Abstract
Neuroimaging studies with adults have begun to reveal the neural bases of empathy; however, this research has focused on empathy for physical pain, rather than empathy for negative social experiences. Moreover, this work has not examined adolescents who may frequently witness and empathize with others who experience negative social experiences like peer rejection. Here, we examined neural activity among early adolescents observing social exclusion compared to observing inclusion, and how this activity related to both trait empathy and subsequent prosocial behavior. Participants were scanned while they observed an individual whom they believed was being socially excluded. At least one day prior to the scan they reported their trait empathy, and following the scan they wrote emails to the excluded victim that were rated for prosocial behavior (e.g., helping, comforting). Observing exclusion compared to inclusion activated regions involved in mentalizing (i.e., dorsomedial prefrontal cortex; DMPFC), particularly among highly empathic individuals. Additionally, individuals who displayed more activity in affective, pain-related regions during observed exclusion compared to inclusion subsequently wrote more prosocial emails to excluded victims. Overall findings suggest that when early adolescents witness social exclusion in their daily lives, some may actually ‘feel the pain’ of the victims and act more prosocially toward them as a result.
Keywords: adolescence, empathy, peer rejection, social exclusion, functional magnetic resonance imaging
The ability to feel empathy allows children to understand others’ feelings, even when they are different from their own, and to form successful social relationships with others. In fact, throughout development, children’s empathic ability has been consistently linked with a variety of prosocial behaviors, such as helping others (Litvack-Miller, McDougall, & Romney, 1997). Given the critical role that empathy plays in promoting successful social relationships, the ability to empathize is likely to be particularly important during early adolescence when maintaining peer relationships becomes central to well-being (e.g., Brown, 1990).
Although emerging research has revealed new insights about the processes that underlie empathic experiences, neuroimaging research on empathy has been dominated by studies examining empathy for physical pain among adults (see Singer, 2006 and Jackson, Rainville, & Decety, 2006 for reviews) and children (Decety, Michalska, & Akitsuki, 2008). In contrast, little work has examined empathy for distressing social situations in adults (c.f., Immordino-Yang, McColl, Damasio, & Damasio, 2009; Masten, Morelli, & Eisenberger, under review), and no neuroimaging research has examined empathy for distressing social situations among children or adolescents. During early adolescence in particular, when individuals place greater value on maintaining social acceptance and may feel particularly threatened by negative experiences like peer rejection (Brown, 1990), observing and feeling empathy for social exclusion is likely to be a more common and salient experience than observing physical pain. In the current study, we used functional magnetic resonance imaging (fMRI) techniques to examine the neural correlates of early adolescents’ empathy for a peer experiencing social exclusion, as well as how these neural processes might relate to the prosocial behaviors that these youth display toward the victims of peer exclusion.
The cognitive and affective components of empathy
Behavioral research on empathy with both children and adults has long suggested that the experience of empathy includes two primary components (Baron-Cohen, 2003; Eisenberg, Spinrad, & Sadovsky, 2006): 1) a cognitive component that involves thinking about and understanding the mental states of others (often termed ‘mentalizing’; Frith, Leslie, & Morton, 1991) and 2) an affective component that involves sharing the emotional experiences of others. However, with behavioral measures alone, it has been difficult to differentiate between these two components of empathy in the moment of the experience, or to examine how these components might differentially relate to prosocial, empathy-induced behavioral responses, such as helping and comforting others.
Fortunately, recent neuroimaging research has shown that the cognitive and affective components do indeed rely on distinct neural networks (Decety & Meyer, 2008; Singer, 2006). For example, studies have indicated that the cognitive component of empathy relies on a network of regions that are associated with mentalizing. Specifically, the posterior superior temporal sulcus (pSTS), the temporal poles, the precuneus/posterior cingulate cortex (PCC), and particularly the medial and dorsomedial prefrontal cortex (MPFC/DMPFC) have been linked with various types of mentalizing processes (Frith & Frith, 1999; Frith & Frith, 2003; Frith & Frith, 2006; Mitchell, Banaji, & Macrae, 2005; Singer, 2006). Moreover, similar networks associated with social understanding of others have also been observed among children thinking about motivations for observed infliction of physical pain on others (Decety, Michalska, & Akitsuki, 2008).
In contrast, the neural regions underlying the affective component of empathy have been shown to overlap with those activated during personally experienced affect such as fear, disgust, and physical pain. For example, the anterior insula is activated by both direct and observed experiences of disgust (Wicker et al., 2003), the amygdala is activated during direct and observed fear (Whalen et al., 2001), and the dACC and anterior insula are activated by direct and observed physical pain in adults (Singer et al., 2004; Singer et al., 2006; Jackson, Bruney, Meltzoff, & Decety, 2005; Botvinick et al., 2005; Morrison, Lloyd, Di Pellegrino, & Roberts, 2004) and children (Decety, Michalska, & Akitsuki, 2008). In addition, similar brain regions are activated when children make or observe others making emotional facial expressions, and the neural circuitry involved in observing others’ emotions is activated more among highly empathic children (Pfeifer, Iacoboni, Mazziotta, & Dapretto, 2008).
Empathy and prosocial behaviors
In trying to understand the processes underlying early adolescents’ empathy for social experiences, one last issue to consider is how empathic processes relate to prosocial behavior in these situations. Behavioral research has shown that feeling more empathy for others is associated with greater concern for the welfare of others (Batson, 1998), and more helping behavior toward others (Batson, 1991; Batson, 1998; Dovidio, Allen & Schroeder, 1990; Schroeder, Dovidio, Sibicky, Mathews & Allen, 1988; Oswald, 1996). Moreover, individuals who display more empathy for others’ problems are also more likely to subsequently aid the victims in these situations (Davis, 1983; Davis et al., 1999). Particularly among children, empathy has also been positively linked with prosocial behavior (Denham et al, 1994; Zahn-Waxler, Radke-Yarrow, Wagner, & Chapman, 1992), and teachers’ ratings of helpfulness (Litvack-Miller, McDougall, & Romney, 1997). Despite this evidence linking empathy and a range of prosocial behaviors and previous neuroimaging work indicating that specific empathy-related neural responses relate to more prosocial behavior in adults (Masten, Morelli, & Eisenberger, under review), links between neural functioning during empathy and early adolescents’ prosocial behavior have not been tested. Thus, it is unknown how individual differences in neural responses to observing another’s experience of social exclusion relate to subsequent prosocial behavior toward the victim of the exclusion.
