Summary
Cyclin D1 elicits transcriptional effects through inactivation of the retinoblastoma protein and direct association with transcriptional regulators. The current work reveals a molecular relationship between cyclin D1/CDK4 kinase and protein arginine methyltransferase 5 (PRMT5), an enzyme associated with histone methylation and transcriptional repression. Primary tumors of a mouse lymphoma model exhibit increased PRMT5 methyltransferase activity and histone arginine methylation. Analyses demonstrate that MEP50, a PRMT5 co-regulatory factor, is a CDK4 substrate, and phosphorylation increases PRMT5/MEP50 activity. Increased PRMT5 activity mediates key events associated with cyclin D1-dependent neoplastic growth including CUL4 repression, CDT1 overexpression, and DNA re-replication. Importantly, human cancers harboring mutations in Fbx4, the cyclin D1 E3 ligase, exhibit nuclear cyclin D1 accumulation and increased PRMT5 activity.
Keywords: Cyclin D1, CDK4, CUL4, CDT1, PRMT5, MEP50, Arginine Methylation
Introduction
Cyclin D1, the allosteric regulator of CDK4 and CDK6, is an integral mediator of growth factor-dependent G1-phase progression. Growth factor stimulation induces cyclin D1 expression, association with CDK4 and nuclear accumulation during mid-G1; active cyclin D1/CDK4 kinase catalyzes phosphorylation-dependent inactivation of the retinoblastoma (RB) family proteins (Diehl, 2002). Following the G1/S transition, cyclin D1 accumulation is opposed by Pro287-directed phosphorylation of threonine-286 (T286) by glycogen synthase kinase 3β (GSK3β), which promotes its nuclear export (Alt et al., 2000). Cytoplasmic, phosphorylated cyclin D1 is poly-ubiquitylated by SCFFbx4 and degraded by the 26S proteasome (Lin et al., 2006).
Overexpression of cyclin D1 occurs in numerous human malignancies including carcinomas of the breast, esophagus, colon, and lung (Bani-Hani et al., 2000; Bartkova et al., 1994a; Bartkova et al., 1995; Bartkova et al., 1994b; Gillett et al., 1994; Herman et al., 1995; Hibberts et al., 1999; Hosokawa and Arnold, 1998; Hosokawa et al., 1999; Jin et al., 2001). While overexpression of cyclin D1 is often observed, overexpression per se is insufficient to drive spontaneous transformation. The primary cellular mechanism that restricts cyclin D1/CDK4 activity is cytoplasmic, ubiquitin-mediated degradation of cyclin D1 during S-phase. Mutations that disrupt this event directly contribute to neoplastic growth (Aggarwal et al., 2007; Alt et al., 2000; Barbash et al., 2008; Benzeno et al., 2006; Lu et al., 2003). Specifically, inhibition of cyclin D1 proteolysis during S-phase via mutations within the cyclin D1 degron or inactivation of the cyclin D1 E3 ligase, Fbx4, triggers constitutive nuclear accumulation of active cyclin D1/CDK4 complexes which, in turn, disrupt temporal regulation of DNA replication, thereby contributing to neoplastic growth. The disruption of S-phase fidelity reflects accumulation of the replication licensing protein CDT1. Inappropriate CDT1 stabilization is a result of nuclear cyclin D1/CDK4-dependent repression of CUL4A and CUL4B, encoding scaffolding proteins for the E3 ligase that directs CDT1 degradation during S-phase (Aggarwal et al., 2007).
Numerous previous studies have linked cyclin D1 with transcriptional repression. The current model focuses on an intrinsic capacity of cyclin D1 to associate directly with transcriptional regulators and either interfere with the recruitment of co-factors that direct gene repression or conversely, facilitate co-activator recruitment as observed in the collaboration between cAMP signaling and cyclin D1 in the activation of estrogen receptor (ER)-mediated transcription in mammary epithelial cells (Lamb et al., 2000). The strongest evidence for cyclin D1 directly contributing to the regulation of gene expression stems from recent work utilizing genome-wide chromatin immune precipitation to identify promoters occupied by cyclin D1 (Bienvenu et al. 2010).
A common theme throughout previous studies is that cyclin D1 modulates gene expression in a kinase-independent manner. However, transcriptional repression of CUL4A and CUL4B by cyclin D1 requires S-phase accumulation of catalytically active cyclin D1/CDK4 (Aggarwal et al., 2007). The current study dissects the molecular mechanism of CUL4A/B transcriptional regulation by cyclin D1 and its contribution to nuclear cyclin D1-driven neoplastic growth.
Results
Association of the PRMT5/MEP50 methyltransferase with cyclin D1T286A/CDK4
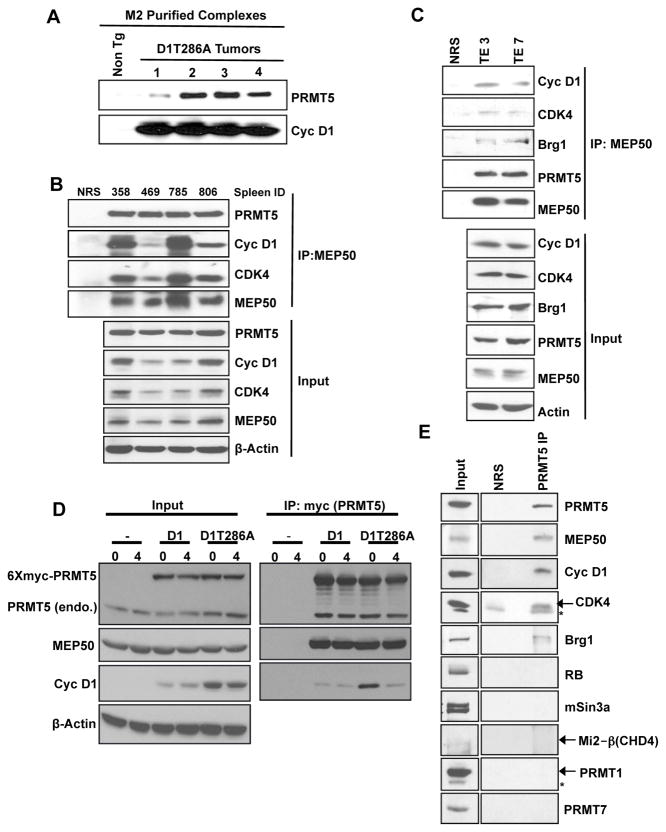
To decipher mechanisms of nuclear cyclin D1-driven neoplasia, we immunopurified cyclin D1T286A complexes from tumors derived from Eμ-D1T286A transgenic mice (Gladden et al., 2006) and identified co-purifying proteins by mass spectrometry (Figure S1A). Given previous reports linking cyclin D1 with transcriptional repression, we were intrigued by co-purification of PRMT5, a Type II methyltransferase, along with MEP50, a WD40 repeat containing protein that contributes to PRMT5 activity and substrate recruitment (peptides recovered Table S1; Krause et al., 2007). Co-precipitation of cyclin D1T286A, CDK4, MEP50 and PRMT5 from B-cell tumors confirmed the interaction (Figures 1A–B). To ascertain the existence of this complex in human cancer cells, we used TE3 and TE7 cell lines that harbor cyclin D1P287A (Benzeno et al., 2006). Cyclin D1P287A is refractory to GSK3β-dependent phosphorylation and is stabilized in the nucleus, analogous to D1T286A. Consistent with the above results, we also observed co-precipitation of cyclin D1P287A along with MEP50, PRMT5, and CDK4 (Figure 1C).
Figure 1. Identification of cyclin D1T286A/PRMT5/MEP50 complexes.
(A) M2 (anti-Flag) purified complexes from Eμ-D1T286A (Flag epitope) transgenic tumors and non-transgenic spleens were blotted for PRMT5 and cyclin D1. (B) MEP50 immunoprecipitates (IP, 1mg whole cell lysate input) prepared from Eμ-D1T286A transgenic tumors were immunoblotted as indicated (C) MEP50 IP (1.5mg whole cell lysate input) from human esophageal cancer cell lines TE3, TE7 were immunoblotted as indicated (Top panel). Input lysates have been shown in the bottom panel. (D) HeLa cells transfected with either wild type cyclin D1 or D1T286A along with myc-PRMT5 and CDK4 were synchronized with aphidicolin. Cells were collected at 0 hours (G1/S boundary) and 4 hours (S-phase) after release. Myc-PRMT5 was pulled down using myc antibody (1mg whole cell lysate input) and immunoblotted as indicated (right panel), input lysates (left panel). (E) Cyclin D1/CDK4 complexes were isolated from Eμ-D1T286A lymphomas using M2-agarose followed by elution with Flag peptide. Eluted complexes were immunoprecipitated with normal rabbit serum (NRS) or PRMT5 antibody. 300μg of eluted complex served as input in NRS or PRMT5 IP, and western analysis was performed as indicated. See also Figure S1 and Table S1.
