Abstract
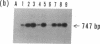
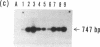
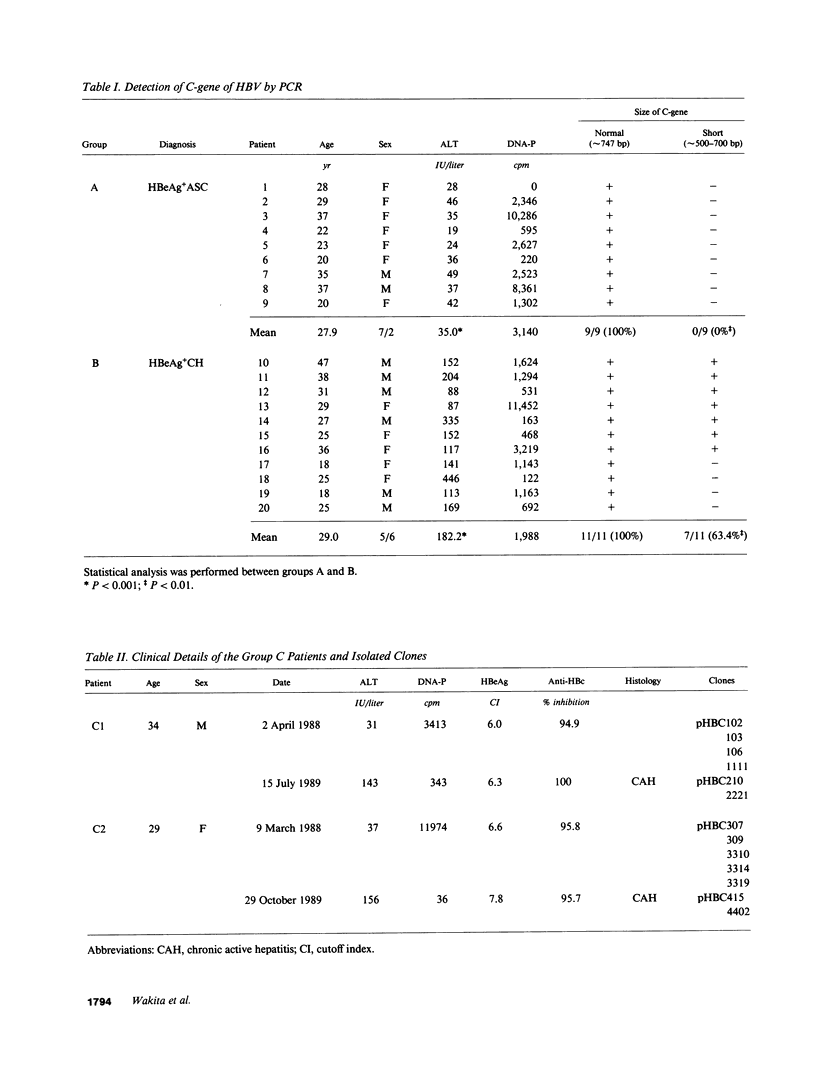
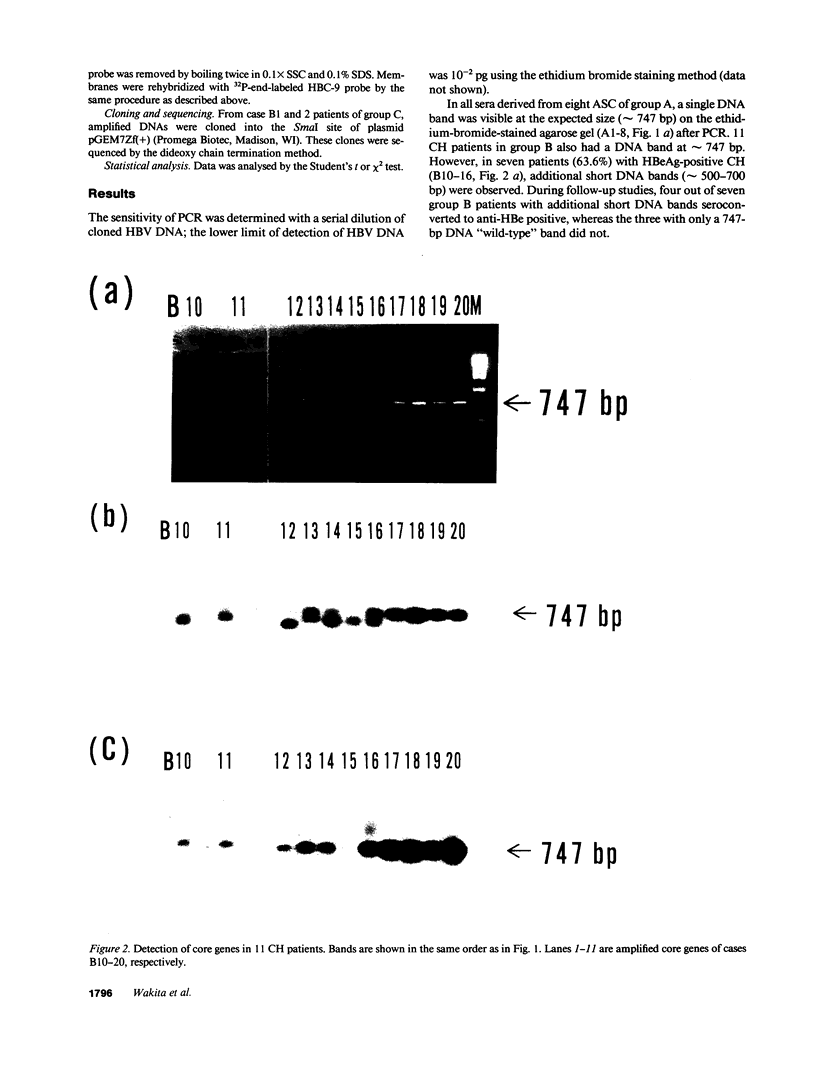
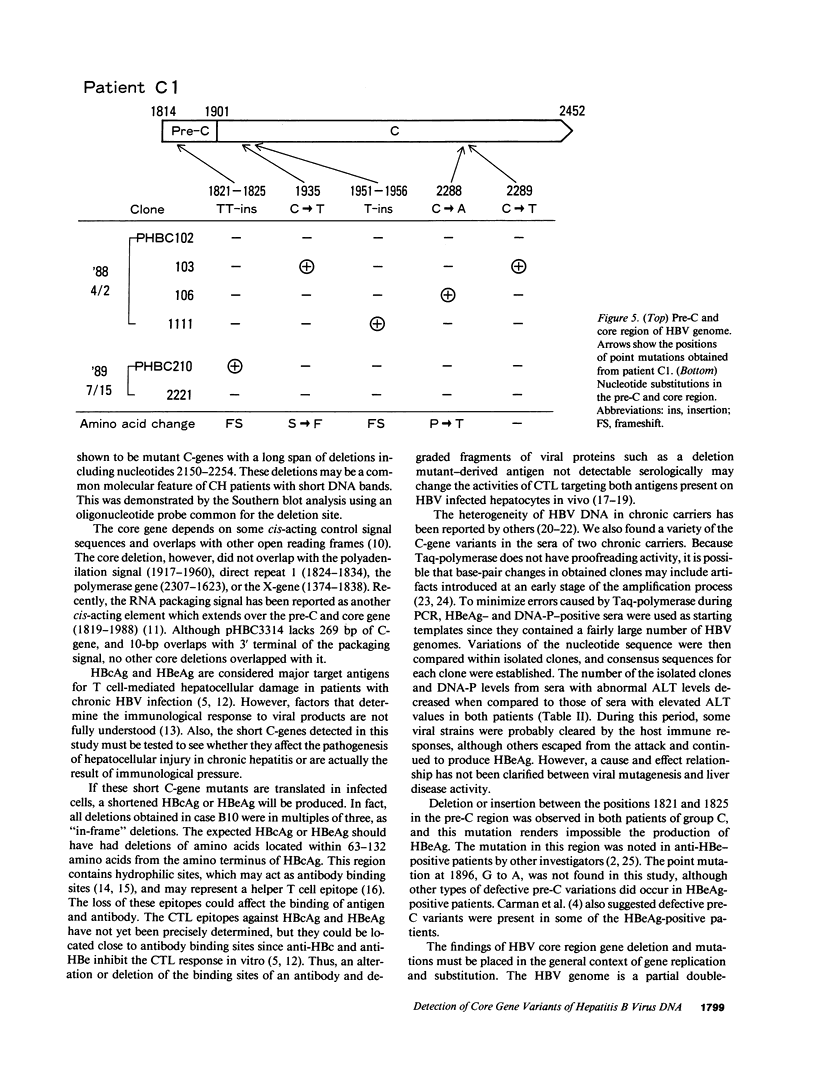
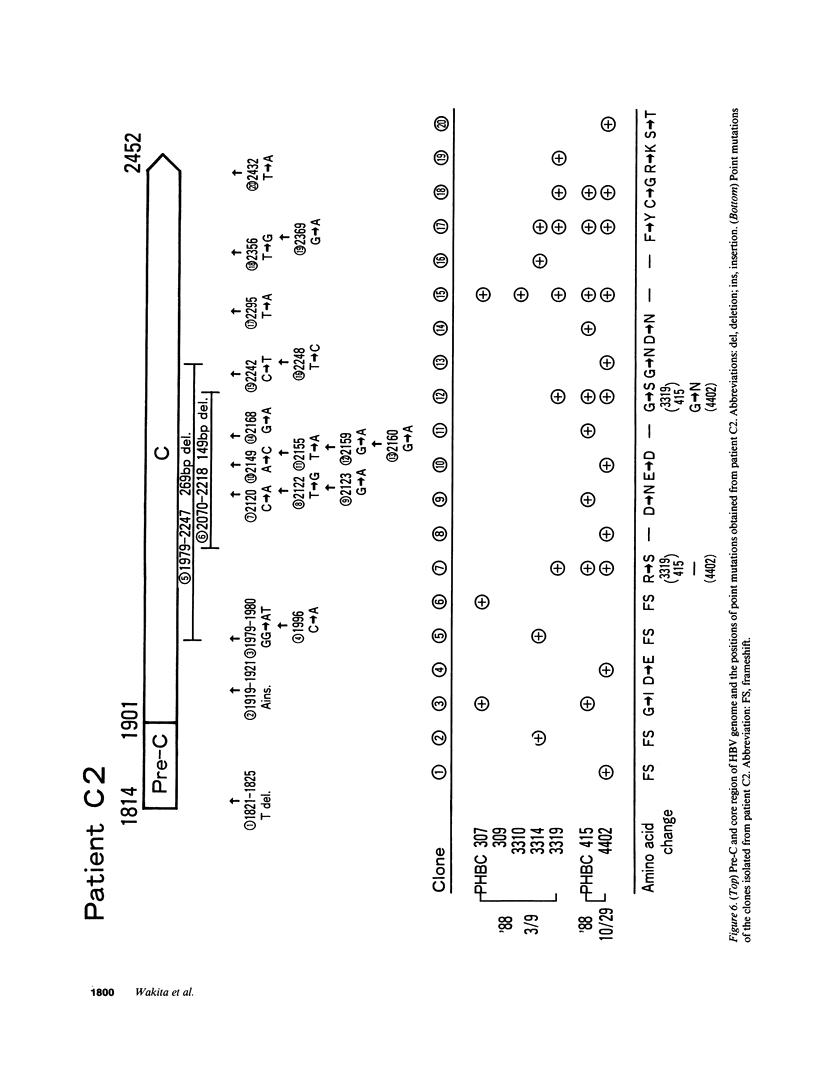
We analyzed the pre-C and core region of hepatitis B virus (HBV) DNA by a polymerase chain reaction in 22 chronic carriers. In 9 hepatitis B e antigen-positive asymptomatic carriers, a single DNA band was detected at the expected size, whereas additional shorter DNA bands were observed in 7 out of 11 patients with chronic hepatitis. The smaller-sized DNAs from one chronic hepatitis patient had various lengths of deletions spanning from 105 to 183 bp in the middle of the core gene, and all deletions included common nucleotide sequences. All of the smaller-sized DNAs from the other patients proved to be variant core genes. They were deleted in similar regions by Southern analysis using oligonucleotide probes. A follow-up study revealed that four out of seven chronic hepatitis patients with a short core gene seroconverted to antibody to hepatitis B e antigen, but those with only a "wild type" did not. In another set of sequence studies, clones isolated from two chronic carriers displayed heterogeneity of the pre-C and core gene which was more often present in sera with normal alanine aminotransferase levels than with abnormal levels. These results suggest that mutant HBV alters the host immune response, and may modulate the clinical course of HBV infection. An alternative possibility is that chronic hepatitis selects for mutant forms.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhat R. A., Ulrich P. P., Vyas G. N. Molecular characterization of a new variant of hepatitis B virus in a persistently infected homosexual man. Hepatology. 1990 Feb;11(2):271–276. doi: 10.1002/hep.1840110218. [DOI] [PubMed] [Google Scholar]
