Abstract
Involvement of the insular cortex is a common finding in neuroanatomical studies of schizophrenia, yet its contribution to disease pathology remains unknown. This review describes the normal function of the insula and examines pathology of this region in schizophrenia. The insula is a cortical structure with extensive connections to many areas of the cortex and limbic system. It integrates external sensory input with the limbic system and is integral to the awareness of the body’s state (interoception). Many deficits observed in schizophrenia involve these functions and may relate to insula pathology. Furthermore, reports describing deficits caused by lesions of the insula parallel deficits observed in schizophrenia. Examples of insula-related functions that are altered in schizophrenia include the processing of both visual and auditory emotional information, pain, and neuronal representations of the self. The last of these functions, processing representations of the self, plays a key role in discriminating between self-generated and external information, suggesting that insula dysfunction may contribute to hallucinations, a cardinal feature of schizophrenia.
Keywords: insula, schizophrenia, neuroimaging, faces, prosody, acetylcholine
1. Introduction
The varied nature of schizophrenia complicates efforts to determine its etiology. The functional and structural neuroanatomical deficits found in the disease have offered clues to the pathology underlying symptoms and have highlighted the potential role of several brain areas and functional networks. Since no single area has been found to be paramount in schizophrenia, expanded knowledge of the role of each area, how they interrelate, and the pathology found within furthers understanding of this complex disorder. The pathology and deficits observed in some areas, such as the dorsolateral prefrontal cortex and the hippocampus, are the subject of extensive and ongoing research. The pathology and deficits in another area, the insula, have only recently come to attention yet may be highly relevant to the symptoms and biology of schizophrenia.
The purpose of this review is to summarize and highlight recent research into the insula’s contribution to the psychopathology of schizophrenia. The review details the function of the insula, describes structural and functional abnormalities observed in the region in schizophrenia, notes similarities between symptoms of the disease and syndromes resulting from lesions to the insula, and hypothesizes how these deficits may together contribute to hallucinations in the disease. A review of the literature was conducted using the search terms “insula” and “schizophrenia” in Pubmed. Results were narrowed to studies on human subjects within the last 15 years. Articles were categorized according to topic, resulting in the subdivisions below. The role of the insula in other psychiatric diseases, including anxiety disorders, has been reviewed elsewhere (Paulus and Stein, 2006; Nagai et al. 2007) and is not included here.
2. The Functional Role of the Insula
The insula plays a major role in processing emotional and sensory stimuli. It is uniquely involved in interoception, the awareness of the body’s internal state. This awareness underlies both the body’s emotional response and complex cognitive states, such the sense of self (James 1890, Craig 2002, Damasio 2003, Critchley et al. 2004). Interoception in the insula is demonstrated by its response during perception of changes in the body’s physiological state, such as heartbeat monitoring (Critchley et al. 2004), biofeedback using changes in skin conductance (Critchley et al. 2002), thermal pain (Kong et al. 2006, Oschner et al. 2008), electrically-induced pain (Singer et al. 2004), and light touch (Lovero et al. 2009). Interoception can be viewed as a mechanism for the James-Lange theory of emotion, which predicts that the body’s physiological response is the basis for emotional experience. Insula involvement in this process is supported by the additive effects of visceral and emotional visual stimuli on the insula (Phillips et al. 2003) and by the association of epileptic foci within the insula with either visceral or emotional symptoms (Dupont et al. 2003). Interoceptive awareness of the body as whole and distinct from the external environment is a key component of the ability to perceive an image of the ‘self,’ and make judgements of ‘self’ versus ‘non-self’ (Damasio 2003, Devue et al. 2007, Kircher et al. 2001).
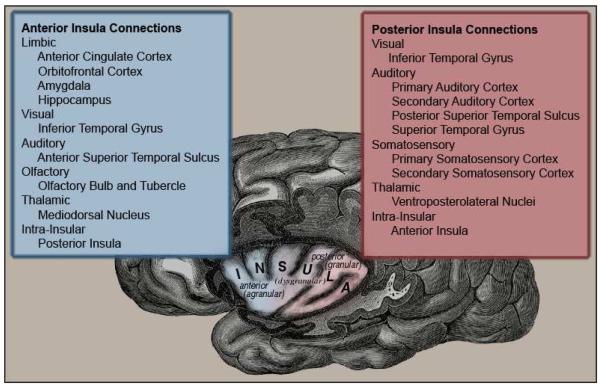
The insula can be subdivided into anterior and posterior sections based on observed connectivity, function and cytoarchitechture (Lovero et al. 2009, Flynn et al. 1996, Craig 2009). Reciprocal connections link the two areas and the transition them between is a gradual change. Cytoarchitecturally, the anterior insula encompasses the agranular region along with part of the adjacent dysgranular region. Its extensive and reciprocal connections to limbic areas, higher-order visual areas, olfactory areas and to the posterior insula are shown in Figure 1 (Mesulam and Mufson 1982a, Augustine 1996, Flynn et al. 1999). Functionally, the anterior region is specialized for processing the emotional component of interoceptive awareness (Craig 2003, Critchley et al. 2002), the anticipation and evaluation of emotional stimuli (Lovero et al. 2009, Kong et al. 2006), and self-awareness (Craig 2009, Devue et al. 2007). The anterior insula is involved in extending self-awareness to other ‘selves’ through action similar to that of mirror neurons (Iacoboni and Dapretto 2006). This process likely is involved in the generation of empathy (Ochsner et al. 2008, Carr et al. 2003) and in the identification of boundaries between the self and other (Oschner et al. 2008, Devue et al. 2007). The posterior insula encompasses the granular and the adjacent part of the dysgranular region. Its reciprocal connections are from higher-order visual areas, auditory processing areas, somatosensory areas and to the anterior insula (Figure 1) (Mesulam and Mufson 1982a, Augustine 1996, Flynn et al. 1999). Functionally, the posterior insula is specialized for directly experienced, multimodal sensory processing; particularly somatosensory processing (Singer et al. 2004, Lovero et al. 2009, Olausson et al. 2002).
Figure 1.
Subdivisions of the insula and their connections to other regions. Cytoarchitechturally, a continuous transition between agranular and granular patterns is observed. The anterior insula, in blue, comprises the agranular and anterioventral dysgranular cytoarchitechture, with connections to limbic and sensory regions of the brain. The posterior insula, in red, comprises the granular and dorsoposterior dysgranular cytoarchitechture, with connections to visual, auditory and somatosensory areas. Both areas have connections to the thalamus and within the insula.
Based on the anatomical connections and function of the insula, disrupted interoception processing within this region would be expected to disrupt the evaluation of emotional stimuli and boundaries of the self. As described below, these deficits have been widely reported in schizophrenia. Correspondingly, grey matter, cytoarchitectural, functional and protein expression abnormalities are observed in the insula in schizophrenia. It is reasonable to hypothesize, therefore, that many of the functional and behavioral deficits observed in schizophrenia may involve pathology of the insula.
3. Pathological Anatomy of the Insula in Schizophrenia
Abnormalities in grey matter volume, cortical thickness, cellular structure, and the expression of proteins within the insula are observed in schizophrenia. Structural imaging techniques using magnetic resonance imaging (MRI) consistently find decreased grey matter in the insula (Honea et al. 2005, Ellison-Wright et al. 2008, Glahn et al. 2008, Fornito et al. 2009, Chan et al. 2009, Kasai et al. 2003, Kim et al. 2003, Takahashi et al. 2004, 2005; Saze et al. 2007, Makris et al. 2006). These deficits are usually bilateral, are present in first-episode schizophrenia, and are progressive in chronic schizophrenia (Ellison-Wright et al. 2008, Chan et al. 2009). It is unclear whether grey matter deficits are localized to a particular subregion of the insula as they have been observed in anterior (Makris et al. 2006), posterior (Saze et al. 2007), or across both areas (Takahashi et al. 2005, Kasai et al. 2003). In monozygotic twins discordant for schizophrenia, the affected twin has decreased grey matter volume in the insula bilaterally (Borgwardt et al. 2009). The bilateral decrease in insular grey matter is present in populations at high risk for developing schizophrenia and progresses further with the transition the psychotic state (Takahashi et al. 2009, Chan et al. 2009). Among the same high-risk population, subjects who go on to develop psychosis have decreased insular grey matter initially compared to those who do not become psychotic (Borgwardt et al. 2007, Takahashi et al. 2009).