Association of cyclin D1T286A with PRMT5 in concert with previous work demonstrating that cyclin D1T286A reduces CUL4A/B expression in a CDK4-dependent manner (Aggarwal et al., 2007) suggested a potential regulatory relationship involving cyclin D1T286A and chromatin modifying proteins. To interrogate this putative relationship, we co-expressed Myc-PRMT5 together with CDK4 and either wild type cyclin D1 or D1T286A in HeLa cells. Following synchronization at the G1/S boundary and release into S-phase, complexes were immune precipitated and associated proteins identified by immunoblot. Cyclin D1T286A was enriched in PRMT5 complexes at the G1/S boundary and declined as cells progressed through S-phase (Figures 1D, S1B). In contrast, low levels of wild type cyclin D1 were associated with PRMT5, with no obvious enrichment. The reduced binding of wild type cyclin D1 reflects both its low abundance, due to proteolysis during S-phase, and cytoplasmic localization of the remaining protein (Alt et al., 2000; Barbash et al., 2008).
To determine whether known components of chromatin remodeling complexes were also present in the cyclin D1T286A-PRMT5 complex, we performed a two-step affinity purification of this complex. Initially, Flag-D1T286A-containing complexes were collected from pooled tumor lysates by M2-affinity chromatography, which was used to isolate flag-tagged cyclin D1. Complexes were eluted with flag peptide then re-precipitated with PRMT5 antibodies. In addition to the expected components (cyclin D1T286A, CDK4, PRMT5, MEP50) we also noted Brg1 (Figure 1E). Importantly, these complexes do not contain RB, PRMT5-related PRMT1 and PRMT7, mSin3a (a component of large multi-subunit histone deacetylase (HDAC) co-repressor complexes), or Mi2 (a nucleosome remodeling and HDAC complex component) (Figure 1E). This result reveals the existence of a cyclin D1T286A, CDK4, MEP50, PRMT5 and Brg1 complex. Consistently, we also noted co-precipitation of Brg1 with endogenous cyclin D1P287A in TE3 and TE7 cells (Figure 1C). Consistently, size exclusion chromatography revealed co-fractionation of cyclin D1T286A, CDK4, PRMT5, MEP50, and Brg1 in murine lymphomas (Figure S1C).
PRMT5/MEP50 mediates cyclin D1T286A/CDK4-dependent CUL4A/B loss and CDT1 stabilization
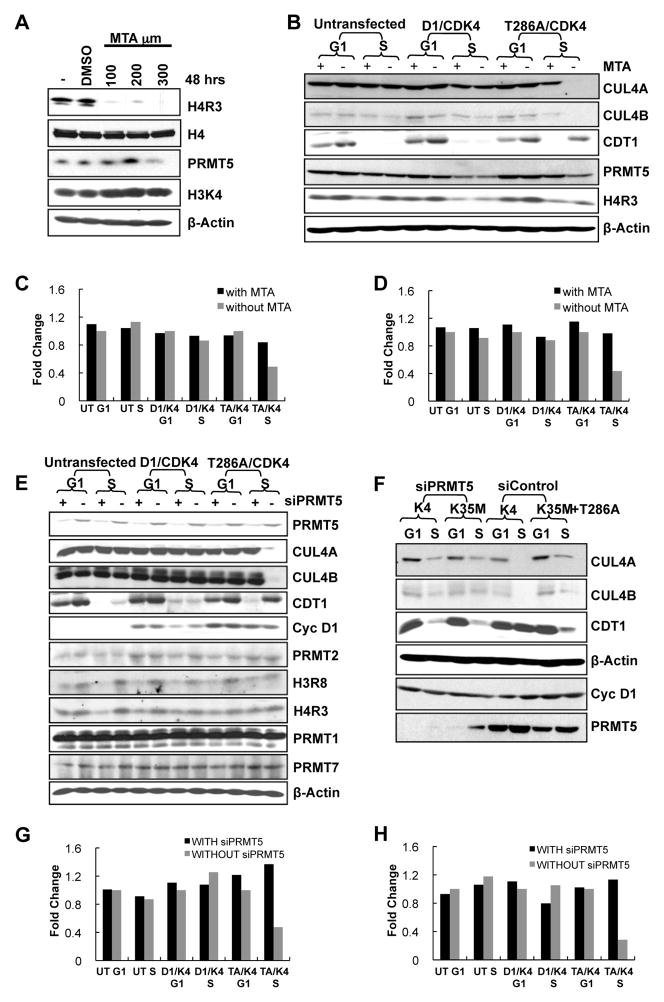
Because PRMT5-dependent dimethylation of histone H3 arginine 8 (H3R8) and histone H4 arginine 3 (H4R3) is associated with transcriptional repression (Pal et al., 2004), we considered whether PRMT5 would mediate cyclin D1T286A/CDK4-dependent repression of CUL4A/B. We first used the general methyltransferase inhibitor 5′ Deoxy-5″-methyl-thioadenosine (MTA; Hou et al., 2008; Iwasaki and Yada, 2007) following identification of a concentration that inhibited H4R3 methylation but not H3K4 methylation (Figure 2A). As previously observed, expression of catalytically active cyclin D1T286A/CDK4 resulted in the maintenance of CDT1 and loss of CUL4A/B proteins and mRNAs specifically during S-phase (Figures 2B–D). Treatment with 100μM MTA restored CUL4A/B proteins and mRNAs (Figures 2B–D) and triggered CDT1 degradation during S-phase (Figure 2B). A similar restoration of CUL4A/B was observed with another PRMT inhibitor, AMI-1 (Cheng et al., 2004) (Figures S2A–D).
Figure 2. PRMT5/MEP50 mediates cyclin D1T286A/CDK4-dependent CUL4 repression and CDT1 stabilization.
(A) HeLa cells were treated with MTA (5′ Deoxy-5″-methyl-thioadenosine) with concentrations ranging from 100 to 300 μM for 48 hours or vehicle DMSO. The cells were analyzed by western blot as indicated. (B) HeLa cells cultured for 24h in the absence or presence of 100μM MTA were transfected with vectors encoding cyclin D1 or D1T286A and CDK4. 24 hours post-transfection, cells were synchronized with nocodazole for 16–18 hrs; mitotic cells were then separated in two dishes. One was harvested 8h after release (G1-phase). Hydroxyurea was added to the second after release and harvested at 14h to obtain cells in S-phase; lysates were subjected to immunoblot as indicated. (C, D) RNA was collected from HeLa cells treated as in (B). The bars illustrate CUL4A (C) and CUL4B (D) mRNA levels with MTA treatment (first bar) and without MTA treatment (second bar) as analyzed by Real time PCR. One representative experiment of three biological independent experiments is presented. (E) HeLa cells were treated with or without siPRMT5 then transfected with wild type cyclin D1 or D1T286A plasmids along with CDK4. Cells were synchronized as in B, and immunoblotted as indicated. (F) HeLa cells were treated with siPRMT5 or si control smartpool for 24 hours, followed by transfection with cyclin D1T286A along with CDK4 or kinase dead CDK4(K35M) plasmids. Cells were synchronized as in (B), and lysates were immunoblotted as indicated. (G, H) Same as (C-D), except that cells were analyzed with and without siPRMT5 treatment. See also Figure S2.
As an independent assessment of PRMT5 function, we utilized siRNA directed at PRMT5 or MEP50. Knockdown of PRMT5 (Figures 2E; G–H) or MEP50 (Figure S2E) restored expression of CUL4A/B and CDT1 loss during S-phase. Knockdown of PRMT5 also reduced H4R3 and H3R8 methylation but did not change levels of PRMT1, 2, and 7 (Figure 2E). We confirmed previous observations that regulation of CUL4 and CDT1 requires the kinase activity of CDK4 as expression of a kinase defective mutant, CDK4(K35M), does not support cyclin D1T286A-dependent CUL4 loss and eliminates the impact of PRMT5 knockdown (Figure 2F). Further supporting a role for PRMT5 activity downstream of cyclin D1, esophageal cancer cells harboring the mutant cyclin D1 P287A exhibited increased CDT1, decreased CUL4A, and increased H4R3 methylation compared to cells with wild type cyclin D1 (Figure S2F).
Since attenuation of PRMT5 relieves CUL4 repression, we questioned whether cyclin D1T286A regulates HDAC activity, contributing to CUL4 repression. Synchronized HeLa cells were treated with the HDAC inhibitors sodium butyrate, trichostatin A and nicotinamide; each inhibitor increased CUL4 expression independent of cyclin D1T286A (Figures S2G–H). Furthermore, acetylation of histone H3 and H4 within the CUL4A promoter (Figure S2I) and CUL4B promoter (data not shown) was not influenced by cyclin D1T286A, suggesting that HDAC activity is not coordinately regulated with histone arginine methylation by cyclin D1T286A/CDK4 at the CUL4 promoters.
