Abstract
Background
Platelet gel is being ever more frequently used to promote healing of cutaneous ulcers. However, the factors that determine the often variable clinical outcome of this procedure are still incompletely understood.
Aims
The aims of this study were to demonstrate that platelet gel, even when obtained under strictly controlled conditions, produces highly variable outcomes in patients with cutaneous ulcers and to propose a method for in vitro standardisation of the biological properties of platelet gel.
Material and methods.
Patients were enrolled on the basis of a pre-defined protocol. Platelet concentrate was produced with standard methods, with a variability in platelet count among the different samples of less than 10%. The platelet gel for clinical use was obtained, under strictly standardized conditions, by adding thrombin and calcium gluconate to the concentrates. For in vitro studies, platelet gel, obtained from platelet-rich plasma from four donors, was frozen and thawed twice so as to increase gel contraction. The supernatant was used to modify cell proliferation, protein synthesis, and the expression of selected genes in cultures of human diploid fibroblasts.
Results
Seventeen patients (aged 44–78 years) with ulcers (4 diabetic, 11 vascular, 1 post-traumatic, 1 decubitus) were treated with platelet gel (4 autologous, 13 homologous). Complete re-epithelialisation of four ulcers (1 diabetic, 1 post-traumatic, 2 vascular) was obtained after applications of platelet gel (2 autologous, 2 homologous); in 11 other cases there was a greater than 50% reduction in the size of the ulcer. Two patients had no benefit. The supernatant of the platelet gel was able to promote dose-dependent proliferation and changes in gene expression as well as in metabolic activities related to protein synthesis.
Conclusions
Although the use of platelet gel in the treatment of cutaneous ulcers is increasing, and conditions for its production are better standardised, very considerable variability of clinical outcomes is still observed, even within single centres, suggesting that there are differences in biological properties of platelet concentrates from individual patients which cannot be readily controlled with current techniques. The biological effects of the platelet gel supernatant described in this article may provide the basis for a simple biological validation of platelet preparations before their clinical use, so as to reduce this potentially important source of variability.
Keywords: platelet gel, fibroblasts, ulcers, cell proliferation
Introduction
In recent years the problem of “cutaneous ulcers” (venous, arterial, diabetic, and pressure sores) has become increasingly important, in particular because of the progressive increase in the elderly population and, therefore, of chronic disorders. Cutaneous ulcers constitute a serious and growing care problem, are often disabling ling and difficult to treat. According to the Italian Association for Cutaneous Ulcers (AIUC), these ulcers cost the Italian National Health Service almost one billion euro each year, besides the loss of almost 500,000 days of work. Among diabetics, the life-long risk of foot ulcers can reach 25% and about 20% of health care costs can be attributed to the diabetic foot1,2.
The most common causes of ulcers are vascular disorders (arterial and venous), trauma, pressure and diabetes. Whatever their cause, cutaneous ulcers are characterised by loss of tissue, involving the epidermis, dermis and, sometimes, adipose tissue and muscle layers, and by a lack of spontaneous repair, perhaps due to aging (that is, cessation of proliferation) of the resident population of mesenchymal stem cells or the incapacity to recruit circulating precursor cells3.
There is a great need for innovative therapeutic approaches to ulcers, and these must be economically feasible and applicable on a large scale. One of the most interesting approaches is the use of platelet gel, a blood component for topical use obtained from a platelet concentrate activated by a preparation of human thrombin in the presence of calcium. Platelet gel has been used for various years in the treatment of skin ulcers4,5, since it is known to have effects of promoting the regeneration of mesenchymal tissues, such as connective tissue, tendons, bone and vessels, which are useful in many situations in which repair processes need to be accelerated6. As far as regards cutaneous ulcers, these effects are manifested clinically first by the appearance of granulation tissue and, subsequently, by re-epithelialisation of the surface of the skin.
The biological rationale for using platelet gel in the treatment of cutaneous ulcers is based on the important role played by platelets in the process of spontaneous healing of lesions. Once activated during the process of haemostasis, platelets release many factors, the most well-studied of which are platelet-derived growth factor, transforming growth factor-beta and vascular endothelial growth factor 7. Besides their effects on cell proliferation, the factors released by platelet granules modify the inflammatory response, have an antibacterial effect, are chemotactic for macrophages and fibroblasts and promote the secretion of proteolytic enzymes that facilitate tissue repair and remodelling.
Despite these solid biological bases, the outcomes of treatment of cutaneous ulcers with platelet gel are varied and there are relatively few controlled studies8,9. The main causes of variability include the methods and equipment used to obtain the platelet concentrate10,11, the protocols used to activate the platelet gel12–14, as well as the characteristics of the patients and of the lesions. The contribution made to the variability of the outcome by the platelets themselves remains to be evaluated: the only certainty on this issue is that the amount of factors released varies in proportion to the number of platelets used15,16.
In this article we describe the experience gained in the Unit of Immunohaematology and Transfusion Medicine in the University Hospital of Parma on the use of platelet gel, obtained with controlled, standardised methods, in the topical treatment of chronic cutaneous ulcers of various causes in the period between January 2006 and October 2008. We also evaluated some short-term effects of platelet gel on fibroblasts which could enable a biological validation of this blood component prior to its clinical use.
