Abstract
Background
Autologous or allogenic platelet gel is a blood component that exploits the effects of the cytokines contained in platelet α granules to stimulate repair processes. The properties of platelet gel were first tested on chronic ulcers to accelerate healing and later in orthopaedic, dental, vascular and cardiothoracic surgery.
In our centre, we have been using platelet gel for 5 years, first for surgical patients with difficult wounds, then for orthopaedic patients undergoing osteosynthesis surgery and patients with ulcers not responding to traditional therapies. Subsequently we decided to extend the use of platelet gel also to amputations or traumatic loss of tissue of fingers.
Materials and methods
In this article we present the results obtained over 5 years concerning 115 patients with finger amputations or wounds treated with platelet gel in our Service of Transfusion Medicine. Platelets were obtained fom allogeneic buffy coats (10 mL) and the gel was produced by adding thrombin to concentrated platelets.
The decision to use homologous platelet gel was based on its limited cost, ease of preparation, almost unlimited availability, the fact that the number of platelets that can be collected is much higher than the therapeutic range and so able to replace the losses due to secondary medication, and last, but not least, it causes no discomfort to patients. The safety of the product was ensured by virology tests including molecular biology studies.
Results
The recovery of soft tissue in all patients ranged from 80 to 100%; the median time for this recovery was 3 weeks (range, 10 days - 6 weeks). Approximately 60% of the patients complained of local hypoaesthesia for some weeks; 30% of the patients developed hyperaesthesia, which resolved completely within 6–8 weeks from starting treatment. Loss of bone tissue represented an obstacle to total tissue recovery, but the aesthetic results were satisfactory in nearly all cases.
Conclusion
All patients showed good compliance, both because of the low frequency of medications (at most, twice a week) and because of the painless platelet gel applications. The only negative aspect was abnormal nail growth in a case of distal partial amputation of a finger.
In conclusion, we believe that platelet gel can be very useful in patients with traumatic or surgical loss of finger tissue, since it can resolve critical situations thus avoiding amputation of residual tissue and compromised joint function.
Keywords: platelet gel, platelet growth factors, tissue regeneration
Introduction
Platelet gel of homologous or autologous derivation is a blood component obtained by mixing platelets, thrombin and/or calcium. Platelets can be obtained from apheresis or from buffy coats after blood separation1. In the last 20 years, several studies showed that the growth of tissues in culture increased when platelets were added2–5. Subsequently, platelet growth factors were identified and isolated and their efficacy was demonstrated first in animals and later on human tissues.
When thrombin are added to platelets, these latter release growth factors into the extracellular environment; the growth factors bind specific receptors, activating a pathway of intracellular tissue signals that start the repair process6. These factors (platelet-derived growth factor, insulin-like growth factors I and II, transforming growth factor-β, epidermal growth factor, platelet-derived angiogenesis factor…) 7,8 stimulate mitosis, chemotaxis, angiogenesis and bone growth and are found in α granules contained in the cytoplasm of platelets.
Platelet-derived growth factors act in synergy with plasma-derived factors to activate a complex network of autocrine functions that modulate healing9. These properties have been exploited through the use of platelets as a gel in several medical specialities: orthopaedics10, dentistry11,12, vascular and cardiothoracic surgery, geriatrics and dermatology13,14. New strategies of platelet gel application are currently under investigation: a recent study evaluated the use of platelet gel in the case of myocardial injury, in order to promote remodelling through regeneration of myocytes, induction of angiogenesis and restoration of a normal extracellular matrix composition15. The use of fat grafting combined with platelet-rich plasma was recently found to be effective in reconstructive surgery of chronic ulcers of the lower limbs16.
In our centre, we have been using platelet gel for over 5 years, at first for surgical patients with difficult wounds, then for orthopaedic patients undergoing osteosynthesis surgery and patients with ulcers not responding to traditional therapy. In July 2004, we needed an alternative therapy to treat partial amputation of a finger with loss of tissue and decided to extend the use of platelet gel to such cases.
In this article we present the results of 5 years of use of platelet gel applications in patients who had loss of finger tissue as a result of trauma or amputation. The patients were treated in our Transfusion Medicine Service, in collaboration with the Orthopaedics and Emergency Departments.