Previous research examining empathy for social exclusion among adults
A recent study examined the neural correlates of the cognitive and affective components of empathy for social exclusion among adults to examine how these neural patterns related to trait empathy and prosocial behaviors (Masten, Morelli, & Eisenberger, under review). Using a task and design similar to the current study, adults were scanned while they observed an individual being ostensibly excluded by others (which was actually a staged computer interaction). After this, they wrote emails to the ‘victim’ of the exclusion, and these emails were later scored for prosocial behavior by outside raters. Results indicated that adults displayed more activity in the mentalizing network (i.e., DMPFC, MPFC, precuneus) when they observed social exclusion compared to inclusion; however, higher levels of trait empathy were associated with a greater difference in activity during observed exclusion relative to inclusion in regions associated with social pain processing as well as those involved in mentalizing. Furthermore, adults who showed more activity in brain regions involved in social pain processing and mentalizing during observed exclusion compared to inclusion subsequently wrote more prosocial emails to the victims of the exclusion, and activity in the MPFC in particular mediated the link between trait empathy and prosocial behavior. As a whole, these findings suggest that both mentalizing and affective processing occur when adults witness and experience empathy for others being socially excluded, and these processes are related to individual differences in trait empathy and resulting behaviors.
The current study
In the current study, we aimed to extend previous research by examining the neural networks underlying early adolescents’ empathy for the experience of peer rejection. We also examined how this neural activity related to their trait empathy and their tendency to act prosocially toward the victim. To accomplish this we scanned 13-year-olds while they believed they were watching three same-age, unfamiliar peers playing an online ball-tossing game from which one player was eventually excluded (although in reality they were observing a computer program). We compared neural activity during the observation of exclusion versus inclusion and examined how differences in trait empathy related to the magnitude of difference in activity between these two conditions. Finally, to examine links between empathic processing and prosocial behavior, we asked participants to email the victim a message about what they had observed. We then examined how neural activity while watching the victim experience exclusion related to how prosocial these emails were judged to be (by a separate set of raters).
Overall, we hypothesized that early adolescents would show neural patterns during empathy for social exclusion similar to those previously seen in adults. For example, we expected that individual differences in trait empathy would specifically relate to the neural engagement of mentalizing regions during this empathic experience. However, we also predicted some development-related patterns among this age group1. Specifically, given that trait empathy among early adolescents can reflect prosocial reasoning ability and related cognitive advancements (e.g., Eisenberg, Miller, Shell, McNalley, & Shea, 1991; Guthrie, Eisenberg, Fabes, Murphy, & Shepard, 1997), these self-reported empathic differences could particularly relate to brain activity in the mentalizing regions that support these cognitive processes. Yet, we also theorized that activity in affective pain-related regions might be particularly influential on early adolescents’ prosocial behaviors. Given the ‘every-day’ nature of witnessing peer rejection for this population, we expected that in contrast to adults, simply mentalizing about victims’ experiences might not elicit prosocial actions or any attempt to ‘interfere’ by early adolescents. However, actual personal distress could provide youth with additional motivation to help and support the victim—potentially to help mitigate their own distress.
Method
Participants
Participants included an ethnically and socioeconomically diverse sample of 20 early adolescents (10 females) from the greater Los Angeles area. All participants had attended at least one year of middle school and ranged in age from 12 to 13 years old (M=13.17); boys and girls did not differ in terms of their mean age. Participants came from a variety of ethnic backgrounds (60% Caucasian, 20% Asian, 10% Latino, 10% African American), and their families were distributed across a large range of socioeconomic status as measured by household income and parental education. Participants were recruited through mass mailings, summer camps, and fliers distributed in the community. All participants and parents provided assent/consent to participate in the study that was approved by UCLA’s Institutional Review Board.
Procedures
To examine participants’ neural and behavioral responses to observing an individual being the victim of peer rejection, participants were scanned while they believed they were observing another individual being socially excluded by others during a computerized ball tossing game. Social exclusion was used as a proxy for peer rejection based on research indicating that during early adolescence, isolating peers from social groups is one of the dominant methods used to reject peers (Coie, Dodge, & Kupersmidt, 1990). Prior to the scan, the experimenter explained that the purpose of the study was to examine neural activity during the observation of social interaction. Participants were then told that three individuals their age, who had previously completed the study, had volunteered to play the game via the internet during their scan, and they were given the first names and genders of these ‘previous participants’ (one male, one female, and a third player, who was to be ‘excluded’ by the first two, whose gender matched that of the participant). Participants were instructed to watch the game closely and think about what the players might be thinking or feeling, how they were treating each other, and what strategies they might have for deciding the recipient of each ball toss.
Participants then observed two rounds of the game Cyberball (Williams, Cheung, & Choi, 2000; Williams et al., 2002), in which three supposed players participate in a computerized ball tossing game that is actually computer-controlled. During the first round of Cyberball, participants observed all three players being included equally in the game (60 throws total), and during the second round they watched as one player was excluded for the majority of the game (after being included for only 10 throws). This paradigm has demonstrated high ecological validity in several previous behavioral and neuroimaging studies in which it was used to simulate social exclusion and produce feelings of distress among adults and children (Eisenberger, Lieberman, & Williams, 2003; Eisenberger, Gable, & Lieberman, 2007; Masten et al., 2009; Van Beest & Williams, 2006; Zadro, Williams, & Richardson, 2004), as well as to create feelings of empathy for observed victims of exclusion (Masten, Morelli, & Eisenberger, under review).
