Summary
Microtubule inhibitors are important cancer drugs that induce mitotic arrest by activating the spindle assembly checkpoint (SAC), which in turn inhibits the ubiquitin ligase activity of the Anaphase-Promoting Complex (APC). Here we report a small molecule, Tosyl-L-Arginine Methyl Ester (TAME), which binds to the APC and prevents its activation by Cdc20 and Cdh1. A prodrug of TAME arrests cells in metaphase without perturbing the spindle, but nonetheless the arrest is dependent on the SAC. Metaphase arrest induced by a proteasome inhibitor is also SAC-dependent, suggesting that APC-dependent proteolysis is required to inactivate the SAC. We propose that mutual antagonism between the APC and the SAC yields a positive feedback loop that amplifies the ability of TAME to induce mitotic arrest.
Introduction
Microtubule inhibitors such as taxanes and the vinca alkaloids represent one of the most important classes of cancer drugs, used in the treatment of breast, ovarian, and lung cancer (Montero et al., 2005). However, the response of cells to microtubule inhibitors is highly variable (Brito et al., 2008; Gascoigne and Taylor, 2008; Orth et al., 2008; Shi et al., 2008), potentially compromising clinical efficacy. How these drugs cause cell death remains unclear, but induction of mitotic arrest appears to be a key aspect of the mechanism (Bekier et al., 2009; Huang et al., 2009). By perturbing the mitotic spindle, these drugs activate the Spindle Assembly Checkpoint (SAC), which delays mitotic exit by inhibiting the ubiquitin ligase activity of the Anaphase-Promoting Complex/Cyclosome (APC). In principle, a compound that directly inhibits APC-dependent proteolysis should arrest cells in mitosis without causing side effects that result from microtubule inhibition such as peripheral neuropathy.
The APC is the most complex ubiquitin ligase known, consisting of more than 11 subunits. The activator proteins Cdh1 and Cdc20 bind to the APC at different cell cycle stages to stimulate APC-dependent ubiquitination of substrates and their subsequent destruction by the 26S proteasome (Peters, 2006). The activators assist in recruitment of APC substrates and may also stimulate ligase activity (Yu, 2007). Cdh1 binds to the APC during G1 to promote degradation of APC substrates during interphase. In contrast, the initiation of anaphase and exit from mitosis require Cdc20-dependent ubiquitination of APC substrates such as securin and mitotic cyclins. Prior to anaphase, the ability of APC-Cdc20 to ubiquitinate certain substrates is inhibited by the SAC (Musacchio and Salmon, 2007). Unattached kinetochores catalyze the formation of an inhibitory protein complex, containing the proteins Mad2, BubR1 and Bub3, that sequesters Cdc20 or interferes with its ability to activate the APC. Attachment of kinetochores to the mitotic spindle diminishes their ability to generate an inhibitory signal. Subsequently, the SAC-inhibited APC-Cdc20 complex is activated, by a mechanism that remains incompletely understood.
Because the APC regulates multiple cell cycle events, it is not clear whether pharmacological inhibition of its activity will lead to selective or prolonged arrest in mitosis as is the case with microtubule inhibitors. Proteasome inhibitors can block APC-dependent proteolysis without perturbing the mitotic spindle (Famulski and Chan, 2007), but they also inhibit the degradation of many other substrates of the ubiquitin-proteasome system, and therefore also cause cell cycle arrest during interphase (Wojcik et al., 1996). It may be difficult to achieve mitotic arrest by pharmacologic APC inhibition, as RNAi approaches indicate that Cdc20 expression must be severely reduced to induce mitotic arrest (Huang et al., 2009; Wolthuis et al., 2008). Even when the SAC is maximally activated by complete microtubule depolymerization, some cells escape mitotic arrest due to residual APC activity (Brito and Rieder, 2006), suggesting that the SAC cannot fully inhibit the APC during mitosis. For this reason, microtubule inhibitors may suffer from limited effectiveness because some cells escape mitotic arrest before dying (Bekier et al., 2009; Huang et al., 2009). Whether an APC inhibitor can better extinguish APC activity and induce a more persistent mitotic arrest is therefore an important question in contemplating development of APC inhibitors as a therapeutic strategy for cancer.
Results
TAME Inhibits APC Activation by Perturbing Activator Protein Binding
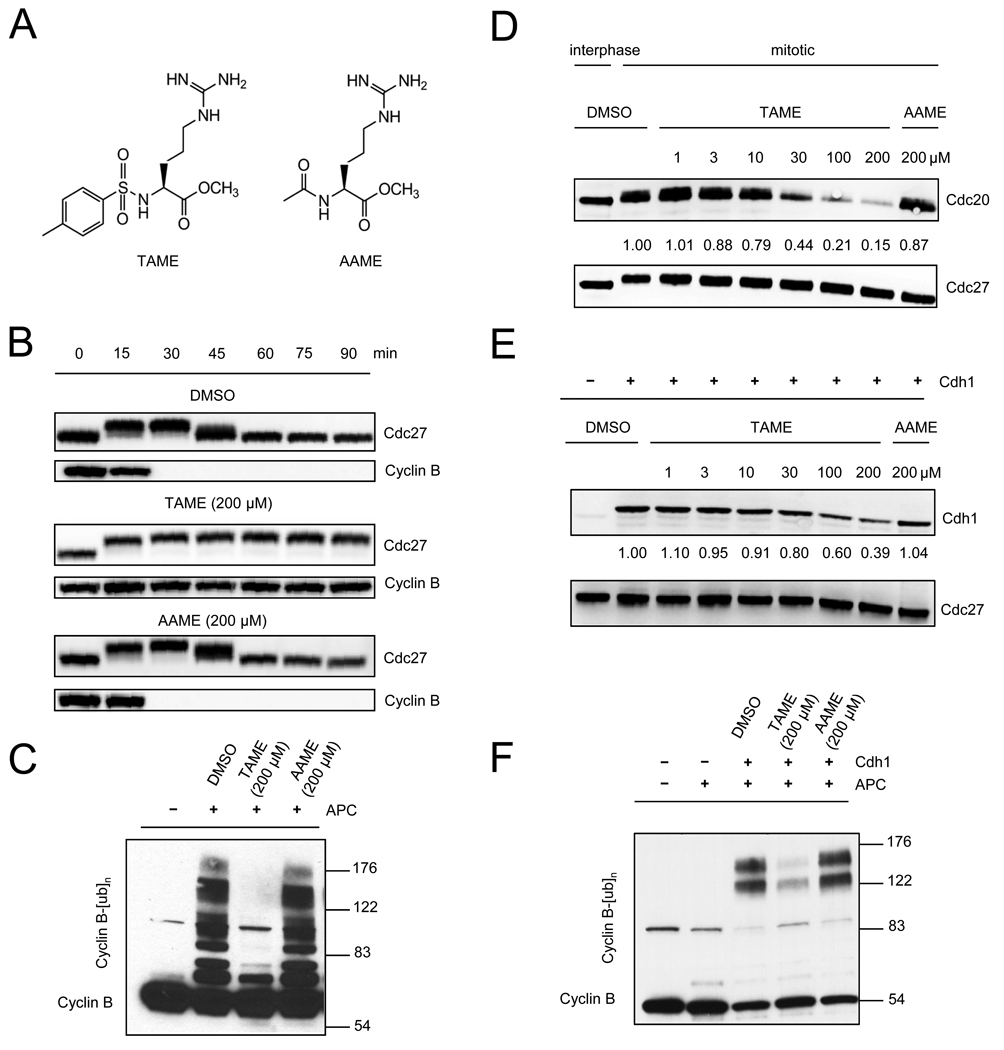
We identified TAME (Figure 1A) in an earlier study (Verma et al., 2004) as an inhibitor of cyclin proteolysis in mitotic Xenopus egg extract (IC50 of 12 µM; Figure S1A), but its mechanism of action has remained unknown. TAME also inhibited cyclin degradation in interphase extract activated by exogenous Cdh1, but had no effect on SCF-dependent proteolysis of β-catenin-luciferase (Verma et al., 2004), indicating that it is not a general inhibitor of the ubiquitin-proteasome system. Testing of TAME derivatives indicated that the tosyl group, arginine, and the methyl ester are each important for activity (Figures S1B and S1C). Acetyl-L-Arginine Methyl Ester (AAME; Figure 1A) showed only low activity, and was therefore used as a negative control in subsequent experiments. When added to interphase extract treated with recombinant cyclin B1/Cdc2 complex, TAME, but not AAME, arrested the extract in mitosis, with stable cyclin B1 and phosphorylated Cdc27 (Figure 1B). Another APC substrate, cyclin A2, was also stabilized by TAME in Xenopus extract (data not shown). TAME had no effect on the ability of Xenopus extract to degrade cyclin B1 that had been preubiquitinated in vitro (Figure S1D), indicating that TAME does not inhibit the proteasome or its ability to recognize ubiquitinated substrates.
Figure 1. TAME inhibits APC activation by perturbing binding of Cdc20 or Cdh1.
(A) Structures of TAME and AAME. (B) TAME induces mitotic arrest in Xenopus extract. Recombinant cyclin B1/Cdk1 was added to interphase extract in the presence of compounds. Cdc27 phosphorylation and cyclin B1 levels were examined by immunoblot. (C) TAME inhibits APC activation. Compounds were added to mitotic Xenopus extract immediately before APC immunoprecipitation. The activity of the isolated APC was measured in a reconstituted assay. (D) TAME inhibits Cdc20 association with mitotic APC. Compounds were added to mitotic Xenopus extract prior to APC immunoprecipitation. Numbers represent CCD-imaging based-intensity quantitation of the immunoblot, and show the relative amount of Cdc20 normalized to Cdc27. (E) TAME inhibits Cdh1 association with interphase APC. Interphase Xenopus extract was pre-incubated with compounds for 30 min prior to adding recombinant Cdh1 and APC immunoprecipitation. (F) TAME inhibits APC activation by Cdh1. Interphase Xenopus extract was pre-incubated with compound for 30 min prior to adding recombinant Cdh1 and APC immunoprecipitation. The activity of the isolated APC was measured in a reconstituted assay. See also Figure S1.
Because the SAC is not active in Xenopus extracts (Minshull et al., 1994), we reasoned that TAME might inhibit cyclin proteolysis by perturbing the APC. Indeed, when TAME was added to mitotic Xenopus extract during APC isolation, the APC showed a dramatic loss of activity in a reconstituted ubiquitination reaction (Figure 1C). Consistent with this finding, TAME addition to extract reduced Cdc20 association with the APC in a dose-dependent manner (Figure 1D), but did not otherwise affect APC composition (Figure S1E). TAME also inhibited the binding of Cdh1 to APC when Cdh1 and TAME were added together to interphase extract (Figure 1E). The reduction in Cdh1 binding was accompanied by a reduction in APC activation (Figure 1F). These findings suggested that TAME might block APC activation by perturbing the interaction between APC and its activator proteins Cdc20 or Cdh1.