Materials and methods
Between January 2004 and December 2008, 115 patients (99 males and 16 females) were enrolled in the study from the Orthopaedics or Emergency Department. The mean age of the patients was 46 years (range, 10–76 years). Of these patients, 70 had a diagnosis of partial amputation with loss of soft tissue; 32 had loss of finger or hand soft tissue; 7 had severe loss of finger or hand substance after a crushing injury, 3 had sequelae of osteotomy surgery after partial amputation and 3 had post-infective necrosis of the finger pad with exposure of the first phalanx. Forty-eight patients had loss of distal bone tissue of the first phalanx.
All patients were treated with homologous group O buffy coat-derived platelets, after having received the proper information on the type of treatment and signed informed consent. Pools were obtained by mixing five or six buffy-coats in Termo Teruflex BP-KIT bags (Terumo®), with an in-line Imugard III-SPL filter (Terumo®), adding 300 mL T-Sol (Baxter®) or Composol (Fresenius®).
An adequate amount of platelets was transferred from the pool to a sterile test-tube (5 mL), then centrifuged to concentrate it to the necessary volume (1–2 mL) and stored in a platelet agitator at a constant temperature until use. The platelet concentration ranged from 5 to 6.3 x 109/μL, which was clearly superior to the therapeutic range (from 1 to 2.5 x 106 platelets/μL), but able to guarantee the same efficacy in consideration of the loss of the product due to its absorption by the secondary medication.
Platelet activation was induced by homologous thrombin obtained in sterile test-tubes starting from fresh apheresis-derived plasma (10 mL) from periodic donors, which, following the addition of 2 mL calcium gluconate, was incubated at 37 °C for 30 minutes and then centrifuged at 3,000 rpm for 20 minutes. Once separated, the platelet-rich supernatant was placed in 1.5 mL Eppendorf test-tubes at a dose of 0.5 or 1 mL and then frozen17,18. This process led to activation of platelets and the formation of an aggregate that was then applied to wounds.
The time of aggregation ranged from 3 to 4 minutes, which is slightly longer than the time of activation of autologous platelet-rich plasma with thrombin and calcium gluconate using the classical method of activation; however, the difference was negligible and in any case we abandoned the practice of adding calcium gluconate to activate the gel about 4 years ago, in order to reduce the burning sensation induced by this substance.
Platelet gels were prepared each morning by technical staff, while applications were performed twice a week in the afternoon. After application, the platelet gel was covered by Adaptic (Johnson & Johnson®) or similar non-adherent gauze which was held in place by an Autofix (Fra-production SpA®) or similar type of bandage. The platelet gel applications were continued until soft tissue recovered; they were discontinued when the recovery was complete or almost complete.
To ensure the quality of the platelet gel preparation and application, defined rules were followed and randomised sterility controls conducted in accordance with good manufacturing practice (GMP) regarding the preparation, conservation and distribution of products. In addition, each patient’s access to the Transfusion Medicine Service and every procedure were recorded in the patient’s clinical chart and photographic documentation of the results was obtained in most cases.
Results
To judge the patients’ outcome, we chose an “observational” approach, comparing the injured fingers with the intact ones on the other hand; such a comparison allowed a direct evaluation of tissue growth and the aesthetic result.
The recovery of soft tissue ranged from 80 to 100%: patients who had had partial bone loss of the first phalanx recovered 80% of the original morphology, while the recovery in patients with preserved bone was between 90% and 100%. There were no significant differences in response in relation to the patients’ age.
The aesthetic result was satisfactory for all patients (Figures 1 to 3) although generally bone loss represented an important obstacle to total tissue recovery (Figure 3); in contrast, patients with preserved bone tissue obtained complete and aesthetically acceptable recovery.
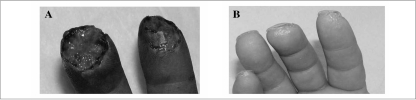
Figure 1.
A) Patient C.A. underwent soft tissue amputation, without bone loss, of the second and third fingers of the right hand. B) One month after trauma and following four applications of platelet gel at a frequency of twice a week, tissue was completely regenerated and the skin covered. In this case the nail grew bending down.
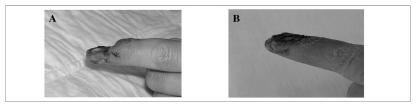
Figure 3.