Following completion of the fMRI scan, participants first completed a manipulation check designed to ensure that they had noticed the exclusion of one player during the game. Then, to examine whether neural responses to observed exclusion would be associated with subsequent behavior, participants were asked to send an email message to the player that they had observed being excluded. The emails that participants wrote to the exclusion victim were later rated for prosocial behavior by a group of outside raters (see below). After completing the scan and all measures, participants were thoroughly debriefed regarding the deception involved in the study.
fMRI data acquisition
Images were collected using a Siemens Allegra 3-Tesla MRI scanner. Extensive instructions and reminders were given to decrease motion, and head motion was restrained with foam padding. For each participant, an initial 2D spin-echo image (TR=4000ms, TE=40 ms, matrix size 256 × 256, 4-mm thick, 1-mm gap) in the sagittal plane was acquired in order to enable prescription of slices obtained in structural and functional scans. In addition, a high-resolution structural scan (echo planar T2-weighted spin-echo, TR=4000 ms, TE=54 ms, matrix size 128 × 128, FOV=20 cm, 36 slices, 1.56-mm in-plane resolution, 3-mm thick) coplanar with the functional scans was obtained for functional image registration during fMRI analysis preprocessing. Each of the two rounds of Cyberball was completed during a functional scan lasting 2 minutes, 48 seconds (echo planar T2*-weighted gradient-echo, TR=2000ms, TE=25ms, flip angle=90°, matrix size 64×64, 36 axial slices, FOV=20-cm; 3-mm thick, skip 1-mm).
Behavioral measures
Trait empathy
Participants self-reported their levels of trait empathy using the Empathy Index (Bryant, 1982), a measure designed to tap empathic processes among children and adolescents. This measure was administered at least one day prior to the scan and was included in a battery of questionnaires that were collected for a separate study. The Empathy Index consists of 22 items assessing different aspects of both perspective taking and concern for others, including “Its hard for me to see why someone else gets upset”, and “Seeing a girl/boy who is crying makes me feel like crying”. This measure has been validated in a range of childhood and adolescent age groups, and has specifically demonstrated good test-retest reliability (r(80)=.83), internal consistency (α=.79), and construct validity (i.e., expected associations with several measures related to empathy) for this particular age group (see Bryant, 1982 for details of psychometric properties). Participants indicated their agreement with each statement using a 9-point scale from 1 (=very strongly disagree) to 9 (=very strongly agree). Items were reverse coded as appropriate and averaged to create an overall score for trait empathy.
Manipulation check
Following completion of the scan, a manipulation check was performed to ensure that participants noticed the social exclusion. Experimenters asked participants to answer a few questions about what happened during the Cyberball game, and specified that this was necessary “because each set of players acts differently”. This measure was comprised of a total of 10 yes/no questions about specific events that had happened during the game that they observed. Five of these 10 items (which were interspersed randomly throughout the list of items) were specifically designed to identify whether participants noticed the exclusion of one player (e.g., “one player was treated unfairly”, “all the players participated in the game the same amount”). All participants indicated that one player had been left out during the second round of the game.
Email to the victim of exclusion
Next, we were particularly interested in whether participants would try to help, support, or comfort the victim of the exclusion in an email, given that these specific types of prosocial behaviors would likely be the primary means available to an adolescent who is trying to make a rejected peer feel better. Participants were told that they could send a message via email to the player that they observed being excluded, since they would not get to meet them in person. Real email accounts were set up for each participant, as well as for the Cyberball player, in order to maintain ecological validity. Participants were told that they could say whatever they wanted to the player about what they observed (but were not specifically instructed to mention the exclusion) and were instructed to send the emails when they finished writing them. Following the final study debriefing, experimenters asked participants for their permission to use the emails that they had sent to the excluded player.
Prosocial ratings of emails
Following completion of data collection, twenty adult raters who did not interact with any of the study participants completed questionnaires designed to assess how prosocial the participant’s emails were to the victim of the exclusion. Raters were told that the participants had observed a social interaction between three other peers and that participants were given the freedom to write whatever they wanted in their emails. Raters read all of the participant’s emails, and answered three questions about each one: “Does it seem like they are trying to comfort the kid?”, “How supportive are they?”, and “How much do they seem like they are trying to help the kid?”. When rating each email, raters were asked to consider their ‘general impression’ and to indicate their answers to each of these questions using to a 7-point scale ranging from 1 (=not at all) to 7 (=very much). The ratings for the three questions were averaged across all the raters to create one total score for each participant indicating how prosocial their email was to the excluded player. Since this is the first time that prosocial behavior has been measured in this way, the high internal consistency of this measure is worth noting (ICC=.88).
fMRI data analysis
Neuroimaging data was preprocessed and analyzed using Statistical Parametric Mapping (SPM5; Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK). Preprocessing included image realignment to correct for head motion, normalization into a standard stereotactic space defined by the Montreal Neurological Institute and the International Consortium for Brain Mapping by averaging across 152 brains, and spatial smoothing using an 8mm Gaussian kernel, full width at half maximum, to increase signal-to-noise ratio. Preprocessing revealed that four participants (3 males and 1 female) moved more than 2mm during the functional scan; these participants were excluded from analyses resulting in a final sample of 16 participants (9 females).
