Abstract
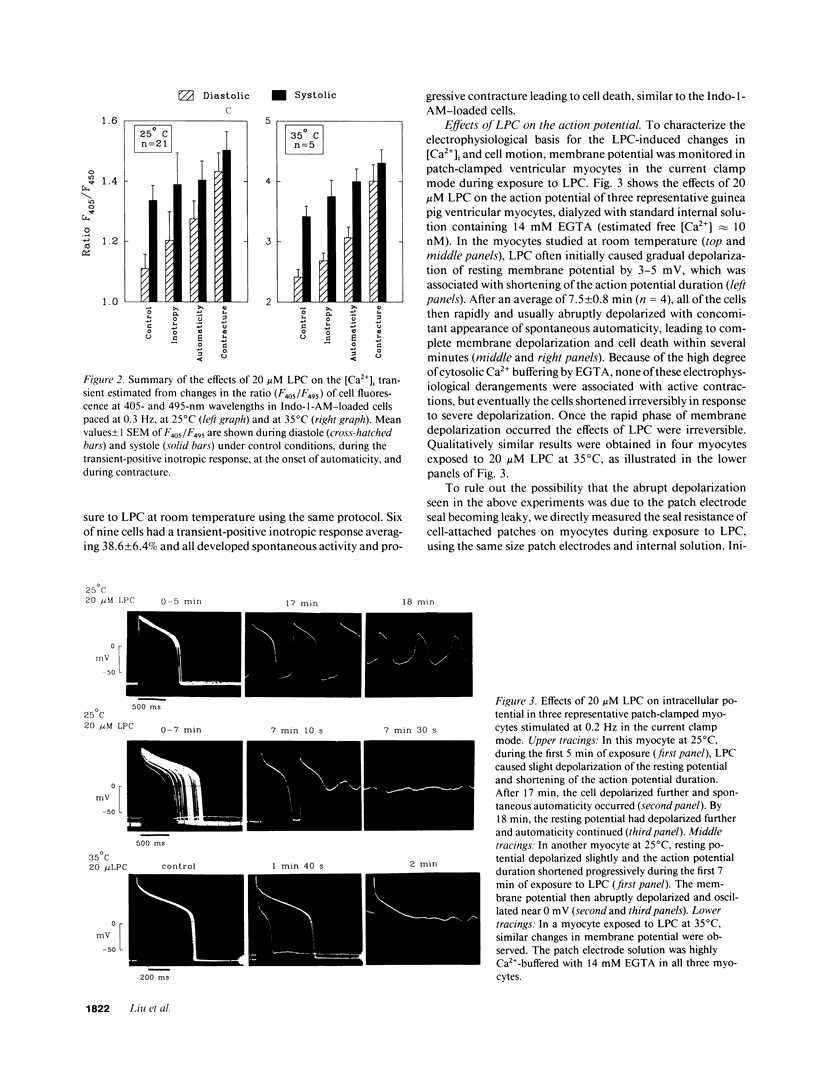
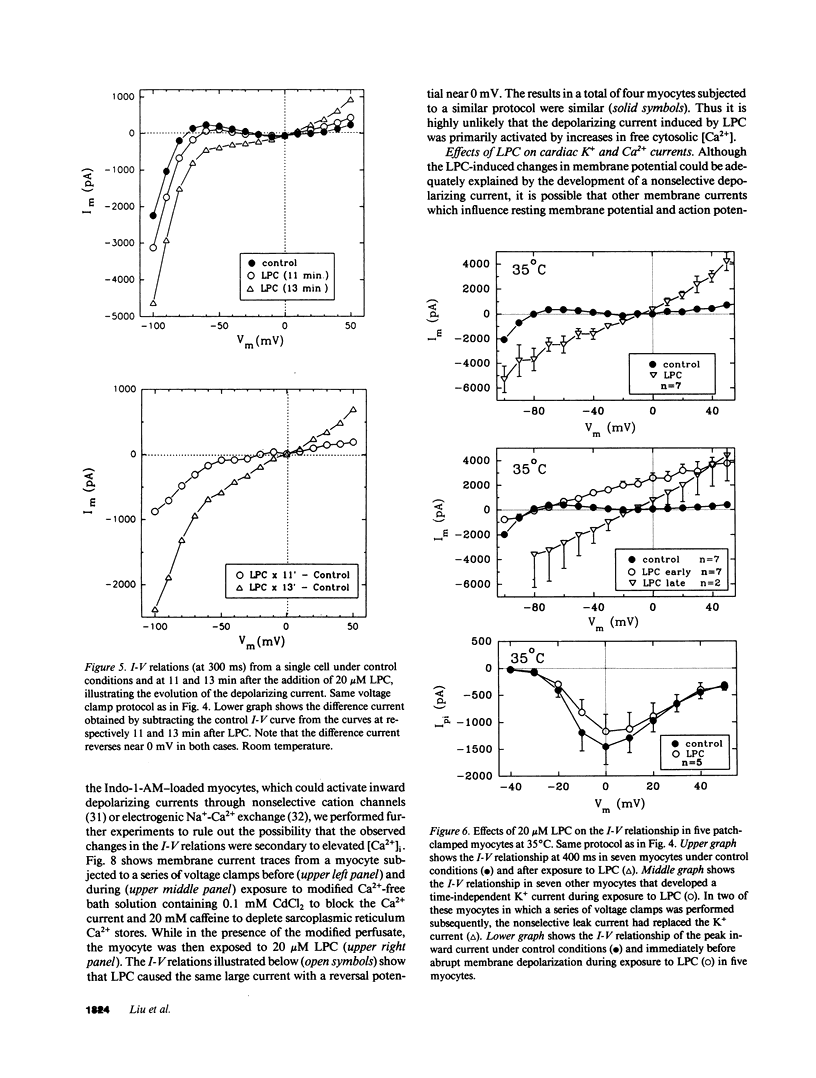
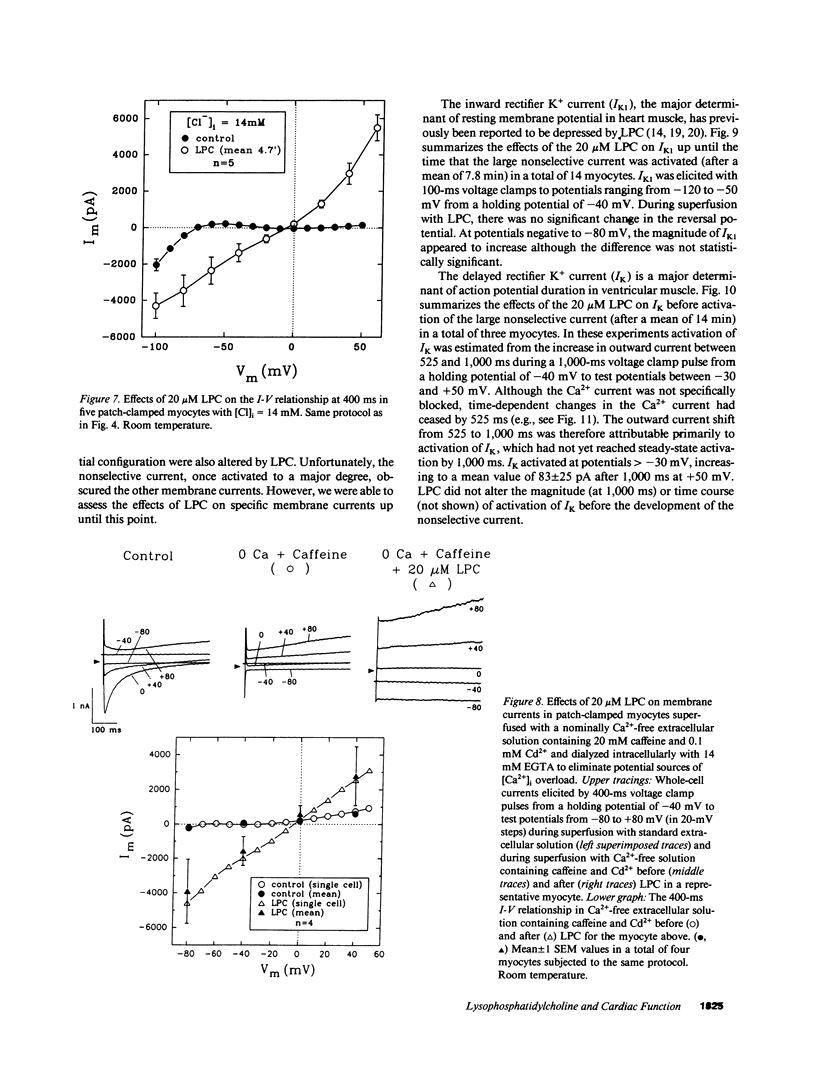
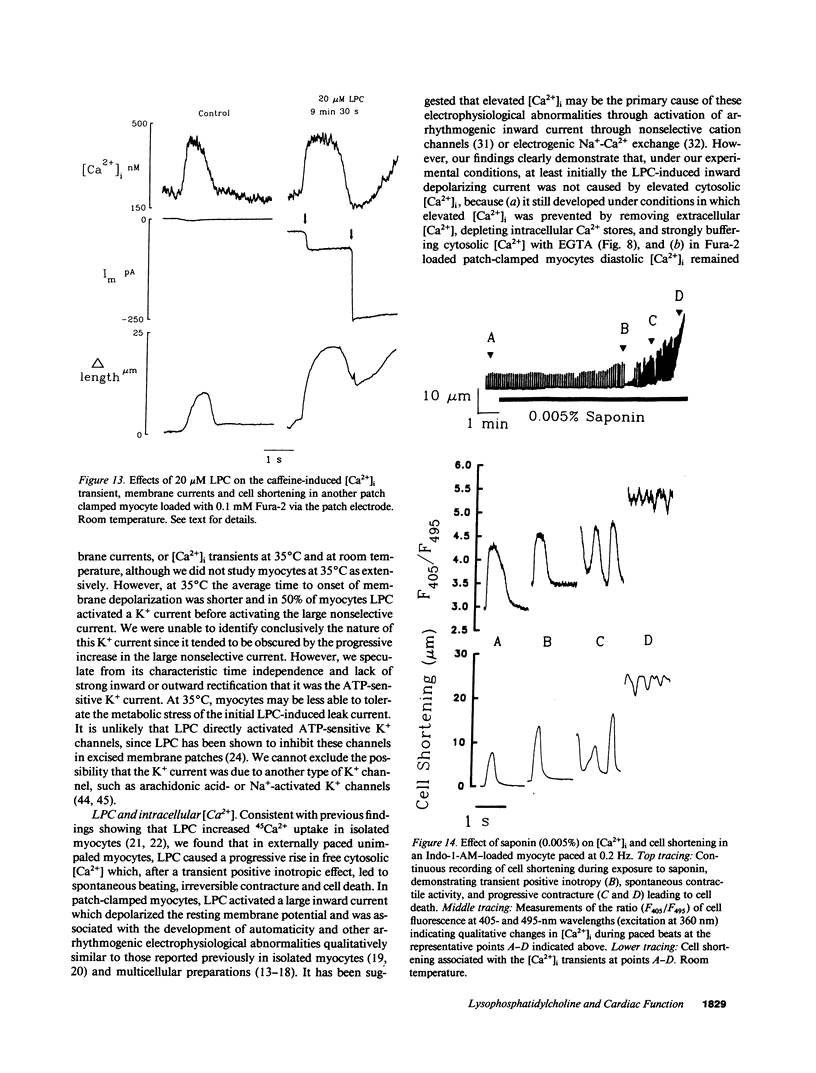
Lysophosphoglyceride accumulation in ischemic myocardium has been implicated as a cause of arrhythmias. We examined the effects of lysophosphatidylcholine (LPC) in isolated guinea pig ventricular myocytes. In paced myocytes loaded with the Ca2+ indicator Indo-1-AM and studied at room temperature, 20 microM LPC caused an initial positive inotropic effect followed by spontaneous automaticity, a decline in active cell shortening, and progressive diastolic shortening (contracture) leading to cell death. These changes were accompanied by a progressive increase in cytosolic [Ca2+]i. In patch-clamped myocytes dialyzed internally with high EGTA concentrations, LPC caused membrane depolarization, shortening of the action potential duration, and abnormal automaticity as seen in multicellular preparations. Voltage clamp experiments revealed the appearance of a nonselective leak conductance without significant changes in the delayed rectifier K+ current, inward rectifier K+ current, L-type Ca2+ current, and T-type Ca2+ current. Pretreatment with 20 mM caffeine and [Ca2+]o-free solution did not prevent the leak current. In patch clamped myocytes loaded with 0.1 mM Fura-2 salt, the [Ca2+]i transient induced by either voltage clamps or brief caffeine exposure remained normal until the nonselective leak current developed. The Na(+)-Ca2+ exchange current elicited during caffeine-induced [Ca2+]i transients also did not appear to be altered by LPC. Qualitatively similar results were obtained in myocytes studied at 35 degrees C. The membrane detergent saponin (0.005% wt/wt) mimicked all of the effects of LPC. We conclude that under these experimental conditions the effects of LPC are most compatible with a detergent action causing membrane leakiness with resultant depolarization, [Ca2+]i overload, and contracture.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambudkar I. S., Abdallah E. S., Shamoo A. E. Lysophospholipid-mediated alterations in the calcium transport systems of skeletal and cardiac muscle sarcoplasmic reticulum. Mol Cell Biochem. 1988 Jan;79(1):81–89. doi: 10.1007/BF00229401. [DOI] [PubMed] [Google Scholar]