To understand how TAME disrupts the interaction between the activator proteins and the APC, we first tested whether TAME binds to the APC. We added 3H-TAME to interphase Xenopus extract, or to extract immunodepleted of APC, and then isolated residual APC with Cdc27 antibodies and measured the amount of radioactivity associated with the beads. We found that binding of 3H-TAME correlated with the amount of immunoprecipitated Cdc27 (Figure 2A). Unlabeled TAME competitively inhibited the binding of 3H-TAME, whereas AAME did not (Figure 2B). Other TAME derivatives competed with 3H-TAME for APC binding in a manner that correlated with their ability to inhibit cyclin-luciferase proteolysis in Xenopus extract (Figure S2A). A similar approach demonstrated that TAME binds to human APC isolated from HeLa cells (Figure 2C). Together these findings indicate that TAME binds to the APC, potentially explaining its ability to perturb activator protein association.
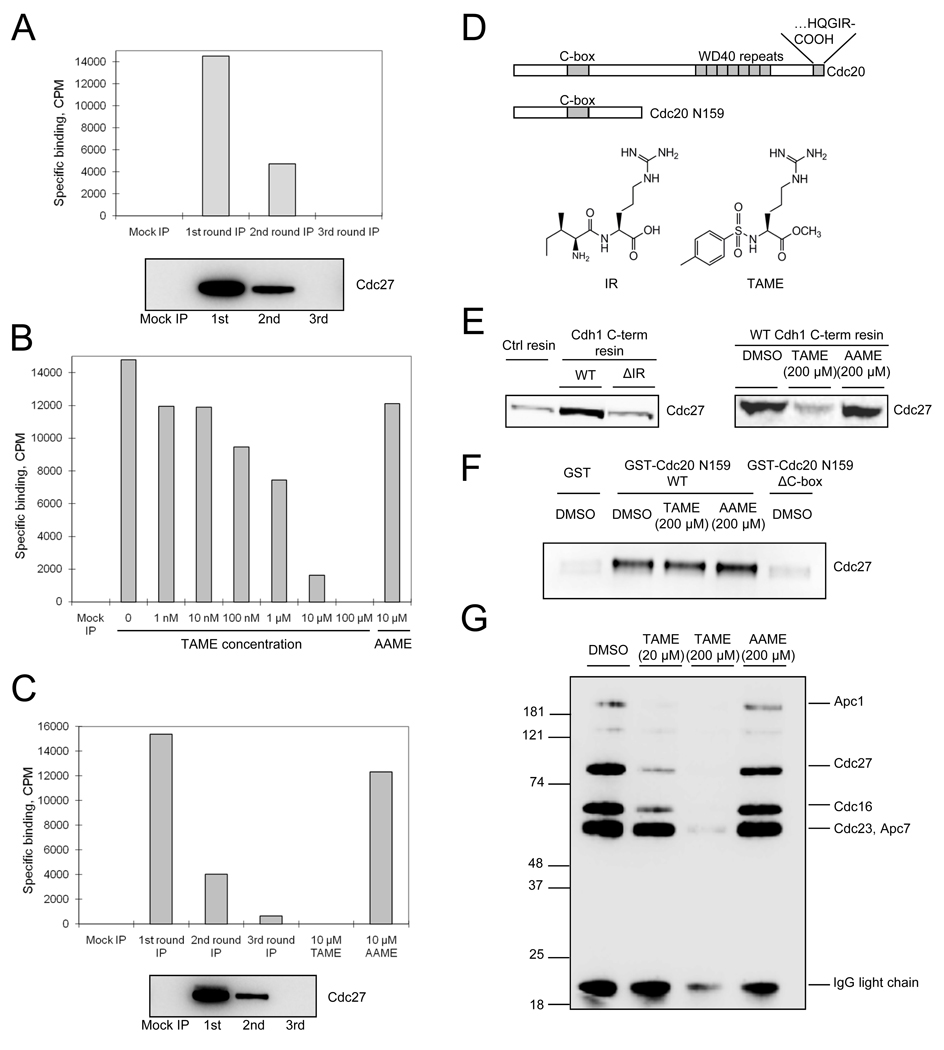
Figure 2. TAME binds to the APC and inhibits binding of the IR tail of activator proteins.
(A) TAME binds Xenopus APC. 3H-TAME was added to interphase extract or to extract that had been partially or completely immunodepleted of APC. Remaining APC was then immunoprecipitated and the associated radioactivity was measured by scintillation counting. Residual APC levels were measured by immunoblot with Cdc27 antibody. Specific binding was calculated as described in the methods. (B) Unlabeled TAME competes with 3H-TAME for binding to Xenopus APC. 3H-TAME was added to interphase extract with unlabeled TAME or AAME prior to APC immunoprecipitation. (C) 3H-TAME binds to human APC. The experiment in 2A was repeated with lysate from asynchronous HeLa cells. (D) Schematic of Cdc20, the C-box containing fragment, and structures of the IR tail and TAME. (E) TAME inhibits the interaction between the Cdh1 C-terminal IR peptide and the APC. Left: Resin coupled with cysteine (Ctrl resin), Cdh1 C-terminal peptide (WT), or the peptide lacking the C-terminal isoleucine and arginine (ΔIR) was incubated with interphase Xenopus extract, washed, and the amount of bound Cdc27 was analyzed by immunoblot. Right: Cdh1 C-terminal resin was incubated with interphase extract in the presence of compounds and the amount of Cdc27 was analyzed as above. (F) TAME does not inhibit the interaction between the C-box and the APC. A 159-amino acid N-terminal fragment of Cdc20 containing the C-box fused to GST (GST-CDC20 N159 WT) or the same fragment lacking the C-box (GST-CDC20 N159 ΔC-box) were bound to glutathione resin and incubated with mitotic Xenopus extract in the presence of compounds. Bound Cdc27 was analyzed by immunoblot. (G) TAME inhibits IR-peptide crosslinking to APC subunits. Purified interphase Xenopus APC was incubated with an IR peptide coupled to a biotin-containing label-transfer reagent, in the presence or absence of compounds, prior to photocrosslinking. Reaction products were detected by streptavidin-HRP. See also Figure S2.
To understand how TAME disrupts activator binding to the APC, we determined whether TAME could inhibit the interaction between APC and motifs of the activator proteins that have been implicated in APC binding, including the C-box (Schwab et al., 2001) and the C-terminal isoleucine-arginine (IR) tail (Figure 2D)(Burton et al., 2005; Vodermaier et al., 2003). Because TAME structurally resembles the IR tail of Cdc20 and Cdh1 (Figure 2D), we hypothesized that TAME might bind to the APC in the same site normally occupied by the IR tail. Previous work has demonstrated that a C-terminal 20 amino acid peptide derived from Cdh1 (“IR peptide”) is sufficient to isolate Xenopus APC from interphase extract (Vodermaier et al., 2003). We confirmed this finding and found that TAME, but not AAME, was sufficient to block APC recruitment by the IR peptide (Figure 2E). In contrast, TAME had no effect on recruitment of APC from mitotic extract by an N-terminal fragment of Cdc20 containing only the C-box interaction motif (Figure 2F), indicating that TAME specifically inhibits the IR-tail-dependent interaction.
The APC subunits Cdc27 and APC7 have been implicated in binding of the IR tail of Cdh1 to the APC (Matyskiela and Morgan, 2009; Vodermaier et al., 2003). To determine whether TAME could competitively inhibit the binding of the IR-tail to these proteins, we conjugated the IR peptide to a photo-affinity reagent and performed crosslinking studies with APC immunopurified from interphase Xenopus extract. Four proteins known to exist in an APC subcomplex, namely Cdc27, Cdc16, Cdc23 and Apc7, were crosslinked in an IR-dependent manner that could be competed by excess unlabeled IR peptide (Figure S2B and S2C). At low concentration (20 µM), TAME efficiently inhibited crosslinking of the IR peptide to Cdc27 and Cdc16 but only slightly reduced crosslinking to Cdc23 and Apc7 (Figure 2G). At high concentration (200 µM), TAME strongly inhibited crosslinking to all APC subunits (Figure 2G). Together these findings support the hypothesis that TAME binds to APC subunits that recruit the IR tail, thereby preventing activator proteins from associating with the APC.
To confirm that TAME specifically antagonizes IR-tail dependent interactions between Cdc20 and the APC, we tested the ability of TAME to inhibit the binding of Cdc20 to the APC in a reconstituted system. APC was purified from mitotic Xenopus extracts and washed with high salt to remove most Cdc20. Purified mitotic APC was then incubated in reticulocyte lysate expressing wild-type or mutant Cdc20, and Cdc20 binding to APC was measured by co-immunoprecipitation. We found that efficient binding of Cdc20 to the APC under these conditions indeed requires the IR-tail, as a mutant lacking these two residues (Cdc20ΔIR) did not bind as efficiently to the APC (Figure 3). TAME also strongly reduced Cdc20 binding to the APC under these conditions (Figure 3). Importantly, addition of TAME had no further effect on binding of the Cdc20ΔIR mutant, confirming that TAME does not perturb other interactions between Cdc20 and the APC.
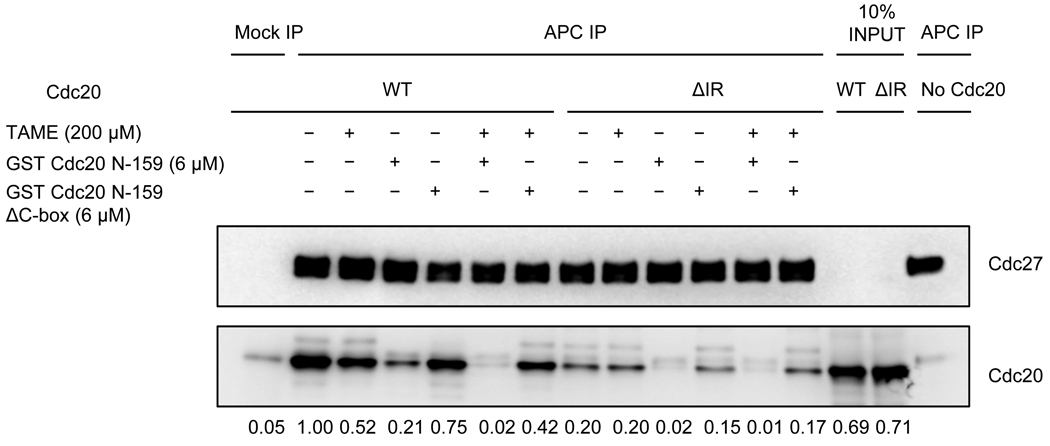
Figure 3. TAME inhibits binding of wild type Cdc20 to the APC, but not binding of a ΔIR mutant.
Mitotic APC immunoprecipitated from Xenopus extract was washed with XB high salt (500 mM KCl) and XB to remove endogenous Cdc20 prior to incubation with in vitro translated wild type Xenopus Cdc20 or the ΔIR mutant. Various competitors were added during incubation as indicated. Unbound proteins were washed away and bound Cdc20 was analyzed by immunoblot. Numbers represent the amount of Cdc20 normalized to Cdc27.