Modeling of contrasts and whole-brain analyses
Cyberball was modeled as a block design. Each round of Cyberball was modeled as a run with each period of inclusion and exclusion modeled as blocks within the run for a total of two inclusion blocks (one during the first run lasting 120 seconds, and one during the shorter period of inclusion in the second run prior to exclusion lasting 60 seconds) and one exclusion block (lasting 50 seconds). After modeling the Cyberball paradigm, linear contrasts were calculated for each planned condition comparison for each participant. These individual contrast images were then used in whole-brain, random-effects analyses. In order to examine the relationships between brain activity during observed exclusion vs. inclusion and each behavioral index, we conducted whole-brain regression analyses that examined how differences in neural activity between exclusion and inclusion correlated with: (a) self-reported trait empathy, and (b) raters’ reports of how prosocial participants’ emails were to the victim of the exclusion. For each behavioral index we performed a single correlational test (the results of which are reported as both t-values and r-values computed from these t-values), to identify regions of the brain in which the behavioral index was significantly associated with the difference in activity between observed exclusion and inclusion.
All group-level regression analyses were thresholded at p<.005 for magnitude, uncorrected, with a minimum cluster size threshold of 10 voxels, for all a priori defined regions in known mentalizing networks (e.g., DMPFC, MPFC, pSTS, PCC, precuneus, temporal poles), and affective/pain networks for both adults (e.g., dACC, anterior and posterior insula) and adolescents (e.g., subgenual anterior cingulate cortex [subACC; Masten et al., 2009]). This threshold is typical of studies examining a priori defined regions and comparable to a false-discovery rate correction of p<.05 in a standard behavioral study (Lieberman & Cunningham, 2009). All other regions of the brain that were not defined a priori were examined at a threshold corrected for multiple comparisons (correction for false detection rate; p<.05 for magnitude, minimum cluster size of 10 voxels), however no significant clusters emerged outside of a priori defined regions. All group-level tests performed using SPM5 were one-tailed, given that all tests were uni-directional. All coordinates are reported in Montreal Neurological Institute (MNI) format.
Results
Behavioral Results
Descriptive information
Participants reported a range of scores (from 4.73 to 6.86) for their levels of trait empathy, using the Empathy Index (M=5.65, SD=.54). In addition, there was also variability in how prosocial (scores ranged from 1.11 to 5.91, M=3.32, SD=1.77) participants’ emails to the victims of the observed exclusion were rated to be by outside raters. Participants’ self-reported trait empathy was negatively but not significantly related to the prosocial ratings of their emails (r=−.31, p=.28). This lack of a relationship could suggest that, in general, young adolescents who believe themselves to be more empathic do not necessarily display more prosocial behaviors toward those they are empathizing with.
Qualitative results of emails to victims
As indicated above, there was substantial variability in how prosocial participants’ emails were to the victims of the exclusion. For example, the following email received the highest rating:
“Hey Adam my name is X, I just saw you play Cyberball with Danny and Erica. I think that you were treated unfairly. Danny and Erica passed the ball more to each other than to you. I know that you might feel left out. If this happens to you again you should tell Danny and Erica that you would like to get the ball passed to you as well. If this happens to you again take my advice and see if it works.”
In contrast, the following email received the lowest rating:
“I saw that the two other people who were playing Cyberball didn’t toss it to you in the second game.”
Thus, there was extensive variability in how the emails were written and how prosocial the participants were toward the victim of the exclusion.
Neuroimaging Results
Neural activity during observed exclusion compared to observed inclusion
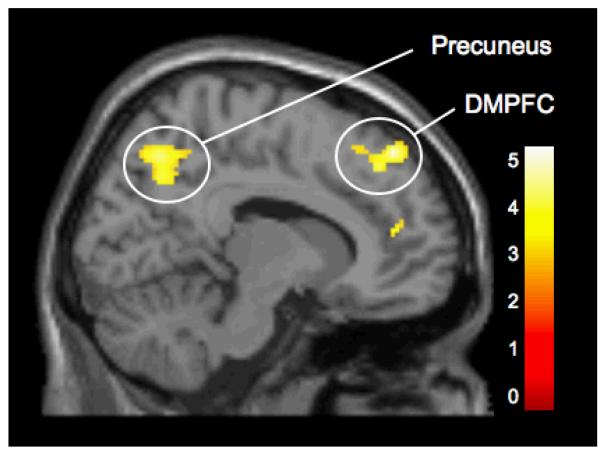
A whole brain contrast revealed that participants displayed a difference in activity in several regions, previously identified as part of the mentalizing network, when they observed another individual being excluded by peers compared to when they observed him/her being included (see Figure 1). Specifically, there were significant clusters of activity in the DMPFC [(12 44 50), t(15)=4.76, p<.0005], MPFC [(16 70 12), t(15)=3.22, p<.005; (14 46 14), t(15)=3.96, p<.001], precuneus [(10 -66 48), t(15)=4.18, p<.0005; (-12 -62 50), t(15)=3.61, p<.005], and pSTS [(58 -44 20), t(15)=3.53, p<.005]. Thus, early adolescents may utilize brain regions that have been previously linked with mentalizing more extensively when they observe another individual being excluded, compared with when they observe a group of peers treating each other equally. Details for these activations are provided in Table 1.
Figure 1.
Activity during observed exclusion vs. observed inclusion in the dorsomedial prefrontal cortex (DMPFC; [12 44 50]) and precuneus ([10 -66 48]).
Table 1.
Regions activated during observed exclusion compared to observed inclusion
| Anatomical Region | BA | x | y | z | t | k | p | |
|---|---|---|---|---|---|---|---|---|
| DMPFC | 8 | R | 12 | 44 | 50 | 4.76 | 194 | <.0005 |
| MPFC | 10 | R | 16 | 70 | 12 | 3.22 | 13 | <.005 |
| 10 | R | 14 | 46 | 14 | 3.96 | 54 | <.001 | |
| Precuneus | 7 | R | 10 | −66 | 48 | 4.18 | 433 | <.0005 |
| 7 | L | −12 | −62 | 50 | 3.61 | 22 | <.005 | |
| pSTS | 22 | R | 58 | −44 | 20 | 3.53 | 311 | <.005 |
NoteBA refers to putative Brodmann’s Area; L and R refer to left and right hemispheres; x, y, and z refer to MNI coordinates in the left-right, anterior-posterior, and interior-superior dimensions, respectively; t refers to the t-score at those coordinates (local maxima). The following abbreviations are used for the names of specific regions: dorsomedial prefrontal cortex (DMPFC), medial prefrontal cortex (MPFC), and posterior superior temporal sulcus (pSTS).