A) Patient G.A. underwent partial amputation of the left thumb after trauma, with distal bone loss. B) Two months after trauma, following five platelet gel applications at a frequency of once a week, tissue was completely regenerated and skin covered. Tissue recovery was not complete but the aesthetic result was satisfactory.
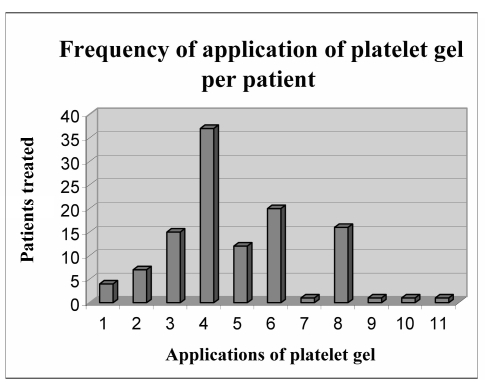
The median time for recovery was 3 weeks (range, 10 days to 6 weeks). The number of platelet gel applications ranged from 1 to 11, with a median of six applications (Figure 4). Skin formation appeared approximately 10–15 days after tissue recovery, following detachment of scabs. Patients who completely lost their nail did not recover it at the end of the platelet gel applications (Figure 2b); in contrast, if the lunule was preserved, nail growth was fully restored (Figure 3).
Figure 4.
The figure shows the number of platelet gel applications in relation to the number of patients. Patients received from 1 to 11 applications. The median number of platelet applications was six; most patients received four applications. Only a few patients received more than eight applications.
Figure 2.
A) Patient K.L. underwent partial amputation of the distal phalanx of the second finger on the left hand after trauma, without bone loss. B) Twenty-five days after trauma, and 7 days after the end of treatment with platelet gel (six applications, at a frequency of twice a week) tissue was almost completely recovered and skin had started to grow. The nail failed to grow, since it was completely removed by the trauma.
Sixty percent of the patients complained of local hypoaesthesia for some weeks; 30% of patients developed hyperaesthesia, which resolved completely within 6–8 weeks of treatment. This hyperaesthesia was probably due to an increased consistency of the regenerated tissue.
Discussion
Overall, the results concerning 115 patients with soft tissue and bone damage of the fingers treated with platelet gel are encouraging. The compliance was good for all the patients, both because of the low frequency of the medications (twice a week, at most) and because the platelet gel applications were painless.
The aesthetic results of lesions treated with platelet gel were better than those of untreated fingers wounds and the recovery time of the former was shorter, in our experience (3 weeks versus 2 months, approximately). We considered such a period as congruous and compatible with proper recovery, with guarantee of functional recovery and early resumption of a working activity as well.
The patients’ satisfaction at the end of treatment represented an important means to judge the therapeutic effects of the treatment. The only regret was the abnormal nail growth in the case of distal partial amputation of a finger: in this case the nail grew from a shortened nail bed, and, therefore, tended to bend down as it grew, worsening the aesthetic result. (Figure 1b).
For the future, we think it is necessary to standardise the techniques for platelet gel application and to perform new randomised studies to determine the effects of this blood component on damaged tissues. The exploration of new fields of application is an important challenge: the use of platelet growth factors in combination with tissue engineering could represent the most promising method for treating bone and/or cartilaginous tissue defects19.
