harper et al., 1983.Harper RM, Frostig Z, Taube D, Hoppenbrouwers T, Hodgman JE. Development of sleep-waking temporal sequencing in infants at risk for the sudden infant death syndrome. Exp Neurol 79: 821–829, 1983 [DOI] [PubMed] [Google Scholar]
- Huang et al., 2007.Huang ZG, Griffioen KJ, Wang X, Dergacheva O, Kamendi H, Gorini C, Mendelowitz D. Nicotinic receptor activation occludes purinergic control of central cardiorespiratory network responses to hypoxia/hypercapnia. J Neurophysiol 98: 2429–2438, 2007 [DOI] [PubMed] [Google Scholar]
- Hunt, 2005.Hunt CE. Gene–environment interactions: implications for sudden unexpected deaths in infancy. Arch Dis Child 90: 48–53, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt and Hauck, 2006.Hunt CE, Hauck FR. Sudden infant death syndrome. Can Med Assoc J 174: 1861–1869, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, 1990.Jones BE. Immunohistochemical study of choline acetyltransferase-immunoreactive processes and cells innervating the pontomedullary reticular formation in the rat. J Comp Neurol 295: 485–514, 1990 [DOI] [PubMed] [Google Scholar]
- Jones and Beaudet, 1987.Jones BE, Beaudet A. Distribution of acetylcholine and catecholamine neurons in the cat brainstem: a choline acetyltransferase and tyrosine hydroxylase immunohistochemical study. J Comp Neurol 261: 15–32, 1987 [DOI] [PubMed] [Google Scholar]
- Kalaria et al., 1993.Kalaria RN, Fiedler C, Hunsaker JC, 3rd, Sparks DL. Synaptic neurochemistry of human striatum during development: changes in sudden infant death syndrome. J Neurochem 60: 2098–2105, 1993 [DOI] [PubMed] [Google Scholar]
- Kamendi et al., 2006.Kamendi H, Stephens C, Dergacheva O, Wang X, Huang ZG, Bouairi E, Gorini C, McIntosh JM, Mendelowitz D. Prenatal nicotine exposure alters the nicotinic receptor subtypes that modulate excitation of parasympathetic cardiac neurons in the nucleus ambiguus from primarily alpha3beta2 and/or alpha6betaX to alpha3beta4. Neuropharmacology 51: 60–66, 2006 [DOI] [PubMed] [Google Scholar]
- Kinney, 2009.Kinney HC. Brainstem mechanisms underlying the sudden infant death syndrome: evidence from human pathologic studies. Dev Psychobiol 51: 223–233, 2009 [DOI] [PubMed] [Google Scholar]
- Kinney et al., 1995.Kinney HC, Filiano JJ, Sleeper LA, Mandell F, Valdes-Dapena M, White WF. Decreased muscarinic receptor binding in the arcuate nucleus in sudden infant death syndrome. Science 269: 1446–1450, 1995 [DOI] [PubMed] [Google Scholar]
- Koehler et al., 1998.Koehler U, Fus E, Grimm W, Pankow W, Schafer H, Stammnitz A, Peter JH. Heart block in patients with obstructive sleep apnoea: pathogenetic factors and effects of treatment. Eur Respir J 11: 434–439, 1998 [DOI] [PubMed] [Google Scholar]
- Kubo et al., 1998.Kubo S, Orihara Y, Gotohda T, Tokunaga I, Tsuda R, Ikematsu K, Kitamura O, Yamamoto A, Nakasono I. Immunohistochemical studies on neuronal changes in brain stem nucleus of forensic autopsied cases. II. Sudden infant death syndrome. Nihon Hoigaku Zasshi 52: 350–354, 1998 [PubMed] [Google Scholar]
- Kumada et al., 1977.Kumada M, Dampney RA, Reis DJ. The trigeminal depressor response: a novel vasodepressor response originating from the trigeminal system. Brain Res 119: 305–326, 1977 [DOI] [PubMed] [Google Scholar]