We found that TAME addition or IR-tail deletion was not sufficient to fully inhibit Cdc20 association under these conditions. We suspected that other interactions, such as C-box-dependent binding, might promote Cdc20 association with the APC, thereby masking the effect of TAME addition or IR-tail deletion. Consistent with this hypothesis, we found that addition of a C-box-containing N-terminal fragment of Cdc20 could competitively inhibit binding of full-length Cdc20 to the APC (Figure 3). In the presence of the C-box fragment, addition of TAME or deletion of the IR-tail was sufficient to completely suppress Cdc20 association with the APC. These results indicate that both C-box-dependent and IR-tail-dependent interactions are important for Cdc20 binding in these conditions, and that TAME specifically disrupts the IR-dependent interaction. We conclude that the target of TAME is the APC, and that it inhibits APC activation by interfering specifically with IR-tail dependent interactions between Cdc20 or Cdh1 and the APC.
A TAME Prodrug Inhibits APC-Cdh1 Activation in Cells
Having established the mechanism by which TAME inhibits APC activation in Xenopus extract, we next wanted to determine whether TAME inhibits APC activation in human cells. Because TAME is not cell permeable, we synthesized a TAME prodrug (proTAME), and its control compound proAAME, by modifying the guanidino group to produce an N,N’-bis(acyloxymethyl carbamate) derivative (Figure 4A). Such prodrugs can be processed by intracellular esterases to yield the parent compound. In Xenopus extract, proTAME was indeed rapidly converted to TAME (Figure S3A), which efficiently inhibited cyclin B-luciferase proteolysis (Figure 4B). ProTAME was also activated efficiently in HeLa cells, but not in MCF10A cells (Figure S3B).
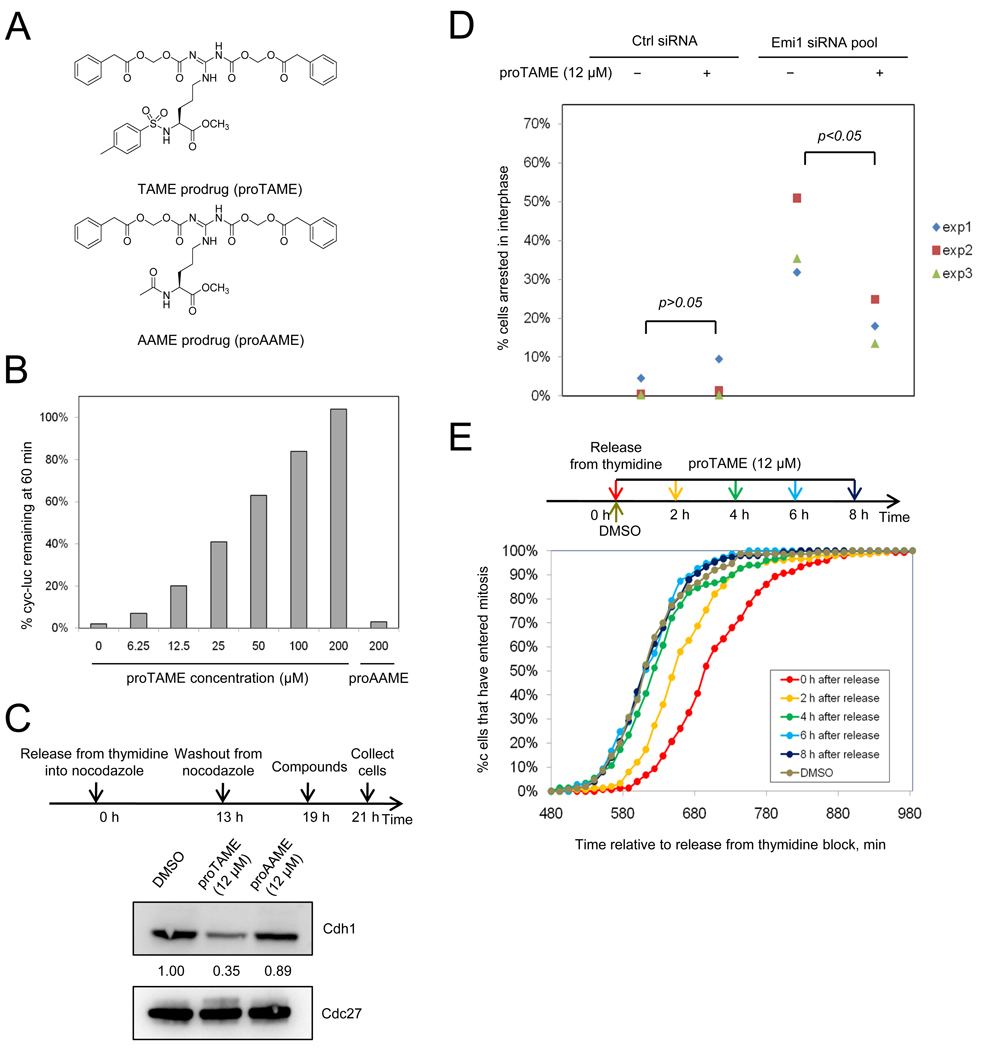
Figure 4. ProTAME inhibits APC activity in Xenopus extract and inhibits Cdh1-dependent APC activity during interphase in HeLa cells.
(A) Structures of proTAME and proAAME. (B) ProTAME inhibits cyclin B-luciferase degradation in mitotic Xenopus extract. Different concentrations of proTAME or proAAME were added to mitotic Xenopus extract containing cyclin B-luciferase reporter. Samples were collected at 60 min and the remaining reporter level was measured by luminescence. (C) ProTAME blocks Cdh1 association with the APC. HeLa cells were released from nocodazole and treated with proTAME in G1. APC was immunoprecipitated from cell lysates and the amount of Cdc27 and Cdh1 was analyzed by immunoblot. (D) ProTAME restores mitotic entry in Emi1-depleted cells. HeLa cells were transfected with control siRNA or Emi1 siRNA and treated with DMSO or proTAME 24 h after transfection and then imaged for 48 h. About 400 cells were analyzed in each experiment, and the proportion that failed to enter mitosis during the 48 h of imaging was calculated. Results of 3 independent experiments are shown. Statistical significance was calculated using an unpaired t-test. (E) ProTAME causes a mitotic entry delay if added during S-phase. HeLa H2B-GFP cells were released from a double thymidine block and proTAME (12 µM) was added at different time points as indicated. Mitotic entry was monitored by time-lapse imaging. Cumulative frequency curves of the time of mitotic entry are shown. Statistical analysis, including mean, median, statistical significance and number of cells analyzed per condition for all experiments is included in Table S1. See also Figure S3.
We first examined whether proTAME could inhibit association of Cdh1 with the APC in cells. We released HeLa cells expressing H2B-GFP from a nocodazole block and added 12 µM proTAME after cells had entered G1, when the APC is activated by Cdh1. We found that addition of proTAME inhibited Cdh1 association with the APC (Figure 4C) but proAAME did not. However, proTAME was not sufficient to cause premature accumulation of endogenous APC substrates in G1 or S phase (Figure S3C). During S phase, when APC substrates are known to be expressed, the effect of proTAME may be masked by Emi1-dependent inhibition of APC-Cdh1 (Hsu et al., 2002). To test this idea, we depleted cells of Emi1, which leads to degradation of APC substrates and prevents mitotic entry (Hsu et al., 2002). We confirmed that Emi1 depletion prevents mitotic entry, and found that addition of 12 µM proTAME substantially rescued the mitotic entry defect caused by depletion of Emi1 (Figure 4D). Therefore, we conclude that proTAME is capable of inhibiting APC-Cdh1 function in cells.
Previous studies have shown that knockdown of Cdh1 induces prolonged S-phase and mitotic entry delay in human cells (Engelbert et al., 2008; Sigl et al., 2009). Consistent with these findings, proTAME caused a 2 h delay in mitotic entry when added during release from a double thymidine block (Figure 4E). However, adding proTAME 6 h or later after release did not delay mitotic entry (Figure 4E), suggesting the delay may be a consequence of inhibiting APC-Cdh1 in S-phase. These findings indicate that although proTAME can inhibit APC-Cdh1 activation, it has only modest effects on cell cycle progression during interphase.
ProTAME Induces Mitotic Arrest in the Absence of Spindle Damage
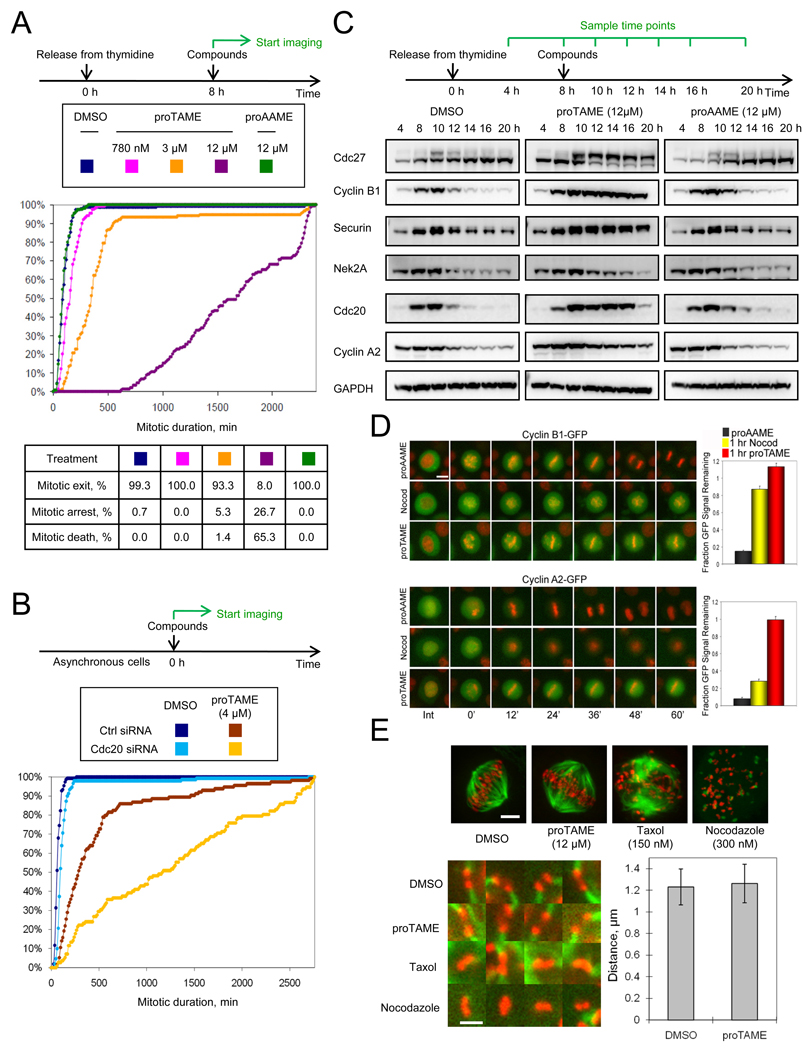
To examine effects of proTAME treatment on mitosis, we released HeLa H2B-GFP cells from a double thymidine block and added proTAME 8 h after release, a time when proTAME addition does not delay mitotic entry (Figure 4E). Mitotic duration was then measured by time-lapse imaging. Cells treated with low doses of proTAME (780 nM or 3 µM) remained in metaphase for as long as five hours, but then proceeded through a normal anaphase, whereas cells treated with 12 µM proTAME arrested in metaphase and subsequently died (Figure 5A). In contrast, treatment of cells with 12 µM proAAME had no effect. ProTAME greatly increased mitotic duration in asynchronous hTERT-RPE1 cells as well, as 6 µM proTAME increased median mitotic duration to over 8 h, compared to 24 min in proAAME-treated cells (Figure S4A). ProTAME had no effect at similar doses in MCF10a cells (data not shown), because the prodrug was not efficiently activated (Figure S3B).