Materials and methods
Platelets
The transfusion specialist, in collaboration with the vascular surgeon or internist, evaluated the clinical condition of the patient and the characteristics of the cutaneous ulcer: aetiology, size, position, time of development, appearance of the bed and margins, effects of any treatments already tried, vascularisation, the presence/absence of infection (evaluated with a preliminary skin swab) and associated pain.
Exclusion criteria were infection in the ulcer, osteomyelitis, necrosis, inadequate vascularisation of the area of the lesion, and ulcers smaller than 2 cm2.
During the first assessment, the patient’s suitability for donation of autologous blood was also evaluated according to the regional guidelines on the collection of autologous blood (Guidelines on Autologous Blood, Region of Emilia-Romagna 31/10/2005). If the patient was not fit to make a self-donation, he or she was considered for treatment with homologous blood components.
The blood components used were subject to haemovigilance: both the autologous and homologous products underwent serological validation. Furthermore, the homologous products had to be ABO compatible and the patient had to sign informed consent prior to starting the treatment.
The criteria used to evaluate the efficacy of treatment with platelet gel were the reduction in the size of the ulcer, the formation of granulation tissue and re-epithelialisation. Additional criteria were reduction of pain, the appearance of the bed of the lesion, and the absence of local infections during the treatment.
Preparation of the platelet gel
a). Platelet concentrate
The autologous platelet concentrates were produced by plateletpheresis using a Hemonetics MCS+ cell separator (Haemonetics Corp., Braintree, MA, USA). Three or four cycles were planned depending on the patient’s initial platelet count. The procedure yielded a platelet concentrate of 120–160 mL. The homologous platelet concentrates were obtained from a unit of 410 mL of whole blood collected in a quadruple bag (Compoflex, Fresenius Kabi Bad Homburg, Germany) and subsequent production of a platelet concentrate of 50–60 mL from platelet-rich plasma. The platelet units produced were maintained under continuous agitation for 24 hours at 22°C 17. Following serological validation, the autologous units were separated, under a sterile closed circuit with TSCD (Terumo Sterile Tubing Welder, Terumo Medical Corporation, Japan) into three or four aliquots of 40 mL. Each aliquot was labelled with labels complying with UNI standards carrying the writing “autologous platelet concentrate” and frozen at −40°C. The homologous units labelled as “homologous platelet concentrate for gel”, were kept as such or separated, again using the TSCD, into two aliquots of 25–30 mL, and stored at −40°C. The platelet content of each aliquot was evaluated in order to determine whether the minimum therapeutic dose of 1 x 106 platelets/μL had been reached. The platelet count in the aliquots of platelet concentrates used ranged from 960 x 105 to 1.35 x 106 platelets/μL.
The homologous platelet concentrates used were obtained from donors who were ABO compatible with the patients to be treated and who had pre-donation platelet counts greater than 250,000/μL
b). Thrombin
Autologous or homologous thrombin was obtained by collecting 20 mL of whole blood from the patient or ABO compatible donor into four Vacutainer test-tubes (Vacuette®, Greiner, Interconsult s.r.l. Medical Division, Caravaggio, BG, Italy); the test-tubes were centrifuged at 3,200 g for 10 min, the serum was separated under a flow hood and 0.2 mL of 10% calcium gluconate were added (Bioindustria L.I.M., Novi Ligure, AL, Italy) before incubation at 37°C for 15–30 min. Finally, the supernatant, containing thrombin precursors, was divided into two or three aliquots and labelled in order to ensure correspondence with the platelet concentrates from the patient or donor. The aliquots of thrombin were then stored at −40°C. The aliquots containing autologous thrombin were placed in the freezer with the corresponding bags, while the aliquots of homologous thrombin were placed in the freezer in a specific container.
c). Activation of the platelet gel
The aliquots of platelet concentrate were frozen at 37°C for 10 min. In the case of treatment with homologous platelet gel, the bag was matched with the patient using our system’s standard procedures of computerised matching of blood components (Cetraplus webä) and subsequently supplied with specific labels to include in the patient’s clinical records in order to be able to ensure the traceability of the blood component used.
The product was activated at the patient’s bedside. This was done by withdrawing 20 mL of the platelet concentrate with a syringe, placing it in a sterile, 10 cm Petri dish and adding 2 mL of thrombin (homologous or autologous) and 2 mL of 10% calcium chloride (Bioindustria L.I.M., Novi Ligure, AL, USA). The solution thus obtained was mixed gently and left to rest for 10–15 min until the platelet gel was formed which, in this case, appeared as a gelatinous disc. Depending on the size of the ulcer, one to two or more platelet gels were used.
Application of the platelet gel
Having washed the base and edges of the ulcer abundantly with 3% boric acid, the platelet gel was detached from the Petri dish with a sterile tongue depressor, placed on a sterile gauze and applied directly onto the ulcer taking care to cover the whole base of the lesion. At this point one or more impregnated gauzes (Atraumanä, Paul Hartmann AG, Heidenheim, Germany) were placed over the lesion and then covered with an occlusive bandage.