Cyclin D1T286A/CDK4 kinase directs increased PRMT5 activity and PRMT5-dependent methylation of CUL4 promoters
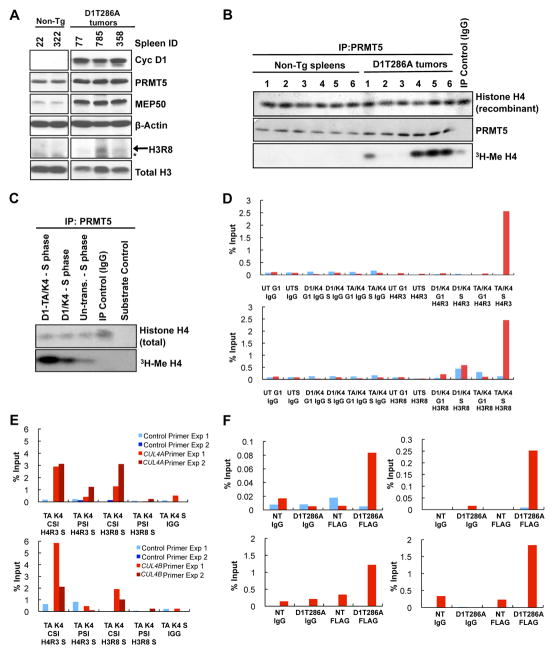
If PRMT5 mediates the action of cyclin D1T286A/CDK4 in vivo, we reasoned that increased H3R8 dimethylation, a PRMT5-specific mark, (Pal et al 2004) should be apparent in tumors derived from Eμ-D1T286A mice. Methylated H3R8 was elevated in tumors compared to non-transgenic controls (Figures 3A and S3A; see Figure S3C for H3R8 antibody specificity). To determine whether increased methylation of histones reflected increased PRMT5 activity, we assessed methyltransferase activity of PRMT5 immuno-purified from control or tumor-burden spleens and found that four of six tumors exhibited an increase in PRMT5 activity (Figure 3B). While PRMT5 levels are increased in cyclin D1T286A tumors, it does not appear sufficient to account for the dramatic increase in PRMT5 catalysis observed. We also observed increased methylation of recombinant H3R8 in vitro (Figure S3B). To more directly determine whether this increase reflects the action of cyclin D1T286A/CDK4, we reconstituted this pathway in HeLa cells. Consistently, PRMT5 isolated from S-phase cells expressing cyclin D1T286A exhibited enhanced methyltransferase activity; a small but significant increase was also observed in cells overexpressing wild type cyclin D1/CDK4 (Figure 3C).
Figure 3. Cyclin D1T286A/CDK4 activity increases PRMT5 methyltransferase activity in vivo.
(A) Non-transgenic spleen or cyclin D1T286A-driven splenic lymphoma lysates were immunoblotted as indicated. (B) Methyltransferase activity of tumor-derived PRMT5 using recombinant histone H4 as the substrate. (C) Same as (B), except that PRMT5 complexes were immuno-purified from HeLa cells transfected with the indicated plasmids and synchronized in S-phase. (D-F) ChIP primer sets (1,2 and 3) were designed to span 1000–2000bp upstream of the first coding exon of either CUL4A or CUL4B respectively (primer design, Figure S3D). Primer set 3 was used for both CUL4A and CUL4B ChIP assays. Control primer sets were designed ~5000bp upstream of first coding exon of CUL4A/B. The CUL4A/B promoter primer (red bar) and control primer (blue bar) are displayed in all graphs. ChIP was performed for CUL4A (Figure 3D) and CUL4B (Figure S3E) using antibodies directed to di-methyl H4R3 (Figure 3D, top panel), H3R8 (Figure 3D, bottom panel; Figure S3E right panel), or normal mouse IgG (first 6 bars of each graph), on chromatin prepared from synchronized HeLa cells expressing wild type cyclin D1/CDK4, D1T286A/CDK4 or untransfected cells. DNA-protein immunoprecipitates and 5% of input chromatin were analyzed by qPCR for both CUL4A and CUL4B promoter regions. PCR values represent percentage of input. One representative experiment of three biological independent experiments is presented. (E) PRMT5 knockdown attenuates methylation of H4R3 and H3R8 of CUL4A (top graph) and CUL4B (bottom graph) promoter regions. ChIP was performed using di-methyl specific H4R3 and H3R8 antibodies as described in D; two representative experiments are shown. (F) Detection of cyclin D1T286A on the Cul4A/B proximal promoter by Flag ChIP from Eμ-D1T286A tumors. Two independent experiments are shown. See also Figure S3.
Given the increase in total H4R3/H3R8 methylation, we determined whether there was an increase in H4R3/H3R8 methylation on the proximal CUL4A/B promoter regions by ChIP using antibodies detecting di-methylated H4R3 and di-methylated H3R8 (see Figure S3D for primer design). We observed an ~2–3 fold increase in methylation of both H4R3 and H3R8 at the proximal promoter regions (~500bp upstream of the first coding exon) of CUL4A (Figure 3D) and CUL4B (Figure S3E) in the presence of cyclin D1T286A/CDK4 as compared to cyclin D1/CDK4 or untransfected controls in S-phase HeLa cells. No enrichment was detected with primers directed to regions corresponding to 1000bp upstream (data not shown) or ~5000bp upstream of first coding exon of CUL4A/B (control/blue bars Figures 3D; S3E). Using PRMT5 specific antibodies for ChIP, we noted PRMT5 occupancy of the same region of the CUL4 promoters (Figure S3F). Critically, siRNA-mediated knockdown of PRMT5 resulted in significant attenuation of cyclin D1T286A/CDK4 dependent H4R3/H3R8 methylation at CUL4A/B promoter regions during S-phase (Figure 3E). In agreement with cyclin D1T286A directly contributing to increased PRMT5 activity, cyclin D1T286A occupancy of both Cul4A/B promoters was observed in cyclin D1T286A expressing primary murine lymphoma cells (Figure 3F). Consistent with the presence of Brg1 in the cyclin D1T286A/PRMT5 complex, Brg1 also occupies the Cul4A and Cul4B promoters (Figure S3G).
Phosphorylation of MEP50 by cyclin D1T286A/CDK4 in vitro and in vivo
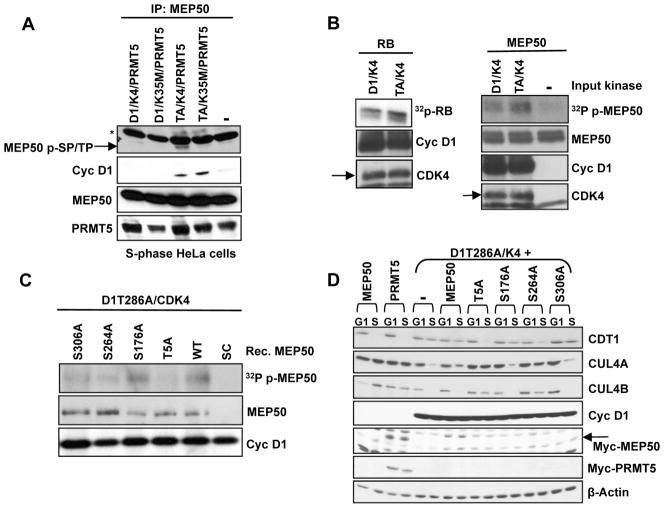
We next considered whether either MEP50 or PRMT5 might be a cyclin D1/CDK4 substrate. PRMT5/MEP50 complexes were precipitated from HeLa cells expressing cyclin D1 or cyclin D1T286A along with either CDK4 or CDK4(K35M) with a MEP50 antibody. Upon immunoblotting with an antibody detecting phosphorylated serine or threonine when either residue is followed by a proline, we noted a band in cells expressing cyclin D1T286A/CDK4 that migrated with the expected mobility of MEP50 (Figure 4A, noted by arrow). No such band was apparent in cyclin D1 expressing cells or in cyclin D1T286A/CDK4(K35M) expressing cells. In addition, we did not detect any apparent p-SP/TP signal corresponding to PRMT5.
Figure 4. Cyclin D1/CDK4 kinase phosphorylates MEP50 in vitro and in vivo.
(A) MEP50 was precipitated from HeLa cells transfected with vectors encoding cyclin D1, D1T286A along with either CDK4 or kinase dead CDK4(K35M) and synchronized in S-phase by sequential nocodazole and HU treatment. MEP50 phosphorylation was assessed using a commercially available phospho-SP/TP antibody. The arrow indicates the mobility of phospho-MEP50, which migrates faster than the (*) indicated non-specific immunoreactive band. Total MEP50, PRMT5 and cyclin D1 present in immune complexes were assessed using appropriate antibodies. (B) Phosphorylation of recombinant MEP50 or RB by cyclin D1/CDK4 or D1T286A/CDK4. Substrate phosphorylation was assessed by SDS-PAGE followed by autoradiography. (C) Cyclin Cyclin D1T286A/CDK4 phosphorylation of MEP50 at Thr-5, Ser-264 and Ser-306 in vitro. (D) Acute expression of MEP50 mutants Thr-5A and Ser-264A inhibit cyclin D1T286A/CDK4-dependent repression of CUL4 and CDT1 stabilization. See also Figure S4.
MEP50 contains four putative CDK phosphorylation sites: Thr-5, Ser-176, Ser-264, and Ser-306 (Figure S4A). Consistent with MEP50 being a direct substrate, recombinant MEP50 was phosphorylated by cyclin D1/CDK4 and D1T286A/CDK4 in vitro (Figure 4B; S4B). To identify phosphorylated residues, recombinant MEP50 was phosphorylated in vitro with purified cyclin D1T286A/CDK4 and subjected to mass spectometry; we also affinity purified MEP50 from cells co-expressing cyclin D1T286A/CDK4 for the same analysis. Both approaches revealed phosphorylation of MEP50 at Thr-5 (Figure S4C–D). We generated recombinant MEP50 proteins harboring alanine substitutions at all four putative CDK phosphorylation sites and used these as substrates. Mutation of Thr-5 dramatically reduced phosphate incorporation; mutation of Ser-264 and Ser-306 also resulted in a moderate reduction while Ser-176A had no apparent effect (Figure 4C).