- Arnsdorf M. F., Sawicki G. J. The effects of lysophosphatidylcholine, a toxic metabolite of ischemia, on the components of cardiac excitability in sheep Purkinje fibers. Circ Res. 1981 Jul;49(1):16–30. doi: 10.1161/01.res.49.1.16. [DOI] [PubMed] [Google Scholar]
- Bean B. P. Two kinds of calcium channels in canine atrial cells. Differences in kinetics, selectivity, and pharmacology. J Gen Physiol. 1985 Jul;86(1):1–30. doi: 10.1085/jgp.86.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann S. R., Ferguson T. B., Jr, Sobel B. E. Effects of amphiphiles on erythrocytes, coronary arteries, and perfused hearts. Am J Physiol. 1981 Feb;240(2):H229–H237. doi: 10.1152/ajpheart.1981.240.2.H229. [DOI] [PubMed] [Google Scholar]
- Bersohn M. M., Philipson K. D., Weiss R. S. Lysophosphatidylcholine and sodium-calcium exchange in cardiac sarcolemma: comparison with ischemia. Am J Physiol. 1991 Mar;260(3 Pt 1):C433–C438. doi: 10.1152/ajpcell.1991.260.3.C433. [DOI] [PubMed] [Google Scholar]
- Blake K., Clusin W. T., Franz M. R., Smith N. A. Mechanism of depolarization in the ischaemic dog heart: discrepancy between T-Q potentials and potassium accumulation. J Physiol. 1988 Mar;397:307–330. doi: 10.1113/jphysiol.1988.sp017003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge J. H., Spitzer K. W., Ershler P. R. Relaxation of isolated ventricular cardiomyocytes by a voltage-dependent process. Science. 1988 Aug 12;241(4867):823–825. doi: 10.1126/science.3406740. [DOI] [PubMed] [Google Scholar]
- Brovkovich V. M., Nikitchenko I. U., Lemeshko V. V. Narushenie Ca2+-transportiruiushchei funktsii i lipidnogo komponenta membran sarkoplazmaticheskogo retikuluma pri total'noi ishemii miokarda krys. Biull Eksp Biol Med. 1987 Nov;104(11):546–548. [PubMed] [Google Scholar]
- Burnashev N. A., Undrovinas A. I., Fleidervish I. A., Rosenshtraukh L. V. Ischemic poison lysophosphatidylcholine modifies heart sodium channels gating inducing long-lasting bursts of openings. Pflugers Arch. 1989 Oct;415(1):124–126. doi: 10.1007/BF00373151. [DOI] [PubMed] [Google Scholar]
