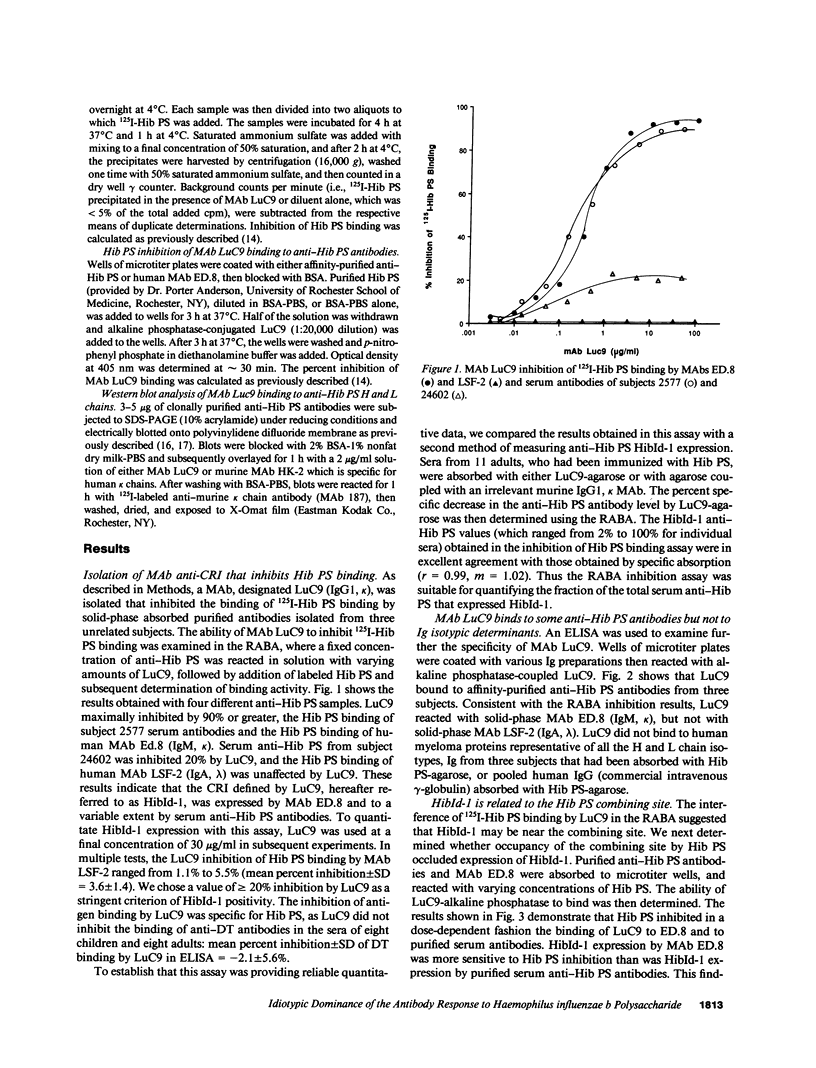
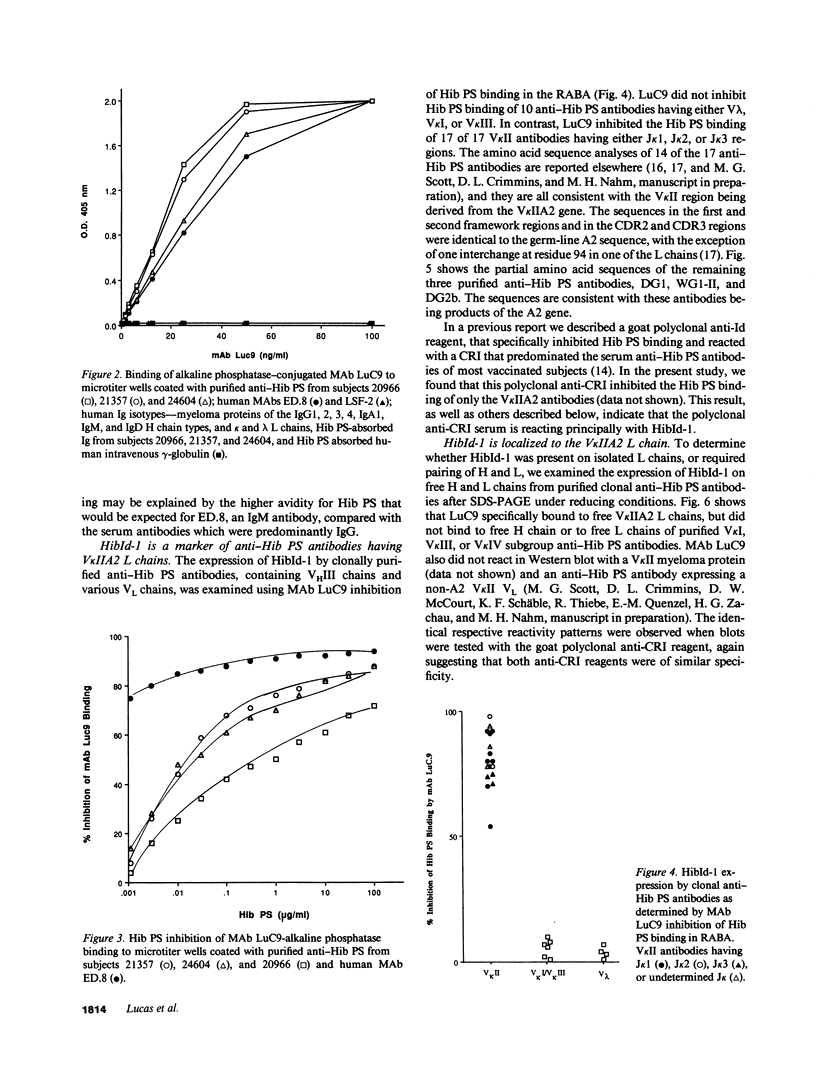
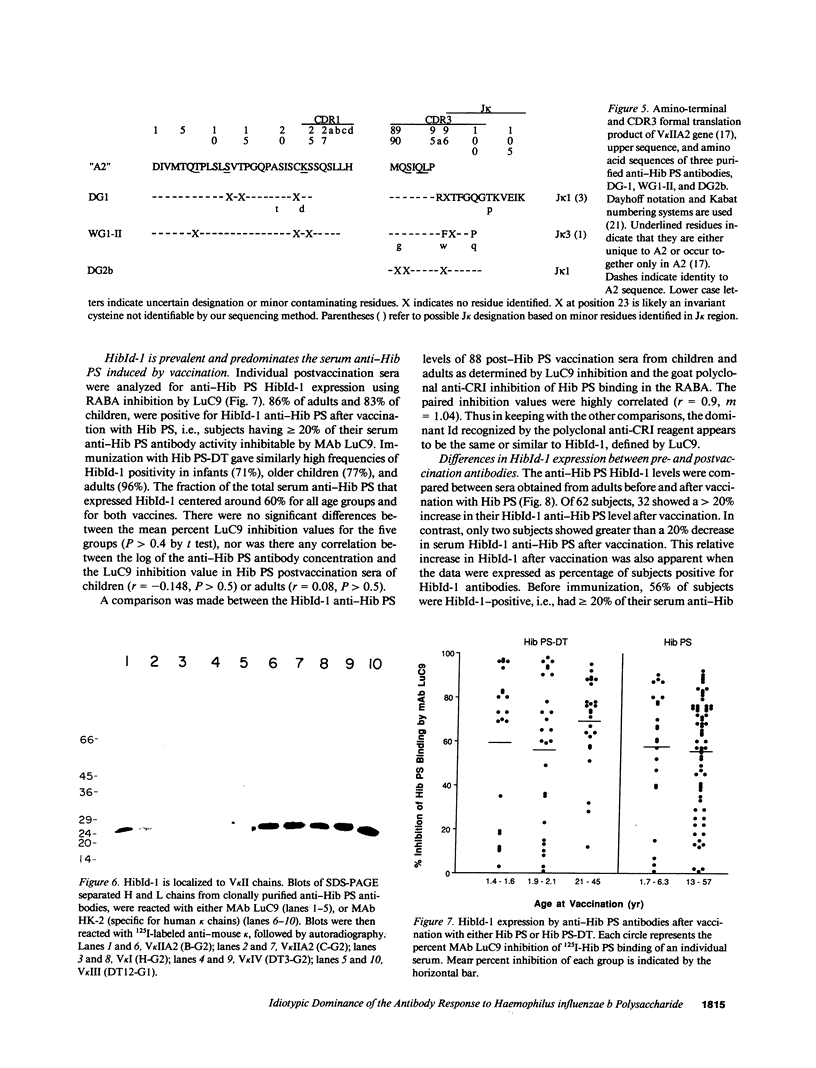
Abstract
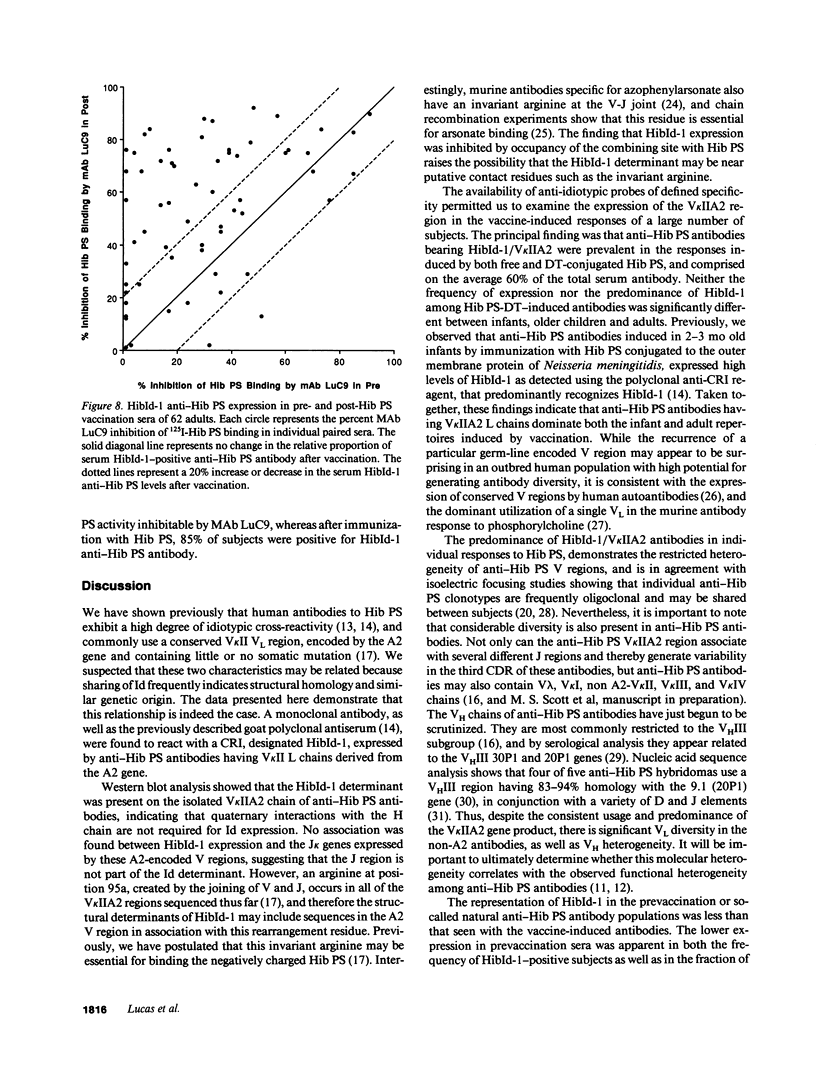
Human antibodies specific for the Haemophilus influenzae b polysaccharide (Hib PS) frequently express a cross-reactive idiotype (CRI), and commonly utilize a VL region that is the product of the V kappa II gene A2. To examine further anti-Hib PS V region expression and to determine whether CRI expression is correlated with the V kappa IIA2 chain, we isolated a monoclonal antibody (MAb) reactive with an idiotypic determinant of anti-Hib PS antibodies. This MAb inhibited Hib PS binding but did not react with Ig isotypic determinants. The CRI recognized by this MAb, designated HibId-1, was associated with the Hib PS-combining site since the reactivity of the MAb with anti-Hib PS antibodies could be inhibited by Hib PS. HibId-1 was expressed by 17 of 17 clonally purified and sequence-defined anti-Hib PS antibodies having V kappa IIA2 L chains. In contrast, 0 of 10 anti-Hib PS antibodies having either V lambda, V kappa I, or V kappa III chains expressed HibId-1. Western blot analysis showed that the MAb anti-CRI reacted with isolated anti-Hib PS V kappa IIA2 L chains but not with H chains or other L chains, indicating that the HibId-1 determinant is localized to the V kappa IIA2 chain, and does not require pairing with H chain for expression. Anti-Hib PS antibodies bearing HibId-1 were present in at least 85% of subjects immunized with either free Hib PS or Hib PS coupled to diphtheria toxoid (Hib PS-DT), and comprised on the average 60% of the total vaccine-induced serum anti-Hib PS. HibId-1 expression was not related to age at vaccination inasmuch as infants, children, and adults had similar distributions of HibId-1-positive anti-Hib PS after vaccination with Hib PS-DT. HibId-1 was expressed at a lower frequency and comprised a smaller fraction of the total anti-Hib PS antibody in adult preimmunization sera as compared to post-Hib PS immunization sera, suggesting that immunization preferentially stimulates HibId-1-positive B cells. These data demonstrate that antibodies bearing HibId-1/V kappa IIA2 comprise a predominant component of the anti-Hib PS response induced by immunization, and that this pattern of VL expression is established early in ontogeny.