Figure 5. ProTAME induces mitotic arrest without disrupting the mitotic spindle.
(A) ProTAME induces mitotic arrest in HeLa cells. Double thymidine synchronized HeLa H2B-GFP cells were treated with compounds and analyzed by time-lapse imaging. Cumulative frequency curves of mitotic duration and cell fate distributions are shown. (B) Partial Cdc20 knockdown sensitizes cells to proTAME treatment. Asynchronous HeLa H2B-GFP cells were transfected with control or Cdc20 siRNA 24 h prior to treatment with compounds. (C) ProTAME stabilizes endogenous APC substrates. Double thymidine synchronized HeLa cells were treated with compounds. (D) ProTAME stabilizes exogenous cyclin B1-GFP and cyclin A2-GFP in HeLa cells. HeLa H2B-RFP cells transduced with cyclin-GFP adenoviruses were treated with 20 µM proTAME or proAAME, or 150 nM nocodazole. Bar: 12 µm. Representative cells are shown. For quantitation, the fraction of GFP intensity remaining at 60 min as compared to the onset of mitosis was determined (n≥ 30 individual cells per treatment). Error bars represent standard error of the mean. (E) ProTAME does not disrupt mitotic spindles or alter interkinetochore distance. Asynchronous HeLa cells were treated with compounds for 2 h, and then stained with anti-tubulin (green) and CREST (red) antibody. Representative images are shown. Bar: 3 µm. Representative images of kinetochore pairs are shown. Bar: 1.2 µm. Inter-kinetochore distance was measured in DMSO or proTAME treated cells (n=55, p=0.23). Error bars represent standard deviation. See also Figure S4 and Movies S1–4.
If proTAME blocks mitotic progression by disrupting the APC-Cdc20 interaction, then reducing Cdc20 expression should enhance the mitotic exit delay induced by proTAME treatment. In control-transfected cells, 4 µM proTAME increased mitotic duration from 1.0 h to 4.8 h (Figure 5B). However, when Cdc20 levels were reduced by 50% using siRNA-mediated knockdown (Figure S4B), proTAME prolonged mitotic duration to 19.4 h (Figure 5B). This effect was synergistic, because Cdc20 knockdown by itself only increased mitotic duration to 1.6 h. These results show that reducing the expression of Cdc20 strongly sensitizes cells to the effect of proTAME, consistent with the APC-Cdc20 interaction as the relevant target of the compound.
We next investigated the effect of proTAME treatment on degradation of APC substrates. Because the SAC does not stabilize all APC substrates during mitosis, some substrates such as cyclin A2, Cdc20, and Nek2A are degraded in cells treated with microtubule inhibitors (den Elzen and Pines, 2001; Hayes et al., 2006; Nilsson et al., 2008). In contrast, substrates such as cyclin B1 and securin are stabilized by SAC activation. If proTAME directly inhibits APC activation, we predicted it would stabilize all APC substrates during mitosis, not just those whose stability depends on the SAC. Consistent with this hypothesis, cells treated with proTAME accumulated cyclin A2, Cdc20 and Nek2A in addition to cyclin B1 and securin (Figure 5C). These results were confirmed in live cell imaging experiments, where proTAME stabilized cyclinA2-GFP but the microtubule depolymerizer nocodazole did not (Figure 5D). Interestingly, proTAME treatment caused greater accumulation of cyclin B1-GFP than nocodazole treatment, consistent with proTAME’s ability to directly inhibit APC activation.
We next assessed the effects of proTAME treatment on mitotic spindle morphology and chromosome congression, and compared this to the effects of treatment of cells with microtubule inhibitors. Compared to DMSO-treated cells, treatment of asynchronous HeLa cells with 12 µM proTAME for 2 h yielded no measurable differences in mitotic spindle morphology or inter-kinetochore distance, indicating that proTAME did not perturb establishment of proper kinetochore tension (Figure 5E). In contrast, treatment of cells with nocodazole or taxol for 2 h strongly perturbed spindle organization (Figure 5E). In live cell imaging experiments, treatment of cells with 3 µM proTAME or 10 µM MG132 caused no delay in chromosome congression (Figure S4C). Treatment of cells with 10 nM nocodazole or 12 µM proTAME caused a similar mild congression delay of 6 min (Figure S4C), but these treatments produced contrasting effects on the metaphase plate. In cells treated with 10 nM nocodazole, the metaphase plate appeared loose and was prone to bending (Movies S1–3), whereas in cells treated with 12 µM proTAME the metaphase plate appeared tight and did not bend (Movie S4). Importantly, 10 nM nocodazole prolonged mitosis by only 20 min (data not shown), whereas 12 µM proTAME induced a mitotic arrest of over 28 h (Figure 5A). Thus, the mild delay in congression is not sufficient to explain the ability of proTAME to arrest cells in mitosis. We conclude that proTAME induces arrest in metaphase without perturbing the morphology or function of the mitotic spindle.
ProTAME-induced Mitotic Arrest is SAC-Dependent
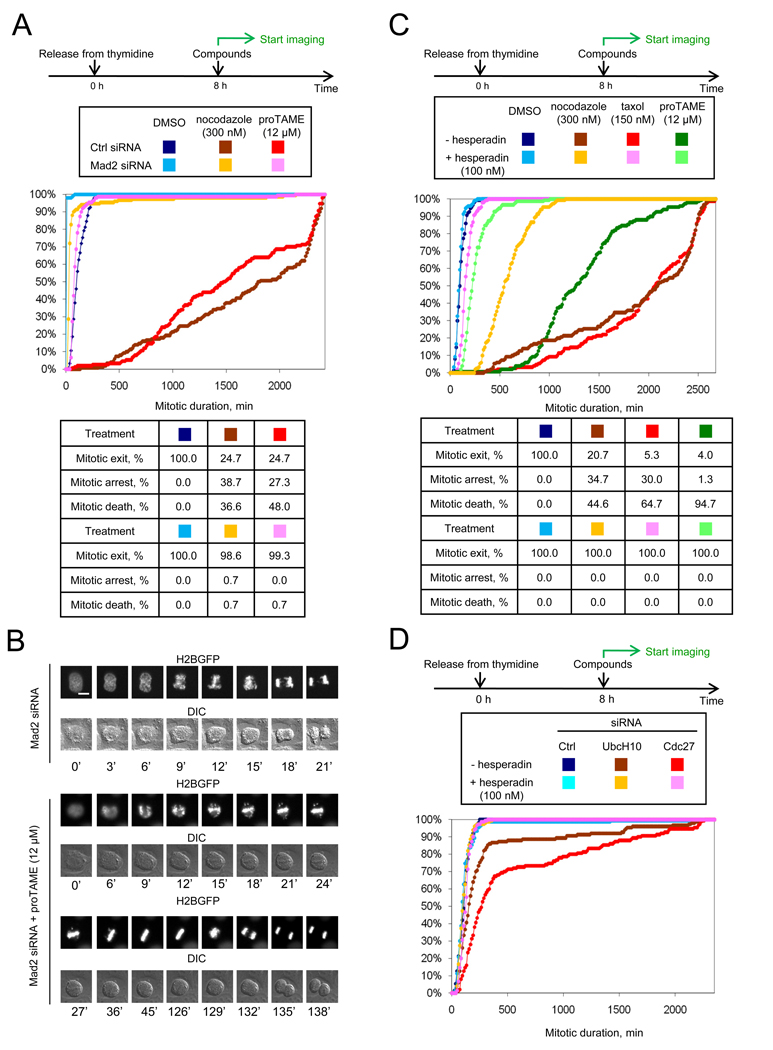
Because TAME directly inhibits the APC, and causes arrest in metaphase with kinetochores that develop tension, we predicted that the proTAME-induced mitotic arrest in human cells would be independent of the SAC. We were therefore surprised to find that the SAC is in fact essential for the prolonged mitotic arrest of cells treated with proTAME. In double-thymidine synchronized cells, Mad2 knockdown greatly shortened the duration of proTAME-induced arrest, from 24.6 h to 1.4 h (Figures 6A and S5A). As expected, Mad2 knockdown abrogated nocodazole-induced arrest, shortening the average mitotic duration from 30 h to 0.6 h. The Mad2-dependence of the proTAME-induced arrest was confirmed by measurement of APC substrate levels in synchronized cells (Figure S5B).
Figure 6. ProTAME-induced mitotic arrest is SAC-dependent.
(A) ProTAME-induced mitotic arrest is Mad2-dependent. HeLa H2B-GFP cells were transfected with indicated siRNAs between rounds of thymidine treatment. Following release, cells were treated with compounds and analyzed by time-lapse imaging. A graph of the same data with an expanded x-axis is shown in Figure S5A. (B) ProTAME rescues the mitotic defect induced by Mad2 knockdown. Asynchronous HeLa H2B-GFP cells were treated with Mad2 siRNA 24 h prior to addition of compound. Bar: 10 µm. (C) ProTAME-induced mitotic arrest is hesperadin-sensitive. Double thymidine synchronized HeLa H2B-GFP cells were treated with compounds 8 h following release. (D) UbcH10 or Cdc27 knockdown induces a hesperadin-sensitive mitotic delay. HeLa H2B-GFP cells were transfected with indicated siRNA between rounds of thymidine synchronization and treated with hesperadin 8 h following release. See also Figure S5 and Movies S5–6.
These experiments also revealed the ability of proTAME to delay mitotic exit independent of Mad2, as expected based on TAME’s ability to directly inhibit APC activation. In Mad2 knockdown cells, proTAME treatment increased median mitotic duration from 12 min to 84 min (Figures 6A and S5A). Strikingly, this mitotic exit delay was sufficient to give Mad2 knockdown cells enough time to build a normal metaphase plate before initiating anaphase, rescuing the chromosome segregation defect caused by Mad2 knockdown (Figures 6B and S5C, Movies S5 and S6). The ability of proTAME to restore normal mitotic division in cells depleted of Mad2 demonstrates that proTAME is unlikely to perturb microtubules or interfere with kinetochore function. The fact that 12 µM proTAME delays mitotic exit by only 72 minutes in the absence of the SAC indicates that this dose of proTAME does not fully inhibit APC activation. The ability of this dose of proTAME to cause mitotic arrest in SAC-proficient cells must therefore arise from significant amplification of APC inhibition by the SAC.
Whereas TAME reduced Cdc20 binding to the APC when added to Xenopus extracts, proTAME treatment did not decrease Cdc20 binding to the APC during mitotic arrest in HeLa cells (data not shown). We suspected that persistent Cdc20 association might result from the ability of the SAC to promote IR-tail independent binding of Cdc20 to the APC. We therefore examined the effect of depleting SAC proteins on the ability of proTAME to disrupt the APC-Cdc20 interaction. Indeed, when we arrested HeLa cells in mitosis by expression of nondegradable cyclin B, proTAME induced significant dissociation of Cdc20 from the APC, but only if SAC proteins were depleted by RNAi (Figure S5D). These results show that a proTAME-induced mitotic arrest occurs without substantial dissociation of Cdc20 from the APC, as a consequence of persistent activity of the SAC.