Associations between trait empathy and neural activity during observed exclusion vs.observed inclusion
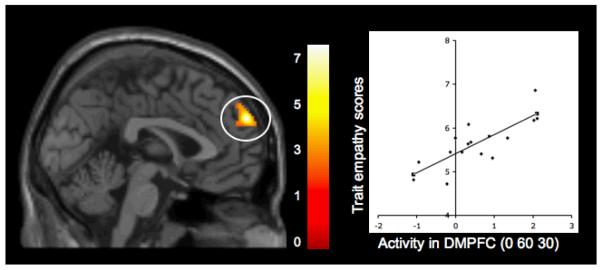
Whole-brain regression analyses revealed significant positive correlations between participants’ self-reported trait empathy and activity during observed exclusion compared to inclusion in neural regions previously linked with mentalizing processes. Specifically, greater trait levels of empathy were related to increased activity during observed exclusion relative to inclusion in two clusters of the DMPFC [(0 60 30), t(14)=7.61, p<.0001, r=.80, see Figure 2; (16 26 56), t(14)=4.06, p<.001, r=.70], as well as one cluster in the temporal pole (54 2 -32), t(14)=4.04, p<.001, r=.72], suggesting that early adolescents who report being more empathic display more activity related to mentalizing when observing another person being excluded by peers, compared to observing peer inclusion. There were no significant negative correlations between self-reported trait empathy and increased activity during observed exclusion compared to inclusion in any neural regions previously linked with social pain processing or mentalizing processes. Details for activations are provided in Table 2 (section A).
Figure 2.
Activity during observed exclusion vs. observed inclusion in the dorsomedial prefrontal cortex (DMPFC) that is positively related to participants’ self-reported levels of trait empathy. Scatterplot is provided to illustrate the relationship between the average difference in activity (exclusion vs. inclusion) across the entire cluster, and trait empathy scores.
Table 2.
Regions activated during observed exclusion compared to observed inclusion that correlated significantly with trait empathy, and prosocial behavior scores
| Anatomical Region | BA | x | y | z | t | r> | k | p | |
|---|---|---|---|---|---|---|---|---|---|
| (A) Positive associations with trait empathy | |||||||||
| DMPFC | 9/10 | 0 | 60 | 30 | 7.61 | .80 | 260 | <.0001 | |
| 8 | R | 16 | 26 | 56 | 4.06 | .70 | 42 | <.001 | |
| Temporal pole | 21 | R | 54 | 2 | −32 | 4.04 | .72 | 60 | <.001 |
| (B) Positive associations with prosocial behavior | |||||||||
| Anterior insula | R | 42 | 16 | −2 | 3.47 | .71 | 11 | <.005 | |
| (C) Negative associations with prosocial behavior | |||||||||
| PCC | 29/30 | R | 6 | −48 | 20 | 3.18 | −.71 | 24 | <.005 |
| Precuneus | 7 | R | 6 | −62 | 38 | 3.32 | −.69 | 10 | <.005 |
NoteBA refers to putative Brodmann’s Area; L and R refer to left and right hemispheres; x, y, and z refer to MNI coordinates in the left-right, anterior-posterior, and interior-superior dimensions, respectively; t refers to the t-score at those coordinates (local maxima); r refers to the correlation coefficient representing the strength of the association between trait empathy or prosocial behavior scores and the average difference between activity during observed exclusion and activity during observed inclusion across the entire cluster in each specified region. The following abbreviations are used for the names of specific regions: dorsomedial prefrontal cortex (DMPFC) and posterior cingulate cortex (PCC).
Associations between prosocial behavior and neural activity during observed exclusion vs. observed inclusion
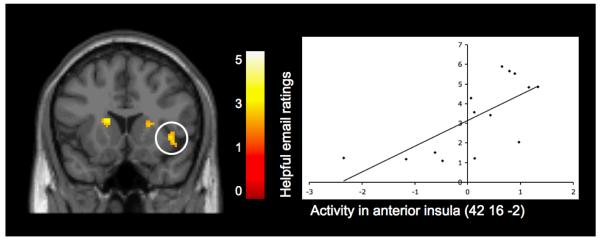
Finally, we wanted to examine whether the difference in neural activation during observed exclusion compared to inclusion correlated with prosocial behavior in response to a victim’s negative social experience. Whole-brain regression analyses revealed that participants’ neural activity in response to observed exclusion relative to inclusion was indeed related to their subsequent behavior toward the victim of the rejection. Participants whose emails were rated as more prosocial displayed greater activity during observed exclusion compared to inclusion, in the anterior insula [(42 16 -2), t(12)=3.47, p<.005, r=.71; see Figure 3], which has been linked with social exclusion (Eisenberger, Lieberman, & Williams, 2003) and empathy for physical pain (Singer, 2006). Thus, it is possible that early adolescents who felt more distress during another individuals experience with peer exclusion, acted more prosocially toward the victims of the exclusion through the emails that they wrote. In addition to these positive correlations, participants who wrote more prosocial emails also displayed less activity during observed exclusion relative to inclusion in the PCC [(6 -48 20), t(12)=3.18, p<.005, r=−.71], as well as in the precuneus [(6 -62 38), t(12)=3.32, p<.005, r=−.69]. Details for activations are provided in Table 2 (sections B and C).
Figure 3.