In vitro studies
A platelet gel derived from a pool of platelet concentrates obtained from six normal, group AB, male blood donors was used for these studies. The donors signed informed consent. The platelet gel was prepared according to the protocol described above. Once obtained, the platelet gel was frozen and thawed twice, to acceleration its contraction. The supernatant obtained at the end was divided into aliquots and stored at −80°C. On average, about 4 mL of supernatant were obtained from one platelet gel of about 50 cm2, prepared in a 10 cm Petri dish.
a). Cell proliferation
Human diploid fibroblasts (not immortalised), originally derived from the foreskin of a 15-year old subject, were used for these investigations18. The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) enriched with 10% foetal bovine serum (FBS), 4 mM glutamine, 100 U/mL penicillin and 100 mg/mL streptomycin. The cells were maintained at 37°C in a 5% CO2 atmosphere at pH 7.4.
In order to evaluate the effects of the platelet gel on proliferation, the fibroblasts were seeded in 24-well plates (Costar Corning, NY, USA) at a density of 10 x 103 cells/well and cultured for 24 hours. The growth medium was then replaced with fresh medium containing FBS or platelet gel supernatant. At predetermined times, the growth rate in culture was determined by counting the number of cells, after trypsinisation, with a Coulter ZM cell counter, or evaluating cell viability using the resazurin method19. This method involves replacing the culture medium, at the selected time, with a solution of resazurin (44 μM) in serum-free culture medium. After 1 h, the fluorescence was measured at 572 nm with a fluorometer (Wallac 1420 Victor2 Multilabel Counter, Perkin Elmer). Cell counts and viability tests were performed in triplicate and the results expressed as the mean ± standard deviation (SD).
b). Protein synthesis and proline transport
Protein synthesis was determined using the incorporation of leucine into the acid-insoluble fraction as an indicator. Briefly, 5 x 104 cells/well were seeded in a 6-well plate. After 24 hours, the medium was replaced with fresh medium supplemented with FBS or platelet gel supernatant. After 3 days the medium was replaced with leucine-free Minimum Essential Medium (MEM), containing L-[4,5-3H]leucine (5 μCi /mL, Amersham). After 30 minutes, the cells were digested by trypsin, centrifuged and incubated with 5% trichloroacetic acid (TCA) at 4°C. The precipitate was washed twice with TCA and the proteins suspended in a solution of 5% sodium deoxycholate in 1N NaOH. After having added the scintillation fluid, the radioactivity present in the protein solution was determined with a scintillation spectrometer (Wallac Microbeta Trilux counter). In parallel, aliquots of the same solution were used to determine the protein content by Lowry’s method. The rate of entry of proline into the cells, an indicator of the activity of the SNAT2 transporter, a parameter related to cell proliferation, was measured as described elsewhere20.
c). Gene expression
One microgram of total RNA, isolated using the RNeasy Mini Kit® (Qiagen S.p.a., Milan, Italy), was reverse transcribed as described elsewhere21. For the real-time polymerase chain reaction (PCR) (40 cycles), cDNA was amplified with 2X Platinum® SYBR® Green qPCR SuperMix-UDG (Invitrogen s.r.l., Milan, Italy) in the presence of the following primers (forward and reverse, 5 pmol of each): 5′ TCA AGA TCA TTG CTC CTC CTG AG 3′ and 5′ ACA TCT GCT GGA AGG TGG ACA 3′ for β-actin; 5′ ACT CTG GAG TAA TGT CAC ACC T 3′ and 5′ GTT GGT CCA CCT TTC ATC TTC A 3′ for MMP1 (matrix metalloproteinase 1); 5′ GTC GAG GGC CAA GAC GAA G 3′ and 5′ CAG ATC ACG TCA TCG CAC AAC 3′ for COL1A1 (collagen, type 1, α1); 5′ TCG TCC AGC GGG ATC TGA A 3′ and 5′ GCC GTT GAA GTA GAG GGC ATT 3′ for serpine 1 (plasminogen activator inhibitor-1, PAI-1).
The primers were designed, with the help of the Primer 3 programme, on the basis of the known sequences deposited in GenBank22. The PCR was carried out in a 36-well Rotor Gene 3000 instrument (Corbett Research, Rotor-Gene™ 3000, version 5.0.60, Mortlake, Australia). Each cycle consisted of denaturation at 95°C for 15 s, followed by annealing (30 s) and extension (30 s, 72°C). Fluorescence was monitored at the end of every extension step. A control without reverse transcriptase and cDNA template was included in every experiment. At the end of the amplification cycles, melting curve analysis was conducted and the data analysed using the Relative Standard Curve Method23; results were expressed as the level of mRNA of the gene of interest normalised to the level of β-actin mRNA determined in the same sample.
Results
Application of platelet gel in patients with cutaneous ulcers
In the period from January 2006 to October 2008 we treated 17 patients (4 males and 13 females) aged between 36 and 78 years old. All patients were treated as inpatients or in the day hospital.
Four patients had been able to pre-deposit their own blood and were treated with autologous platelet gel, whereas the other 13 were treated with ABO-compatible homologous gel. The aetiology of the ulcers was varied: diabetic (n=4), vascular (n=11), post-traumatic (n=1) and decubitus (n=1). The mean initial size of the treated cutaneous ulcers was 63 cm2 (range, 36 – 90 cm2) and the mean depth was 1.35 cm (range, 0.5 – 2.2 cm). Sixteen of the 17 lesions were on the lower limbs and only one, a pressure sore, was on the sacral region.