To further understand the SAC-dependence of the proTAME arrest, we pharmacologically inactivated SAC signaling by treating cells with hesperadin (Hauf et al., 2003), an inhibitor of Aurora B kinase. This kinase phosphorylates proteins at kinetochores that are not under tension, leading to destabilization of microtubule-kinetochore interactions and activation of the SAC (Biggins and Murray, 2001; Cheeseman et al., 2006; DeLuca et al., 2006). Recent work using phosphospecific antibodies that recognize Aurora B substrates indicates that kinetochore proteins remain phosphorylated at a basal rate during metaphase (Welburn et al., 2010). We hypothesized that this basal rate of Aurora B-dependent phosphorylation may produce a persistent SAC signal during metaphase that contributes to the proTAME-induced arrest. Three observations are consistent with this hypothesis. First, hesperadin treatment dramatically shortened proTAME-induced mitotic arrest (Figure 6C), and led to dissociation of Mad2 and BubR1 from the APC in proTAME-arrested cells (Figure S5E). As expected, hesperadin also substantially shortened taxol-induced mitotic arrest, with a less pronounced effect on nocodazole-induced arrest (Figure 6C). Second, hesperadin treatment caused deformation of the metaphase plate in proTAME-arrested cells (Figure S5F), suggesting that Aurora B-dependent phosphorylation is required to maintain proper kinetochore-microtubule attachments in metaphase. Third, knockdown of the APC component Cdc27 or the APC-specific E2 UbcH10 caused a mitotic exit delay that could be completely suppressed by hesperadin treatment (Figure 6D). Together, these experiments are consistent with the idea that the SAC remains active at a basal rate during metaphase, despite the presence of properly attached chromosomes, and that kinetochore-dependent SAC signaling is important for the prolonged mitotic arrest induced by APC inhibition.
One possible explanation for the SAC-dependence of the proTAME arrest is that proTAME stabilizes APC substrates such as Nek2A or cyclin A that are normally degraded in early mitosis. For example, overexpression of cyclin A has been reported to delay chromosome congression (den Elzen and Pines, 2001). To test whether stabilization of these substrates is important for the proTAME-induced arrest, we released HeLa cells from double thymidine block into nocodazole for 15 h to allow degradation of cyclin A and other APC substrates that are not efficiently stabilized by the SAC. We then washed cells out of nocodazole into proTAME. Under this condition, proTAME remained capable of inducing a prolonged mitotic arrest that was highly hesperadin-sensitive (Figure S5G). This result indicates that the SAC-dependence of proTAME-induced mitotic arrest is unlikely to be caused by stabilization of APC substrates that are normally degraded in a SAC-independent fashion.
Metaphase Arrest Induced by a Proteasome Inhibitor is SAC-Dependent
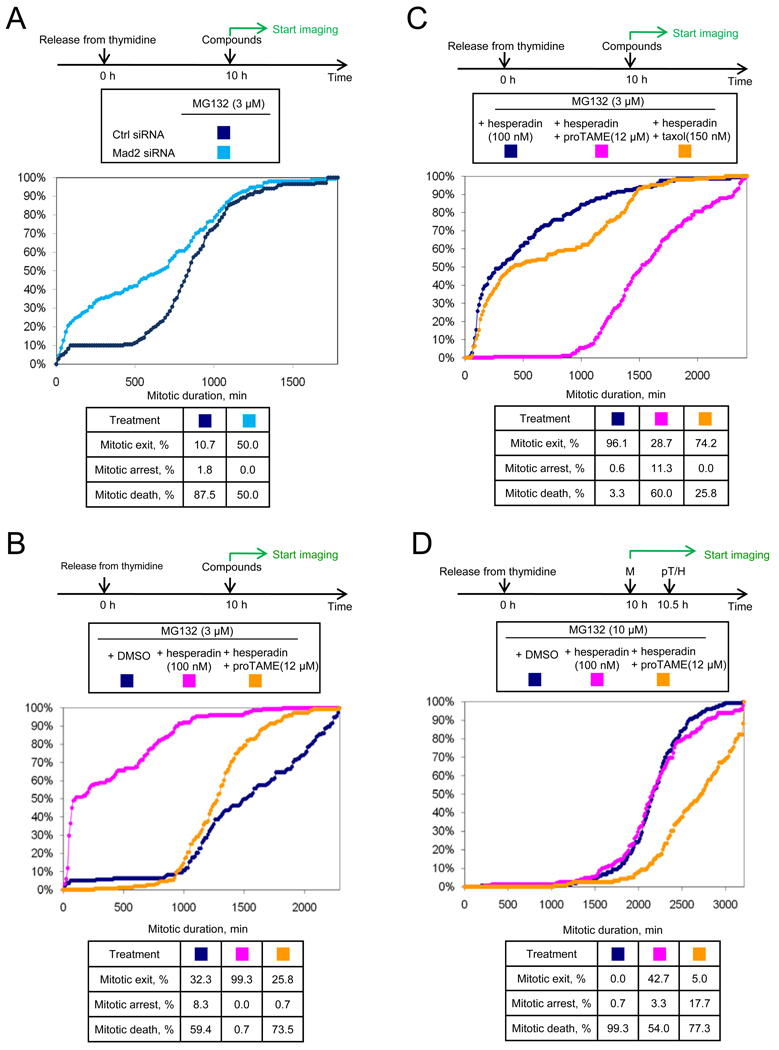
Previous work has shown that APC-dependent ubiquitination promotes SAC inactivation in cell lysates (Reddy et al., 2007). In this system, APC-dependent ubiquitination of Cdc20, but not APC-dependent proteolysis, was suggested to be important for release of Cdc20 from SAC proteins (Reddy et al., 2007). However, a recent study found that proteasome activity is required for dissociation of the Mad2-Cdc20 complex in cells (Visconti et al., 2010). Together with our findings, these studies suggested that APC-dependent proteolysis could be important for SAC inactivation. A prediction of this model is that mitotic arrest induced by treatment with a low dose of proteasome inhibitor should be SAC-dependent. To test this idea, we treated cells with a dose of MG132 (3 µM) that was just sufficient to arrest cells in mitosis (median duration of 15 h). At this concentration, the duration of arrest was limited by cell death rather than mitotic exit, as only 10% of cells exited mitosis over 30h (Figure 7A). In MG132-treated cells depleted of Mad2 by RNAi, we observed that 50% of the cells exited mitosis (Figure 7A), indicating that the SAC is indeed required for efficient induction of mitotic arrest by proteasome inhibition.
Figure 7. MG132-induced mitotic arrest is SAC-dependent.
(A) MG132-induced arrest is Mad2-dependent. HeLa H2B-GFP cells were transfected with indicated siRNAs between rounds of thymidine synchronization, treated with compounds, and followed by time-lapse imaging. (B) MG132-induced arrest is hesperadin-sensitive, but mitotic exit can be suppressed by proTAME. Double thymidine synchronized HeLa cells were treated with compounds. (C) Taxol cannot restore mitotic arrest in the presence of MG132 and hesperadin. Double thymidine synchronized HeLa cells were treated with compounds. (D) Mitotic arrest induced by a higher concentration of MG132 remains hesperadin-sensitive. Double thymidine synchronized HeLa cells were treated with compounds. M: MG132; pT: proTAME; H: hesperadin. See also Figure S6.
Like proTAME-treated cells, MG132-treated cells arrest in metaphase with kinetochores that develop normal tension (Famulski and Chan, 2007). If metaphase chromosomes are indeed competent to generate a checkpoint signal, we predicted that the MG132-induced arrest should be hesperadin-sensitive. To test this idea, ten hours following thymidine release, HeLa H2B-GFP cells were treated with 3 µM MG132 in the presence or absence of hesperadin. Strikingly, hesperadin induced rapid mitotic exit in half of the cells, with the remainder exiting mitosis more slowly (Figure 7B). These distinct behaviors correlated with the timing of drug administration: cells that encountered drug while in mitosis exited mitosis quickly, whereas cells that encountered drug before mitosis exited slowly (Figure S6A). Hesperadin treatment induced dephosphorylation of Cdc27 and reduced levels of Mad2 and BubR1 bound to the APC compared to cells treated with MG132 alone (Figure S6B). Co-addition of proTAME to MG132 abrogated the ability of hesperadin to drive mitotic exit (Figure 7B), indicating that mitotic exit remains dependent on APC-dependent ubiquitination. In contrast, co-addition of taxol to MG132 did not efficiently suppress hesperadin-induced mitotic exit (Figure 7C), underscoring the distinct mechanisms underlying taxol and proTAME-induced mitotic arrests. Similar results were obtained when the proteasome was more fully inhibited by increasing the MG132 concentration to 10 µM (Figure 7D), indicating that the SAC continues to be important for complete inhibition of APC-dependent proteolysis even when the proteasome is more completely inhibited by drug.
The hesperadin sensitivity of the MG132-induced arrest suggested that Aurora B activity could be important for maintaining the metaphase plate, as we observed in proTAME treated cells. We found that treatment of MG132-arrested cells with hesperadin induced deformation of the metaphase plate within 30 min, whereas cells arrested with MG132 alone maintained a normal-appearing metaphase plate for over 5 h (Figure S6C). These findings provide further support for the idea that Aurora B-dependent pathways remain active in metaphase. Together our findings indicate that mitotic arrest induced by a low concentration of proteasome inhibitor is not a simple consequence of direct inhibition of the proteasome by the drug, but also depends on continued inhibition of APC-dependent ubiquitination by the SAC.