- Callewaert G., Cleemann L., Morad M. Caffeine-induced Ca2+ release activates Ca2+ extrusion via Na+-Ca2+ exchanger in cardiac myocytes. Am J Physiol. 1989 Jul;257(1 Pt 1):C147–C152. doi: 10.1152/ajpcell.1989.257.1.C147. [DOI] [PubMed] [Google Scholar]
- Callewaert G., Cleemann L., Morad M. Epinephrine enhances Ca2+ current-regulated Ca2+ release and Ca2+ reuptake in rat ventricular myocytes. Proc Natl Acad Sci U S A. 1988 Mar;85(6):2009–2013. doi: 10.1073/pnas.85.6.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone E., Lux H. D. Kinetics and selectivity of a low-voltage-activated calcium current in chick and rat sensory neurones. J Physiol. 1987 May;386:547–570. doi: 10.1113/jphysiol.1987.sp016551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson C. W., Ten Eick R. E. On the mechanism of lysophosphatidylcholine-induced depolarization of cat ventricular myocardium. Circ Res. 1983 May;52(5):543–556. doi: 10.1161/01.res.52.5.543. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Neher E., Reuter H., Stevens C. F. Inward current channels activated by intracellular Ca in cultured cardiac cells. Nature. 1981 Dec 24;294(5843):752–754. doi: 10.1038/294752a0. [DOI] [PubMed] [Google Scholar]
- Corr P. B., Cain M. E., Witkowski F. X., Price D. A., Sobel B. E. Potential arrhythmogenic electrophysiological derangements in canine Purkinje fibers induced by lysophosphoglycerides. Circ Res. 1979 Jun;44(6):822–832. doi: 10.1161/01.res.44.6.822. [DOI] [PubMed] [Google Scholar]
- Corr P. B., Gross R. W., Sobel B. E. Amphipathic metabolites and membrane dysfunction in ischemic myocardium. Circ Res. 1984 Aug;55(2):135–154. doi: 10.1161/01.res.55.2.135. [DOI] [PubMed] [Google Scholar]
- Corr P. B., Gross R. W., Sobel B. E. Arrhythmogenic amphiphilic lipids and the myocardial cell membrane. J Mol Cell Cardiol. 1982 Nov;14(11):619–626. doi: 10.1016/0022-2828(82)90159-6. [DOI] [PubMed] [Google Scholar]
- Corr P. B., Saffitz J. E., Sobel B. E. Lysophospholipids, long chain acylcarnitines and membrane dysfunction in the ischaemic heart. Basic Res Cardiol. 1987;82 (Suppl 1):199–208. doi: 10.1007/978-3-662-08390-1_24. [DOI] [PubMed] [Google Scholar]