Some studies have reported negative correlations between positive symptoms and grey matter volume (Crespo-Facorro et al. 2000, Duggal et al. 2005, Pressler et al. 2005) but other studies have not found these effects (Crespo-Facorro et al. 2010, Takahashi et al. 2005, Kasai et al. 2003). Bilateral decreases in grey matter volumes are associated with schizophrenia with hallucinations (Shapleske et al. 2002). Studies of other forms of psychosis are inconclusive. Grey matter deficits in affective psychosis have been observed in one study (Morgan et al. 2007) but not in another (Kasai et al. 2003). Another study of insula grey matter volume in schizophrenia-spectrum disorders did not find any deficit (Crespo-Facorro et al. 2010).
Not all studies have found insula grey matter deficits in schizophrenia and not all deficits found have been bilateral (Crespo-Facorro et al. 2010, Honea et al. 2005). In one meta-analysis, of the thirteen studies that examined the insula, seven found grey matter decreases in the region. Of the seven, two studies found a deficit localized to the right side, one found left-sided localization, and four found a bilateral deficit (Honea et al. 2005). Other meta-analyses, however, have more consistently found bilateral grey matter deficits in the insula in schizophrenia (Ellison-Wright et al. 2008, Glahn et al. 2008, Fornito et al. 2009, Chan et al. 2009). It should be noted that grey matter deficits in other regions, such as the superior temporal gyrus, medial frontal, anterior cingulate, and parahippocampal areas are as consistently or more frequently found in schizophrenia in these same studies (Honea et al. 2005, Ellison-Wright et al. 2008, Glahn et al. 2008, Fornito et al. 2009, Chan et al. 2009).
Related to grey matter volume deficits, cortical thickness in the insula also is reduced in schizophrenia. Decreases in the left (Kuperberg et al. 2003), right (Nesvag et al. 2008) or bilateral insula (Roiz-Santianez et al. 2010) are reported. These decreases do not correlate with antipsychotic medication use or clinical measures (Nesvag et al. 2008, Roiz-Santianez 2010).
Cellular and molecular abnormalities within the insula also are observed in schizophrenia. Post-mortem studies show poor development and heterogeneity in the upper layers of the insular cortex, decreases in the number of neurons in layers II & III by 20-30%, and reductions in neuronal and glial somal sizes in layer II (Pennington et al. 2008a, Jakob and Beckmann 1986). Proteomic analysis shows differential expression of 57 proteins in layer II of the insular cortex of schizophrenic patients. These abnormally expressed proteins broadly relate to neuronal plasticity through their involvement in neuronal outgrowth, cellular morphogenesis and synaptic function (Pennington et al. 2008b).
4. Pathological Function of the Insula in Schizophrenia
4.1 Facial Affect Processing
The pathology of the insula in schizophrenia may contribute to the difficulty in recognizing emotional facial expressions found in the disease. Higher-order visual information needed to process faces is relayed to the insula through the inferior temporal gyrus (Mesulam and Mufson 1982a). Functional imaging studies, both functional magnetic resonance imaging (fMRI) and positron emission topography (PET), show involvement of the insula in healthy subjects when evaluating facial expressions (summarized in table 1). The exact type of stimuli that engage the insula is not well established but seems to relate more to negative emotions such as disgust. Lesion studies also support the involvement of the insula in facial affect processing. Patients with insula lesions experience difficulty in recognizing facial expressions, particularly expressions of disgust (table 1).
Table 1.
Facial Affect Studies
| Study: | Method | Design: | Insula Involvement: |
|---|---|---|---|
| Arce et al. (2008) | Imaging, PET | Angry, fearful and happy faces vs. non-face shapes |
Increases in bilateral anterior and posterior insula responses |
| Morris et al. (1998) | Imaging, PET | Parametric analysis of the response to fearful faces |
L anterior Insula response correlates with Intensity of emotion |
| Wicker et al. (2003) | Imaging, fMRI | Disgusted faces vs. rest | Increase in L anterior insula response |
| Sprengelmeyer (1998) | Imaging, fMRI | Disgusted faces vs. neutral faces | Increase in L anterior insula response |
| Phillips et al. (1997) | Imaging, fMRI | Disgusted faces vs. neutral faces | R anterior insula response correlates with intensity of emotion, Increase in L anterior insula response at higher emotional intensity |
| Carr et al. (2003) | Imaging, fMRI | Viewing and imitating emotional expressions vs. rest |
Increase in R anterior insula response in both conditions |
| Adolphs et al. (2000) | Lesion study, quantitative |
Recognition of facial affect | Insula lesions associated with a deficit in recognition of happy, surprised, afraid, angry, sad, or disgusted faces |
| Calder et al. (2000) | Lesion case-study | Recognition of facial and auditory expressions of disgust |
Infarct in L insula and putamen |
| Adolphs et al. (2003) | Lesion case-study | Recognition of static facial expressions of surprise, fear, anger, sadness and disgust |
Extensive lesions including insula bilaterally |
| Recognition of videos of disgust reactions | Extensive lesions including insula bilaterally |
Paralleling the symptoms of neurological damage to the insula, subjects with schizophrenia have difficulty recognizing facial emotions. Responses to specific facial expressions vary, but difficulties in recognizing negative emotions like sadness, anger, fear or disgust are most common (Bediou et al. 2005a, Bediou et al. 2005b, Leppanen et al. 2008, van’t Wout et al. 2007, Schneider et al. 2006). Scores on facial recognition tests averaging across all emotions correlate with negative symptom severity (Martin et al. 2005, Turetsky et al. 2007, Sachs et al. 2004, Gur et al. 2006). Patients with paranoid schizophrenia may perform better in some cases (van’t Wout et al. 2007, Phillips et al. 1999). The difficulty in recognizing angry faces extends to the unaffected siblings of schizophrenic patients, suggesting a genetic contribution to the deficit (Leppanen et al. 2008).
Functional imaging has shown that the insula responds abnormally to emotional faces in schizophrenia. Patients with schizophrenia have a decreased hemodynamic response in the left anterior insula when viewing facial expressions of disgust (Phillips et al. 1999). This deficit is limited to non-paranoid patients, with paranoid patients showing a smaller decrease compared to non-paranoid patients (Phillips et al. 1999, Williams et al. 2007). Early-onset schizophrenia is associated with hypoactivation in the left insula to sad facial expressions (Seiferth et al. 2009). Response in the right insula to fearful faces is observed in a healthy control group but not in schizophrenia, but this decrease does not reach significance when compared directly (Michalopoulou et al. 2008).
In healthy subjects, the processing of facial affect involves a network of regions that includes the insula as well as the amygdala, medial prefrontal cortex, and anterior cingulate gyrus (Arce et al. 2008, Morris et al. 1998, Sprengelmeyer et al. 1998, Phillips et al. 1997). These areas also show decreased responses in schizophrenia (Baas et al. 2008, Phillips et al. 1999, Williams et al. 2007) and have dense reciprocal connections with the insula (Mesulam and Mufson 1982a).
In summary, the insula response observed during the processing of emotional facial expressions is abnormal in schizophrenia. This response deficit may contribute to the difficulty in recognizing emotional expressions found in the disease. This process involves evaluating emotions, empathy and theory of mind, all functions subserved by the anterior insula (Schneider et al. 2006, Carr et al. 2003). Anger, fear and especially disgust are the emotions which most strongly engage the anterior insula. These same emotions are the expressions subjects with schizophrenia have the most difficulty recognizing. Lesions to the insula result in difficulties evaluating emotional expressions similar to those experienced by patients with schizophrenia. All of this evidence suggests that these symptoms of schizophrenia may be associated with disruptions in normal insula function.