The applications of platelet gel were started for ulcers present for at least 30 days, which had proven resistant to traditional or advanced treatments. In almost all cases, preliminary surgical debridement was necessary. The mean number of platelet gel applications was 14.5 (range, 4–25) (Table I).
Table I.
Characteristics and results of the treatments.
| Patients treated | Ulcers causes | Surface area (cm2)* | Depth (mm)* | Type of platelet gel | Number of treatments/week | Complete response healed | Partial response recovery >50% | No response | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diabetic vascular | Vascular | Post-traumatic | Pressure | Autologous | Homologous | |||||||
| 17 | 4 | 11 | 1 | 1 | 63 (36–90) | 135 (50–200) | 4 | 13 | 14.5 (4–25)* | 4 | 11 | 2 |
Median (range).
Complete re-epithelialisation of the ulcer was achieved in four cases, in 11 cases there was a greater than 50% reduction in the size of the ulcer and in two cases (a sacral pressure sore and a diabetic ulcer) treatment was ineffective. Of the four cases in which complete re-epithelialisation occurred, two were treated with autologous gel (a diabetic ulcer and a traumatic ulcer), while the other two, treated with homologous gel, had ulcers of vascular aetiology.
Of the 11 cases in which there was a significant reduction in the size of the ulcer, four subsequently underwent autologous skin grafting (not previously possible given the large size of the ulcers) whereas the other seven continued with traditional medications. In all cases with a positive response to treatment, there was rapid formation of granulation tissue (already after the first application of gel) and a notable reduction of pain and improvement of compliance of the patients. No infections occurred during the treatments.
We describe one case, included in the study, in which complete re-epithelialisation was observed.
A 78-year old male with dilated cardiomyopathy, chronic atrial fibrillation receiving oral anticoagulation therapy, and type 2 diabetes mellitus was admitted to the Angiology and Haemostasis section of the Unit of Internal Medicine of the University Hospital of Parma because of suspected deep vein thrombosis in the presence of an infected, necrotic, 6 cm2 cutaneous ulcer of the posterior surface of the right lower leg and fever. Doppler ultrasound studies excluded deep vein thrombosis but revealed stage IV arterial disease of the lower limbs. After medical therapy with antibiotics and surgical toilette of the skin ulcer in the Plastic Surgery department, the patient underwent a right distal femoro-popliteal by-pass with the stripped contralateral saphenous vein in the Vascular Surgery Unit. After the operation the patient received blood transfusions because of bleeding from the surgical drains. Subsequently he started traditional treatment with Ilaprost, which produced only a modest improvement in the chronic skin ulcer. At this point, our Unit was contacted and, after a clinical evaluation (see Materials and Methods section), the patient started treatment according to the above-described protocol.
Considering the clinical condition of the patient, the applications were carried out with homologous platelet gel from a donor with the same blood group as that of the patient, following an explanation of the treatment and the patient’s signed, informed consent.
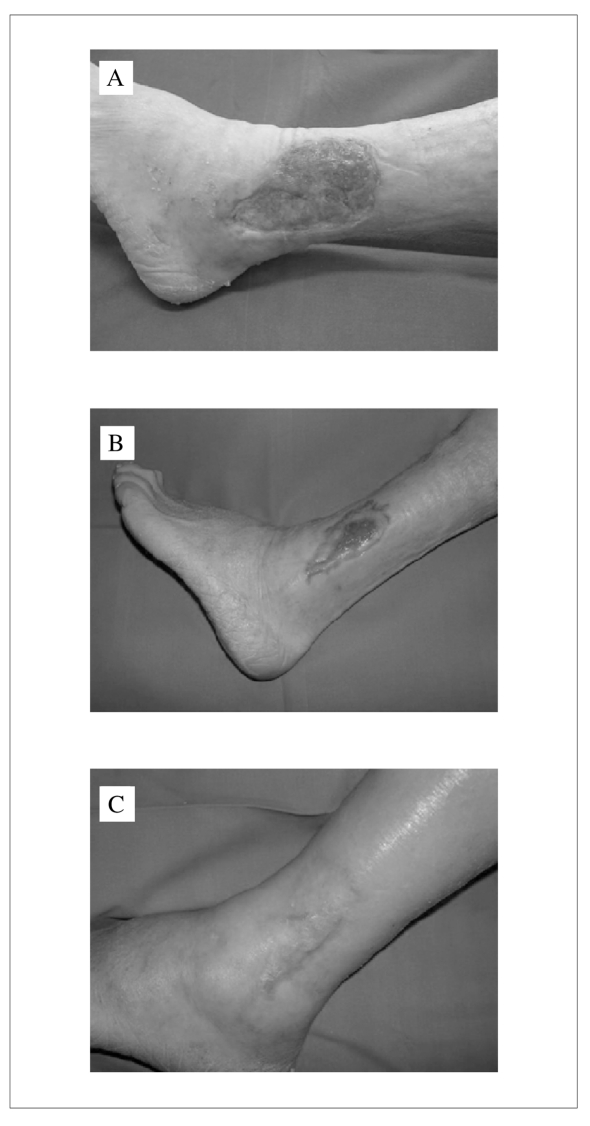
The patient underwent weekly treatment with homologous platelet gel. After four applications the size of the ulcer decreased and initial re-epithelialisation of the margins of the lesion were observed together with abundant granulation tissue (Figure 1A). After ten applications the dimensions of the ulcer had decreased by more than 50% (Figure 1B) and after 20 applications complete re-epithelialisation of the skin lesion was observed (Figure 1C).