- Blum H. E., Galun E., Liang T. J., von Weizsäcker F., Wands J. R. Naturally occurring missense mutation in the polymerase gene terminating hepatitis B virus replication. J Virol. 1991 Apr;65(4):1836–1842. doi: 10.1128/jvi.65.4.1836-1842.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunetto M. R., Stemler M., Bonino F., Schodel F., Oliveri F., Rizzetto M., Verme G., Will H. A new hepatitis B virus strain in patients with severe anti-HBe positive chronic hepatitis B. J Hepatol. 1990 Mar;10(2):258–261. doi: 10.1016/0168-8278(90)90062-v. [DOI] [PubMed] [Google Scholar]
- Carman W. F., Jacyna M. R., Hadziyannis S., Karayiannis P., McGarvey M. J., Makris A., Thomas H. C. Mutation preventing formation of hepatitis B e antigen in patients with chronic hepatitis B infection. Lancet. 1989 Sep 9;2(8663):588–591. doi: 10.1016/s0140-6736(89)90713-7. [DOI] [PubMed] [Google Scholar]
- Colucci G., Beazer Y., Cantaluppi C., Tackney C. Identification of a major hepatitis B core antigen (HBcAg) determinant by using synthetic peptides and monoclonal antibodies. J Immunol. 1988 Dec 15;141(12):4376–4380. [PubMed] [Google Scholar]
- Dudley F. J., Fox R. A., Sherlock S. Cellular immunity and hepatitis-associated, Australia antigen liver disease. Lancet. 1972 Apr 1;1(7753):723–726. doi: 10.1016/s0140-6736(72)90234-6. [DOI] [PubMed] [Google Scholar]
- Ferrari C., Penna A., DegliAntoni A., Fiaccadori F. Cellular immune response to hepatitis B virus antigens. An overview. J Hepatol. 1988 Aug;7(1):21–33. doi: 10.1016/s0168-8278(88)80503-8. [DOI] [PubMed] [Google Scholar]
- Junker-Niepmann M., Bartenschlager R., Schaller H. A short cis-acting sequence is required for hepatitis B virus pregenome encapsidation and sufficient for packaging of foreign RNA. EMBO J. 1990 Oct;9(10):3389–3396. doi: 10.1002/j.1460-2075.1990.tb07540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko S., Miller R. H., Feinstone S. M., Unoura M., Kobayashi K., Hattori N., Purcell R. H. Detection of serum hepatitis B virus DNA in patients with chronic hepatitis using the polymerase chain reaction assay. Proc Natl Acad Sci U S A. 1989 Jan;86(1):312–316. doi: 10.1073/pnas.86.1.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko S., Miller R. H. Heterogeneity of the core gene sequence in a patient chronically infected with hepatitis B virus. J Infect Dis. 1989 Nov;160(5):903–904. doi: 10.1093/infdis/160.5.903. [DOI] [PubMed] [Google Scholar]
- Kwok S., Higuchi R. Avoiding false positives with PCR. Nature. 1989 May 18;339(6221):237–238. doi: 10.1038/339237a0. [DOI] [PubMed] [Google Scholar]
- Liang T. J., Blum H. E., Wands J. R. Characterization and biological properties of a hepatitis B virus isolated from a patient without hepatitis B virus serologic markers. Hepatology. 1990 Aug;12(2):204–212. doi: 10.1002/hep.1840120205. [DOI] [PubMed] [Google Scholar]
- Liang T. J., Isselbacher K. J., Wands J. R. Rapid identification of low level hepatitis B-related viral genome in serum. J Clin Invest. 1989 Oct;84(4):1367–1371. doi: 10.1172/JCI114308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McChesney M., Tanneau F., Regnault A., Sansonetti P., Montagnier L., Kieny M. P., Rivière Y. Detection of primary cytotoxic T lymphocytes specific for the envelope glycoprotein of HIV-1 by deletion of the env amino-terminal signal sequence. Eur J Immunol. 1990 Jan;20(1):215–220. doi: 10.1002/eji.1830200131. [DOI] [PubMed] [Google Scholar]
- Milich D. R., McLachlan A., Moriarty A., Thornton G. B. Immune response to hepatitis B virus core antigen (HBcAg): localization of T cell recognition sites within HBcAg/HBeAg. J Immunol. 1987 Aug 15;139(4):1223–1231. [PubMed] [Google Scholar]