- Corr P. B., Snyder D. W., Cain M. E., Crafford W. A., Jr, Gross R. W., Sobel B. E. Electrophysiological effects of amphiphiles on canine purkinje fibers. Implications for dysrhythmia secondary to ischemia. Circ Res. 1981 Aug;49(2):354–363. doi: 10.1161/01.res.49.2.354. [DOI] [PubMed] [Google Scholar]
- Corr P. B., Snyder D. W., Lee B. I., Gross R. W., Keim C. R., Sobel B. E. Pathophysiological concentrations of lysophosphatides and the slow response. Am J Physiol. 1982 Aug;243(2):H187–H195. doi: 10.1152/ajpheart.1982.243.2.H187. [DOI] [PubMed] [Google Scholar]
- Corr P. B., Yamada K. A., Creer M. H., Sharma A. D., Sobel B. E. Lysophosphoglycerides and ventricular fibrillation early after onset of ischemia. J Mol Cell Cardiol. 1987 Oct;19 (Suppl 5):45–53. doi: 10.1016/s0022-2828(87)80609-0. [DOI] [PubMed] [Google Scholar]
- Downar E., Janse M. J., Durrer D. The effect of "ischemic" blood on transmembrane potentials of normal porcine ventricular myocardium. Circulation. 1977 Mar;55(3):455–462. doi: 10.1161/01.cir.55.3.455. [DOI] [PubMed] [Google Scholar]
- Fedida D., Noble D., Rankin A. C., Spindler A. J. The arrhythmogenic transient inward current iTI and related contraction in isolated guinea-pig ventricular myocytes. J Physiol. 1987 Nov;392:523–542. doi: 10.1113/jphysiol.1987.sp016795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hagiwara N., Irisawa H., Kameyama M. Contribution of two types of calcium currents to the pacemaker potentials of rabbit sino-atrial node cells. J Physiol. 1988 Jan;395:233–253. doi: 10.1113/jphysiol.1988.sp016916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Janse M. J., Wit A. L. Electrophysiological mechanisms of ventricular arrhythmias resulting from myocardial ischemia and infarction. Physiol Rev. 1989 Oct;69(4):1049–1169. doi: 10.1152/physrev.1989.69.4.1049. [DOI] [PubMed] [Google Scholar]
- Kameyama M., Kakei M., Sato R., Shibasaki T., Matsuda H., Irisawa H. Intracellular Na+ activates a K+ channel in mammalian cardiac cells. Nature. 1984 May 24;309(5966):354–356. doi: 10.1038/309354a0. [DOI] [PubMed] [Google Scholar]