Figure 1.
Effect of platelet gel on a cutaneous ulcer. The images show the appearance of the lesion after 4 (A), 10 (B) or 20 applications of the platelet gel (C). See text for details on the patient.
Quantitative effects of the platelet gel supernatant
A). Stimulation of the growth of human cultured fibroblasts
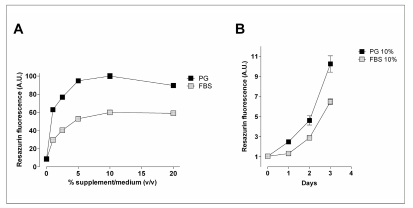
In order to evaluate the capacity of the platelet gel supernatant to stimulate the proliferation of human fibroblasts, cell cultures were incubated for 72 hours in increasing concentrations of this blood derivative. The effects of the supernatant were compared with those of comparable concentrations of FBS.
The results (Figure 2, Panel A) show that the supernatant had a marked dose-dependent effect on cell proliferation, causing significant stimulation of growth already at a concentration of 0.5%. The effect observed in the resazurin test, a standard method for evaluating the viability of cell populations, was consistent with the cell counts (results not shown). It is interesting to note that the platelet gel supernatant was more potent than FBS, with the concentrations at which the half-maximal stimulatory effect was observed being 0.4% and 1.5%, respectively. The kinetics of the effect (panel B) indicate that, at the maximum concentration of 10%, the platelet gel supernatant had a greater proliferative effect than FBS already after 24 hours of treatment.
Figure 2.
Effects of platelet gel supernatant on the growth of human cultured fibroblasts. The cells were seeded in 24-well plates in complete growth medium (DMEM + 10% FBS). (Panel A) After 24 hours, the medium was replaced by serum-free DMEM (0) or DMEM with FBS (grey squares) or platelet gel supernatant (black squares), at the concentrations indicated. Cell viability was evaluated after 72 hours, as described in the Materials and Methods. (Panel B) After 24 hours, the medium was replaced with DMEM supplemented with FBS (10%, grey squares) or platelet gel supernatant (10%, black squares). Cell viability was evaluated at the indicated times. For both panels, the data are the mean of six independent experiments with S.E.M. shown when larger than the size of the point.
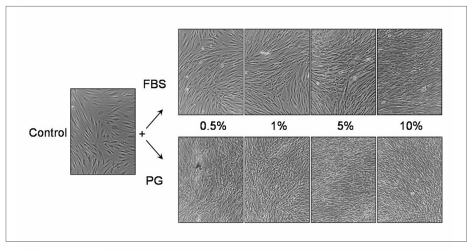
After 72 hours of treatment, the cell cultures appeared clearly different for the two conditions, particularly at the lower concentrations of FBS or platelet gel supernatant (Figure 3). In fact, while the cells treated with 0.5% FBS had the characteristic arrangement of parallel layers of clearly distinct cells, the cells treated with 0.5% platelet gel supernatant were greatly increased in density and arranged in irregular bundles.
Figure 3.
Effect of platelet gel supernatant on the morphology of the human fibroblast cultures. The cells were seeded in six-well plates in complete growth medium (DMEM + 10% FBS). After 24 hours, the medium was replaced with serum-free DMEM (control) or with DMEM supplemented with FBS (upper row) or platelet gel supernatant (lower row) at the indicated concentrations. The incubation was then continued for 72 hours and the cultures photographed by phase contrast microscopy. Representative fields are shown (original magnification x100).
B). Stimulation of protein synthesis, proline transport and gene expression
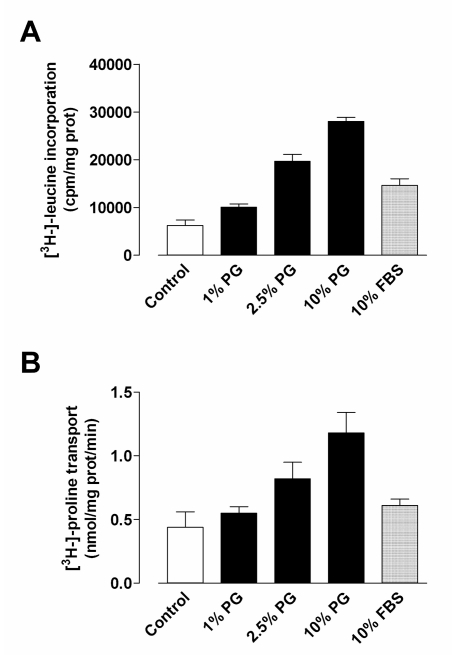
Figure 4 shows the results of the tests carried out to evaluate the metabolic effects of the platelet gel supernatant on human cultured fibroblasts. After 3 days of treatment, the blood derivative produced marked dose-dependent stimulation of protein synthesis, evaluated by the incorporation of L-leucine into the acid-insoluble fraction (Panel A) or by the entry of the L-proline amino acid into the cells (Panel B), an indicator of the activity of the SNAT2 transporter24 and, consequently, of the size of the intracellular pool of amino acids in human cultured fibroblasts25. In both cases, the effect of supplementing the culture medium with platelet gel supernatant was greater than that observed after incubation with a comparable dose of FBS.