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adderson E. E., Shackelford P. G., Quinn A., Carroll W. L. Restricted Ig H chain V gene usage in the human antibody response to Haemophilus influenzae type b capsular polysaccharide. J Immunol. 1991 Sep 1;147(5):1667–1674. [PubMed] [Google Scholar]
- Alt F. W., Blackwell T. K., Yancopoulos G. D. Development of the primary antibody repertoire. Science. 1987 Nov 20;238(4830):1079–1087. doi: 10.1126/science.3317825. [DOI] [PubMed] [Google Scholar]
- Amir J., Liang X., Granoff D. M. Variability in the functional activity of vaccine-induced antibody to Haemophilus influenzae type b. Pediatr Res. 1990 Apr;27(4 Pt 1):358–364. doi: 10.1203/00006450-199004000-00008. [DOI] [PubMed] [Google Scholar]
- Amir J., Scott M. G., Nahm M. H., Granoff D. M. Bactericidal and opsonic activity of IgG1 and IgG2 anticapsular antibodies to Haemophilus influenzae type b. J Infect Dis. 1990 Jul;162(1):163–171. doi: 10.1093/infdis/162.1.163. [DOI] [PubMed] [Google Scholar]
- Anderson P., Pichichero M., Edwards K., Porch C. R., Insel R. Priming and induction of Haemophilus influenzae type b capsular antibodies in early infancy by Dpo20, an oligosaccharide-protein conjugate vaccine. J Pediatr. 1987 Nov;111(5):644–650. doi: 10.1016/s0022-3476(87)80237-8. [DOI] [PubMed] [Google Scholar]
- Berman J. E., Mellis S. J., Pollock R., Smith C. L., Suh H., Heinke B., Kowal C., Surti U., Chess L., Cantor C. R. Content and organization of the human Ig VH locus: definition of three new VH families and linkage to the Ig CH locus. EMBO J. 1988 Mar;7(3):727–738. doi: 10.1002/j.1460-2075.1988.tb02869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briles D. E., Forman C., Hudak S., Claflin J. L. Anti-phosphorylcholine antibodies of the T15 idiotype are optimally protective against Streptococcus pneumoniae. J Exp Med. 1982 Oct 1;156(4):1177–1185. doi: 10.1084/jem.156.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einhorn M. S., Weinberg G. A., Anderson E. L., Granoff P. D., Granoff D. M. Immunogenicity in infants of Haemophilus influenzae type B polysaccharide in a conjugate vaccine with Neisseria meningitidis outer-membrane protein. Lancet. 1986 Aug 9;2(8502):299–302. doi: 10.1016/s0140-6736(86)90001-2. [DOI] [PubMed] [Google Scholar]