If CUL4 repression and CDT1 stabilization depends upon MEP50 phosphorylation by cyclin D1T286A/CDK4, we reasoned that overexpression of key non-phosphorylatable MEP50 mutants should be inhibitory. Indeed, expression of myc-tagged MEP50-T5A and to a lesser degree MEP50-S264A interfered with cyclin D1T286A-dependent CDT1 stabilization and CUL4 loss during S-phase (Figure 4D). Collectively, these results reveal that MEP50 is a CDK4 substrate and that phosphorylation of MEP50 predominantly on Thr-5 dominantly mediates downstream function.
Cyclin D1T286A/CDK4 regulates PRMT5 methyltransferase activity via MEP50 phosphorylation
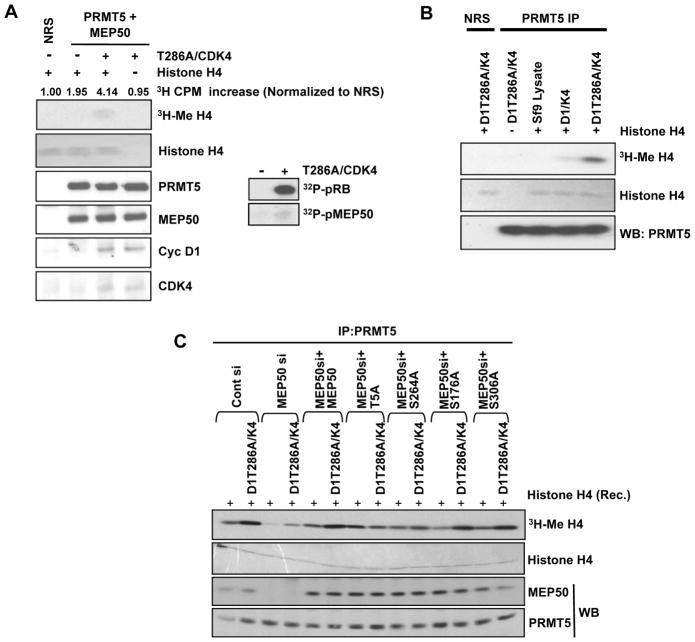
To determine whether phosphorylation of MEP50 can directly regulate PRMT5 methyltransferase activity independent of association with high molecular weight chromatin remodeling complexes, coupled in vitro kinase/methyltransferase reactions were performed with purified recombinant PRMT5/MEP50 produced in Sf9 cells. PRMT5-dependent methyltransferase activity was increased following a kinase reaction with purified cyclin D1T286A/CDK4 (Figure 5A). Additionally, increased PRMT5/MEP50 SAM-dependent methyltransferase activity directed towards histone H4 was readily apparent following incubation of PRMT5/MEP50 complexes purified from HeLa cells with cyclin D1T286A/CDK4 and to a lesser degree with cyclin D1/CDK4 (Figure 5B). To evaluate the role of MEP50 phosphorylation, we expressed either wild type MEP50 or mutant MEP50 alleles in cells treated with MEP50 siRNA. Expression of MEP50-T5A maintained basal methyltransferase activity in vitro, but was refractory to the CDK4-dependent increase of PRMT5 activation (Figure 5C). Serine-264 to alanine substitution also attenuated the response to cyclin D1T286A/CDK4 whereas alanine substitution at 176/306 was without influence. These results demonstrate that cyclin D1/CDK4 can increase histone methyltransferase activity of PRMT5/MEP50 through phosphorylation of Thr-5 and perhaps through Ser-264.
Figure 5. Cyclin D1T286A/CDK4 increases PRMT5 methyltransferase activity through MEP50.
(A, B) Purified cyclin D1/CDK4 and D1T286A/CDK4 kinases increase catalytic activity of PRMT5/MEP50 in vitro. Purified cyclin/CDK4 complexes from Sf9 cells were mixed with purified recombinant PRMT5/MEP50 produced in Sf9 cells (A) or PRMT5 complexes purified from HeLa cells (B) and incubated for 30 min with ATP at 30°C. PRMT5 complexes were washed and methyltransferase activity assessed using 3H-Me (SAM) and recombinant Histone H4. (C) Same as (B), except PRMT5 was purified from HeLa cells following MEP50 knockdown and re-expression of the indicated MEP50 mutants.
PRMT5/MEP50 mediates cyclin D1T286A-dependent re-replication, transformation and survival of cyclin D1T286A expressing lymphoma cells
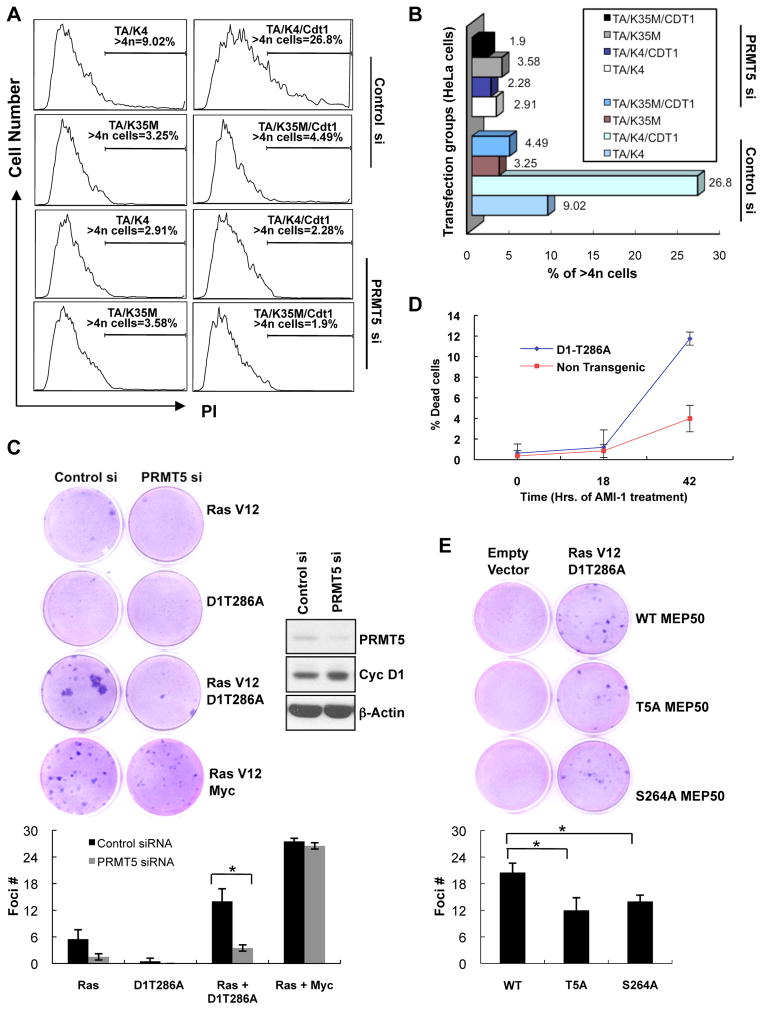
Cyclin D1T286A/CDK4 expression induces re-replication in a CDT1-dependent fashion (Aggarwal et al., 2007). If PRMT5/MEP50 mediates re-replication, knockdown of PRMT5 should be inhibitory. Consistent with this hypothesis, we observed a dramatic reduction of >4N population from 26.8% to 2.28% with PRMT5 knockdown in T286A/CDK4/CDT1 transfected HeLa cells (Figure 6A–B).
Figure 6. PRMT5 knockdown inhibits cyclin D1T286A-dependent DNA re-replication, cell transformation and increases death of tumor cells.
(A) Following transfection of HeLa cells with the indicated expression plasmids, TA (D1T286A), K4 (CDK4), K35M (CDK4(K35M)), CDT1, cell cycle profile was assessed by FACS after PI staining. (B) Graphical representation of the flow cytometry data from (A). The bars show the percentage of cells showing >4N DNA content. (C) Foci formation assay was performed by concurrent knockdown of murine PRMT5 and overexpression of the indicated proteins in NIH3T3 cells. Cells were grown for 14 days and stained with Giemsa to visualize foci. Quantification of data has been shown. Error bars represent ±SD and * indicates p-value < 0.05. (D) Single cell suspensions of splenocytes prepared from non-transgenic or malignant Eμ-D1T286A transgenic tumor burdened spleens were treated with 200μM arginine methyltransferase inhibitor AMI-1. The cells were analyzed for the inhibitor sensitivity by measuring the % cell death via AnnexinV –PI staining/flow cytometry. Values represent the mean of three independent experiments with error bars indicating ±SD. (E) Foci formation assay was performed by transfection of the indicated MEP50 constructs along with empty vector control or RasV12 plus cyclin D1T286A in NIH 3T3 cells. Cells were grown for 14 days and foci were analyzed as in (C). See also Figure S5.
We next determined whether PRMT5 mediates cyclin D1T286A-dependent transformation by knockdown of PRMT5 in murine fibroblasts, concurrent with expression of oncogenic RasV12 and cyclin D1T286A. Indeed, while co-transfection of both RasV12 and cyclin D1T286A induced focus formation (Figure 6C), foci number was reduced by 50% following knockdown of PRMT5. PRMT5 knockdown was not accompanied by cell cycle arrest (Figure S5A) suggesting that foci reduction is not a consequence of cell cycle arrest. In addition, PRMT5 knockdown did not significantly attenuate transformation mediated by Ras plus c-Myc, suggesting mechanistic specificity between PRMT5-dependent methyltransferase activity and constitutively nuclear cyclin D1 (Figure 6C).