Protein Synthesis is Required for Mitotic Arrest Induced by Microtubule Inhibitors but not for APC or Proteasome Inhibitors
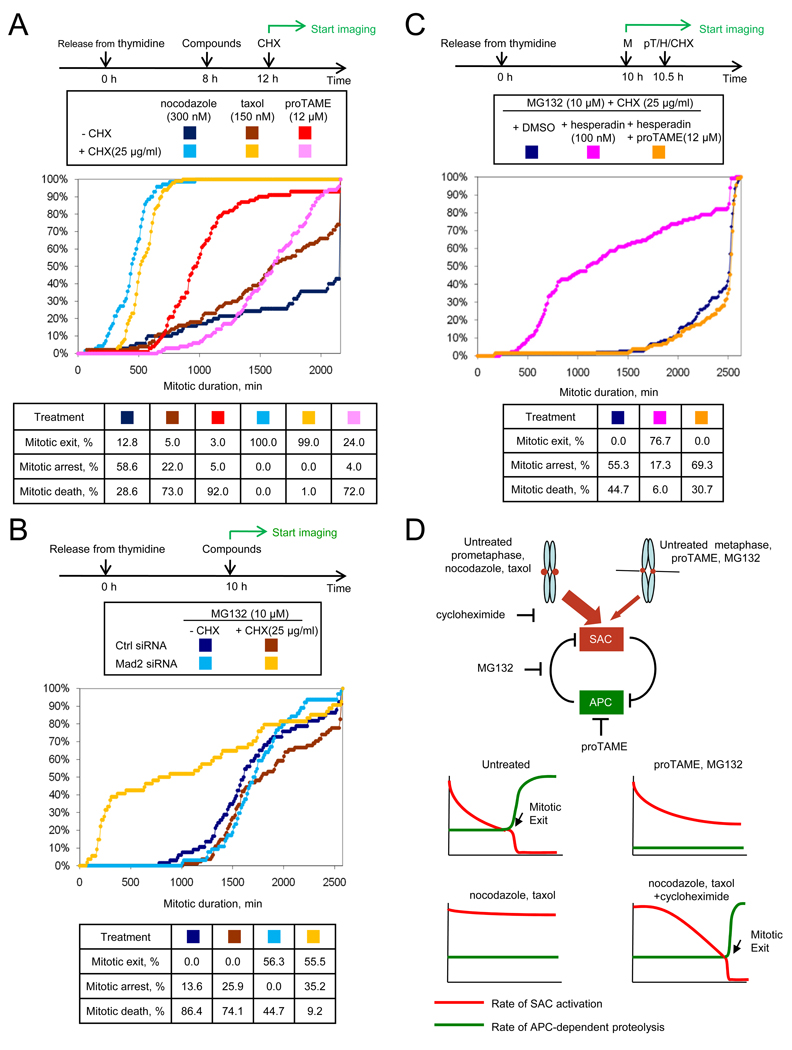
Our data support a model in which APC-dependent proteolysis is required to inactivate the SAC. However, this model yields a paradox: How could the APC initiate SAC inactivation if it is fully inhibited by the SAC? One possibility is that SAC inhibition of APC is never complete, with residual APC remaining active to initiate SAC inactivation. Indeed, it has been shown that cyclin B1 and securin are slowly degraded even in the presence of a fully active SAC (Brito and Rieder, 2006; Nilsson et al., 2008). Prolonged arrest in mitosis might therefore require the continued synthesis of APC substrates during mitosis. Consistent with this hypothesis, we found that cycloheximide promoted mitotic exit of nocodazole- or taxol-arrested cells (Figure 8A). In striking contrast, cycloheximide did not accelerate mitotic exit in proTAME-treated cells, but rather extended mitotic arrest by delaying cell death (Figure 8A). Cycloheximide addition produced similar effects in MG132-treated cells, suppressing cell death without promoting mitotic exit (Figure 8B). Consistent with these findings, labeling experiments demonstrated that known APC substrates such as cyclin B1 and BubR1 are translated during mitotic arrest (Figure S7). Together these findings indicate that ongoing mitotic protein synthesis is essential to maintain a SAC-dependent mitotic arrest, perhaps by replenishing components that are degraded by residual APC-dependent proteolysis.
Figure 8. Microtubule inhibitors require protein synthesis for mitotic arrest whereas proTAME and MG132 do not.
(A) Mitotic arrest induced by microtubule inhibitors requires protein synthesis but proTAME-induced arrest does not. Double thymidine synchronized HeLa-H2B-GFP cells were treated with compounds and followed by time-lapse imaging. CHX: cycloheximide. (B) MG132 (10 µM)-induced arrest is cycloheximide-resistant but Mad2-dependent. HeLa cells were transfected with indicated siRNAs between rounds of thymidine synchronization. (C) MG132 (10 µM)-induced arrest is cycloheximide-resistant but hesperadin-sensitive. Double thymidine synchronized HeLa cells were treated with compounds. (D) Model. In the bottom panels, the x-axis indicates time from mitotic entry. See also Figure S7.
We next wanted to understand why the MG132-induced arrest is resistant to cycloheximide. We hypothesized that persistent SAC activity cooperates with direct pharmacologic inhibition of the proteasome to slow the rate of APC-dependent proteolysis to such a great extent that mitotic arrest no longer depends upon protein synthesis. If this hypothesis is correct, then inactivating the SAC should make the MG132-induced arrest sensitive to cycloheximide, as protein synthesis would now be required to balance the increased rate of APC-dependent degradation. This was indeed the case, as depletion of Mad2 (Figure 8B) or inactivation of the SAC with hesperadin (Figure 8C) led to mitotic exit in cells treated with cycloheximide and 10 µM MG132. Addition of proTAME suppressed the effect of hesperadin (Figure 8C), indicating that mitotic exit remains dependent on APC-mediated ubiquitination. Together these results indicate that the ability of proTAME or MG132 to induce mitotic arrest independent of protein synthesis requires persistent inhibition of the APC by the SAC.
Discussion
Here we identify the mechanism of action of a small molecule inhibitor of cyclin proteolysis discovered in a phenotypic screen in Xenopus extract (Verma et al., 2004). TAME binds to the APC and displaces the IR tail of Cdc20 or Cdh1, preventing efficient APC activation. In human cells, proTAME treatment causes arrest in metaphase without perturbing the mitotic spindle. Despite development of normal kinetochore tension that should silence the SAC, the SAC is required for proTAME to induce mitotic arrest. Similar results were obtained using a proteasome inhibitor. We propose that kinetochore-dependent SAC signaling persists at a low rate in metaphase, and is inactivated by residual APC-dependent proteolysis, creating a positive feedback loop between the APC and the SAC (Figure 8D). The ability of low doses of proTAME or MG132 to induce metaphase arrest is strongly enhanced by this feedback loop, enabling mitotic arrest to be achieved at drug concentrations below those necessary to fully inhibit the APC or the proteasome.
TAME interferes with IR-tail dependent APC activation
Our findings indicate that TAME prevents APC activation by perturbing the binding of the IR-tail of Cdc20 and Cdh1 to the APC. The importance of the IR motif in promoting Cdh1 association with yeast and human APC is well-established (Burton et al., 2005; Kraft et al., 2005; Matyskiela and Morgan, 2009; Vodermaier et al., 2003). However, the role of the Cdc20 IR motif is less clear, because the Cdc20 IR tail is not essential in budding yeast (Thornton et al., 2006) and a Cdc20ΔIR mutant can support APC-dependent degradation of Nek2A in Xenopus extract (Kimata et al., 2008). Our data show that the IR motif of Cdc20 indeed contributes significantly to APC-association in vitro, as Cdc20ΔIR binds the APC with lower affinity than the wild type protein, and TAME competes with wild type Cdc20 for APC association. Moreover, TAME induces significant dissociation of Cdc20 from the APC in Xenopus extract, and proTAME can antagonize Cdc20 binding in human cells if the SAC is inactivated. Functionally, TAME stabilizes APC substrates in Xenopus extract and proTAME inhibits both Cdc20 and Cdh1-dependent degradation in HeLa cells. Taken together, these data show that proper engagement of the IR motif of Cdc20 or Cdh1 is critical for APC activation.
TAME Exploits A Positive Feedback Loop Between the SAC and the APC
We found that proTAME-induced mitotic arrest requires sustained SAC activity. This finding was unexpected, because proTAME-treated cells arrest in metaphase with kinetochores that develop normal tension, a condition that should inactivate the SAC. In principle, the requirement for the SAC in the proTAME arrest could be explained in one of two ways. First, proTAME treatment could produce defects in microtubule-kinetochore interactions that generate an abnormally high degree of checkpoint signal compared to normal metaphase kinetochores. Alternatively, proTAME may hamper SAC inactivation, despite normal microtubule-kinetochore interactions. We favor the latter model because the degree of checkpoint dependence far exceeds the degree of kinetochore-microtubule perturbation that we observe.
Defects in microtubule-kinetochore attachment could arise from an off-target effect of TAME on microtubules, or be a consequence of specific APC inhibition. We found that knockdown of Cdc27 or UbcH10 each produced a mitotic exit delay that was SAC-dependent. Furthermore, treatment of cells with a proteasome inhibitor yielded a SAC-dependent mitotic arrest, consistent with a recent study showing that MG132-treated mitotic cells show persistent Mad2-Cdc20 interaction (Visconti et al., 2010), and work in S. pombe showing that Mad2 and Mad3 remain APC-bound in proteasome mutants (Ohi et al., 2007). Together these findings suggest that if defective microtubule-kinetochore interactions are indeed present in proTAME-treated cells, they are likely to result from specific inhibition of APC-dependent proteolysis rather than from nonspecific effects of proTAME on microtubules.
If defective microtubule-kinetochore interactions exist in proTAME-treated cells, they must be subtle. Cells treated with 12 µM proTAME arrest in mitosis until they die, yet form a normal-appearing metaphase plate and develop normal kinetochore tension. Furthermore, cells treated with 12 µM proTAME undergo a normal-appearing anaphase when the SAC is inactivated, indicating that the mitotic spindle functions properly in the presence of proTAME. The only change in chromosome behavior caused by this dose of proTAME is a slight delay in chromosome congression. A lower dose of proTAME (3 µM) causes no delay in chromosome congression, yet still extends mitotic duration to 5 hours. Although we cannot completely rule out subtle defects in microtubule-kinetochore interactions in proTAME-treated cells, we believe such defects are not of sufficient magnitude to explain the strong dependence of the proTAME arrest on the SAC.
The alternative explanation for the SAC-dependence of the proTAME arrest is that APC-dependent ubiquitination or proteolysis is required to inactivate the SAC. Such mutual antagonism between the APC and the SAC is predicted to create a positive feedback loop that would amplify the inhibitory effects of proTAME or a proteasome inhibitor in a SAC-dependent manner. This is what we observed. If the SAC is inactivated by Mad2 depletion, 12 µM proTAME extends mitotic duration by only 72 minutes, indicating that this dose only partially inhibits APC activation (consistent with the measured IC50 of 12 µM in Xenopus extract). However, when the same dose of proTAME is used in cells with an intact SAC, proTAME extends mitotic duration by 23 hours, indicating that the effect of proTAME is greatly amplified by the SAC. This degree of amplification cannot be explained by the mild effect of proTAME on chromosome congression, because a dose of nocodazole (10 nM) that causes a similar delay in chromosome congression extends mitotic duration by only 20 minutes in SAC-proficient cells. Because we obtained similar results with a proteasome inhibitor, we believe this amplification is best explained by a requirement for APC-dependent proteolysis to inactivate the SAC.
It is unclear which APC substrates play the most important role in mediating the mutual antagonism between the APC and the SAC. APC-dependent ubiquitination of Cdc20 has been proposed to release the APC from the inhibitory effects of the SAC (Reddy et al., 2007; Stegmeier et al., 2007). However, this process does not require proteasome activity in cell lysates (Reddy et al., 2007), and others argue that Cdc20 ubiquitination targets Cdc20 for proteasomal degradation in a manner that sustains the SAC (Ge et al., 2009; Nilsson et al., 2008). Alternatively, many SAC proteins are APC substrates, and may need to be degraded to inactivate the SAC. Consistent with this possibility, expression of a stable BubR1 mutant induces a mitotic arrest (Choi et al., 2009). Another candidate is cyclin B1, because it is degraded prior to anaphase (Clute and Pines, 1999) and cyclin-dependent kinase activity is required to maintain the SAC (Chung and Chen, 2003; D'Angiolella et al., 2003). Other SAC proteins, including Mps1, Bub1, and Aurora B are also APC substrates, but their bulk population is not degraded until after anaphase (Palframan et al., 2006; Qi and Yu, 2007; Stewart and Fang, 2005). It is possible that degradation of these proteins prior to anaphase is masked by their resynthesis. The mutual antagonism between the APC and the SAC may reflect a system-level behavior that is regulated by small changes in the abundance of multiple SAC proteins prior to anaphase. If so, confirmation of our model will require quantitative measurements of the relative rates of synthesis and degradation of APC substrates that regulate SAC activity.