- Kumada et al., 1978.Kumada M, Dampney RA, Whitnall MH, Reis DJ. Hemodynamic similarities between the trigeminal and aortic vasodepressor responses. Am J Physiol Heart Circ Physiol 234: H67–H73, 1978 [DOI] [PubMed] [Google Scholar]
- Lobban, 1991.Lobban CD. The human dive reflex as a primary cause of SIDS. A review of the literature. Med J Aust 155: 561–563, 1991 [PubMed] [Google Scholar]
- Lobban, 1995.Lobban CD. The oxygen-conserving dive reflex re-examined as the principal contributory factor in sudden infant death. Med Hypotheses 44: 273–277, 1995 [DOI] [PubMed] [Google Scholar]
- Mallard et al., 1999.Mallard C, Tolcos M, Leditschke J, Campbell P, Rees S. Reduction in choline acetyltransferase immunoreactivity but not muscarinic-m2 receptor immunoreactivity in the brainstem of SIDS infants. J Neuropathol Exp Neurol 58: 255–264, 1999 [DOI] [PubMed] [Google Scholar]
- Matturri et al., 2004.Matturri L, Ottaviani G, Alfonsi G, Crippa M, Rossi L, Lavezzi AM. Study of the brainstem, particularly the arcuate nucleus, in sudden infant death syndrome (SIDS) and sudden intrauterine unexplained death (SIUD). Am J Forensic Med Pathol 25: 44–48, 2004 [DOI] [PubMed] [Google Scholar]
- Matturri et al., 2005.Matturri L, Ottaviani G, Lavezzi AM. Sudden infant death triggered by dive reflex. J Clin Pathol 58: 77–80, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelowitz and Kunze, 1991.Mendelowitz D, Kunze DL. Identification and dissociation of cardiovascular neurons from the medulla for patch clamp analysis. Neurosci Lett 132: 217–221, 1991 [DOI] [PubMed] [Google Scholar]
- Meny et al., 1994.Meny RG, Carroll JL, Carbone MT, Kelly DH. Cardiorespiratory recordings from infants dying suddenly and unexpectedly at home. Pediatrics 93: 44–49, 1994 [PubMed] [Google Scholar]
- Milner et al., 1989.Milner TA, Pickel VM, Reis DJ. Ultrastructural basis for interactions between central opioids and catecholamines. I. Rostral ventrolateral medulla. J Neurosci 9: 2114–2130, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morpurgo et al., 2004.Morpurgo CV, Lavezzi AM, Ottaviani G, Rossi L. Bulbo-spinal pathology and sudden respiratory infant death syndrome. Eur J Anaesthesiol 21: 589–593, 2004 [DOI] [PubMed] [Google Scholar]
- Nachmanoff et al., 1998.Nachmanoff DB, Panigrahy A, Filiano JJ, Mandell F, Sleeper LA, Valdes-Dapena M, Krous HF, White WF, Kinney HC. Brainstem 3H-nicotine receptor binding in the sudden infant death syndrome. J Neuropathol Exp Neurol 57: 1018–1025, 1998 [DOI] [PubMed] [Google Scholar]
- Oda, 1999.Oda Y. Choline acetyltransferase: the structure, distribution and pathologic changes in the central nervous system. Pathol Int 49: 921–937, 1999 [DOI] [PubMed] [Google Scholar]
- Panigrahy et al., 2000.Panigrahy A, Filiano J, Sleeper LA, Mandell F, Valdes-Dapena M, Krous HF, Rava LA, Foley E, White WF, Kinney HC. Decreased serotonergic receptor binding in rhombic lip-derived regions of the medulla oblongata in the sudden infant death syndrome. J Neuropathol Exp Neurol 59: 377–384, 2000 [DOI] [PubMed] [Google Scholar]