To investigate therapeutic potential of targeting PRMT5, we determined whether inhibition of PRMT5 would compromise survival of primary Eμ-D1T286A lymphoma cells ex vivo. Because we were unable to efficiently introduce siRNA into these primary tumor cells, we utilized AMI-1. AMI-1 treatment increased death of tumor cells relative to normal controls (Figure 6D). In addition, treatment of primary lymphoma cells with MTA also triggered increased cell death relative to untreated tumor cells or MTA treated control lymphocytes (Figure S5B). Together, these observations suggest that PRMT5 plays an important role in promoting cyclin D1T286A/CDK4-dependent DNA re-replication, cell transformation and survival of cells harboring constitutively active cyclin D1/CDK4.
To examine the role of MEP50 phosphorylation in cyclin D1T286A-dependent transformation, wild type or phosphorylation-deficient T5A or S264A mutant MEP50 was co-expressed with RasV12 and cyclin D1T286A in NIH3T3 cells. Compared to wild type MEP50, expression of the T5A or S264A MEP50 mutant significantly reduced foci number, suggesting that phosphorylation of MEP50 and subsequent increase in PRMT5 activity is a driving force for cellular transformation in the presence of cyclin D1T286A (Figure 6E).
Fbx4 inactivation promotes increased PRMT5-dependent histone methylation
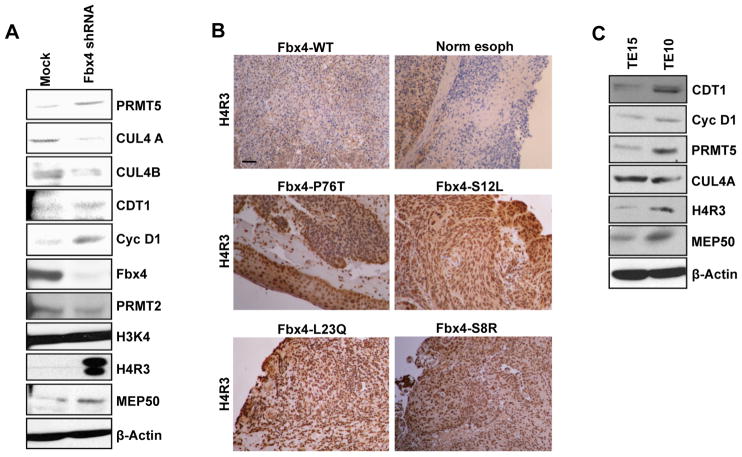
While mutations directly targeting cyclin D1 do occur in human cancer (Benzeno et al., 2006), they occur infrequently relative to inactivation of the cyclin D1 E3 ligase (Barbash et al., 2008). Because inactivation of Fbx4 also drives nuclear accumulation of cyclin D1 kinase during S-phase, we postulated that Fbx4 loss should also impact PRMT5 function. We initially assessed this hypothesis in NIH3T3 cells harboring Fbx4 shRNA (Lin et al., 2006). Fbx4 knockdown increased cyclin D1 and CDT1 and decreased CUL4A/B; critically, the level of H4R3 methylation was dramatically increased (Figure 7A). Previous work identified esophageal tumors harboring inactivating mutations in Fbx4 that resulted in strong cyclin D1 overexpression (Barbash et al., 2008). We chose these same tumors to ascertain whether loss of Fbx4 function and overexpression of wild type cyclin D1 kinase also correlated with increased H4R3 methylation. Immunohistochemical staining on tumor sections revealed a marked increase in H4R3 methylation relative to an esophageal tumor harboring wild type Fbx4 or normal esophageal epithelium (Figure 7B). Because our analysis was restricted to tumors with known Fbx4 mutations and tissue availability for IHC (n=4), our analysis did not achieve statistical significance. To support this analysis, we analyzed an esophageal tumor cell line harboring a mutant Fbx4, Fbx4(S8R) (TE10; Barbash et al., 2008) and another expressing both wild type Fbx4 and cyclin D1 (TE15) and found that inactive Fbx4 correlates with increased CDT1 and methylated H4R3 (Figure 7C). Together, these observations demonstrate that stabilized nuclear cyclin D1 resulting from either cyclin D1 mutation or inactivation of Fbx4 results in increased PRMT5-dependent histone methylation.
Figure 7. Knockdown of Fbx4 stabilizes nuclear cyclin D1 resulting in increased PRMT5-dependent histone methylation.
(A) Direct western analysis of the protein lysates prepared from control NIH3T3 or NIH3T3 harboring Fbx4 knockdown. (B) Representative immunohistochemistry images showing di-methyl H4R3 staining of human esophageal tumor sections. Scale bar = 100μm. (C) Direct western analysis of the protein lysates prepared from TE15 and TE10 cell lines.
Discussion
Cyclin D1 is subject to intricate post-translational control to ensure its temporal regulation. Cyclin D1 nuclear export followed by Fbx4-dependent ubiquitylation and degradation during S-phase are key features that serve to restrain nuclear cyclin D1/CDK4 activity and ensure normal cell division (Alt et al., 2000; Benzeno et al., 2006; Lin et al., 2006). Previous work revealed that aberrant nuclear accumulation of cyclin D1 during S-phase promotes transformation in vitro (Alt et al., 2000) and drives both B-cell lymphomas and mammary carcinomas in mice (Gladden et al., 2006; Lin et al., 2008). Molecular analysis of these tumors revealed a potential mechanism wherein the cyclin D1-dependent kinase interferes with CUL4-dependent CDT1 proteolysis during S-phase (Higa et al., 2006; Sansam et al., 2006), due to transcriptional repression of CUL4A and CUL4B; the resulting overexpression of CDT1 during S-phase triggers DNA re-replication, enhancing genomic instability and the acquisition of fortuitous mutations (Aggarwal et al., 2007). A significant feature of the regulatory pathway leading to loss of CUL4 expression is the noted dependence upon cyclin D1/CDK4 activity. The current work revealing that phosphorylation of MEP50 on threonine 5 by cyclin D1T286A/CDK4 mediates PRMT5-dependent transcriptional repression provides key mechanistic insights into the neoplastic activities of the cyclin D1/CDK4 enzyme.
While PRMT5/MEP50 was identified through affinity purification of D1T286A complexes, it is the demonstration that MEP50 is a direct substrate for D-type cyclin dependent kinase that contributes a key advance in our understanding of cyclin D1-driven tumorigenesis. Phosphorylation of MEP50 on Thr-5 by D1T286A/CDK4 is necessary and sufficient to increase the intrinsic methyltransferase activity of PRMT5, resulting in increased H4R3/H3R8 methylation and repression of the CUL4A/B expression. Notably, although we did not detect phosphorylation of Ser-264 in vivo, mutation of Ser-264 to alanine in MEP50 reduced cyclin D1-dependent induction of PRMT5 activity, suggesting that phosphorylation of this site may also contribute. The fact that inhibition of PRMT5/MEP50 activity by siRNA or overexpression of non-phosphorylatable MEP50 attenuates CUL4 repression is consistent with a model where PRMT5/MEP50 represents a key molecular target of nuclear cyclin D1/CDK4 during S-phase.
Regulation of PRMT5 by the cyclin D1-dependent kinase
PRMT5 methylates arginines in multiple proteins including myelin basic protein (Ghosh et al., 1988), SM proteins (Friesen et al., 2001) and p53 (Jansson et al., 2008). However, it is PRMT5-dependent histone di-methylation that is associated with transcriptional repression. Of the eleven PRMTs, PRMT5 is closely associated with transcriptional repression (Pal and Sif, 2007; Pal et al., 2004); its repressive function is attributed to symmetric di-methylation of histone 4 (arginine 3) and histone 3 (arginine 8) (Krause et al., 2007). These histone modifications were recently linked with the subsequent recruitment of DNMT3, linking histone methylation with DNA methylation (Zhao et al., 2009).
Regulation of PRMT5 function remains poorly understood and modification of PRMT5 by phosphorylation, acetylation or ubiquitylation has not been reported. In contrast, association of PRMT5 with MEP50 is an activating event (Krause et al., 2007). In addition, association of COPR5 with PRMT5 changes the balance of activity from H3R8 towards H4R3 (Lacroix et al., 2008). While PRMT5 is not detectably modified in a CDK4-dependent manner, a multiplicity of approaches revealed MEP50 to be a direct substrate. The data provided demonstrate that phosphorylation of Thr-5, and perhaps Ser-264, of MEP50 contributes to increased PRMT5/MEP50 methyltransferase activity in cells harboring nuclear cyclin D1/CDK4. As addition of purified MEP50 to recombinant PRMT5 is sufficient to activate PRMT5 methyltransferase function (Krause et al., 2007), the most direct model is that cyclin D1-dependent phosphorylation functions as a switch to facilitate MEP50-dependent, structural alterations in PRMT5 that contribute to enhanced catalysis or perhaps increased affinity for substrates which translates into more efficient methylation.