Activity during observed exclusion vs. observed inclusion in the insula that is positively related to ratings of how prosocial participants’ emails were to the victims of the observed exclusion. Scatterplot is provided to illustrate the relationship between the average difference in activity (exclusion vs. inclusion) across each specified cluster, and the ratings of how helpful the emails were. For display purposes only, activation shown in this figure is thresholded at p=.01 to better depict the location and nature of this activation.
Discussion
As a whole, findings from this study demonstrated that early adolescents engaged a network of neural regions implicated in mentalizing when they observed a peer being socially excluded and that increased activity in these mentalizing regions during observed exclusion relative to inclusion was associated with higher levels of trait empathy. Interestingly, however, it was greater affective neural activity during observed exclusion relative to inclusion that related to early adolescents displaying more prosocial behavior toward this victim. Together these findings extend current knowledge of how the brain responds to negative social interactions during early adolescence, and what processes youth engage in when they observe peer rejection in their daily lives.
First, when contrasting early adolescents neural responses during observed peer exclusion compared to observed peer inclusion, findings revealed a network of regions that have been previously linked with mentalizing behaviors among adults and specifically adults empathy for social pain, including the DMPFC and MPFC, the precuneus, and the pSTS (Masten, Morelli, & Eisenberger, under review). The involvement of these regions in early adolescents’ experiences of observed exclusion suggest that, similar to adults, early adolescents may engage in mentalizing behaviors more so when they observe another individual being excluded, than when they observe a group of peers treating each other equally. This is interesting given that observing any social interaction among peers (not necessarily a negative one) could be expected to activate the mentalizing network. It is possible that seeing one person being excluded could focus individuals attention on this person, which could result in greater mentalizing activity associated with thinking specifically about the experience of one individual rather than the general interactions of a group. Alternatively, witnessing an interaction in which a peer is treated negatively may heighten individuals’ efforts to reason about the situation, speculate about the intentions of the victim and other players, and reflect on their own similar experiences.
Next, consistent with hypotheses, we found that individual differences in trait levels of empathy were also associated with activity in neural regions that are part of the mentalizing network. Specifically, early adolescents’ self-reports of trait empathy were positively correlated with increased activity during observed exclusion relative to inclusion in the DMPFC and right temporal pole, suggesting that early adolescents who self-report greater empathic tendencies display more neural evidence of mentalizing when they witness a peer being rejected. This finding is consistent with previous work implicating the DMPFC and temporal poles in empathic and mentalizing processes (see Singer, 2006), as well as developmental research suggesting that children with greater trait levels of empathy will experience greater amounts of empathy for others in negative social situations (Davis, 1983; Nezlek, Feist, Wilson, & Plesko, 2001). In fact, it is possible that more highly empathic individuals become more engaged upon witnessing peer rejection, and are more sensitive to the thoughts of the players involved.
Next, findings from this study suggest that the neural responses that early adolescents display when they observe peer rejection are related to their prosocial behavior toward the victims of this rejection. Specifically, results indicated that early adolescents who wrote more prosocial emails to the excluded player had displayed greater activity during observed exclusion compared to inclusion in the insula, an area that has been consistently linked with experiences of social exclusion in adults (Eisenberger, Lieberman, & Williams, 2003) and early adolescents (Masten et al., 2009), as well as both direct and empathic disgust (Wicker et al., 2003) and physical pain (e.g., Singer et al., 2004; Singer et al., 2006). This finding is consistent with our hypothesis that early adolescents who experience a greater degree of distress (as evidenced by increased insula activity) when watching others experiencing rejection may subsequently act in a more helpful and supportive manner toward these rejected individuals. This is particularly interesting because it suggests that while early adolescents, on average, may not always ‘feel the pain’ of others who are socially excluded (as suggested by the lack of social pain-related neural activity while observing social exclusion vs. inclusion), those who do show differential pain related neural activity may be the ones who are most compelled to help and comfort those being treated unfairly. Thus, in early adolescence, feelings of distress or pain during a witnessed interaction may be the driving force behind behaviors aimed to mitigate the situation.
Implications & Conclusion
Findings from the current study suggest that empathy for social pain may be quite different from empathy for physical pain. For example, in both the current study with early adolescents and the previous study on empathy for social exclusion with adults (Masten, Morelli, & Eisenberger, under review), there was evidence of differential neural activity in the mentalizing network, but no evidence of social pain-related neural activity, when comparing observed exclusion to observed inclusion. This type of activity in mentalizing regions has not been consistently found in studies examining physical pain, which have focused primarily on pain-related regions (e.g., Singer et al., 2004; Singer et al., 2006; Jackson, Bruney, Meltzoff, & Decety, 2005; Botvinick et al., 2005; Morrison, Lloyd, Di Pellegrino, & Roberts, 2004; Decety, Michalska, & Akitsuki, 2008; see Singer, 2006 and Jackson, Rainville, & Decety, 2006 for reviews). This could suggest that empathy for social situations is less automatic than empathy for physical pain, and that observing negative social situations may require individuals to think more about why the situation happened and the intentions of those involved, rather than just experiencing an immediate aversive response.
Next, although the current study did not directly compare adults’ and early adolescents’ experiences of empathy during social exclusion, it is likely that there are developmental differences in how individuals experience empathy for social situations. One possibility is that adults and early adolescents could respond to victims of exclusion differently because of a difference in the meaning and frequency of these occurrences. For example, early adolescents may observe peer rejection on a daily basis, and may view these occurrences as mainstream and common. They may even feel that it is “uncool” to try to interfere, or that it may jeopardize their own peer status. Thus, their empathic ability may have little bearing on their actions in these situations, and instead feeling actual pain or distress (e.g., as evidenced by greater activation in pain/affective neural regions) might drive subsequent prosocial behaviors. In contrast, adults who tend to feel greater empathy for others may consistently make efforts to help or support victims of exclusion when it overtly occurs because exclusion is no longer accepted as a common, unavoidable part of life. In other words, adults may generally view social exclusion as unacceptable and unfair, whereas early adolescents may believe that these distressing occurrences are unavoidable and it is usually better not to interfere.