Our results indicate that it is possible to induce mitotic arrest without fully inhibiting the APC or the proteasome pharmacologically. This result was unexpected, because RNAi-based experiments indicated that Cdc20 must be reduced to very low levels to induce mitotic arrest (Wolthuis et al., 2008). Unlike the proTAME-induced arrest, the mitotic arrest induced by Cdc20 knockdown does not depend on the SAC (Huang et al., 2009). One possible explanation for the lack of SAC-dependence in the context of Cdc20 depletion is that Cdc20 is the target of the SAC (Yu, 2007). Therefore, when Cdc20 levels are reduced, the SAC is no longer required to inhibit Cdc20 function. In contrast, other methods of perturbing APC function, including knockdown of core APC subunits or the E2 enzyme UbcH10, or proTAME treatment, all produce an arrest that is SAC-dependent. This is likely a consequence of the fact that Cdc20 remains present under each of these conditions.
A Model for Regulation of Mitotic Exit
Based on our findings, we propose the following model (Figure 8D). A positive feedback loop between the SAC and the APC has the potential to adopt one of two stable states: high SAC activity (mitotic arrest) or high APC activity (mitotic exit). During normal division, it is important that cells do not become permanently arrested in mitosis. We propose that the SAC does not fully inhibit the APC during mitosis because residual APC activity must be preserved to prevent cells from becoming locked in mitosis. This residual APC activity may explain why cyclin B1 is degraded prior to the initiation of anaphase (Clute and Pines, 1999) and during prolonged SAC-dependent mitotic arrest (Brito and Rieder, 2006; Gascoigne and Taylor, 2008; Huang et al., 2009; Nilsson et al., 2008). To remain in mitosis for a prolonged period, a cell may need to continue to resynthesize APC substrates that are degraded by residual APC-dependent proteolysis.
During normal mitosis, the development of kinetochore tension reduces the rate of SAC activation, but SAC activation is unlikely to be completely suppressed during metaphase. Anaphase is triggered when the rate of SAC activation falls below the rate at which APC-dependent proteolysis inactivates the SAC, tipping the feedback loop toward rapid APC activation and mitotic exit. The timing of anaphase initiation therefore depends not only on how kinetochore attachment controls SAC activation, but also on the level of residual APC activity.
During nocodazole or taxol treatment, the rate of SAC activation remains above the rate at which the APC inactivates the SAC, tipping the loop in the direction of APC inhibition thereby preventing mitotic exit. Because APC-dependent proteolysis is not fully inhibited by the SAC, mitotic arrest is dependent on protein synthesis to resupply APC substrates. If the rate of protein synthesis is not sufficient, the rate of SAC signal production will fall below the rate at which it is inactivated by the APC, leading to rapid APC activation and mitotic slippage. Therefore, the rate of protein synthesis in mitosis may be an important determinant of the duration of mitotic arrest in cells treated with microtubule inhibitors.
In contrast to microtubule inhibitors, proTAME and MG132 induce mitotic arrest by inhibiting residual APC-dependent proteolysis rather than by stimulating SAC activation. The rate of SAC signal production by kinetochores may decline normally in proTAME- or MG132-treated cells because kinetochores develop proper tension. However, because the rate of residual APC-dependent proteolysis is lowered by proTAME or MG132, the rate of SAC signal production cannot fall below the rate at which it is inactivated by APC-dependent proteolysis, leading to mitotic arrest. The strong hesperadin sensitivity of both proTAME and MG132-induced arrests indicates the importance of metaphase kinetochores in generating a SAC signal to sustain mitotic arrest. Compared to microtubule inhibitors, this mechanism of mitotic arrest shows reduced dependence on protein synthesis because the rate of residual APC activity is lower in proTAME and MG132-treated cells, yielding a lower requirement for protein synthesis to replenish APC substrates.
An Opportunity for Antimitotic Cancer Therapy
Our study has identified a potential explanation for the variability in cellular responses to microtubule inhibitors that could limit their therapeutic effectiveness. Because the SAC does not completely inhibit the APC, mitotic arrest induced by microtubule inhibition depends on protein synthesis. As a result, variation in the rates of protein synthesis among cells may be one factor that explains the highly variable response of cells to microtubule inhibitors. In contrast, cells treated with an APC inhibitor may be less prone to mitotic slippage because residual APC activity is inhibited. APC inhibitors may therefore be more effective in promoting mitotic arrest, inducing a greater pro-apoptotic effect. Furthermore, low doses of an APC inhibitor may be useful in combination with microtubule inhibitors to sustain mitotic arrest and enhance cell death.
Experimental Procedures
A list of reagents, methods of synthesis of proTAME and additional experimental procedures are provided in the Supplemental Information.
3H-TAME binding assay
3H-TAME (200 nM; 15 Ci/mmol) was added to 100 µl interphase Xenopus extract or HeLa cell lysate. APC was immunoprecipitated with Cdc27 antibody (Santa Cruz, AF3.1) coupled to affiprep beads (Bio-Rad) as previously described (Kirkpatrick et al., 2006).
The beads were washed with XB and radioactivity measured by scintillation counting. Alternatively, 3H-TAME was added and Cdc27 immunoprecipitation was performed after one or two rounds of APC immunodepletion. Specific binding was calculated as the difference between counts associated with Cdc27 antibody beads compared to beads lacking antibody (mock IP).
APC isolation by IR peptide or C-box fragment and crosslinking
A cysteine-containing 20 amino acid peptide derived from the C-terminus of Cdh1, or a control peptide lacking the C-terminal isoleucine and arginine residues, was reduced with TCEP at RT for 15 min and coupled to Ultralink iodoacetyl resin (Pierce). Ten µl of resin was mixed with 100 µl interphase Xenopus egg extract and incubated on a rotator for 30 min at 4 °C. The resin was then washed with XB (100 mM KCl, 0.1 mM CaCl2, 1 mM MgCl2 and 10 mM HEPES, pH 7.7) and bound Cdc27 was analyzed by immunoblot. To investigate the effect of TAME on C-box interactions, a GST fusion protein containing the N-terminal 159 residues of Xenopus Cdc20, or the same protein lacking the C-box, were expressed and purified as described previously (Kimata et al., 2008). The proteins (10 µg) were preloaded on 5 µl Glutathione-Sepharose 4B resin (GE Healthcare) and incubated with cyclin B1Δ90-arrested mitotic Xenopus extract at RT for 30 min in the presence of 1% DMSO, 200 µM TAME or 200 µM AAME. The resin was then washed with XB and bound Cdc27 was analyzed by immunoblot. For crosslinking studies, the Cdh1-derived C-terminal peptide was conjugated to Profound Mts-Atf-Biotin label transfer reagent (Pierce) and crosslinked as described in the supplemental experimental procedures.
APC-Cdc20/Cdh1 association assay
APC was immunoprecipitated from cyclin B1Δ90-arrested mitotic Xenopus extract or interphase extract supplemented with 0.5 µg/ml recombinant Cdh1 as previously described (Kirkpatrick et al., 2006). Compounds were added to mitotic extract immediately before immunoprecipitating the APC. Interphase extracts were pre-incubated with compounds for 30 min before adding recombinant Cdh1 and immunoprecipitating the APC. The beads were washed with XB high salt (XB with 500 mM KCl) and then XB, and bound Cdc27 and Cdc20/Cdh1 were analyzed by immunoblot. Alternatively, Cdc20 was expressed using an in vitro coupled transcription/translation reticulocyte lysate system following the manufacturer’s instruction (Promega L1170). The lysate was diluted with XB so that the concentration of Cdc20 was approximately equal to that of the endogenous Cdc20 in Xenopus extract. APC was immunoprecipitated from mitotic extract as described above and the beads were washed with XB high salt and XB. For each binding assay, 5 µl beads were mixed with 50 µl diluted lysate plus 1 µM okadaic acid, 0.05% IPEGAL CA-630 and various competitors as indicated for 30 min with constant shaking. The beads were then washed with XB + 0.05% IPEGAL CA-630 and bound Cdc27 and Cdc20 were analyzed by immunoblot.
Significance
The Anaphase-Promoting Complex (APC) is required for mitotic exit, making the APC a potential target for antimitotic chemotherapy. Here we identify TAME as a small molecule inhibitor of the APC and develop a cell-permeable derivative, proTAME. Treatment of cells with proTAME causes a surprisingly robust mitotic arrest because APC-dependent proteolysis is required for inactivation of the spindle assembly checkpoint (SAC). In contrast, SAC-activating compounds such as microtubule inhibitors do not suppress APC activity as completely. As a result, cells rely on continued protein synthesis to maintain mitotic arrest, providing an explanation for the known variability in cellular response to microtubule inhibitors. Direct APC inhibitors may therefore provide a more uniform and specific method for inducing mitotic arrest.
Supplementary Material
Acknowledgments
We thank Mike Aguiar and Steve Gygi for assistance with mass spectrometry and Jonathan Iaconelli for technical assistance. We thank Daniel Finley, David Pellman and Tom Rapoport for comments on the manuscript. This work was supported by NIH Grant GM66492 to RWK and a grant from the Stewart Trust. RWK is a member of the Dana Farber-Harvard Cancer Center Breast Cancer SPORE, supported by NIH grant CA089393. KLP was supported by fellowship GM085923. Immunofluorescence microscopy data for this study were acquired in the Nikon Imaging Center at Harvard Medical School.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no financial conflict of interest.