Transcriptional regulation of CUL4A/B by cyclin D1
Previous work demonstrated that nuclear, active cyclin D1/CDK4 during S-phase leads to reduced CUL4A/B expression (Aggarwal et al., 2007). While the underlying mechanism remained elusive, a large body of work associating cyclin D1 with transcriptional regulation suggested that regulation could be direct. For example, cyclin D1 was first noted to associate with the estrogen receptor (ER) to coordinate ligand-independent expression of ER targets independent of CDK activation (Lamb et al., 2000; McMahon et al., 1999; Zwijsen et al., 1998; Zwijsen et al., 1997). Subsequently, cyclin D1 was shown to bind and regulate the activities of androgen receptor (Knudsen et al., 1999), DMP1 (Hirai and Sherr, 1996), and C/EBPβ (Lamb et al., 2003). Our current work demonstrates that regulation reflects CDK4-dependent phosphorylation of MEP50, which in turn increases PRMT5-dependent histone methylation of target genes, reducing their expression. While previous work supports a model where cyclin D1 functions as a molecular bridge between DNA-bound transcription factors and co-activators/repressors, our data reveal not only that cyclin D1 occupies promoter regions of a target gene, but also that regulation reflects CDK-dependent phosphorylation of a recruited epigenetic regulatory enzyme. Consistently, recent work utilizing genome-wide ChIP analysis demonstrated that cyclin D1 is enriched in regions near the transcriptional start site of a large number of genes (Bienvenu et al., 2010), providing additional evidence for cyclin D1 directly regulating gene expression. It is intriguing to note that the strongest H4R3 methylation occurs near the predicted transcription start site of both CUL4A and CUL4B.
The role of CDK4, while perhaps unexpected in the context of transcription, is not totally unexpected in the context of tumorigenesis given that CDK activation is central to the function of cyclin D1 during neoplastic transformation. That MEP50 is a substrate for cyclin D1/CDK4 makes the PRMT5/MEP50 complex one of a limited number of cyclin D1-dependent substrates. Previously, only the RB family members (Kato et al., 1993) and Smad3, a key mediator for TGF-β anti-proliferative responses (Liu and Matsuura, 2005; Matsuura et al., 2004), have been established as substrates in vitro and in vivo.
While increased cyclin D1T286A/CDK4-dependent PRMT5/MEP50 function is required for CUL4 loss and CDT1 overexpression, we cannot rule out the contribution of additional mechanisms that contribute to CUL4 regulation. For example, neither cyclin D1 nor PRMT5/MEP50 has intrinsic DNA binding properties. Thus, it remains unclear how cyclin D1T286A/CDK4 promotes the targeting to this locus. In addition, we questioned the possibility that cyclin D1T286A might result in the recruitment of histone deacetylases and thereby impact CUL4 expression. Our results indicated that HDAC inhibition resulted in increased CUL4 expression regardless of cyclin D1T286A/CDK4 suggesting a non-specific effect (Figure S2G–H).
Signaling through PRMT5/MEP50 is not limited to lymphoid tumors harboring mutant cyclin D1 alleles
Cyclin D1T286A/CDK4 increases PRMT5 methyltransferase activity in vivo through MEP50, revealing a pathway where PRMT5 facilitates cyclin D1T286A/CDK4 dependent re-replication and survival of murine tumors harboring a lymphoid-specific D1T286A transgene. However, it is important to note that this mechanism is not restricted to this model system or this particular cyclin D1 allele for the following reasons. First, knockdown of Fbx4, the specificity component of the cyclin D1 E3 ligase, stabilizes nuclear cyclin D1/CDK4 complexes in the nucleus during S-phase and thereby increases PRMT5-dependent histone methylation resulting in CUL4 loss. Second, human tumors harboring inactivating mutations in Fbx4 and subsequent nuclear accumulation of endogenous cyclin D1-dependent kinase, exhibit a dramatic increase in H4R3 methylation. Finally, cyclin D1/CDK4-MEP50/PRMT5 complexes are observed in esophageal carcinoma cell lines harboring cyclin D1P287A. These independent findings implicate aberrant nuclear cyclin D1 in transcriptional repression of CUL4.
Collectively, our current work supports a model wherein accumulation of nuclear cyclin D1/CDK4 during S-phase triggers increased PRMT5/MEP50 activity, thereby specifically reducing CUL4 expression (and triggering downstream stabilization of CUL4 ligase targets such as CDT1). Our functional analysis revealed that PRMT5 is necessary for cyclin D1-mediated cell transformation and further that tumors harboring dysregulated cyclin D1 exhibit increased PRMT5-dependent histone methylation. Future efforts to determine the global gene expression pattern altered through cyclin D1-dependent regulation of PRMT5 and which of these specifically contribute to neoplastic growth will be of significant importance.
Experimental Procedures
Cell Culture, Transfections and Plasmids
Cell culture conditions and transfections were performed as previously noted (Aggarwal et al., 2007). The human PRMT5 Myc-tagged vector was generated by PCR using primers designed for directional cloning into pCS2-MT plasmid (in frame with six Myc epitope tags at the N terminus of PRMT5), using PRMT5 cDNA (Open Biosystems) as the template. Human GST-tagged MEP50 vector was generated by PCR of human MEP50 with primers designed for directional cloning into pGEX-4T-1 plasmid (in frame with GST tag at the N terminus of MEP50), using pOTB7-MEP50 (Open Biosystems) as the template. Human MEP50 Myc-tagged vector was generated by PCR of human MEP50 with primers designed for directional cloning into pcDNA3 plasmid (in frame with 1X Myc tag at the N terminus of MEP50). Site directed mutagenesis was performed using QuikChange Site-Directed Mutagenesis Kit (Stratagene) according to the manufacturer’s instructions. All clones were sequenced in their entirety.
Chromatin Immunoprecipitation Assay
For ChIP, cells were cross-linked in 0.75% formaldehyde for 10 min and quenched with 0.18mM glycine for 5 min. Cells were harvested in cell lysis buffer (50mM HEPES, pH 7.5, 140mM NaCl, 1mM EDTA pH8.0, 1.0% Triton-X-100, 0.1% SDS, 0.1% deoxycholate, fresh protease inhibitors) and chromatin sheared to an average size of 350–600 bp by sonication (3×10min cycles of 30×30 sec each) at constant output on high setting (Bioruptor). Sonicated DNA was diluted 3-fold in dilution buffer (20mM Tris-HCl pH 8.0, 2mM EDTA pH 8.0, 1% Triton X-100, 150mM NaCl, protease inhibitors) and pre-cleared with protein A plus-agarose (Millipore). Lysates were rotated at 4°C overnight with 5μg of polyclonal antibodies specific for H4R3 (Abcam) or H3R8 anti-sera as described below, PRMT5 (Abcam), M2 monoclonal, (Sigma), Brg1 (Santa Cruz) or normal rabbit IgG (Cell Signaling). After washing, complexes were eluted in 130μl of Elution buffer (1% SDS + 100mM NaHCO3), followed by addition of 100μg of proteinase K at 65°C overnight for cross-link reversal. The DNA was purified by phenol-chloroform extraction and precipitated DNA was analyzed by qPCR. The primers used for CUL4A and CUL4B have been described in Figure S3D.
Immunoprecipitation and Immunoblot Analysis
Cells were harvested in Tween-20 buffer (50mM HEPES (pH 8.0), 150mM NaCl, 2.5mM EGTA, 1mM EDTA, 0.1% Tween 20, protease, and phosphatase inhibitors (1mM PMSF, 20 U/ml aprotinin, 5μg/ml leupeptin, 1mM DTT, 0.4μM NaF, and 10μM β-glycerophosphate), and protein concentration of samples was determined by BCA assay. Cyclin D1, PRMT5 and MEP50 were precipitated using M2 anti-flag agarose (to recognize flag-tagged proteins, Sigma-Aldrich), PRMT5 rabbit polyclonal antibody (Abcam) or c-myc (9E10) and MEP50 rabbit polyclonal antibody (Bethyl Lab. Inc) respectively. Proteins were resolved by SDS-PAGE, transferred to nitrocellulose membranes, and analyzed by immunoblotting. Antibodies used in these studies were as follows: Fbx4 rabbit polyclonal antibody (Rockland Immunochemicals), cyclin D1 mouse monoclonal antibody D1-72-13G, cyclin D1 mouse anti-human (Calbiochem); β-actin mouse monoclonal (Sigma-Aldrich); Cul4A rabbit polyclonal (Bethyl Lab. Inc); Cul4B rabbit polyclonal (ProteinTech Group Inc); pSP/TP mouse monoclonal (16B4, Biomol); CDK4 (C-22 or H-22), Cdt1, Brg1, RB, PRMT5 mouse monoclonal, PRMT2, mSin3a (Santa Cruz); H4R3 rabbit polyclonal-ChIP grade, H3K4 rabbit polyclonal, Histone H3 rabbit polyclonal, PRMT1 and PRMT7, Histone H4 rabbit polyclonal (Abcam). Anti-H3R8 (corresponding to symmetric di-methylated R8 of human histone H3) antibody was generated by immunizing rabbits with peptide containing modified histone H3 (YenZym Antibodies, LLC) followed by affinity purification. Antibody binding was visualized by chemiluminescence (Perkin Elmer). Figure S3A–C reveals the specificity of this antibody.
Immunohistochemistry
Human esophageal tumors harboring Fbx4 mutations and normal esophageal tissue were obtained with informed consent and with Institutional Review Board approval from the University of Pennsylvania (IRB #803902), Fox Chase Cancer Center (IRB #98-53), and University of Fukui, Japan (IRB #H190228-155). Tissue was fixed in 10% buffered formalin and subsequently dehydrated, paraffin embedded and sectioned. Tissue sections were immunostained as described previously (Aggarwal et al., 2007), using rabbit polyclonal H4R3 (Abcam) at 1:500 dilution as the primary antibody.