- Katz A. M. Membrane-derived lipids and the pathogenesis of ischemic myocardial damage. J Mol Cell Cardiol. 1982 Nov;14(11):627–632. doi: 10.1016/0022-2828(82)90160-2. [DOI] [PubMed] [Google Scholar]
- Katz A. M., Messineo F. C. Lipid-membrane interactions and the pathogenesis of ischemic damage in the myocardium. Circ Res. 1981 Jan;48(1):1–16. doi: 10.1161/01.res.48.1.1. [DOI] [PubMed] [Google Scholar]
- Kim D., Clapham D. E. Potassium channels in cardiac cells activated by arachidonic acid and phospholipids. Science. 1989 Jun 9;244(4909):1174–1176. doi: 10.1126/science.2727703. [DOI] [PubMed] [Google Scholar]
- Kim D., Duff R. A. Regulation of K+ channels in cardiac myocytes by free fatty acids. Circ Res. 1990 Oct;67(4):1040–1046. doi: 10.1161/01.res.67.4.1040. [DOI] [PubMed] [Google Scholar]
- Kinnaird A. A., Choy P. C., Man R. Y. Lysophosphatidylcholine accumulation in the ischemic canine heart. Lipids. 1988 Jan;23(1):32–35. doi: 10.1007/BF02535301. [DOI] [PubMed] [Google Scholar]
- Kiyosue T., Aomine M., Arita M. Lysophosphatidylcholine decreases single channel conductance of inward rectifier K channel in mammalian ventricular myocytes. Jpn J Physiol. 1984;34(2):369–373. doi: 10.2170/jjphysiol.34.369. [DOI] [PubMed] [Google Scholar]
- Kiyosue T., Arita M. Effects of lysophosphatidylcholine on resting potassium conductance of isolated guinea pig ventricular cells. Pflugers Arch. 1986 Mar;406(3):296–302. doi: 10.1007/BF00640917. [DOI] [PubMed] [Google Scholar]
- Kléber A. G. Resting membrane potential, extracellular potassium activity, and intracellular sodium activity during acute global ischemia in isolated perfused guinea pig hearts. Circ Res. 1983 Apr;52(4):442–450. doi: 10.1161/01.res.52.4.442. [DOI] [PubMed] [Google Scholar]
- Lee Y., Chan S. I. Effect of lysolecithin on the structure and permeability of lecithin bilayer vesicles. Biochemistry. 1977 Apr 5;16(7):1303–1309. doi: 10.1021/bi00626a010. [DOI] [PubMed] [Google Scholar]
- Liedtke A. J. Alterations of carbohydrate and lipid metabolism in the acutely ischemic heart. Prog Cardiovasc Dis. 1981 Mar-Apr;23(5):321–336. doi: 10.1016/0033-0620(81)90019-0. [DOI] [PubMed] [Google Scholar]