Figure 4.
Effect of platelet gel supernatant on protein synthesis and proline transport in human fibroblast cultures. The cultured human fibroblasts were incubated for 72 hours in DMEM + 0.1% FBS (control) or in DMEM supplemented with 10% FBS or with platelet gel supernatant at the indicated concentrations. At the end of this period, L-leucine incorporation into the acid-insoluble fraction (panel A) and L-proline transport (panel B) were determined in parallel, as described in the Materials and Methods. The data are the means ± SD of three independent determinations.
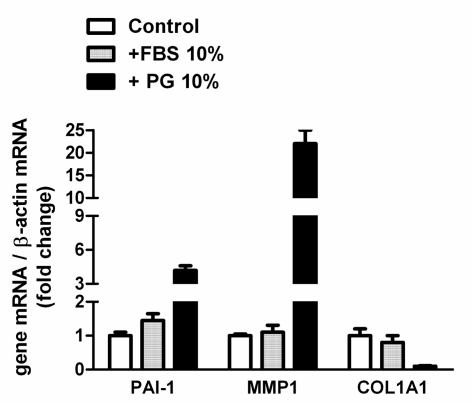
The platelet gel supernatant also produced evident changes in gene expression in human fibroblasts. It is interesting to note that, in this case, the effect seemed qualitatively different from that observed after incubation in the presence of FBS (Figure 5). In particular, after 3 days of treatment with supernatant, there was clear induction of the MMP1 and PAI1 genes, encoding, respectively, for matrix metalloproteinase 1 and plasminogen activator inhibitor. In contrast, induction of these genes was not observed in cells treated with FBS. It is interesting to note that in the same experimental conditions, the expression of COL1A1, which codes for the a1 subunit of type 1 collagen, was almost completely inhibited in cells incubated with supernatant, while it was evident in cells maintained in the presence of 10% FBS.
Figure 5.
Effects of platelet gel supernatant on gene expression of cultured human fibroblasts. The fibroblasts were incubated for 72 hours in DMEM + 0.1% FBS (control) or in DMEM supplemented with FBS or platelet gel supernatant (both at a concentration of 10%), as indicated. At the end of this period, the relative abundance of the mRNA of PAI-1, MMP1 and COL1A1, normalised to the level of b-actin mRNA, was determined by RT-PCR and expressed as a ratio with respect to the value obtained in control conditions. The data are means ± SD of four determination in two different experiments.
Discussion
The pro-regenerative action of platelet gel appears to be related to three main components. First of all, platelet gel release growth factors and cytokines able to stimulate the proliferation, migration and differentiation of fibroblasts and endothelial cells26,27. Unlike treatments based on “pure” factors or platelet lysates, the application of platelet gel leads to “temporally controlled” exposure28 to a complex mixture of factors (the main ones being platelet-derived growth factor, transforming growth factor-beta, epidermal growth factor, fibroblast growth factor, vascular endothelial growth factor and insulin-like growth factor, each with different biological properties. The second component is the mesh of fibrin that constitutes the “structure” of the platelet gel: this mesh forms a biological scaffold that helps and guides the migration of the mesenchymal cells, derived from populations of resident stem cells or circulating precursors, from the base and the margins of the wound. At the same time it protects the damaged skin surface and provides a structure for the storage and gradual release of the growth factors and other substances. The third component is represented by the antibacterial properties of the factors released by the platelets and, probably, by the leucocytes contained in the platelet-rich plasma, which remain viable for prolonged periods29.
Platelet gel, therefore, constitutes a medium that enables the growth factors necessary to stimulate physiological repair of the tissue to be delivered to the site of the lesion. In this light, the application of platelet gel can be considered as an adjuvant treatment in a multidisciplinary programme of therapy for chronic cutaneous ulcers: such a programme cannot be effective without close collaboration between the internist, diabetes specialist, dermatologist, vascular surgeon, plastic surgeon and the transfusion medicine specialist.
From our experience we can state that topical application of platelet gel to chronic cutaneous ulcers, not responsive to other therapies, was effective: in most cases it stimulated rapid formation of granulation tissue and reduced the size of the ulcers by at least 50% in a satisfactory period (8–12 weeks) and, in about a quarter of the cases, even healed the lesions with complete re-epithelialisation.
It is important to note that pain decreased rapidly in all the treated patients, with relief of physical and psychological distress and consequent improvement of quality of life. Furthermore, there were no adverse reactions to applications of either autologous or homologous platelet gel, so it can be stated that homologous platelet gel is a biologically very safe blood component, comparable to the autologous product.