- Granoff D. M., Cates K. L. Haemophilus influenzae type b polysaccharide vaccines. J Pediatr. 1985 Sep;107(3):330–336. doi: 10.1016/s0022-3476(85)80502-3. [DOI] [PubMed] [Google Scholar]
- Granoff D. M., Chacko A., Lottenbach K. R., Sheetz K. E. Immunogenicity of Haemophilus influenzae type b polysaccharide-outer membrane protein conjugate vaccine in patients who acquired Haemophilus disease despite previous vaccination with type b polysaccharide vaccine. J Pediatr. 1989 Jun;114(6):925–933. doi: 10.1016/s0022-3476(89)80432-9. [DOI] [PubMed] [Google Scholar]
- Granoff D. M., Munson R. S., Jr Prospects for prevention of Haemophilus influenzae type b disease by immunization. J Infect Dis. 1986 Mar;153(3):448–461. doi: 10.1093/infdis/153.3.448. [DOI] [PubMed] [Google Scholar]
- Granoff D. M., Shackelford P. G., Pandey J. P., Boies E. G. Antibody responses to Haemophilus influenzae type b polysaccharide vaccine in relation to Km(1) and G2m(23) immunoglobulin allotypes. J Infect Dis. 1986 Aug;154(2):257–264. doi: 10.1093/infdis/154.2.257. [DOI] [PubMed] [Google Scholar]
- Griswold W. R., Lucas A. H., Bastian J. F., Garcia G. Functional affinity of antibody to the Haemophilus influenzae type b polysaccharide. J Infect Dis. 1989 Jun;159(6):1083–1087. doi: 10.1093/infdis/159.6.1083. [DOI] [PubMed] [Google Scholar]
- Holmberg D., Andersson A., Carlsson L., Forsgren S. Establishment and functional implications of B-cell connectivity. Immunol Rev. 1989 Aug;110:89–103. doi: 10.1111/j.1600-065x.1989.tb00028.x. [DOI] [PubMed] [Google Scholar]
- Insel R. A., Anderson P. W., Jr Cross-reactivity with Escherichia coli K100 in the human serum anticapsular antibody response to Haemophilus influenzae type B. J Immunol. 1982 Mar;128(3):1267–1270. [PubMed] [Google Scholar]
- Insel R. A., Kittelberger A., Anderson P. Isoelectric focusing of human antibody to the Haemophilus influenzae b capsular polysaccharide: restricted and identical spectrotypes in adults. J Immunol. 1985 Oct;135(4):2810–2816. [PubMed] [Google Scholar]