4.2 Auditory Affect Processing
Similar to emotional facial expressions, the pathology in the insula in schizophrenia may contribute to the difficulties in evaluating and creating emotional vocal expressions observed in the disease. The emotional aspects of speech are expressed as variations in rhythm, stress and intonation, collectively referred to as the prosody of speech. Although typically strongly tied to words, prosody itself is non-verbal. For example, the emotional content of a heated argument behind a closed door is obvious without the need to discern words. The auditory information necessary to processes prosody arrives at the posterior insula directly through connections with the primary auditory and association cortices. From there it is passed to the anterior insula for more abstract and higher-order processing involving the subregion’s dense reciprocal connections to the amygdala, hippocampal and prefrontal areas of the limbic system (Mesulam and Mufson 1982a, Flynn et al. 1999).
A summary of the studies demonstrating the insula’s involvement in evaluating prosody in healthy subjects is shown in table 2. Negative emotions in particular engage the insula, but responses to positive emotions or a global response across all emotions have been observed. Lesions of the insula disrupt both the comprehension and the production of prosody, leading to either hypoprosody or aprosody syndromes (table 2).
Table 2.
Prosody Studies
| Study: | Method: | Design: | Insula Involvement: |
|---|---|---|---|
| Beaucousin et al. (2007) | Imaging, fMRI | Sentences spoken with emotional inflection vs. sentences lacking inflection |
Increase in bilateral anterior insula response |
| Bach et al. (2008) | Imaging, fMRI | Sentences of pseudo-words spoken with emotional inflection vs. pseudo-words with neutral inflection |
Increase in L insula response |
| Meyer et al. (2002) | Imaging, fMRI | Grammatically uninterpretable sentences without lexical information vs. meaningless but syntactically correct sentences of pseudo-words |
Increase in bilateral insula response |
| Morris et al. (1999) | Imaging, PET | Non-verbal emotional vocalizations (fearful, sad, happy) vs. emotionally neutral vocalizations |
Increase in bilateral anterior insula response |
| Imaging, PET | Non-verbal vocalizations of fear vs. neutral vocalizations |
Increase in L anterior insula and decrease in R anterior insula responses |
|
| Kotz et al. (2003) | Negative intonations vs. neutral prosody | Increase in bilateral insula response | |
| Johnstone et al. (2006) | Imaging, fMRI | Happy vs. angry short phrases | Increase in L insula response |
| Quadflieg et al. (2008) | Imaging, fMRI | Anger prosody vs. neutral prosody | Increase in bilateral anterior insula |
| Shuren et al. (1993) | Lesion case-study | Production of speech prosody | Lesin in L anterior insula |
| Calder et al. (2000) | Lesion case-study | Recognition of disgust prosody | Lesin in L anterior insula |
| Cancelliere and Kertesz (1990) | Lesion study, quantitative |
Hypoprosody symptoms | Associated with lesions to either left or right insula |
Deficits in prosody perception and expression are observed in schizophrenia (Edwards et al. 2002, Hoekert et al. 2007, Ross et al. 2001, Leitman et al. 2005). Prosody deficits affect 84% of patients and are comparable to prosodic deficits from ischemic stroke (Ross et al. 2001). This finding applies to perceiving the prosody of sentences, words, syllables and non-syllable sounds (Leitman et al. 2005, Ross et al. 2001). It is present in first-episode schizophrenia (Edwards et al. 2001) and worsens with chronic schizophrenia (Kucharska-Pietura et al. 2005). It extends to fundamental components of prosody comprehension such as the patterns of internal pitch within sentences, but excludes terminal pitch changes such as used for questions (Matsumoto et al. 2006). It is most pronounced for the discrimination of negative emotions such as anger, fear and sadness (Bozikas et al. 2006, Pijnenborg et al. 2007, Edwards et al. 2001).
Emotional blunting, the decreased spontaneous generation of vocal prosody, is the most notable prosodic symptom in schizophrenia (Leentjens et al. 1998, Murphy and Cutting 1990). Deficits in prosody discrimination correlate with severity of negative symptoms (Leitman et al. 2005), while the deficit in discriminating the internal pitch component of prosody correlates with positive symptoms (Matsumoto et al. 2006). Deficits in facial affect recognition and vocal prosody in schizophrenia correlate with each other and with poor social functioning (Kucharska-Pietura et al. 2005, Hooker and Park 2002). In stable patients, the deficit is less severe in paranoid compared to non-paranoid subtypes of schizophrenia (Chan et al. 2008). In acute inpatients, the deficit in prosody recognition is greater in patients with auditory hallucinations compared to those without auditory hallucinations (Rossell and Boundy 2005).
One functional imaging study has connected the prosody deficits in schizophrenia to the insula. Compared to healthy controls, patients with schizophrenia have an increased response in the left insula when attending to prosody (Mitchell et al. 2004). This finding may reflect insula disinhibition or a hypersensitivity of the region to attentional modulation (Mitchell et al. 2004).
In summary, the production and comprehension of prosody is abnormal in schizophrenia. Functional and lesion studies show that the processing of prosody involves the anterior insula. The prosodic deficits associated with schizophrenia are most pronounced for negative emotions, the valence that most engages in the anterior insula. The aprosody and hypoprosody resulting from anterior insula lesions resemble the aprosodic deficits found in schizophrenia. The insula in schizophrenia responds abnormally when patients evaluate prosody. All of this evidence suggests that these symptoms of schizophrenia may be associated with disruptions in the normal function of the insula.
4.3 Pain Processing
Insula pathology in schizophrenia may contribute to the pain insensitivity observed in the disease. The perception of pain is strongly associated with the insula (table 3). The posterior insula receives sensory input from the somatosensory cortices and ventroposteriolateral thalamic nuclei. The anterior insula, with its connections to the limbic system and prefrontal cortex, is involved in evaluating the emotional elements of pain (Mesulam and Mufson 1982a). The functions of the two subregions are complementary: the posterior insula bilaterally encodes the experience of thermal pain while the bilateral anterior insula in involved in evaluating the intensity of the pain (Kong et al. 2006). Directly experienced pain elicits response in the contralateral posterior insula along with bilateral anterior insula. Pain that is witnessed, empathic pain, engages the anterior insula bilaterally (Singer et al. 2004, Ochsner et al. 2008). Distracting the subject from the pain decreases the response in the anterior insula (Brooks et al. 2002). Asymbolia for pain is a neurologic condition with normal recognition of pain but decreased emotional response to and decreased withdrawal from painful stimuli. Lesions to the insula may lead to this condition (Berthier 1988).
Table 3.
Somatosensory Studies
| Study: | Method: | Design: | Insula Involvement: |
|---|---|---|---|
| Peyron et al. (2000) | Meta-analysis of imaging studies |
Articles about pain processing published on or before 1999 |
Involvement of insula in 90% of studies |
| Apkarian et al. (2005) | Meta-analysis of imaging studies |
Articales about pain processing published on or before 2004 |
Involvement of insula in 94% of studies |
| Farrell et al. (2005) | Meta-analysis using activation likelihood estimations (ALE) |
Noxious heat applied to either upper limb |
Increses in posterior insula contralateral to site of stimulation along with anterior insula response bilaterally |
| Berthier (1988) | Lesion study, qualitative |
Asymbolia for pain | Associated with Involvement of insula, particular posterior subdivision |
Pain insensitivity is observed in schizophrenia, dating back to the writings of Kraeplin and Blueler (Potvin and Marchand 2008, Singh et al. 2006). Schizophrenic patients with very painful surgical conditions may present with minimal or absent pain. Additionally, there is a reduced prevalence of schizophrenia in patient populations with pain syndromes (Singh et al. 2006). Neuroleptic-free patients show reduced responses to pain, suggesting the effect is not related to medication effects. (Potvin and Marchand 2008). The deficit is described as a loss of the “meaning of pain” (Marchand et al. 1959, Geschwind 1977), and has been described as asymbolia for pain (Singh et al. 2006). Pain insensitivity also is found in the healthy relatives of patients with schizophrenia, suggesting a genetic contribution to the trait (Hooley and Delgado 2001).
Despite the above provocative findings, no imaging studies have yet evaluated the possible role of the insula in pain processing in schizophrenia. With the strong association between the insula and pain, and the condition of asymbolia associated with both insula lesions and as a symptom of schizophrenia, abnormality in the insula response to either direct or empathic pain is predicted.