The topical use of platelet gel is, therefore, an effective support to stimulate the healing of chronic cutaneous ulcers. However, multicentre, randomised studies are necessary to evaluate the therapeutic efficacy of platelet gel in relation to ulcers of different aetiologies, to standardise further the methods for its preparation and application, and to determine the appropriate therapeutic dose for stimulating tissue regeneration taking into account, primarily, the platelet concentration, a parameter obviously related to the amount of growth factors released15. Furthermore, it seems necessary to establish “end points” which, depending on the characteristics of the skin lesions, could lead to the introduction of protocols indicating the correct ratio between efficacy/number of applications and the best interval between the applications.
It is important to note that there was marked variability in outcome also in our series, despite careful standardisation and uniformity in the amount of platelets employed and the methods used to obtain the platelet concentrates and activate the platelet gel. We hypothesise that this variability is due to different biological activity of platelets between individuals. In fact, it is known that the amount of growth factors released by platelets from different subjects is extremely variable16, although this variability is not taken into account at all during the preparation of platelet gel.
The initial characterisation of the in vitro effects of platelet gel supernatant that we present in this study highlight at least three parameters that could form the basis for a biological assay of the activity of platelet gel preparations prior to their clinical use.
The first potential parameter is growth-promoting activity. A second parameter that could be used is related to the metabolic effects of the treatment and, in particular, the stimulation of protein synthesis and related events (such as proline transport). A third parameter of potential use could be the transcriptional response specifically related to the platelet gel: characteristic changes in gene expression are demonstrable in fibroblasts treated with platelet gel and these are qualitatively different from those observed in cell cultures stimulated with FBS.
In these in vitro experiments, the biological effects of the platelet gel were studied using the supernatant released from the gel during its contraction, accelerated by a double cycle of freezing and thawing. Although this supernatant may not fully represent the diverse biological activities of the platelet gel, the pleiotrophic effects that we demonstrated and the ease of its preparation indicate that the supernatant could be useful for evaluating the biological activity of platelet gel prior to its clinical use.
We also believe that a “composite” biological assay (in particular as regards proliferative activity and gene expression) could better reflect the complex activities of platelet gel compared to assays of single factors present in the granules and released during the formation of the gel. In fact, the platelet secretory proteome is particularly complex30 and an approach based on assaying single factors, with the hope that these would be representative, would be technically complicated and extremely expensive.
In conclusion, we believe that it would definitely be interesting to evaluate the clinical efficacy of the application of platelet gel, relating this to the biological properties of the platelet gel to use. Although platelet gel has been widely used for years on an empirical basis for numerous therapeutic indications, an approach of this type could significantly decrease the clinical variability associated with its use and lead to its recognition as an economically valid therapeutic strategy with precise indications in the context of regenerative medicine.
References
- 1.Reiber GE, Raugi GJ. Preventing foot ulcers and amputations in diabetes. Lancet. 2005;366:1676–7. doi: 10.1016/S0140-6736(05)67674-X. [DOI] [PubMed] [Google Scholar]
- 2.Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, et al. The global burden of diabetic foot disease. Lancet. 2005;366:1719–24. doi: 10.1016/S0140-6736(05)67698-2. [DOI] [PubMed] [Google Scholar]
- 3.Vande Berg JS, Robson MC. Arresting cell cycles and the effect on wound healing. Surg Clin North Am. 2003;83:509–20. doi: 10.1016/S0039-6109(02)00195-0. [DOI] [PubMed] [Google Scholar]
- 4.Crovetti G, Martinelli G, Issi M, et al. Platelet gel for healing cutaneous chronic wounds. Transfus Apher Sci. 2004;30:145–51. doi: 10.1016/j.transci.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Mazzucco L, Medici D, Serra M, et al. The use of autologous platelet gel to treat difficult-to-heal wounds: a pilot study. Transfusion. 2004;44:1013–8. doi: 10.1111/j.1537-2995.2004.03366.x. [DOI] [PubMed] [Google Scholar]
- 6.Everts PA, Overdevest EP, Jakimowicz JJ, et al. The use of autologous platelet-leukocyte gels to enhance the healing process in surgery, a review. Surg Endosc. 2007;21:2063–8. doi: 10.1007/s00464-007-9293-x. [DOI] [PubMed] [Google Scholar]
- 7.Caloprisco G, Borean A, Mele A. Lezioni del VII Corso di aggiornamento in Emaferesi. SIdEM. 2002 [Google Scholar]