- Jeske D. J., Jarvis J., Milstein C., Capra J. D. Junctional diversity is essential to antibody activity. J Immunol. 1984 Sep;133(3):1090–1092. [PubMed] [Google Scholar]
- Kaushik A., Schulze D. H., Bona C., Kelsoe G. Murine V kappa gene expression does not follow the VH paradigm. J Exp Med. 1989 May 1;169(5):1859–1864. doi: 10.1084/jem.169.5.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Kearney J. F., Vakil M. Idiotype-directed interactions during ontogeny play a major role in the establishment of the adult B cell repertoire. Immunol Rev. 1986 Dec;94:39–50. doi: 10.1111/j.1600-065x.1986.tb01163.x. [DOI] [PubMed] [Google Scholar]
- Korsmeyer S. J., Hieter P. A., Sharrow S. O., Goldman C. K., Leder P., Waldmann T. A. Normal human B cells display ordered light chain gene rearrangements and deletions. J Exp Med. 1982 Oct 1;156(4):975–985. doi: 10.1084/jem.156.4.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas A. H. Expression of crossreactive idiotypes by human antibodies specific for the capsular polysaccharide of Hemophilus influenzae B. J Clin Invest. 1988 Feb;81(2):480–486. doi: 10.1172/JCI113345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas A. H., Granoff D. M. A major crossreactive idiotype associated with human antibodies to the Haemophilus influenzae b polysaccharide. Expression in relation to age and immunoglobulin G subclass. J Clin Invest. 1990 Apr;85(4):1158–1166. doi: 10.1172/JCI114548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meindl A., Klobeck H. G., Ohnheiser R., Zachau H. G. The V kappa gene repertoire in the human germ line. Eur J Immunol. 1990 Aug;20(8):1855–1863. doi: 10.1002/eji.1830200834. [DOI] [PubMed] [Google Scholar]
- Moxon E. R., Rappuoli R. Haemophilus influenzae infections and whooping cough. Lancet. 1990 Jun 2;335(8701):1324–1329. doi: 10.1016/0140-6736(90)91200-t. [DOI] [PubMed] [Google Scholar]
- Perlmutter R. M., Crews S. T., Douglas R., Sorensen G., Johnson N., Nivera N., Gearhart P. J., Hood L. The generation of diversity in phosphorylcholine-binding antibodies. Adv Immunol. 1984;35:1–37. doi: 10.1016/s0065-2776(08)60572-6. [DOI] [PubMed] [Google Scholar]