- Paterson et al., 2006.Paterson DS, Trachtenberg FL, Thompson EG, Belliveau RA, Beggs AH, Darnall R, Chadwick AE, Krous HF, Kinney HC. Multiple serotonergic brainstem abnormalities in sudden infant death syndrome. J Am Med Assoc 296: 2124–2132, 2006 [DOI] [PubMed] [Google Scholar]
- Poets et al., 1999.Poets CF, Meny RG, Chobanian MR, Bonofiglo RE. Gasping and other cardiorespiratory patterns during sudden infant deaths. Pediatr Res 45: 350–354, 1999 [DOI] [PubMed] [Google Scholar]
- Robertson et al., 1998.Robertson BS, Satterfield BE, Said SI, Dey RD. N-Methyl-d-aspartate receptors are expressed by intrinsic neurons of rat larynx and esophagus. Neurosci Lett 244: 77–80, 1998 [DOI] [PubMed] [Google Scholar]
- Sawaguchi et al., 2002.Sawaguchi T, Kato I, Franco P, Kadhim H, Groswasser J, Sottiaux M, Togari H, Kobayashi M, Nishida H, Sawaguchi A, Kahn A. Arousal deficiency theory in sudden infant death syndrome with reference to neuronal plasticity. Sleep Med 3, Suppl. 2: S57–S60, 2002 [DOI] [PubMed] [Google Scholar]
- Schaller, 2004.Schaller B. Trigeminocardiac reflex. A clinical phenomenon or a new physiological entity? J Neurol 251: 658–665, 2004 [DOI] [PubMed] [Google Scholar]
- Schaller, 2005a.Schaller B. Trigemino-cardiac reflex during microvascular trigeminal decompression in cases of trigeminal neuralgia. J Neurosurg Anesthesiol 17: 45–48, 2005a [PubMed] [Google Scholar]
- Schaller, 2005b.Schaller B. Trigemino-cardiac reflex during transsphenoidal surgery for pituitary adenomas. Clin Neurol Neurosurg 107: 468–474, 2005b [DOI] [PubMed] [Google Scholar]
- Schaller et al., 1999.Schaller B, Probst R, Strebel S, Gratzl O. Trigeminocardiac reflex during surgery in the cerebellopontine angle. J Neurosurg 90: 215–220, 1999 [DOI] [PubMed] [Google Scholar]
- Schaller and Buchfelder, 2006.Schaller BJ, Buchfelder M. Trigemino-cardiac reflex in skull base surgery: from a better understanding to a better outcome? Acta Neurochir 148: 1029–1031, 2006 [DOI] [PubMed] [Google Scholar]
- Schechtman et al., 1992.Schechtman VL, Raetz SL, Harper RK, Garfinkel A, Wilson AJ, Southall DP, Harper RM. Dynamic analysis of cardiac R-R intervals in normal infants and in infants who subsequently succumbed to the sudden infant death syndrome. Pediatr Res 31: 606–612, 1992 [DOI] [PubMed] [Google Scholar]
- Shiromani et al., 1988.Shiromani PJ, Armstrong DM, Berkowitz A, Jeste DV, Gillin JC. Distribution of choline acetyltransferase immunoreactive somata in the feline brainstem: implications for REM sleep generation. Sleep 11: 1–16, 1988 [DOI] [PubMed] [Google Scholar]
- Slotkin et al., 1999.Slotkin TA, Epps TA, Stenger ML, Sawyer KJ, Seidler FJ. Cholinergic receptors in heart and brainstem of rats exposed to nicotine during development: implications for hypoxia tolerance and perinatal mortality. Brain Res Dev Brain Res 113: 1–12, 1999 [DOI] [PubMed] [Google Scholar]
- Sparks and Hunsaker, 1991.Sparks DL, Hunsaker JC., 3rd Sudden infant death syndrome: altered aminergic-cholinergic synaptic markers in hypothalamus. J Child Neurol 6: 335–339, 1991 [DOI] [PubMed] [Google Scholar]
- Spyer and Gilbey, 1988.Spyer KM, Gilbey MP. Cardiorespiratory interactions in heart-rate control. Ann NY Acad Sci 533: 350–357, 1988 [DOI] [PubMed] [Google Scholar]