- Liedtke A. J. Lipid burden in ischemic myocardium. J Mol Cell Cardiol. 1988 Mar;20 (Suppl 2):65–74. doi: 10.1016/0022-2828(88)90333-1. [DOI] [PubMed] [Google Scholar]
- Mak I. T., Kramer J. H., Weglicki W. B. Potentiation of free radical-induced lipid peroxidative injury to sarcolemmal membranes by lipid amphiphiles. J Biol Chem. 1986 Jan 25;261(3):1153–1157. [PubMed] [Google Scholar]
- Mitra R., Morad M. Two types of calcium channels in guinea pig ventricular myocytes. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5340–5344. doi: 10.1073/pnas.83.14.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B., Hess P., Lansman J. B., Tsien R. W. A novel type of cardiac calcium channel in ventricular cells. Nature. 1985 Aug 1;316(6027):443–446. doi: 10.1038/316443a0. [DOI] [PubMed] [Google Scholar]
- Noireaud J., Bright C. M., Ellis D. Effects of saponin on contractile force and intracellular ion activities of cardiac tissues. J Mol Cell Cardiol. 1989 Mar;21(3):291–298. doi: 10.1016/0022-2828(89)90744-x. [DOI] [PubMed] [Google Scholar]
- Oishi K., Zheng B., Kuo J. F. Inhibition of Na,K-ATPase and sodium pump by protein kinase C regulators sphingosine, lysophosphatidylcholine, and oleic acid. J Biol Chem. 1990 Jan 5;265(1):70–75. [PubMed] [Google Scholar]
- Pogwizd S. M., Onufer J. R., Kramer J. B., Sobel B. E., Corr P. B. Induction of delayed afterdepolarizations and triggered activity in canine Purkinje fibers by lysophosphoglycerides. Circ Res. 1986 Oct;59(4):416–426. doi: 10.1161/01.res.59.4.416. [DOI] [PubMed] [Google Scholar]
- Reiss-Husson F. Structure des phases liquide-cristallines de différents phospholipides, monoglycérides, sphingolipides, anhydres ou en présence d'eau. J Mol Biol. 1967 May 14;25(3):363–382. doi: 10.1016/0022-2836(67)90192-1. [DOI] [PubMed] [Google Scholar]
- Sedlis S. P., Corr P. B., Sobel B. E., Ahumada G. G. Lysophosphatidyl choline potentiates Ca2+ accumulation in rat cardiac myocytes. Am J Physiol. 1983 Jan;244(1):H32–H38. doi: 10.1152/ajpheart.1983.244.1.H32. [DOI] [PubMed] [Google Scholar]