- Robbins J. B., Parke J. C., Jr, Schneerson R., Whisnant J. K. Quantitative measurement of "natural" and immunization-induced Haemophilus influenzae type b capsular polysaccharide antibodies. Pediatr Res. 1973 Mar;7(3):103–110. doi: 10.1203/00006450-197303000-00001. [DOI] [PubMed] [Google Scholar]
- Schneerson R., Barrera O., Sutton A., Robbins J. B. Preparation, characterization, and immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugates. J Exp Med. 1980 Aug 1;152(2):361–376. doi: 10.1084/jem.152.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneerson R., Bradshaw M., Whisnant J. K., Myerowitz R. L., Parke J. C., Jr, Robbins J. B. An Escherichia coli antigen cross-reactive with the capsular polysaccharide of Haemophilus influenzae type b: occurrence among known serotypes, and immunochemical and biologic properties of E. coli antisera toward H. influenzae type b. J Immunol. 1972 Jun;108(6):1551–1562. [PubMed] [Google Scholar]
- Schroeder H. W., Jr, Hillson J. L., Perlmutter R. M. Early restriction of the human antibody repertoire. Science. 1987 Nov 6;238(4828):791–793. doi: 10.1126/science.3118465. [DOI] [PubMed] [Google Scholar]
- Schroeder H. W., Jr, Wang J. Y. Preferential utilization of conserved immunoglobulin heavy chain variable gene segments during human fetal life. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6146–6150. doi: 10.1073/pnas.87.16.6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M. G., Crimmins D. L., McCourt D. W., Zocher I., Thiebe R., Zachau H. G., Nahm M. H. Clonal characterization of the human IgG antibody repertoire to Haemophilus influenzae type b polysaccharide. III. A single VKII gene and one of several JK genes are joined by an invariant arginine to form the most common L chain V region. J Immunol. 1989 Dec 15;143(12):4110–4116. [PubMed] [Google Scholar]
- Scott M. G., Tarrand J. J., Crimmins D. L., McCourt D. W., Siegel N. R., Smith C. E., Nahm M. H. Clonal characterization of the human IgG antibody repertoire to Haemophilus influenzae type b polysaccharide. II. IgG antibodies contain VH genes from a single VH family and VL genes from at least four VL families. J Immunol. 1989 Jul 1;143(1):293–298. [PubMed] [Google Scholar]