4.4 Self versus Non-self
Insula pathology in schizophrenia may contribute to the poor perception of the self found in the disease. Patients with schizophrenia often describe their reflections as being independently alive, sinister, physically in flux and belonging to another person (Harrington et al. 1989). They show decreased accuracy in recognizing pictures of their own face from that of strangers (Kircher et al. 2007, Irani et al. 2006). When tasked with correcting their body image in an adjustable distorted mirror, they show a substantial deficit in the perception of their bodies. Yet when asked to correct the image of an inanimate object in the same mirror, their performance is substantially closer to that of controls (Orbach et al. 1966, Traub et al. 1967). These studies point to a deficit in the perception of the self in schizophrenia.
Schizophrenia is associated with difficulties in discriminating between self-generated and externally-generated sources of sensory stimuli. When listening to distorted speech, patients with schizophrenia make more errors in attributing their own speech to that of others (Cahill et al. 1996, Allen et al. 2004, Stephane et al. 2009). Patients actively experiencing hallucinations make more external attribution errors than patients without hallucinations (Johns et al. 2001, Costafreda et al. 2008).
The insula is involved in self-recognition and discriminating self from non-self sources of sensory input. The anterior insula responds to viewing self-portrait pictures, compared to both viewing pictures of unknown faces or of the subject’s partner (Sugiura et al. 2000, Kircher et al. 2000). Partners’ faces are almost as well-known to the subject as their own, suggesting this effect is from more than familiarity. Reading aloud with distorted feedback of the subject’s voice increases the response in the left insula, compared to reading aloud with feedback of a different person’s voice (McGuire et al. 1996). Misattribution of speech to a non-self source involves processing in the insula. While listening to speech and judging whether the stimuli was the subject’s own pre-recorded, distorted voice or the distorted voice of another person, activation of the left insula is associated with correct attributions (Allen et al. 2005).
The insula is involved in the self-attribution of actions. The left insula responds to auditory feedback directly resulting from the subject’s actions, compared to randomly timed feedback, during a motor task (Blakemore et al. 1998). The bilateral anterior insula responds to visual feedback resulting directly from the subject’s actions while driving a virtual race car around a track, compared to unrelated, random feedback (Farrer and Frith 2002). More abstractly, the left posterior insula responds to imagining carrying out an action in the first person compared to imagining the same action in the third (Ruby and Decety 2001). The left insula responds to imagined speech in the first versus third person (Shergill et al. 2001). These results suggest the insula plays a role in discerning self-associated and self-attributed sensory stimuli.
Known syndromes resulting from lesions of the insula include distortions of the boundaries of the self and self-awareness of actions. Somatoparaphrenia is a neurologic condition where patients attribute the ownership of their limb and it’s actions to another person. It is a very common complication of anosognosia for hemiparesis/hemiplegia, where a patient denies that there is anything wrong with a limb paralyzed by stroke (Baier and Karnath 2008). Both anosognosia for hemiparesis/hemiplagia generally and somatoparaphrenia in particular are associated with lesions to the right posterior insula (Karnath et al. 2005, Baier and Karnath 2008, Cereda et al. 2002). This suggests that damage to the insula disrupts conceptions of the self and the self-awareness of actions.
In summary, patients with schizophrenia have a distorted perception of self and difficulties properly attributing self-generated sensory stimuli. The insula, especially the anterior part, plays a role in processing representations of self-generated sensory stimuli. Abnormalities in this region may contribute to these difficulties. Misperception of the self as a distinct entity from the external world has been suggested as a possible mechanism of hallucinations and is discussed below.
4.5 Neurotransmitter Action within the Insula in Schizophrenia
To date, few studies have examined the involvement of specific neurotransmitter systems in the insula. Some evidence points to the involvement of cholinergic neurotransmission in the insula, and possible disruption of this system in schizophrenia. Involvement of the insula in cigarette addiction is suggested by that fact that damage to the region leads to immediate, easy, and relapse-free tobacco cessation (Naqvi et al. 2007). Anatomically, the insula receives cholinergic projections from the nucleus basalis of Meynert (Selden et al. 1998). It contains a high concentration of acetylcholinesterase, an indicator of cholinergic neurons, especially in the ventrorostral agranular region (Mesulam and Mufson 1982b, Sutoo et al. 1994). Alpha-4 and beta-2 nictonic acetylcholine receptor subunits are enriched in the insula. These two subunits combine to form the major high affinity nicotinic receptor in the human brain (Agulhon et al. 1998, Rubboli et al. 1994). In a healthy smoker, radiolabeled [11C]nicotine injections show the highest concentration in the bilateral insula (Nyback et al. 1989). Nicotine injection increases baseline hemodynamic response in the bilateral anterior and left posterior insula (Stein et al. 1998). Cholinergic systems in the insula modulate affect processing, as evidenced by the ability of physostigmine, a reversible acetylcholinesterase inhibitor, to alter bilateral insula hemodynamic activity in response to viewing fearful or neutral affect faces in healthy subjects (Bentley et al. 2003).
Disruption of cholinergic neurotransmission previously has been hypothesized in schizophrenia. This theory is supported by observations of exceedingly high rate of smoking in patients, information processing deficits in the disease that are corrected with nicotine, and the genetic linkage of polymorphisms in the promoter of the alpha-7 nicotinic receptor to the disease (Hughes et al. 1986, Adler et al. 1993, Freedman et al. 2001). Considering the established role of acetylcholine in modulating sensory information deficits in schizophrenia and connection between sensory and emotional information processing (Yamashita et al. 2005), it is possible that abnormalities in cholinergic neurotransmission could contribute to insula-related emotional processing deficits in schizophrenia. Additionally, the relation between cholinergic agonists and pharmacologically-induced hallucinations suggests a possible role of the neurotransmitter in the etiology of hallucinations (Perry and Perry 1995). Given the role of the insula in hallucinations (discussed below), it is possible that dysfunction of cholinergic systems in this region contributes to this symptom in the disease.
Experimental tasks that are modulated by acetylcholine and are abnormal in schizophrenia involve the insula. Oddball tasks, in which infrequent deviant stimuli are intermixed within regularly presented stimuli, are dependent on cholinergic modulation and are disrupted in schizophrenia (Javitt et al. 2008). Auditory oddball tasks elicit bilateral insula response in healthy subjects. In schizophrenia, this response is present but reduced (Kiehl et al. 2005). Compared to controls, patients with schizophrenia performing the oddball task also exhibit decreased neuronal synchrony between the bilateral insula and the medial frontal, superior temporal, thalamic, parahippocampal, and anterior cingulate regions (Kim et al. 2008). Prepulse inhibition of the startle response (PPI) is another abnormal physiological response in schizophrenia that improves with nicotine treatment. Direct subcutaneous nicotine improves PPI and increases the response of the right insula of patients with schizophrenia (Postma et al. 2006). A similar effect is seen with olfactory identification, which also is impaired in schizophrenia (Crespo-Facorro et al. 2008) and involves the insula (Royet et al. 2003). Smoking normalizes olfactory function in patients with schizophrenia while worsening it in healthy controls (McLean et al. 2004). These studies suggest that the abnormal cholinergic system in schizophrenia extends to the insula, disrupting processing of emotional stimuli and contributing to the affect-related deficits associated with the disease.
Other neurotransmitter systems relevant to schizophrenia also may be disrupted in the insula. The bilateral agranular anterior insula in the rat has an unusually high utilization of dopamine, significantly greater than the next highest area in the cortex, the medial prefrontal cortex (Jones et al. 1986). In humans, D1 receptors are rich in the insula and the grey matter volume of the insula bilaterally correlates with D2 receptor density (Hurd et al. 2001, Woodward et al. 2009). Both of these receptors are targets of neuroleptics and exposure to these drugs correlates with insula volume (Pressler et al. 2005). D4 receptors and 5HT1a receptors, both targets of the atypical antipsychotic clozapine, are enriched in the insula compared to the other regions of the cortex (Lahti et al. 1998, Rabiner et al. 2002). Disruption of these neurotransmitter systems in the insula could contribute to the symptoms of schizophrenia directly, or indirectly by interacting with cholinergic neurotransmission (Sato et al. 2007, Mameli-Engvall et al. 2006).
5. Putting it all together: The Insula and the Etiology of Hallucinations
Given the insula’s role in processing external and internal sensory information, and the related cognitive constructs of ‘self’ versus ‘non-self,’ it is possible to hypothesize that hallucinations, which likely relate to dysfunction of these processes, involve the insula. The sections below describe evidence for the involvement of the insula in hallucinations, and propose a mechanism for how insula dysfunction may lead to this cardinal feature of schizophrenia.