- 8.Driver VR, Hanft J, Fylling CP, et al. A prospective, randomized, controlled trial of autologous platelet-rich plasma gel for the treatment of diabetic foot ulcers. Ostomy Wound Manage. 2006;52:68–70. 72. 74 passim. [PubMed] [Google Scholar]
- 9.Dougherty EJ. An evidence-based model comparing the cost-effectiveness of platelet-rich plasma gel to alternative therapies for patients with nonhealing diabetic foot ulcers. Adv Skin Wound Care. 2008;21:568–75. doi: 10.1097/01.ASW.0000323589.27605.71. [DOI] [PubMed] [Google Scholar]
- 10.Everts PA, Hoffmann J, Weibrich G, et al. Differences in platelet growth factor release and leucocyte kinetics during autologous platelet gel formation. Transfus Med. 2006;16:363–8. doi: 10.1111/j.1365-3148.2006.00708.x. [DOI] [PubMed] [Google Scholar]
- 11.Mazzucco L, Balbo V, Cattana E, et al. Not every PRP-gel is born equal Evaluation of growth factor availability for tissues through four PRP-gel preparations: Fibrinet, RegenPRP-Kit, Plateltex and one manual procedure. Vox Sang. 2009;97:110–8. doi: 10.1111/j.1423-0410.2009.01188.x. [DOI] [PubMed] [Google Scholar]
- 12.Waters JH, Roberts KC. Database review of possible factors influencing point-of-care platelet gel manufacture. J Extra Corpor Technol. 2004;36:250–4. [PubMed] [Google Scholar]
- 13.Valeri CR, Saleem B, Ragno G. Release of platelet-derived growth factors and proliferation of fibroblasts in the releasates from platelets stored in the liquid state at 22 degrees C after stimulation with agonists. Transfusion. 2006;46:225–9. doi: 10.1111/j.1537-2995.2006.00705.x. [DOI] [PubMed] [Google Scholar]
- 14.Kevy SV, Jacobson MS. Comparison of methods for point of care preparation of autologous platelet gel. J Extra Corpor Technol. 2004;36:28–35. [PubMed] [Google Scholar]
- 15.Eppley BL, Woodell JE, Higgins J. Platelet quantification and growth factor analysis from platelet-rich plasma: implications for wound healing. Plast Reconstr Surg. 2004;114:1502–8. doi: 10.1097/01.prs.0000138251.07040.51. [DOI] [PubMed] [Google Scholar]
- 16.Wildemann B, Kadow-Romacker A, Haas NP, et al. Quantification of various growth factors in different demineralized bone matrix preparations. J Biomed Mater Res A. 2007;81:437–42. doi: 10.1002/jbm.a.31085. [DOI] [PubMed] [Google Scholar]
- 17.Ficarelli E, Bernuzzi G, Tognetti E, et al. Treatment of chronic venous leg ulcers by platelet gel. Dermatol Ther. 2008;21(Suppl 1):S13–7. doi: 10.1111/j.1529-8019.2008.00196.x. [DOI] [PubMed] [Google Scholar]
- 18.Franchi-Gazzola R, Visigalli R, Dall’Asta V, et al. Amino acid depletion activates TonEBP and sodium-coupled inositol transport. Am J Physiol Cell Physiol. 2001;280:C1465–74. doi: 10.1152/ajpcell.2001.280.6.C1465. [DOI] [PubMed] [Google Scholar]
- 19.O’Brien J, Wilson I, Orton T, et al. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur J Biochem. 2000;267:5421–6. doi: 10.1046/j.1432-1327.2000.01606.x. [DOI] [PubMed] [Google Scholar]
- 20.Franchi-Gazzola R, Gaccioli F, Bevilacqua E, et al. The synthesis of SNAT2 transporters is required for the hypertonic stimulation of system A transport activity. Biochim Biophys Acta. 2004;1667:157–66. doi: 10.1016/j.bbamem.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 21.Bianchi MG, Gazzola GC, Tognazzi L, et al. C6 glioma cells differentiated by retinoic acid overexpress the glutamate transporter excitatory amino acid carrier 1 (EAAC1) Neuroscience. 2008;151:1042–52. doi: 10.1016/j.neuroscience.2007.11.055. [DOI] [PubMed] [Google Scholar]
- 22.Rozen SSH. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–86. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 23.Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol. 2000;25:169–93. doi: 10.1677/jme.0.0250169. [DOI] [PubMed] [Google Scholar]
- 24.Gaccioli F, Huang CC, Wang C, et al. Amino acid starvation induces the SNAT2 neutral amino acid transporter by a mechanism that involves eukaryotic initiation factor 2alpha phosphorylation and cap-independent translation. J Biol Chem. 2006;281:17929–40. doi: 10.1074/jbc.M600341200. [DOI] [PubMed] [Google Scholar]
- 25.Franchi-Gazzola R, Dall’Asta V, Sala R, et al. The role of the neutral amino acid transporter SNAT2 in cell volume regulation. Acta Physiol (Oxf) 2006;187:273–83. doi: 10.1111/j.1748-1716.2006.01552.x. [DOI] [PubMed] [Google Scholar]
- 26.Steed DL. The role of growth factors in wound healing. Surg Clin North Am. 1997;77:575–86. doi: 10.1016/s0039-6109(05)70569-7. [DOI] [PubMed] [Google Scholar]
- 27.Greenhalgh DG. The role of growth factors in wound healing. J Trauma. 1996;41:159–67. doi: 10.1097/00005373-199607000-00029. [DOI] [PubMed] [Google Scholar]
- 28.Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 2009;17:153–62. doi: 10.1111/j.1524-475X.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- 29.Bielecki TM, Gazdzik TS, Arendt J, et al. Antibacterial effect of autologous platelet gel enriched with growth factors and other active substances: an in vitro study. J Bone Joint Surg Br. 2007;89:417–20. doi: 10.1302/0301-620X.89B3.18491. [DOI] [PubMed] [Google Scholar]
- 30.Della Corte A, Maugeri N, Pampuch A, et al. Application of 2-dimensional difference gel electrophoresis (2D-DIGE) to the study of thrombin-activated human platelet secretome. Platelets. 2008;19:43–5. doi: 10.1080/09537100701609035. [DOI] [PubMed] [Google Scholar]