- Sedlis S. P., Sequeira J. M., Ahumada G. G., el Sherif N. Effects of lysophosphatidylcholine on cultured heart cells: correlation of rate of uptake and extent of accumulation with cell injury. J Lab Clin Med. 1988 Dec;112(6):745–754. [PubMed] [Google Scholar]
- Shaikh N. A., Downar E. Time course of changes in porcine myocardial phospholipid levels during ischemia. A reassessment of the lysolipid hypothesis. Circ Res. 1981 Aug;49(2):316–325. doi: 10.1161/01.res.49.2.316. [DOI] [PubMed] [Google Scholar]
- Snyder D. W., Crafford W. A., Jr, Glashow J. L., Rankin D., Sobel B. E., Corr P. B. Lysophosphoglycerides in ischemic myocardium effluents and potentiation of their arrhythmogenic effects. Am J Physiol. 1981 Nov;241(5):H700–H707. doi: 10.1152/ajpheart.1981.241.5.H700. [DOI] [PubMed] [Google Scholar]
- Stafford R. E., Fanni T., Dennis E. A. Interfacial properties and critical micelle concentration of lysophospholipids. Biochemistry. 1989 Jun 13;28(12):5113–5120. doi: 10.1021/bi00438a031. [DOI] [PubMed] [Google Scholar]
- Steadman B. W., Moore K. B., Spitzer K. W., Bridge J. H. A video system for measuring motion in contracting heart cells. IEEE Trans Biomed Eng. 1988 Apr;35(4):264–272. doi: 10.1109/10.1375. [DOI] [PubMed] [Google Scholar]
- Weiss J., Shine K. I. Effects of heart rate on extracellular [K+] accumulation during myocardial ischemia. Am J Physiol. 1986 Jun;250(6 Pt 2):H982–H991. doi: 10.1152/ajpheart.1986.250.6.H982. [DOI] [PubMed] [Google Scholar]
- Weltzien H. U. Cytolytic and membrane-perturbing properties of lysophosphatidylcholine. Biochim Biophys Acta. 1979 Aug 20;559(2-3):259–287. doi: 10.1016/0304-4157(79)90004-2. [DOI] [PubMed] [Google Scholar]
- van der Vusse G. J., Prinzen F. W., van Bilsen M., Engels W., Reneman R. S. Accumulation of lipids and lipid-intermediates in the heart during ischaemia. Basic Res Cardiol. 1987;82 (Suppl 1):157–167. doi: 10.1007/978-3-662-08390-1_20. [DOI] [PubMed] [Google Scholar]
- van der Vusse G. J., Stam H. Lipid and carbohydrate metabolism in the ischaemic heart. Basic Res Cardiol. 1987;82 (Suppl 1):149–153. doi: 10.1007/978-3-662-08390-1_18. [DOI] [PubMed] [Google Scholar]