5.1 Evidence for Involvement in Hallucinations
Insula grey matter volume abnormalities are associated with both hallucinations and positive symptoms. Compared to non-hallucinating patients with schizophrenia, hallucinating patients have decreases in gray matter volume in the left insula. This decrease is above and beyond the bilateral grey-matter decrease in the insula found in all patients with schizophrenia in the same population of subjects (Shapleske et al. 2002). Bilateral insula gray matter volume negatively correlates with positive symptoms using the Scale for the Assessment of Positive Symptoms (SAPS) (Crespo-Facorro et al. 2000, Duggal et al. 2005). Hallucination and delusion scores for the same scale negatively correlate with right insula grey matter volume (Pressler et al. 2005). Hallucination severity correlates with bilateral insula grey matter volume (Modinos et al. 2009). These clinical measures suggest a relationship between decreasing grey matter volumes in the insula and an increased hallucinations and delusions.
Abnormal insula response is associated directly with hallucinations. Auditory hallucinations in schizophrenia increase the hemodynamic response in the bilateral insula (Shergill et al. 2000, Sommer et al. 2008, Jardri et al. 2009). Response of the left insula has been reported to occur shortly before a hallucination or as the subject becomes aware of it (Hoffman et al. 2008, Shergill et al. 2004). Somatic hallucinations in schizophrenia also are associated with bilateral insula response in one case-study (Shergill 2001). There is an overlap in activity encompassing the left and right anterior insula between auditory hallucinations in schizophrenia and silent word generation in the same subjects (Sommer et al. 2008).
5.2 Hallucinations and Internal (self) versus External (non-self)
Hallucinations have been conceptualized as a failure to differentiate an internally generated from an external sensory experience (Frith and Done 1988, Ford and Mathalon 2004). The insula, with its extensive connections to areas that process external sensory information and its role processing visceral sensation, is ideally connected to accomplish this function (Mesulam and Mufson 1982a, Damasio et al. 2000). A dysfunction in the insula could lead to confusion of the two sources, allowing internal sensory information to be attributed to an external source. A similar idea is that hallucinations are the result of a breakdown in the boundaries of the self, with self-generated sensory experience attributed to an alien, non-self source (Churchland 2002). With the role of the anterior insula in self-representation (Kircher et al. 2001, McGuire et al. 1996) and perception of other ‘selves’ (Oschner et al. 2008, Devue et al. 2007), a breakdown or blurring of these functions of the insula could lead to the perception of an alien non-self attached to the internally-created sensory experience.
A similar syndrome results from lesions to the posterior insula. As discussed above (in section 4.4), somatoparaphrenia involves a disruption in the sense of self as it relates to the body, where ownership of the affected limb is attributed to another person. Even more relevant, somatoparaphrenia involves a disruption in the self-awareness, where actions taken by the affected limb are attributed to an external force (kinaesthetic hallucinations). This syndrome results from lesions to the posterior insula and is likely related to the area’s association with somatosensory processing (Baier and Karnath 2008). Disruptions in the anterior insula may result in a similar phenomenon. However, with this subregion’s association with self-awareness (Craig 2009, Devue et al. 2007), a more abstract disruption in the concept of self would be expected.
A possible mechanism for these related theories is found in the differential response of the anterior insula to empathic (or non-self) sensory experience and direct (or self) sensory experience: both conditions engage the anterior insula but the degree of response differs. Empathic pain, witnessed but not experienced, elicits a hemodynamic response in the anterior insula bilaterally. The same areas respond similarly to pain directly experienced, but to a greater degree (Singer et al. 2004, Oschner et al. 2008). Right anterior insula response is observed when viewing both a partner’s face and one’s own face, but is greater in the self condition (Kircher et al. 2001). The same observation applies to images of one’s own face and body compared to a colleague’s face and body. Both show activity in the right anterior insula, but the degree of activity in the self condition is greater (Devue et al. 2007). Imitation of facial expressions compared to observation of the same facial expressions show the same result: anterior insula activation in both conditions but greater activation in the imitation condition, where the emotion is directly experienced (Carr et al. 2003, Hennenlotter et al. 2005). Given these findings, it is possible to hypothesize that self/non-self attribution is related to a threshold phenomenon: the anterior insula response observed in hallucinations (Shergill et al. 2000, Sommer et al. 2008) is too weak to identify the sensory information as having an internal source and results in it being attributed to an external, alien source.
6. Conclusion
Disruption of processing in the insula or a network involving the region could explain or contribute to many of the sensory deficits found in schizophrenia. The identification of emotional expressions on faces, the emotional content of speech, the emotional content of pain, and the neuronal representation of the self are impaired in the disease. People with schizophrenia have deficits comparable to subjects with lesions in the insula and show abnormal insula response in tasks that assess these functions. These deficits suggest a polymodal disruption in sensory-affect processing in schizophrenia, consistent with impaired insula function. With the overlap between sensory processing and sensory-affect processing, disruptions in cholinergic modulation could contribute to the observed deficits. As part of its role in sensory-affect processing, the anterior insula is involved in identifying self-generated from externally-generated sensory information. Failure of this process may lead to internally generated sensory information being attributed to an external source, contributing to hallucinations in schizophrenia. Future research focusing on insula function may further our understanding of disease pathology and improve therapeutic development.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler LE, Hoffer LD, Wiser A, Freedman R. Normalization of Auditory Physiology by Cigarette Smoking in Schizophrenic Patients. American Journal of Psychiatry. 1993;150(12):1856–1861. doi: 10.1176/ajp.150.12.1856. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Damasio H, Tranel D, Cooper G, Damasio A. A role for somatosensory cortices in the visual recognition of emotion as revealed by three-dimensional lesion mapping. The Journal of Neuroscience. 2000;20(7):2683–2690. doi: 10.1523/JNEUROSCI.20-07-02683.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio A. Dissociable neural systems for recognizing emotions. Brain and Cognition. 2003;52:61–69. doi: 10.1016/s0278-2626(03)00009-5. [DOI] [PubMed] [Google Scholar]
- Algulhon C, Charnay Y, Vallet P, Bertrand D, Malafosse A. Distribution of mRNA for the alpha-4 subunit of the nicotinic acetylcholine receptor in the human fetal brain. Molecular Brain Research. 1998;58:123–131. doi: 10.1016/s0169-328x(98)00113-2. [DOI] [PubMed] [Google Scholar]