References
- 1.Zimmermann R, Jakubiets R, Jakubiets M, et al. Different preparation methods to obtain platelet components as a source of growth factors for local application. Transfusion. 2001;41:1217–24. doi: 10.1046/j.1537-2995.2001.41101217.x. [DOI] [PubMed] [Google Scholar]
- 2.D’Agostino E, Fratellanza G, Caloprisco G, et al. Effetto del gel piastrinico sulla proliferazione cellulare in vitro. La Trasf del Sangue. 2002;47:429–33. [Google Scholar]
- 3.Weiser L, Bhargava M, Attia E, Torzilli PA. Effect of serum and platelet-derived growth factor on chondrocytes grown in collagen gels. Tissue Eng. 1999;5:533–44. doi: 10.1089/ten.1999.5.533. [DOI] [PubMed] [Google Scholar]
- 4.Lucarelli E, Beccheroni A, Donati D, et al. Platelet-derived growth factors enhance proliferation of human stromal stem cells. Biomaterials. 2003;24:3095–100. doi: 10.1016/s0142-9612(03)00114-5. [DOI] [PubMed] [Google Scholar]
- 5.Krasna M, Domanović D, Tomsic A, et al. Platelet gel stimulates proliferation of human dermal fibroblasts in vitro. Acta Dermatovenerol Alp Panonica Adriat. 2007;16:105–10. [PubMed] [Google Scholar]
- 6.Everts PA, Overdest EP, Jakimowicz JJ, et al. The use of autologous platelet-leukocyte gels to enhance the healing process in surgery, a review. Surg Endosc. 2007;21:2063–68. doi: 10.1007/s00464-007-9293-x. [DOI] [PubMed] [Google Scholar]
- 7.Mazzucco L, Medici D, Serra M, et al. The use of autologous platelet gel to treat difficult-to-heal wounds: a pilot study. Transfusion. 2004;44:1013–8. doi: 10.1111/j.1537-2995.2004.03366.x. [DOI] [PubMed] [Google Scholar]
- 8.Borzini P, Mazzucco L. Tissue regeneration and in loco administration of platelet derivates: outcome, heterogeneous products, and heterogeneity of the effector mechanisms. Transfusion. 2005;45:1759–67. doi: 10.1111/j.1537-2995.2005.00600.x. [DOI] [PubMed] [Google Scholar]
- 9.Borzini P, Mazzucco L. Platelet gel and releases. Curr Opin Hematol. 2005;12:473–9. doi: 10.1097/01.moh.0000177831.70657.e8. [DOI] [PubMed] [Google Scholar]
- 10.Rughetti A, Flamini S, Colafarina O, et al. Closed surgery: autologous platelet gel for the treatment of pseudoarthrosis. Blood Transfus. 2004;2:37–43. [Google Scholar]
- 11.Fontana S, Olmedo ER, Limares JA, et al. Effect of platelet-rich plasma on the peri-implant bone response: an experimental study. Implant Dent. 2004;13:73–8. doi: 10.1097/01.id.0000116455.68968.29. [DOI] [PubMed] [Google Scholar]
- 12.Marx RE, Carlson ER, Eichstaedt RM, et al. Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638–46. doi: 10.1016/s1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
- 13.Borzini P, Mazzucco L, Panizza R, et al. Regarding “Randomized trial and local biological effect of autologous platelets used as adjuvant therapy for chronic venous leg ulcers”. J Vasc Surg. 2004;39:1146–7. doi: 10.1016/j.jvs.2004.01.047. [DOI] [PubMed] [Google Scholar]
- 14.Fecarelli E, Bernuzzi G, Tognetti E, et al. Treatment of chronic venous leg ulcers by platelet gel. Dermatol Ther. 2008;21:S13–7. doi: 10.1111/j.1529-8019.2008.00196.x. [DOI] [PubMed] [Google Scholar]
- 15.Mogan C, Larson DF. Rationale of platelets gel to augment adaptive remodelling of the injured heart. J Extra Corpor Technol. 2004;36:191–6. [PubMed] [Google Scholar]
- 16.Cervelli V, Gentile P, Scioli MG, et al. Application of platelet-rich plasma to fat grafting during plastic surgical procedures: clinical and in vitro evaluation. Tissue Eng Part C Methods. 2009;15:625–34. doi: 10.1089/ten.TEC.2008.0518. [DOI] [PubMed] [Google Scholar]
- 17.Martineau I, Lacoste E, Ganon G. Effects of calcium and thrombin on growth factor release from platelet concentrates: kinetics and regulation of endothelial cell proliferation. Biomaterials. 2004;25:4489–02. doi: 10.1016/j.biomaterials.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 18.Mazzucco L. Tecniche di preparazione del gel piastrinico. 4° Convegno di Aggiornamenti di Medicina Trasfusionale, Savigliano - 24/10/2003.
- 19.Wrotniak M, Bielecki T, Gaždzik TS. Current opinion about using the platelet-rich gel in orthopaedics and trauma surgery. Ortop Traumatol Rehabil. 2007;9:227–38. [PubMed] [Google Scholar]