In vitro kinase assay and in vitro methyltransferase assay
Purified GST-MEP50 wild type, GST-T5A, S176A, S264A, S306A mutants and GST-RB were used as substrates for in vitro kinase reactions (Matsushime et al., 1994). Cyclin D1/CDK4 kinase complexes were purified from Sf9 cells. For assessment of PRMT5 methyltransferase activity, PRMT5 complexes were collected from the indicated sources by immune precipitation. Beads were washed in Tween-20 buffer followed by methyltransferase buffer (15mM HEPES (pH7.9), 100mM KCl, 5mM MgCl2, 20% Glycerol, 1mM EDTA, 0.25mM DTT and 0.5mM PMSF). The methylation reaction included PRMT5 immune complexes on beads, 1μg recombinant Histone H4 (NEB), and 2.75μCi S-adenosyl-L-(methyl-3H)methionine (Amersham Pharmacia) in a total volume of 25μl for 1.5 hours at 30°C. The reaction mixture was resolved on a SDS-polyacrylamide gel, and modified histone H4 was detected by fluorography. For the coupled kinase assay/methyltransferase assay, cyclin complexes were purified from Sf9 cells and mixed with purified recombinant PRMT5/MEP50 complex (produced in Sf9 cells, BPS Bioscience) or PRMT5 immune precipitated from HeLa cells immobilized on beads. In vitro kinase assays were performed for 30 minutes at 30°C in kinase buffer. PRMT5-containing beads were washed into methyltransferase buffer, followed by in vitro methylation reaction for 2 hours at 30°C with 2μg H4 substrate and 2.75μCi 3H-SAM. Methylated H4 was visualized by fluorography or scintillation counting for 3H incorporation.
Foci Formation
Low passage NIH3T3 cells were transfected with control or murine PRMT5 siRNA (Dharmacon) using HiPerfect (Qiagen). 24 hours post siRNA delivery, cells were transfected with 2ug of the indicated plasmids (pBabe Ras V12 or pBabe cyclin D1T286A) or empty vector (pBabe puro) using Lipofectamine/Plus Reagent (Invitrogen). Transfected cells were counted after 48 hours, and 2×105 cells were plated in duplicate on 35mm plates in DMEM containing 5% FBS. Cells were re-fed with DMEM plus 5% FBS every 2 days for 14 days, followed by staining with Giemsa to visualize foci formation. Foci were counted for duplicate plates and error bars represent ± standard deviation and * indicates p-value < 0.05, as determined by student’s t-test. For MEP50 overexpression assays, NIH3T3 cells were transfected with 2ug of wt MEP50 or phosphorylation-deficient MEP50 mutant T5A or S264A, along with empty vector or Ras V12 and cyclin D1T286A constructs as indicated. Cells were seeded and foci formation was analyzed as described above.
Real time quantitative PCR analysis of gene expression
RNA isolation was performed using standard protocols. DNA fragments were generated by reverse transcriptase PCR (RT-PCR; Superscript™, Invitrogen). Mixed primer/probe sets for human CUL4A/B and 18S rRNA were used to measure the levels of these transcripts using the Applied Biosystems 7900HT sequence detection system according to the manufacturer’s instructions. Primers used for detection of human CUL4A and CUL4B were described previously (Aggarwal et al., 2007).
siRNA and Methyltransferase Inhibitor experiments
siGENOME SMARTpool targeting PRMT5 and MEP50 or control siRNA were purchased from Dharmacon. HeLa cells were treated with indicated siRNA or methyltransferase inhibitors, MTA (Sigma) or AMI-1 (Calbiochem), for 24 hours followed by transfection and synchronization of cells in G1 and S-phases. For targeting 3′ UTR region of human MEP50, siRNA (5′ CUCCUUACCAUUAAACUGA 3′) was designed and purchased from Sigma.
Highlights.
Nuclear cyclin D1 accumulation promotes increased PRMT5 methyltransferase activity
Cyclin D1/CDK4 phosphorylates the PRMT5 co-factor MEP50
PRMT5 mediates cyclin D1-dependent transcriptional repression of CUL4A/B
PRMT5 inhibition reduces survival of cyclin D1-driven lymphoma cells
Significance.
Cyclin D1 contributes to cell growth through phosphorylation of the retinoblastoma protein and direct association with transcriptional regulators. Our work demonstrates that direct regulation of critical target genes associated with neoplastic transformation depends upon cyclin D1 activation of CDK4. The identification of a CDK4 substrate that contributes to epigenetic regulation of target genes associated with cancer establishes a paradigm where cyclin D1 regulation of gene expression occurs independently of RB, yet remains kinase-dependent. The association of cyclin D1 with transcriptional repression in concert with the repressive nature of PRMT5-dependent histone methylation supports a model wherein PRMT5/MEP50 is one key effector of cyclin D1-dependent gene expression during neoplastic growth.
Supplementary Material
Acknowledgments
The authors wish to thank Margarita Romero for excellent technical assistance and Chao Lu for assistance in profiling the H3R8 antibody. This work was supported by grants from the NIH (CA93237) and a Leukemia & Lymphoma Scholar award (JAD); P01-CA098101 (AKR; MH; JAD); CA090465, CA098172 and funds from the Commonwealth Universal Research Enhancement Program, Pennsylvania Department of Health (SBM). Support was also provided by the Morphology Core Facility (S. Mitchell).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggarwal P, Lessie MD, Lin DI, Pontano L, Gladden AB, Nuskey B, Goradia A, Wasik MA, Klein-Szanto AJ, Rustgi AK, et al. Nuclear accumulation of cyclin D1 during S phase inhibits Cul4-dependent Cdt1 proteolysis and triggers p53-dependent DNA rereplication. Genes Dev. 2007;21:2908–2922. doi: 10.1101/gad.1586007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alt JR, Cleveland JL, Hannink M, Diehl JA. Phosphorylation-dependent regulation of cyclin D1 nuclear export and cyclin D1-dependent cellular transformation. Genes Dev. 2000;14:3102–3114. doi: 10.1101/gad.854900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bani-Hani K, Martin IG, Hardie LJ, Mapstone N, Briggs JA, Forman D, Wild CP. Prospective study of cyclin D1 overexpression in Barrett’s esophagus: association with increased risk of adenocarcinoma. J Natl Cancer Inst. 2000;92:1316–1321. doi: 10.1093/jnci/92.16.1316. [DOI] [PubMed] [Google Scholar]
- Barbash O, Zamfirova P, Lin DI, Chen X, Yang K, Nakagawa H, Lu F, Rustgi AK, Diehl JA. Mutations in Fbx4 inhibit dimerization of the SCF(Fbx4) ligase and contribute to cyclin D1 overexpression in human cancer. Cancer Cell. 2008;14:68–78. doi: 10.1016/j.ccr.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartkova J, Lukas J, Muller H, Lutzhoft D, Strauss M, Bartek J. Cyclin D1 protein expression and function in human breast cancer. Int J Cancer. 1994a;57:353–361. doi: 10.1002/ijc.2910570311. [DOI] [PubMed] [Google Scholar]
- Bartkova J, Lukas J, Muller H, Strauss M, Gusterson B, Bartek J. Abnormal patterns of D-type cyclin expression and G1 regulation in human head and neck cancer. Cancer Res. 1995;55:949–956. [PubMed] [Google Scholar]
- Bartkova J, Lukas J, Strauss M, Bartek J. The PRAD-1/cyclin D1 oncogene product accumulates aberrantly in a subset of colorectal carcinomas. Int J Cancer. 1994b;58:568–573. doi: 10.1002/ijc.2910580420. [DOI] [PubMed] [Google Scholar]
- Benzeno S, Lu F, Guo M, Barbash O, Zhang F, Herman JG, Klein PS, Rustgi A, Diehl JA. Identification of mutations that disrupt phosphorylation-dependent nuclear export of cyclin D1. Oncogene. 2006;25:6291–6303. doi: 10.1038/sj.onc.1209644. [DOI] [PubMed] [Google Scholar]
- Bienvenu F, Jirawatnotai S, Elias JE, Meyer CA, Mizeracka K, Marson A, Frampton GM, Cole MF, Odom DT, Odajima J, et al. Transcriptional role of cyclin D1 in development revealed by a genetic-proteomic screen. Nature. 2010;463:374–378. doi: 10.1038/nature08684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng D, Yadav N, King RW, Swanson MS, Weinstein EJ, Bedford MT. Small molecule regulators of protein arginine methyltransferases. J Biol Chem. 2004;279:23892–23899. doi: 10.1074/jbc.M401853200. [DOI] [PubMed] [Google Scholar]
- Diehl JA. Cycling to Cancer with Cyclin Dl. Cancer Biol Ther. 2002;1:226–231. doi: 10.4161/cbt.72. [DOI] [PubMed] [Google Scholar]