- Allen PP, Amaro E, Fu CHY, Williams SCR, Brammer M, Johns LC, McGuire PK. Neural Correlates of the Misattribution of Self-Generated Speech. Human Brain Mapping. 2005;26:44–53. doi: 10.1002/hbm.20120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen PP, Johns LC, Fu CHY, Broome MR, Vythelingum GN, McGuire PK. Misattribution of External Speech in Patients with Hallucinations and Delusions. Schizophrenia Research. 2004;69:277–287. doi: 10.1016/j.schres.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Bushnell MC, Treede R, Zubieta J. Human Brain Mechanisms of Pain Perception and Regulation in Health and Disease. European Journal of Pain. 2005;9:463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Arce E, Simmons A, Lovero K, Stein M, Paulus M. Escitalopram effects on insula and amygdala BOLD activation during emotional processing. Psychopharmacology. 2008;196:661–672. doi: 10.1007/s00213-007-1004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine J. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Research Reviews. 1996;22:229–244. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- Baas D, Aleman A, Vink M, Ramsey NF, de Haan EHR, Kahn RS. Evidence of altered cortical and amygdala activation during social decision-making in schizophrenia. Neuroimage. 2008;40:719–727. doi: 10.1016/j.neuroimage.2007.12.039. [DOI] [PubMed] [Google Scholar]
- Bach DR, Grandjean D, Sander D, Herdener M, Strik WK, Seifritz E. The Effect of Appraisal Level on Processing of Emotional Prosody in Meaningless Speech. Neuroimage. 2008;42:919–927. doi: 10.1016/j.neuroimage.2008.05.034. [DOI] [PubMed] [Google Scholar]
- Baier B, Karnath H. Tight Link Between Our Sense of Limb Ownership and Self-Awareness of Actions. Stroke. 2008;39:486–488. doi: 10.1161/STROKEAHA.107.495606. [DOI] [PubMed] [Google Scholar]
- Beaucousin V, Lacheret A, Turbelin M, Morel M, Mazoyer B, Tzourio-Mazoyer N. fMRI Study of Emotional Speech Comprehension. Cerebral Cortex. 2007;17:339–352. doi: 10.1093/cercor/bhj151. [DOI] [PubMed] [Google Scholar]
- Bediou B, Franck N, Saoud M, Baudouin J, Tiberghien G, Dalery J, d’ Amato T. Effects of emotion and identity on facial affect processing in schizophrenia. Psychiatry Research. 2005;133:149–157. doi: 10.1016/j.psychres.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Bediou B, Krolak-Salmon P, Saoud M, Henaff M, Burt M, Dalery J, d’ Amato T. Facial expression and sex recognition in schizophrenia and depression. Canadian Journal of Pyschiatry. 2005;50(9):525–533. doi: 10.1177/070674370505000905. [DOI] [PubMed] [Google Scholar]
- Bentley P, Vuilleumier P, Thiel CM, Driver J, Dolan RJ. Cholinergic enhancement modulates neural correlates of selective attention and emotional processing. Neuroimage. 2003;20:58–70. doi: 10.1016/s1053-8119(03)00302-1. [DOI] [PubMed] [Google Scholar]
- Berthier M, Starkstein S, Leiguarda R. Asymbolia for pain: A sensory-limbic disconnection syndrome. Annals of Neurology. 1988;24:41–49. doi: 10.1002/ana.410240109. [DOI] [PubMed] [Google Scholar]
- Blakemore S, Rees G, Frith CD. How do we predict the consequences of our actions? A functional imaging study. Neuropsychologia. 1998;36(6):521–529. doi: 10.1016/s0028-3932(97)00145-0. [DOI] [PubMed] [Google Scholar]
- Borgwardt SJ, Picchioni MM, Ettinger U, Toulopoulou T, Murray R, McGuire PK. Regional Gray Matter Volume in Monozygotic Twins Concordant and Discordant for Schizophrenia. Biological Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.10.026. doi:10.1016/j.biopsych.2009.10.1026. [DOI] [PubMed] [Google Scholar]
- Borgwardt SJ, Riecher-Rossler A, Dazzan P, Chitnis X, Aston J, Drewe M, Gschwandtner U, et al. Regional Grey Matter Volume Abnormalities in the At Risk Mental State. Biological Psychiatry. 2007;61:1148–1156. doi: 10.1016/j.biopsych.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Bozikas V, Kosmidis MH, Anezoulaki D, Giannakou C, Karavatos A. Impaired Perception of Affective Prosody in Schizophrenia. Journal of Neuropsychiatry and Clinical Neurosciences. 2006;18(1):81–85. doi: 10.1176/jnp.18.1.81. [DOI] [PubMed] [Google Scholar]
- Brooks JCW, Nurmikko TJ, Bimson WE, Singh KD, Roberts N. fMRI of Thermal Pain: Effects of Stimulus Laterality and Attention. Neuroimage. 2002;15:293–301. doi: 10.1006/nimg.2001.0974. [DOI] [PubMed] [Google Scholar]
- Cahill C, Silbersweig D, Frith C. Psychotic Experiences Induced in Deluded Patients Using Distorted Auditory Feedback. Cognitive Neuropsychiatry. 1996;1(3):201–211. doi: 10.1080/135468096396505. [DOI] [PubMed] [Google Scholar]
- Calder A, Keane J, Manes F, Antoun N, Young A. Impaired recognition and experience of disgust following brain injury. Nature Neuroscience. 2000;3(11):1077–1078. doi: 10.1038/80586. [DOI] [PubMed] [Google Scholar]
- Cancelliere AEB, Kertesz A. Lesion localization in acquired deficits of emotional expression and comprehension. Brain and Cognition. 1990;13:133–147. doi: 10.1016/0278-2626(90)90046-q. [DOI] [PubMed] [Google Scholar]
- Carr L, Iacoboni M, Dubeau M, Mazziotta JC, Lenzi GL. Neural Mechanisms of Empathy in Humans: A relay from Neural Systems for Imitation to Limbic Areas. Proceedings of the National Academy of Sciences. 2003;100(9):5497–5502. doi: 10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr L, Iacobonni M, Dubeau M, Mazziotta JC, Lenzi GL. Neural Mechanisms of Empathy in Humans: A Relay from Neural Systems for Imitation to Limbic Areas. Proceedings of the National Academy of Sciences. 2003;100(9):5497–5502. doi: 10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereda C, Ghika J, Maeder P, Bogousslavsky J. Strokes Restricted to the Insular Cortex. Neurology. 2002;59:1950–1955. doi: 10.1212/01.wnl.0000038905.75660.bd. [DOI] [PubMed] [Google Scholar]
- Chan CCH, Wong R, Wang K, Lee TMC. Emotion recognition in Chinese people with schizophrenia. Psychiatry Research. 2008;157:67–76. doi: 10.1016/j.psychres.2006.03.028. [DOI] [PubMed] [Google Scholar]
- Chan R, Di X, McAlonan G, Gong Q. Brain Anatomical Abnormalities in High-Risk Individuals, First-Episode, and Chronic Schizophrenia: An activation likelihood estimation meta-analysis of illness progression. Schizophrenia Bulletin. doi: 10.1093/schbul/sbp073. (n.d.) doi:10.1093/schbul/sbp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland PS. Self-Representations in Nervous Systems. Science. 2002;296:308–310. doi: 10.1126/science.1070564. [DOI] [PubMed] [Google Scholar]
- Costafreda SG, Brebion G, Allen P, McGuire PK, Fu CHY. Affective Modulation of External Misattribution Bias in Source Monitoring in Schizophrenia. Psychological Medicine. 2008;38:821–824. doi: 10.1017/S0033291708003243. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: The Sense of the Physiological Condition of the Body. Current Opinion in Neurobiology. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Craig AD. How Do You Feel-Now? The Anterior Insula and Human Awareness. Nature Reviews Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Crespo-Facorro B, Kim J, Andreasen NC, O’Leary DS, Bockholt J, Magnotta V. Insular cortex abnormalities in schizophrenia: A structural magnetic resonance imaging study of first-episode patients. Schizophrenia Research. 2000;46:35–43. doi: 10.1016/s0920-9964(00)00028-1. [DOI] [PubMed] [Google Scholar]
- Crespo-Facorro B, Paradiso S, Andreasen NC, O’Leary DS, Watkins GL, Ponto LLB, Hichwa RD. Neural mechanisms of anhedonia in schizophrenia. Journal of the American Medical Association. 2001;286(4):427–435. doi: 10.1001/jama.286.4.427. [DOI] [PubMed] [Google Scholar]