- Miller R. H., Kaneko S., Chung C. T., Girones R., Purcell R. H. Compact organization of the hepatitis B virus genome. Hepatology. 1989 Feb;9(2):322–327. doi: 10.1002/hep.1840090226. [DOI] [PubMed] [Google Scholar]
- Mondelli M., Vergani G. M., Alberti A., Vergani D., Portmann B., Eddleston A. L., Williams R. Specificity of T lymphocyte cytotoxicity to autologous hepatocytes in chronic hepatitis B virus infection: evidence that T cells are directed against HBV core antigen expressed on hepatocytes. J Immunol. 1982 Dec;129(6):2773–2778. [PubMed] [Google Scholar]
- Okamoto H., Imai M., Kametani M., Nakamura T., Mayumi M. Genomic heterogeneity of hepatitis B virus in a 54-year-old woman who contracted the infection through materno-fetal transmission. Jpn J Exp Med. 1987 Aug;57(4):231–236. [PubMed] [Google Scholar]
- Okamoto H., Imai M., Shimozaki M., Hoshi Y., Iizuka H., Gotanda T., Tsuda F., Miyakawa Y., Mayumi M. Nucleotide sequence of a cloned hepatitis B virus genome, subtype ayr: comparison with genomes of the other three subtypes. J Gen Virol. 1986 Nov;67(Pt 11):2305–2314. doi: 10.1099/0022-1317-67-11-2305. [DOI] [PubMed] [Google Scholar]
- Okamoto H., Yotsumoto S., Akahane Y., Yamanaka T., Miyazaki Y., Sugai Y., Tsuda F., Tanaka T., Miyakawa Y., Mayumi M. Hepatitis B viruses with precore region defects prevail in persistently infected hosts along with seroconversion to the antibody against e antigen. J Virol. 1990 Mar;64(3):1298–1303. doi: 10.1128/jvi.64.3.1298-1303.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Salfeld J., Pfaff E., Noah M., Schaller H. Antigenic determinants and functional domains in core antigen and e antigen from hepatitis B virus. J Virol. 1989 Feb;63(2):798–808. doi: 10.1128/jvi.63.2.798-808.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su T. S., Lai C. J., Huang J. L., Lin L. H., Yauk Y. K., Chang C. M., Lo S. J., Han S. H. Hepatitis B virus transcript produced by RNA splicing. J Virol. 