- Friesen WJ, Paushkin S, Wyce A, Massenet S, Pesiridis GS, Van Duyne G, Rappsilber J, Mann M, Dreyfuss G. The methylosome, a 20S complex containing JBP1 and pICln, produces dimethylarginine-modified Sm proteins. Mol Cell Biol. 2001;21:8289–8300. doi: 10.1128/MCB.21.24.8289-8300.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh SK, Paik WK, Kim S. Purification and molecular identification of two protein methylases I from calf brain. Myelin basic protein- and histone-specific enzyme. J Biol Chem. 1988;263:19024–19033. [PubMed] [Google Scholar]
- Gillett C, Fantl V, Smith R, Fisher C, Bartek J, Dickson C, Barnes D, Peters G. Amplification and overexpression of cyclin D1 in breast cancer detected by immunohistochemical staining. Cancer Res. 1994;54:1812–1817. [PubMed] [Google Scholar]
- Gladden AB, Woolery R, Aggarwal P, Wasik MA, Diehl JA. Expression of constitutively nuclear cyclin D1 in murine lymphocytes induces B-cell lymphoma. Oncogene. 2006;25:998–1007. doi: 10.1038/sj.onc.1209147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JG, Merlo A, Mao L, Lapidus RG, Issa JP, Davidson NE, Sidransky D, Baylin SB. Inactivation of the CDKN2/p16/MTS1 gene is frequently associated with aberrant DNA methylation in all common human cancers. Cancer Res. 1995;55:4525–4530. [PubMed] [Google Scholar]
- Hibberts NA, Simpson DJ, Bicknell JE, Broome JC, Hoban PR, Clayton RN, Farrell WE. Analysis of cyclin D1 (CCND1) allelic imbalance and overexpression in sporadic human pituitary tumors. Clin Cancer Res. 1999;5:2133–2139. [PubMed] [Google Scholar]
- Higa LA, Banks D, Wu M, Kobayashi R, Sun H, Zhang H. L2DTL/CDT2 interacts with the CUL4/DDB1 complex and PCNA and regulates CDT1 proteolysis in response to DNA damage. Cell Cycle. 2006;5:1675–1680. doi: 10.4161/cc.5.15.3149. [DOI] [PubMed] [Google Scholar]
- Hirai H, Sherr CJ. Interaction of D-type cyclins with a novel myb-like transcription factor, DMP1. Mol Cell Biol. 1996;16:6457–6467. doi: 10.1128/mcb.16.11.6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa Y, Arnold A. Mechanism of cyclin D1 (CCND1, PRAD1) overexpression in human cancer cells: analysis of allele-specific expression. Genes Chromosomes Cancer. 1998;22:66–71. doi: 10.1002/(sici)1098-2264(199805)22:1<66::aid-gcc9>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Hosokawa Y, Joh T, Maeda Y, Arnold A, Seto M. Cyclin D1/PRAD1/BCL-1 alternative transcript [B] protein product in B-lymphoid malignancies with t(11;14)(q13;q32) translocation. Int J Cancer. 1999;81:616–619. doi: 10.1002/(sici)1097-0215(19990517)81:4<616::aid-ijc18>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Hou Z, Peng H, Ayyanathan K, Yan KP, Langer EM, Longmore GD, Rauscher FJ., 3rd The LIM protein AJUBA recruits protein arginine methyltransferase 5 to mediate SNAIL-dependent transcriptional repression. Mol Cell Biol. 2008;28:3198–3207. doi: 10.1128/MCB.01435-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki H, Yada T. Protein arginine methylation regulates insulin signaling in L6 skeletal muscle cells. Biochem Biophys Res Commun. 2007;364:1015–1021. doi: 10.1016/j.bbrc.2007.10.113. [DOI] [PubMed] [Google Scholar]
- Jansson M, Durant ST, Cho EC, Sheahan S, Edelmann M, Kessler B, La Thangue NB. Arginine methylation regulates the p53 response. Nat Cell Biol. 2008;10:1431–1439. doi: 10.1038/ncb1802. [DOI] [PubMed] [Google Scholar]
- Jin M, Inoue S, Umemura T, Moriya J, Arakawa M, Nagashima K, Kato H. Cyclin D1, p16 and retinoblastoma gene product expression as a predictor for prognosis in non-small cell lung cancer at stages I and II. Lung Cancer. 2001;34:207–218. doi: 10.1016/s0169-5002(01)00225-2. [DOI] [PubMed] [Google Scholar]
- Kato J, Matsushime H, Hiebert SW, Ewen ME, Sherr CJ. Direct binding of cyclin D to the retinoblastoma gene product (pRB) and pRB phosphorylation by the cyclin D-dependent kinase CDK4. Genes Dev. 1993;7:331–342. doi: 10.1101/gad.7.3.331. [DOI] [PubMed] [Google Scholar]
- Knudsen KE, Cavenee WK, Arden KC. D-type cyclins complex with the androgen receptor and inhibit its transcriptional transactivation ability. Cancer Res. 1999;59:2297–2301. [PubMed] [Google Scholar]
- Krause CD, Yang ZH, Kim YS, Lee JH, Cook JR, Pestka S. Protein arginine methyltransferases: evolution and assessment of their pharmacological and therapeutic potential. Pharmacol Ther. 2007;113:50–87. doi: 10.1016/j.pharmthera.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Lacroix M, Messaoudi SE, Rodier G, Le Cam A, Sardet C, Fabbrizio E. The histone-binding protein COPR5 is required for nuclear functions of the protein arginine methyltransferase PRMT5. EMBO Rep. 2008;9:452–458. doi: 10.1038/embor.2008.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb J, Ladha MH, McMahon C, Sutherland RL, Ewen ME. Regulation of the functional interaction between cyclin D1 and the estrogen receptor. Mol Cell Biol. 2000;20:8667–8675. doi: 10.1128/mcb.20.23.8667-8675.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb J, Ramaswamy S, Ford HL, Contreras B, Martinez RV, Kittrell FS, Zahnow CA, Patterson N, Golub TR, Ewen ME. A mechanism of cyclin D1 action encoded in the patterns of gene expression in human cancer. Cell. 2003;114:323–334. doi: 10.1016/s0092-8674(03)00570-1. [DOI] [PubMed] [Google Scholar]
- Lin DI, Barbash O, Kumar KG, Weber JD, Harper JW, Klein-Szanto AJ, Rustgi A, Fuchs SY, Diehl JA. Phosphorylation-dependent ubiquitination of cyclin D1 by the SCF(FBX4-alphaB crystallin) complex. Mol Cell. 2006;24:355–366. doi: 10.1016/j.molcel.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DI, Lessie MD, Gladden AB, Bassing CH, Wagner KU, Diehl JA. Disruption of cyclin D1 nuclear export and proteolysis accelerates mammary carcinogenesis. Oncogene. 2008;27:1231–1242. doi: 10.1038/sj.onc.1210738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Matsuura I. Inhibition of Smad antiproliferative function by CDK phosphorylation. Cell Cycle. 2005;4:63–66. doi: 10.4161/cc.4.1.1366. [DOI] [PubMed] [Google Scholar]
- Lu F, Gladden AB, Diehl JA. An alternatively spliced cyclin D1 isoform, cyclin D1b, is a nuclear oncogene. Cancer Res. 2003;63:7056–7061. [PubMed] [Google Scholar]
- Matsushime H, Quelle DE, Shurtleff SA, Shibuya M, Sherr CJ, Kato JY. D-type cyclin-dependent kinase activity in mammalian cells. Mol Cell Biol. 1994;14:2066–2076. doi: 10.1128/mcb.14.3.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura I, Denissova NG, Wang G, He D, Long J, Liu F. Cyclin-dependent kinases regulate the antiproliferative function of Smads. Nature. 2004;430:226–231. doi: 10.1038/nature02650. [DOI] [PubMed] [Google Scholar]
- McMahon C, Suthiphongchai T, DiRenzo J, Ewen ME. P/CAF associates with cyclin D1 and potentiates its activation of the estrogen receptor. Proc Natl Acad Sci U S A. 1999;96:5382–5387. doi: 10.1073/pnas.96.10.5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S, Sif S. Interplay between chromatin remodelers and protein arginine methyltransferases. J Cell Physiol. 2007;213:306–315. doi: 10.1002/jcp.21180. [DOI] [PubMed] [Google Scholar]
- Pal S, Vishwanath SN, Erdjument-Bromage H, Tempst P, Sif S. Human SWI/SNF-associated PRMT5 methylates histone H3 arginine 8 and negatively regulates expression of ST7 and NM23 tumor suppressor genes. Mol Cell Biol. 2004;24:9630–9645. doi: 10.1128/MCB.24.21.9630-9645.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansam CL, Shepard JL, Lai K, Ianari A, Danielian PS, Amsterdam A, Hopkins N, Lees JA. DTL/CDT2 is essential for both CDT1 regulation and the early G2/M checkpoint. Genes Dev. 2006;20:3117–3129. doi: 10.1101/gad.1482106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Rank G, Tan YT, Li H, Moritz RL, Simpson RJ, Cerruti L, Curtis DJ, Patel DJ, Allis CD, et al. PRMT5-mediated methylation of histone H4R3 recruits DNMT3A, coupling histone and DNA methylation in gene silencing. Nat Struct Mol Biol. 2009;16:304–311. doi: 10.1038/nsmb.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwijsen RM, Buckle RS, Hijmans EM, Loomans CJ, Bernards R. Ligand-independent recruitment of steroid receptor coactivators to estrogen receptor by cyclin D1. Genes Dev. 1998;12:3488–3498. doi: 10.1101/gad.12.22.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwijsen RM, Wientjens E, Klompmaker R, van der Sman J, Bernards R, Michalides RJ. CDK-independent activation of estrogen receptor by cyclin D1. Cell. 1997;88:405–415. doi: 10.1016/s0092-8674(00)81879-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.