- Crespo-Facorro B, Roiz-Santianez R, Quintero C, Perez-Iglesias R, Tordesillas-Gutierrez D, Mata I, Rodriguez-Sanchez JM, et al. Insular cortex morphology in first-episode schizophrenia-spectrum patients: Diagnostic specificity and clinical correlations. Journal of Psychiatric Research. 2010;44:314–320. doi: 10.1016/j.jpsychires.2009.08.014. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Melmed RN, Featherstone E, Mathias CJ, Dolan RJ. Volitional Control of Autonomic Arousal: A Functional Magnetic Resonace Study. Neuroimage. 2002;16:909–919. doi: 10.1006/nimg.2002.1147. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural Systems Supporting Interoceptive Awareness. Nature Neuroscience. 2004;7(2):189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Damasio A. The Person Within. Nature. 2003;423:227. doi: 10.1038/423227a. [DOI] [PubMed] [Google Scholar]
- Damasio A, Grabowksi T, Bechara A, Damasio H, Ponto L, Parvizi J, Hichwa R. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nature Neuroscience. 2000;3(10):1049–1056. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- Devue C, Collette F, Balteau E, Degueldre C, Luxen A, Maquet P, Bredart S. Here I Am: The Cortical Correlates of Visual Self-Recognition. Brain Research. 2007;1143:169–182. doi: 10.1016/j.brainres.2007.01.055. [DOI] [PubMed] [Google Scholar]
- Duggal HS, Muddasani S, Keshavan MS. Insular volumes in first-episode schizophrenia: gender effect. Schizophrenia Research. 2005;73:113–120. doi: 10.1016/j.schres.2004.08.027. [DOI] [PubMed] [Google Scholar]
- Dupont S, Bouilleret V, Hasboun D, Semah F, Baulac M. Functional Anatomy of the Insula: New Insights from Imaging. Surgical and Radiologic Anatomy. 2003;25:113–119. doi: 10.1007/s00276-003-0103-4. [DOI] [PubMed] [Google Scholar]
- Edwards J, Jackson HJ, Pattison PE. Emotion Recognition via Facial Expression and Affective Prosody in Schizophrenia: A Methodological Review. Clinical Psychology Review. 2002;22:789–832. doi: 10.1016/s0272-7358(02)00130-7. [DOI] [PubMed] [Google Scholar]
- Edwards J, Pattison PE, Jackson HJ, Wales RJ. Facial Affect and Affective Prosody Recognition in First-episode Schizophrenia. Schizophrenia Research. 2001;48:235–253. doi: 10.1016/s0920-9964(00)00099-2. [DOI] [PubMed] [Google Scholar]
- Ellison-Wright I, Glahn D, Laird A, Thelen S, Bullmore E. The anatomy of first-episode and chronic schizophrenia: An anatomical likelihood estimation meta-analysis. American Journal of Psychiatry. 2008;165(8):1015–1023. doi: 10.1176/appi.ajp.2008.07101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell MJ, Laird AR, Egan GF. Brain Activity Associated with Painfully Hot Stimuli Appliet to the Upper Limb: A Meta-Analysis. Human Brain Mapping. 2005;25:129–139. doi: 10.1002/hbm.20125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrer C, Frith CD. Experiencing oneself vs another person as being the cause of an action: the neural correlates of the experience of agency. Neuroimage. 2002;15:596–603. doi: 10.1006/nimg.2001.1009. [DOI] [PubMed] [Google Scholar]
- Flynn F, Benson DF, Ardila A. Anatomy of the insula-functional and clinical correlates. Aphasiology. 1999;13(1):55–78. doi:10.1080/026870399402325. [Google Scholar]
- Ford JM, Mathalon DH. Electrophysiological Evidence of Corollary Discharge Dysfunction in Schizophrenia During Talking and Thinking. Journal of Psychiatric Research. 2004;38:37–46. doi: 10.1016/s0022-3956(03)00095-5. [DOI] [PubMed] [Google Scholar]
- Fornito A, Yucel M, Patti J, Wood SJ, Pantelis C. Mapping Grey Matter Reductions in Schizophrenia: An anatomical estimation analysis of voxel-based morphometry studies. Schizophrenia Research. 2009;108:104–113. doi: 10.1016/j.schres.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Freedman R, Leonard S, Gault JM, Hopkins J, Cloninger CR, Kaufmann CA, Tsuang MT, et al. Linkage Disequilibrium for Schizophrenia at the Chromosome 15q13-14 Locus of the alpha7-Nicotinic Acetylcholine Receptor Subunit Gene (CHRNA7) American Journal of Medical Genetics. 2001;105:20–22. [PubMed] [Google Scholar]
- Frith CD, Done J. Towards a Neuropsychology of Schizophrenia. British Journal of Psychiatry. 1988;153:437–443. doi: 10.1192/bjp.153.4.437. [DOI] [PubMed] [Google Scholar]
- Geschwind N. Insensitivity to pain in psychotic patients. The New England Journal of Medicine. 1977;296(25):1480. doi: 10.1056/nejm197706232962520. [DOI] [PubMed] [Google Scholar]
- Glahn D, Laird A, Ellison-Wright I, Thelen S, Robinson J, Lancaster J, Bullmore E, et al. Meta-analysis of gray matter anomalies in schizophrenia: Application of anatomic likelihood estimation and network analysis. Biological Psychiatry. 2008 doi: 10.1016/j.biopsych.2008.03.031. doi:10.1016/j.biopsych.2008.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RE, Kohler CG, Ragland JD, Siegel SJ, Lesko K, Bilker WB, Gur RC. Flat affect in schizophrenia: Relation to emotion processing and neurocognitive measures. Schizophrenia Bulletin. 2006;32(2):279–287. doi: 10.1093/schbul/sbj041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington A, Oepen G, Spitzer M. Disordered Recognition and Perception of Human Faces in Acute Schizophrenia and Experimental Psychosis. Comprehensive Psychiatry. 1989;30(5):376–384. doi: 10.1016/0010-440x(89)90003-5. [DOI] [PubMed] [Google Scholar]
- Hennenlotter A, Schroeder U, Erhard P, Castrop F, Haslinger B, Stoecker D, Lange KW, et al. A Common Neural Basis for Receptive and Expressive Communication of Pleasant Facial Affect. Neuroimage. 2005;26:581–591. doi: 10.1016/j.neuroimage.2005.01.057. [DOI] [PubMed] [Google Scholar]
- Hoekert M, Kahn RS, Pijnenborg M, Aleman A. Impaired recognition and expression of emotional prosody in schizophrenia: Review and meta-analysis. Schizophrenia Research. 2007;96:135–145. doi: 10.1016/j.schres.2007.07.023. [DOI] [PubMed] [Google Scholar]
- Hoffman RE, Anderson AW, Varanko M, Gore JC, Hampson M. Time Course of Regional Brain Activation Associated with Onset of Auditory/Verbal Hallucinations. The British Journal of Psychiatry. 2008;193:424–425. doi: 10.1192/bjp.bp.107.040501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honea R, Crow T, Passingham D, Mackay C. Regional deficits in brain volume in schizophrenia: A meta-analysis of voxel-based morphometry studies. American Journal of Psychiatry. 2005;162(12):2233–2245. doi: 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- Hooker C, Park S. Emotion processing and its relationship to social functioning in schizophrenia patients. Psychiatry Research. 2002;112:41–50. doi: 10.1016/s0165-1781(02)00177-4. [DOI] [PubMed] [Google Scholar]
- Hooley JM, Delgado ML. Pain insensitivity in the relatives of schizophrenic patients. Schizophrenia Research. 2001;47:265–273. doi: 10.1016/s0920-9964(00)00064-5. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami DK, Mitchell JE, Dahlgren LA. Prevalence of Smoking Amoung Psychiatric Oupatients. American Journal of Psychiatry. 1986;143(8):993–997. doi: 10.1176/ajp.143.8.993. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Suzuki M, Sedvall G. D1 and D2 Dopamine Receptor mRNA Expression in Whole Hemisphere Sections of the Human Brain. Journal of Chemical Neuroanatomy. 2001;22:127–137. doi: 10.1016/s0891-0618(01)00122-3. [DOI] [PubMed] [Google Scholar]
- Iacobonni M, Dapretto M. The Mirror Neuron System and the Consequences of its Dysfunction. Nature Reviews Neuroscience. 2006;7:942–951. doi: 10.1038/nrn2024. [DOI] [PubMed] [Google Scholar]
- Irani F, Platek SM, Panyavin IS, Calkins ME, Kohler C, Siegel SJ, Schachter M, et al. Self-Face Recognition and Theory of Mind in Patients with Schizophrenia and First-Degree Relatives. Schizophrenia Research. 2006;88:151–160. doi: 10.1016/j.schres.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Jakob H, Beckmann H. Prenatal developmental disturbances in the limbic allocortex in schizophrenics. Journal of Neural Transmission. 1986;65:303–326. doi: 10.1007/BF01249090. [DOI] [PubMed] [Google Scholar]