1989 Sep;63(9):4011–4018. doi: 10.1128/jvi.63.9.4011-4018.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers J., Mason W. S. Replication of the genome of a hepatitis B--like virus by reverse transcription of an RNA intermediate. Cell. 1982 Jun;29(2):403–415. doi: 10.1016/0092-8674(82)90157-x. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Masui N., Kajino K., Saito I., Miyamura T. Detection and mapping of spliced RNA from a human hepatoma cell line transfected with the hepatitis B virus genome. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8422–8426. doi: 10.1073/pnas.86.21.8422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tindall K. R., Kunkel T. A. Fidelity of DNA synthesis by the Thermus aquaticus DNA polymerase. Biochemistry. 1988 Aug 9;27(16):6008–6013. doi: 10.1021/bi00416a027. [DOI] [PubMed] [Google Scholar]
- Tiollais P., Pourcel C., Dejean A. The hepatitis B virus. Nature. 1985 Oct 10;317(6037):489–495. doi: 10.1038/317489a0. [DOI] [PubMed] [Google Scholar]
- Tong S. P., Li J. S., Vitvitski L., Trépo C. Active hepatitis B virus replication in the presence of anti-HBe is associated with viral variants containing an inactive pre-C region. Virology. 1990 Jun;176(2):596–603. doi: 10.1016/0042-6822(90)90030-u. [DOI] [PubMed] [Google Scholar]
- Townsend A. R., Bastin J., Gould K., Brownlee G. G. Cytotoxic T lymphocytes recognize influenza haemagglutinin that lacks a signal sequence. Nature. 1986 Dec 11;324(6097):575–577. doi: 10.1038/324575a0. [DOI] [PubMed] [Google Scholar]
- Townsend A., Bastin J., Gould K., Brownlee G., Andrew M., Coupar B., Boyle D., Chan S., Smith G. Defective presentation to class I-restricted cytotoxic T lymphocytes in vaccinia-infected cells is overcome by enhanced degradation of antigen. J Exp Med. 1988 Oct 1;168(4):1211–1224. doi: 10.1084/jem.168.4.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]