References
- Bekier ME, Fischbach R, Lee J, Taylor WR. Length of mitotic arrest induced by microtubule-stabilizing drugs determines cell death after mitotic exit. Mol Cancer Ther. 2009;8:1646–1654. doi: 10.1158/1535-7163.MCT-08-1084. [DOI] [PubMed] [Google Scholar]
- Biggins S, Murray AW. The budding yeast protein kinase Ipl1/Aurora allows the absence of tension to activate the spindle checkpoint. Genes Dev. 2001;15:3118–3129. doi: 10.1101/gad.934801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito DA, Rieder CL. Mitotic checkpoint slippage in humans occurs via cyclin B destruction in the presence of an active checkpoint. Curr Biol. 2006;16:1194–1200. doi: 10.1016/j.cub.2006.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito DA, Yang Z, Rieder CL. Microtubules do not promote mitotic slippage when the spindle assembly checkpoint cannot be satisfied. J Cell Biol. 2008;182:623–629. doi: 10.1083/jcb.200805072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton JL, Tsakraklides V, Solomon MJ. Assembly of an APC-Cdh1-substrate complex is stimulated by engagement of a destruction box. Mol Cell. 2005;18:533–542. doi: 10.1016/j.molcel.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Cheeseman IM, Chappie JS, Wilson-Kubalek EM, Desai A. The conserved KMN network constitutes the core microtubule-binding site of the kinetochore. Cell. 2006;127:983–997. doi: 10.1016/j.cell.2006.09.039. [DOI] [PubMed] [Google Scholar]
- Choi E, Choe H, Min J, Choi JY, Kim J, Lee H. BubR1 acetylation at prometaphase is required for modulating APC/C activity and timing of mitosis. EMBO J. 2009;28:2077–2089. doi: 10.1038/emboj.2009.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung E, Chen RH. Phosphorylation of Cdc20 is required for its inhibition by the spindle checkpoint. Nat Cell Biol. 2003;5:748–753. doi: 10.1038/ncb1022. [DOI] [PubMed] [Google Scholar]
- Clute P, Pines J. Temporal and spatial control of cyclin B1 destruction in metaphase. Nat Cell Biol. 1999;1:82–87. doi: 10.1038/10049. [DOI] [PubMed] [Google Scholar]
- D'Angiolella V, Mari C, Nocera D, Rametti L, Grieco D. The spindle checkpoint requires cyclin-dependent kinase activity. Genes Dev. 2003;17:2520–2525. doi: 10.1101/gad.267603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca JG, Gall WE, Ciferri C, Cimini D, Musacchio A, Salmon ED. Kinetochore microtubule dynamics and attachment stability are regulated by Hec1. Cell. 2006;127:969–982. doi: 10.1016/j.cell.2006.09.047. [DOI] [PubMed] [Google Scholar]
- den Elzen N, Pines J. Cyclin A is destroyed in prometaphase and can delay chromosome alignment and anaphase. J Cell Biol. 2001;153:121–136. doi: 10.1083/jcb.153.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelbert D, Schnerch D, Baumgarten A, Wasch R. The ubiquitin ligase APC(Cdh1) is required to maintain genome integrity in primary human cells. Oncogene. 2008;27:907–917. doi: 10.1038/sj.onc.1210703. [DOI] [PubMed] [Google Scholar]
- Famulski JK, Chan GK. Aurora B kinase-dependent recruitment of hZW10 and hROD to tensionless kinetochores. Curr Biol. 2007;17:2143–2149. doi: 10.1016/j.cub.2007.11.037. [DOI] [PubMed] [Google Scholar]
- Gascoigne KE, Taylor SS. Cancer cells display profound intra- and interline variation following prolonged exposure to antimitotic drugs. Cancer Cell. 2008;14:111–122. doi: 10.1016/j.ccr.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Ge S, Skaar JR, Pagano M. APC/C- and Mad2-mediated degradation of Cdc20 during spindle checkpoint activation. Cell Cycle. 2009;8:167–171. doi: 10.4161/cc.8.1.7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauf S, Cole RW, LaTerra S, Zimmer C, Schnapp G, Walter R, Heckel A, van Meel J, Rieder CL, Peters JM. The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore-microtubule attachment and in maintaining the spindle assembly checkpoint. J Cell Biol. 2003;161:281–294. doi: 10.1083/jcb.200208092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes MJ, Kimata Y, Wattam SL, Lindon C, Mao G, Yamano H, Fry AM. Early mitotic degradation of Nek2A depends on Cdc20-independent interaction with the APC/C. Nat Cell Biol. 2006;8:607–614. doi: 10.1038/ncb1410. [DOI] [PubMed] [Google Scholar]
- Hsu JY, Reimann JD, Sorensen CS, Lukas J, Jackson PK. E2F-dependent accumulation of hEmi1 regulates S phase entry by inhibiting APC(Cdh1) Nat Cell Biol. 2002;4:358–366. doi: 10.1038/ncb785. [DOI] [PubMed] [Google Scholar]
- Huang HC, Shi J, Orth JD, Mitchison TJ. Evidence that mitotic exit is a better cancer therapeutic target than spindle assembly. Cancer Cell. 2009;16:347–358. doi: 10.1016/j.ccr.2009.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimata Y, Baxter JE, Fry AM, Yamano H. A role for the Fizzy/Cdc20 family of proteins in activation of the APC/C distinct from substrate recruitment. Mol Cell. 2008;32:576–583. doi: 10.1016/j.molcel.2008.09.023. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick DS, Hathaway NA, Hanna J, Elsasser S, Rush J, Finley D, King RW, Gygi SP. Quantitative analysis of in vitro ubiquitinated cyclin B1 reveals complex chain topology. Nat Cell Biol. 2006;8:700–710. doi: 10.1038/ncb1436. [DOI] [PubMed] [Google Scholar]
- Kraft C, Vodermaier HC, Maurer-Stroh S, Eisenhaber F, Peters JM. The WD40 propeller domain of Cdh1 functions as a destruction box receptor for APC/C substrates. Mol Cell. 2005;18:543–553. doi: 10.1016/j.molcel.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Matyskiela ME, Morgan DO. Analysis of activator-binding sites on the APC/C supports a cooperative substrate-binding mechanism. Mol Cell. 2009;34:68–80. doi: 10.1016/j.molcel.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshull J, Sun H, Tonks NK, Murray AW. A MAP kinase-dependent spindle assembly checkpoint in Xenopus egg extracts. Cell. 1994;79:475–486. doi: 10.1016/0092-8674(94)90256-9. [DOI] [PubMed] [Google Scholar]
- Montero A, Fossella F, Hortobagyi G, Valero V. Docetaxel for treatment of solid tumours: a systematic review of clinical data. Lancet Oncol. 2005;6:229–239. doi: 10.1016/S1470-2045(05)70094-2. [DOI] [PubMed] [Google Scholar]
- Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol. 2007;8:379–393. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- Nilsson J, Yekezare M, Minshull J, Pines J. The APC/C maintains the spindle assembly checkpoint by targeting Cdc20 for destruction. Nat Cell Biol. 2008;10:1411–1420. doi: 10.1038/ncb1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohi MD, Feoktistova A, Ren L, Yip C, Cheng Y, Chen JS, Yoon HJ, Wall JS, Huang Z, Penczek PA, et al. Structural organization of the anaphase-promoting complex bound to the mitotic activator Slp1. Mol Cell. 2007;28:871–885. doi: 10.1016/j.molcel.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth JD, Tang Y, Shi J, Loy CT, Amendt C, Wilm C, Zenke FT, Mitchison TJ. Quantitative live imaging of cancer and normal cells treated with Kinesin-5 inhibitors indicates significant differences in phenotypic responses and cell fate. Mol Cancer Ther. 2008;7:3480–3489. doi: 10.1158/1535-7163.MCT-08-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palframan WJ, Meehl JB, Jaspersen SL, Winey M, Murray AW. Anaphase inactivation of the spindle checkpoint. Science. 2006;313:680–684. doi: 10.1126/science.1127205. [DOI] [PubMed] [Google Scholar]
- Peters JM. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat Rev Mol Cell Biol. 2006;7:644–656. doi: 10.1038/nrm1988. [DOI] [PubMed] [Google Scholar]
- Qi W, Yu H. KEN-box-dependent degradation of the Bub1 spindle checkpoint kinase by the anaphase-promoting complex/cyclosome. J Biol Chem. 2007;282:3672–3679. doi: 10.1074/jbc.M609376200. [DOI] [PubMed] [Google Scholar]
- Reddy SK, Rape M, Margansky WA, Kirschner MW. Ubiquitination by the anaphase-promoting complex drives spindle checkpoint inactivation. Nature. 2007;446:921–925. doi: 10.1038/nature05734. [DOI] [PubMed] [Google Scholar]
- Schwab M, Neutzner M, Mocker D, Seufert W. Yeast Hct1 recognizes the mitotic cyclin Clb2 and other substrates of the ubiquitin ligase APC. EMBO J. 2001;20:5165–5175. doi: 10.1093/emboj/20.18.5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Orth JD, Mitchison T. Cell type variation in responses to antimitotic drugs that target microtubules and kinesin-5. Cancer Res. 2008;68:3269–3276. doi: 10.1158/0008-5472.CAN-07-6699. [DOI] [PubMed] [Google Scholar]
- Sigl R, Wandke C, Rauch V, Kirk J, Hunt T, Geley S. Loss of the mammalian APC/C activator FZR1 shortens G1 and lengthens S phase but has little effect on exit from mitosis. J Cell Sci. 2009;122:4208–4217. doi: 10.1242/jcs.054197. [DOI] [PubMed] [Google Scholar]
- Stegmeier F, Rape M, Draviam VM, Nalepa G, Sowa ME, Ang XL, McDonald ER, 3rd, Li MZ, Hannon GJ, Sorger PK, et al. Anaphase initiation is regulated by antagonistic ubiquitination and deubiquitination activities. Nature. 2007;446:876–881. doi: 10.1038/nature05694. [DOI] [PubMed] [Google Scholar]
- Stewart S, Fang G. Destruction box-dependent degradation of aurora B is mediated by the anaphase-promoting complex/cyclosome and Cdh1. Cancer Res. 2005;65:8730–8735. doi: 10.1158/0008-5472.CAN-05-1500. [DOI] [PubMed] [Google Scholar]
- Thornton BR, Ng TM, Matyskiela ME, Carroll CW, Morgan DO, Toczyski DP. An architectural map of the anaphase-promoting complex. Genes Dev. 2006;20:449–460. doi: 10.1101/gad.1396906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma R, Peters NR, D'Onofrio M, Tochtrop GP, Sakamoto KM, Varadan R, Zhang M, Coffino P, Fushman D, Deshaies RJ, King RW. Ubistatins inhibit proteasome-dependent degradation by binding the ubiquitin chain. Science. 2004;306:117–120. doi: 10.1126/science.1100946. [DOI] [PubMed] [Google Scholar]
- Visconti R, Palazzo L, Grieco D. Requirement for proteolysis in spindle assembly checkpoint silencing. Cell Cycle. 2010;9:564–569. doi: 10.4161/cc.9.3.10581. [DOI] [PubMed] [Google Scholar]
- Vodermaier HC, Gieffers C, Maurer-Stroh S, Eisenhaber F, Peters JM. TPR subunits of the anaphase-promoting complex mediate binding to the activator protein CDH1. Curr Biol. 2003;13:1459–1468. doi: 10.1016/s0960-9822(03)00581-5. [DOI] [PubMed] [Google Scholar]
- Welburn JP, Vleugel M, Liu D, Yates JR, 3rd, Lampson MA, Fukagawa T, Cheeseman IM. Aurora B phosphorylates spatially distinct targets to differentially regulate the kinetochore-microtubule interface. Mol Cell. 2010;38:383–392. doi: 10.1016/j.molcel.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcik C, Schroeter D, Stoehr M, Wilk S, Paweletz N. An inhibitor of the chymotrypsin-like activity of the multicatalytic proteinase complex (20S proteasome) induces arrest in G2-phase and metaphase in HeLa cells. Eur J Cell Biol. 1996;70:172–178. [PubMed] [Google Scholar]
- Wolthuis R, Clay-Farrace L, van Zon W, Yekezare M, Koop L, Ogink J, Medema R, Pines J. Cdc20 and Cks direct the spindle checkpoint-independent destruction of cyclin A. Mol Cell. 2008;30:290–302. doi: 10.1016/j.molcel.2008.02.027. [DOI] [PubMed] [Google Scholar]
- Yu H. Cdc20: a WD40 activator for a cell cycle degradation machine. Mol Cell. 2007;27:3–16. doi: 10.1016/j.molcel.2007.06.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.