- Staines, 2005.Staines D. Are vasoactive neuropeptide autoimmune fatigue-related disorders mediated via G protein-coupled receptors? Med Hypotheses 65: 29–31, 2005 [DOI] [PubMed] [Google Scholar]
- Staines, 2006.Staines DR. Postulated vasoactive neuropeptide autoimmunity in fatigue-related conditions: a brief review and hypothesis. Clin Dev Immunol 13: 25–39, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun et al., 2009.Sun DW, Zhou R, Li N, Zhang QG, Zhu FG. An increase in intracelluar free calcium ions modulated by cholinergic receptors in rat facial nucleus. Chin Med J (Engl) 122: 1049–1055, 2009 [PubMed] [Google Scholar]
- Thiriez et al., 2009.Thiriez G, Bouhaddi M, Mourot L, Nobili F, Fortrat JO, Menget A, Franco P, Regnard J. Heart rate variability in preterm infants and maternal smoking during pregnancy. Clin Auton Res 19: 149–156, 2009 [DOI] [PubMed] [Google Scholar]
- Vazquez and Baghdoyan, 2004.Vazquez J, Baghdoyan HA. GABAA receptors inhibit acetylcholine release in cat pontine reticular formation: implications for REM sleep regulation. J Neurophysiol 92: 2198–2206, 2004 [DOI] [PubMed] [Google Scholar]
- Verret et al., 2005.Verret L, Leger L, Fort P, Luppi PH. Cholinergic and noncholinergic brainstem neurons expressing Fos after paradoxical (REM) sleep deprivation and recovery. Eur J Neurosci 21: 2488–2504, 2005 [DOI] [PubMed] [Google Scholar]
- Verrier et al., 1998.Verrier RL, Lau TR, Wallooppillai U, Quattrochi J, Nearing BD, Moreno R, Hobson JA. Primary vagally mediated decelerations in heart rate during tonic rapid eye movement sleep in cats. Am J Physiol Regul Integr Comp Physiol 274: R1136–R1141, 1998 [DOI] [PubMed] [Google Scholar]
- Wang et al., 2001.Wang J, Irnaten M, Neff RA, Venkatesan P, Evans C, Loewy AD, Mettenleiter TC, Mendelowitz D. Synaptic and neurotransmitter activation of cardiac vagal neurons in the nucleus ambiguus. Ann NY Acad Sci 940: 237–246, 2001 [DOI] [PubMed] [Google Scholar]
- Wang et al., 2003.Wang J, Wang X, Irnaten M, Venkatesan P, Evans C, Baxi S, Mendelowitz D. Endogenous acetylcholine and nicotine activation enhances GABAergic and glycinergic inputs to cardiac vagal neurons. J Neurophysiol 89: 2473–2481, 2003 [DOI] [PubMed] [Google Scholar]
- Wess et al., 2007.Wess J, Eglen RM, Gautam D. Muscarinic acetylcholine receptors: mutant mice provide new insights for drug development. Nat Rev Drug Discov 6: 721–733, 2007 [DOI] [PubMed] [Google Scholar]
- Winzer-Serhan and Leslie, 1997.Winzer-Serhan UH, Leslie FM. Codistribution of nicotinic acetylcholine receptor subunit alpha3 and beta4 mRNAs during rat brain development. J Comp Neurol 386: 540–554, 1997 [DOI] [PubMed] [Google Scholar]
- Yavari et al., 1996.Yavari P, McCulloch PF, Panneton WM. Trigeminally-mediated alteration of cardiorespiratory rhythms during nasal application of carbon dioxide in the rat. J Auton Nerv Syst 61: 195–200, 1996 [DOI] [PubMed] [Google Scholar]
- Zhu et al., 1998.Zhu J, Taniguchi T, Konishi Y, Mayumi M, Muramatsu I. Nicotine administration decreases the number of binding sites and mRNA of M1 and M2 muscarinic receptors in specific brain regions of rat neonates. Life Sci 62: 1089–1098, 1998 [DOI] [PubMed] [Google Scholar]