It would be useful for future studies to directly compare adults’ and adolescents’ empathy for social exclusion, as well as to take into account other developmentally-relevant biological factors such as pubertal status, pubertal timing, and increasing levels of sex hormones during the adolescent period. Examining these types of factors will help further clarify the developmental implications of adolescents’ neural responses to empathy for social experiences, and extend our knowledge about how social exclusion differentially impacts the daily lives of adolescents and adults. In addition, it would be useful for future studies to include larger samples of adolescents that would enable the examination of gender differences in the neural correlates of empathy for social exclusion. While the current sample size was too small to permit meaningful tests of differential neural processing among boys and girls, this would likely be a fruitful avenue for future research given known biological and psychosocial differences between boys and girls at this age.
As a whole, findings from the current study contribute to our understanding of how youth respond in situations where they witness peer rejection, and extend the current empathy literature by providing new information about how neural mechanisms of empathy may influence prosocial behavior and impact daily interactions with peers during early adolescence. Our hope is that future neuroimaging studies will continue to examine empathy for social situations among adolescents, and potentially make new discoveries about how these experiences impact adolescents social development as well as the cognitive and affective mechanisms that drive their interactions with others.
Acknowledgements
This work was supported by the Santa Fe Institute Consortium, as well as by an Elizabeth Munsterberg Koppitz Award and a Ruth L. Kirschstein National Research Service Award to C. Masten. For generous support the authors also wish to thank the Brain Mapping Medical Research Organization, Brain Mapping Support Foundation, Pierson-Lovelace Foundation, Ahmanson Foundation, Tamkin Foundation, Jennifer Jones-Simon Foundation, Capital Group Companies Charitable Foundation, Robson Family, William M. and Linda R. Dietel Philanthropic Fund at the Northern Piedmont Community Foundation, and Northstar Fund. This project was in part also supported by grants (RR12169, RR13642 and RR00865) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH); its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCR or NIH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Data for the current sample of early adolescents and the previously collected sample of adults (Masten, Morelli, & Eisenberger, under review) could not be directly compared because the data for each of these groups was collected on two different MRI scanners. As a result, only qualitative comparisons are made in the current paper, given that quantitative comparisons were not possible.
References
- Baron-Cohen S. The essential difference. Basic Books; New York: 2003. [Google Scholar]
- Batson CD. Altruism and prosocial behavior. In: Gilbert DT, Fiske ST, Lindzey G, editors. The Handbook of Social Psychology. Vols. 1 and 2. McGraw-Hill; New York, NY: 1998. pp. 282–316. [Google Scholar]
- Batson CD. The altruism question: Toward a social-psychological answer. Erlbaum; Hillsdale, NJ: 1991. [Google Scholar]
- Botvinick M, et al. Viewing facial expressions of pain engages cortical areas involved in the direct experience of pain. Neuroimage. 2005;25:312–319. doi: 10.1016/j.neuroimage.2004.11.043. [DOI] [PubMed] [Google Scholar]
- Brown BB. Peer groups and peer cultures. In: Feldman SS, Elliot GR, editors. At the threshold: The developing adolescent. Harvard University Press; Cambridge, MA: 1990. pp. 171–196. [Google Scholar]
- Bryant B. An index of empathy children and adolescents. Child Development. 1982;53:413–425. [Google Scholar]
- Coie JD, Dodge KA, Kupersmidt JB. Peer group behavior and social status. In: Asher SR, Coie JD, editors. Peer rejection in childhood. Cambridge studies in social and emotional development. Cambridge University Press; New York, NY: 1990. pp. 17–59. [Google Scholar]
- Davis MH. Measuring individual differences in empathy: Evidence for a multidimensional approach. Journal of Personality and Social Psychology. 1983;44:113–126. [Google Scholar]
- Davis MH, Mitchell KV, Hall JA, Lothert J, Snapp T, Meyer M. Empathy, expectations, and situational preferences: Personality influences on the decision to participate in volunteer helping behaviors. Journal of Personality. 1999;67:469–503. doi: 10.1111/1467-6494.00062. [DOI] [PubMed] [Google Scholar]
- Decety J, Meyer M. From emotion resonance to empathic understanding: A social developmental neuroscience account. Development and Psychopathology. 2008;20:1053–1080. doi: 10.1017/S0954579408000503. [DOI] [PubMed] [Google Scholar]
- Decety J, Michalska KJ, Akitsuki Y. Who caused the pain? An fMRI investigation of empathy and intentionality in children. Neuropsychologia. 2008;46:2607–2614. doi: 10.1016/j.neuropsychologia.2008.05.026. [DOI] [PubMed] [Google Scholar]
- Denham SA, Renwick-DeBardi S, Hewes S. Emotional communication between mothers and preschoolers: relations with emotional competence. Merrill-Palmer Quarterly. 1994;40:488–508. [Google Scholar]
- Dovidio JF, Allen JL, Schroeder DA. Specificity of empathy-induced helping: Evidence for altruistic motivation. Journal of Personality and Social Psychology. 1990;59:249–260. [Google Scholar]
- Eisenberg N, Miller PA, Shell R, McNally S, Shea C. Prosocial development in adolescence: a longitudinal study. Developmental Psychology. 1991;27:849–857. [Google Scholar]