- Siber G. R., Santosham M., Reid G. R., Thompson C., Almeido-Hill J., Morell A., deLange G., Ketcham J. K., Callahan E. H. Impaired antibody response to Haemophilus influenzae type b polysaccharide and low IgG2 and IgG4 concentrations in Apache children. N Engl J Med. 1990 Nov 15;323(20):1387–1392. doi: 10.1056/NEJM199011153232005. [DOI] [PubMed] [Google Scholar]
- Siegelman M., Capra J. D. Complete amino acid sequence of light chain variable regions derived from five monoclonal anti-p-azophenylarsonate antibodies differing with respect to a crossreactive idiotype. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7679–7683. doi: 10.1073/pnas.78.12.7679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman G. J., Lucas A. H. Variable region diversity in human circulating antibodies specific for the capsular polysaccharide of Haemophilus influenzae type b. Preferential usage of two types of VH3 heavy chains. J Clin Invest. 1991 Sep;88(3):911–920. doi: 10.1172/JCI115394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. H., Peter G., Ingram D. L., Harding A. L., Anderson P. Responses of children immunized with the capsular polysaccharide of Hemophilus influenzae, type b. Pediatrics. 1973 Nov;52(5):637–644. [PubMed] [Google Scholar]
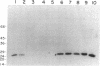
- Tarrand J. J., Scott M. G., Takes P. A., Nahm M. H. Clonal characterization of the human IgG antibody repertoire to Haemophilus influenzae type B polysaccharide. Demonstration of three types of V regions and their association with H and L chain isotypes. J Immunol. 1989 Apr 1;142(7):2519–2526. [PubMed] [Google Scholar]
- Ward J., Brenneman G., Letson G. W., Heyward W. L. Limited efficacy of a Haemophilus influenzae type b conjugate vaccine in Alaska Native infants. The Alaska H. influenzae Vaccine Study Group. N Engl J Med. 1990 Nov 15;323(20):1393–1401. doi: 10.1056/NEJM199011153232006. [DOI] [PubMed] [Google Scholar]
- Weinberg G. A., Granoff D. M. Polysaccharide-protein conjugate vaccines for the prevention of Haemophilus influenzae type b disease. J Pediatr. 1988 Oct;113(4):621–631. doi: 10.1016/s0022-3476(88)80369-x. [DOI] [PubMed] [Google Scholar]