- Eisenberg N, Spinrad TL, Sadovsky A. Empathy-related responding in children. In: Killen M, Smetana J, editors. Handbook of moral development. Lawrence Erlbaum Associates; Mahwah, NJ: 2006. pp. 517–549. [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An fMRI study of social exclusion. Science. 2003;302:290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Gable SL, Lieberman MD. fMRI responses relate to differences in real-world social experience. Emotion. 2007;7:745–754. doi: 10.1037/1528-3542.7.4.745. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. Interacting minds – A biological basis. Science. 1999;286:1692–1695. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. The neural basis of mentalizing. Neuron. 2006;50:531–534. doi: 10.1016/j.neuron.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Frith U, Frith CD. Development and neurophysiology of mentalizing. Philosophical Transactions of the Royal Society of Biological Sciences. 2003;358:459–473. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith U, Leslie AM, Morton J. The cognitive basis of a biological disorder: Autism. Trends in Neurosciences. 1991;14:433–438. doi: 10.1016/0166-2236(91)90041-r. [DOI] [PubMed] [Google Scholar]
- Guthrie IK, Eisenberg N, Fabes RA, Murphy B, Shepard S. The relations of regulation and emotionality to childrens situational empathy-related responding. Motivation and Emotion. 1997;21:87–108. [Google Scholar]
- Immordino-Yang MH, McColl A, Damasio H, Damasio A. Neural correlates of compassion. Proceedings of the National Academy of Sciences. 2009;106:8021–8026. doi: 10.1073/pnas.0810363106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson PL, Bruney E, Meltzoff AN, Decety J. How do we perceive the pain of others? A window into the neural processes involved in empathy. Neuroimage. 2005;24:771–779. doi: 10.1016/j.neuroimage.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Jackson PL, Rainville P, Decety J. To what extent do we share the pain of others? Insight from the neural bases of pain empathy. Pain. 2006;125:5–9. doi: 10.1016/j.pain.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: Re-balancing the scale. Social Cognitive and Affective Neuroscience. 2009;4:423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvak-Miller W, McDougall D, Romney DM. The structure of empathy during middle childhood and its relationship to prosocial behavior. Genetic, Social, and General Psychology Monographs. 1997;123:303–324. [PubMed] [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, Pfeifer JH, McNealy K, Mazziotta J, Dapretto M. Neural correlates of social exclusion during adolescence: Understanding the distress of peer rejection. Social Cognitive and Affective Neuroscience. 2009;4:143–157. doi: 10.1093/scan/nsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten CL, Morelli S, Eisenberger NI. Feeling others’ social pain: An fMRI investigation of empathy for social exclusion. under review. [DOI] [PubMed]
- Mitchell JP, Banaji MR, Macrae CN. The link between social cognition and self-referential thought in the medial prefrontal cortex. Journal of Cognitive Neuroscience. 2005;17:1306–1315. doi: 10.1162/0898929055002418. [DOI] [PubMed] [Google Scholar]
- Morrison I, Lloyd D, Di Pellegrino G, Roberts N. Vicarious responses to in anterior cingulate cortex: Is empathy a multisensory issue? Cognitive, Affective & Behavioral Neuroscience. 2004;4:270–278. doi: 10.3758/cabn.4.2.270. [DOI] [PubMed] [Google Scholar]
- Nezlek JB, Feist GJ, Wilson FC, Plesko RM. Day-to-day variability in empathy as a function of daily events and mood. Journal of Research in Personality. 2001;35:401–423. [Google Scholar]
- Oswald PA. The effects of cognitive and affective perspective taking on empathic concern and altruistic helping. Journal of Social Psychology. 1996;136:613–623. doi: 10.1080/00224545.1996.9714045. [DOI] [PubMed] [Google Scholar]
- Pfeifer JH, Iacoboni M, Mazziotta JC, Dapretto M. Mirroring others’ emotions related to empathy and interpersonal competence in children. Neuroimage. 2008;39:2076–2085. doi: 10.1016/j.neuroimage.2007.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder DA, Dovidio JF, Sibicky ME, Matthews LL, Allen JL. Empathic concern and helping behavior: Egoism or altruism? Journal of Experimental Social Psychology. 1988;24:333–353. [Google Scholar]
- Singer T. The neuronal basis and ontogeny of empathy and mind reading: Review of literature and implications for future research. Neuroscience & Biobehavioral Reviews. 2006;30:855–863. doi: 10.1016/j.neubiorev.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty JP, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty JP, Stephan KE, Dolan RJ, Frith CD. Empathic neural responses are modulated by the perceived fairness of others. Nature. 2006;439:466–469. doi: 10.1038/nature04271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beest I, Williams KD. When inclusion costs and ostracism pays, ostracism still hurts. Journal of Personality and Social Psychology. 2006;91:918–928. doi: 10.1037/0022-3514.91.5.918. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Shin LM, McInerney SC, Fischer H, Wright CI, Rauch SL. A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion. 2001;1:70–83. doi: 10.1037/1528-3542.1.1.70. [DOI] [PubMed] [Google Scholar]
- Wicker B, et al. Both of us disgusted in My insula: the common neural basis of seeing and feeling disgust. Neuron. 2003;40:655–664. doi: 10.1016/s0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]
- Williams KD, Cheung CK, Choi W. Cyberostracism: Effects of being ignored over the Internet. Journal of Personality and Social Psychology. 2000;79:748. doi: 10.1037//0022-3514.79.5.748. [DOI] [PubMed] [Google Scholar]
- Williams KD, Govan CL, Croker V, Tynan D, Cruickshank M, Lam A. Investigations into differences between social- and cyberostracism. Group Dynamics: Theory Research, and Practice. Special Issue: Groups and Internet. 2002;6:65–77. [Google Scholar]
- Zadro L, Williams KD, Richardson R. How low can you go? Ostracism by a computer is sufficient to lower self-reported levels of belonging, control, self-esteem, and meaningful existence. Journal of Experimental Social Psychology. 2004;40:560–567. [Google Scholar]
- Zahn-Waxler C, Radke-Yarrow M, Wagner E, Chapman M. Development of concern for others. Developmental Psychology. 1992;28:126–136. [Google Scholar]