- James W. The Principles of Psychology. Harvard University Press; Cambridge, MA: 1890. [Google Scholar]
- Jardri R, Pins D, Bubrovszky M, Lucas B, Lethuc V, Delmaire C, Vantyghem V, et al. Neural Functional Organization of Hallucinations in Schizophrenia: Multisensory Dissolution of Pathological Emergence in Consciousness. Consciousness and Cognition. 2009;18:449–457. doi: 10.1016/j.concog.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Spencer KM, Thaker GK, Winterer G, Hajos M. Neurophysiological biomarkers for drug development in schizophrenia. Nature Reviews Drug Discovery. 2008;7:68–83. doi: 10.1038/nrd2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns LC, Rossell S, Frith C, Ahmad F, Hemsley D, Kuipers E, McGuire PK. Verbal Self-Monitoring and Auditory Verbal Hallucinations in Patients with Schizophrenia. Psychological Medicine. 2001;31:705–715. doi: 10.1017/s0033291701003774. [DOI] [PubMed] [Google Scholar]
- Johnstone T, van Reekum CM, Oakes TR, Davidson RJ. The Voice of Emotion: An fMRI Study of Neural Responses to Angry and Happy Vocal Expressions. Social Cognitive and Affective Neuroscience. 2006;1:242–249. doi: 10.1093/scan/nsl027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MW, Kilpatrick IC, Phillipson OT. The Agranular Insular Cortex: A Site of Unusually High Dopamine Utilisation. Neuroscience Letters. 1986;72:330–334. doi: 10.1016/0304-3940(86)90536-7. [DOI] [PubMed] [Google Scholar]
- Karnath H, Baier B, Nagele T. Awareness of the Functioning of One’s Own Limbs Mediated by the Insular Cortex? The Journal of Neuroscience. 2005;25(31):7134–7138. doi: 10.1523/JNEUROSCI.1590-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai K, Shenton M, Salisbury D, Onitsuka T, Toner S, Yurgelun-Todd D, Kikinis R, et al. Differences and similarities in insular and temporal pole MRI gray matter volume abnormalities in first-episode schizophrenia and affective psychosis. 2003 doi: 10.1001/archpsyc.60.11.1069. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Stevens MC, Celone K, Kurtz M, Krystal JH. Abnormal hemodynamics in schizophrenia during an auditory oddball task. Biological Psychiatry. 2005;57:1029–1040. doi: 10.1016/j.biopsych.2005.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Pearlson GD, Kiehl KA, Bedrick E, Demirci O, Calhoun VD. A method for multi-group inter-participant correlation: Abnormal synchrony in patients with schizophrenia during auditory target detection. Neuroimage. 2008;39:1129–1141. doi: 10.1016/j.neuroimage.2007.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Youn T, Lee JM, Kim IY, Kim SI, Kwon JS. Morphometric abnormality of the insula in schizophrenia: A comparison with obsessive-compulsive disorder and normal control using MRI. Schizophrenia Research. 2003;60:191–198. doi: 10.1016/s0920-9964(02)00306-7. [DOI] [PubMed] [Google Scholar]
- Kircher TT, Seiferth NY, Plewnia C, Baar S, Schwabe R. Self-Face Recognition in Schizophrenia. Schizophrenia Research. 2007;94:264–272. doi: 10.1016/j.schres.2007.04.029. [DOI] [PubMed] [Google Scholar]
- Kircher TTJ, Senior C, Phillips ML, Benson PJ, Bullmore ET, Brammer M, Simmons A, et al. Towards a functional neuroanatomy of self processing: Effects of faces and words. Cognitive Brain Research. 2000;10:133–144. doi: 10.1016/s0926-6410(00)00036-7. [DOI] [PubMed] [Google Scholar]
- Kircher TTJ, Senior C, Phillips ML, Rabe-Hesketh S, Benson PJ, Bullmore ET, Brammer M, et al. Recognizing One’s Own Face. Cognition. 2001;78:B1–B15. doi: 10.1016/s0010-0277(00)00104-9. [DOI] [PubMed] [Google Scholar]
- Kong J, White NS, Kwong KK, Vangel MG, Rosman IS, Gracely RH, Gollub RL. Using fMRI to Dissociate Sensory Encoding from Cognitive Evaluation of Heat Pain Intensity. Human Brain Mapping. 2006;27:715–721. doi: 10.1002/hbm.20213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotz SA, Meyer M, Alter K, Besson M, von Cramon DY, Friederici AD. On the Lateralization of Emotional Prosody: An Event-related Functional MR Investigation. Brain and Language. 2003;86:366–376. doi: 10.1016/s0093-934x(02)00532-1. [DOI] [PubMed] [Google Scholar]
- Kucharska-Pietura K, David AS, Masiak M, Phillips ML. Perception of Facial and Vocal Affect by People with Schizophrenia in Early and Late Stages of Illness. British Journal of Psychiatry. 2005;187:523–528. doi: 10.1192/bjp.187.6.523. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, Goff D, et al. Regionally Localized Thinning of the Cerebral Cortex in Schizophrenia. Archives of General Psychiatry. 2003;60:878–888. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- Lahti RA, Roberts RC, Cochrane EV, Primus RJ, Gallager DW, Conley RR, Tamminga CA. Direct Determination of Dopamine D4 Receptors in Normal and Schizophrenic Postmortem Brain Tissue: A [3H]NGD-94-1 Study. Molecular Psychiatry. 1998;3:528–533. doi: 10.1038/sj.mp.4000423. [DOI] [PubMed] [Google Scholar]
- Leentjens AFG, Wielaert SM, von Harskamp F, Wilmink FW. Disturbances of Affective Prosody in Patients with Schizophrenia; A Cross Sectional Study. Journal of Neurology, Neurosurgery, and Psychiatry. 1998;64:375–378. doi: 10.1136/jnnp.64.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitman DI, Foxe JJ, Butler PD, Saperstein A, Revheim N, Javitt DC. Sensory contributions to impaired prosodic processing in schizophrenia. Biological Psychiatry. 2005;58:56–61. doi: 10.1016/j.biopsych.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Leppanen J, Niehaus D, Koen L, Toit ED, Schoeman R, Emsley R. Deficits in facial affect recognition in unaffected siblings of Xhosa schizophrenia patients: Evidence for a neurocognitive endophenotype. Schizophrenia Research. 2008;99:270–273. doi: 10.1016/j.schres.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Lovero KL, Simmons AN, Aron JL, Paulus MP. Anterior Insular Cortex Anticipates Impending Stimulus Significance. Neuroimage. 2009;45:976–983. doi: 10.1016/j.neuroimage.2008.12.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Goldstein JM, Kennedy D, Hodge SM, Caviness VS, Faraone SV, Tsuang MT, et al. Decreased volume of left and total anterior insular lobule in schizophrenia. Schizophrenia Research. 2006;83:155–171. doi: 10.1016/j.schres.2005.11.020. [DOI] [PubMed] [Google Scholar]
- Mameli-Engvall M, Evrard A, Pons S, Maskos U, Svensson T, Changeux J, Faure P. Hierarchical control of dopamine neuron-firing patterns by nicotinic receptors. Neuron. 2006;50:911–921. doi: 10.1016/j.neuron.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Marchand WE, Sarota B, Marble HC, Leary TM, Burbank CB, Bellinger MJ. Occurrence of painless acute surgical disorders in psychotic patients. The New England Journal of Medicine. 1959;260(12):580–585. doi: 10.1056/NEJM195903192601203. [DOI] [PubMed] [Google Scholar]
- Martin F, Baudouin J, Tiberghien G, Franck N. Processing emotional expression and facial identity in schizophrenia. Psychiatry Research. 2005;134:43–53. doi: 10.1016/j.psychres.2003.12.031. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Samson GT, O’Daly OD, Tracy DK, Patel AD, Shergill SS. Prosodic Discrimination in Patients with Schizophrenia. British Journal of Psychiatry. 2006;189:180–181. doi: 10.1192/bjp.bp.105.009332. [DOI] [PubMed] [Google Scholar]
- McGuire PK, Silbersweig DA, Frith CD. Functional neuroanatomy of verbal self-monitoring. Brain. 1996;119:907–917. doi: 10.1093/brain/119.3.907. [DOI] [PubMed] [Google Scholar]
- McLean D, Feron F, Mackay-Sim A, McCurdy R, Hirning M, Chant D, McGrath J. Paradoxical Association Between Smoking and Olfactory Identification in Psychosis Versus Controls. Australian and New Zealand Journal of Psychiatry. 2004;38:81–83. doi: 10.1111/j.1440-1614.2004.01301.x. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson E. Insula of the old world monkey. III: Efferent cortical output and comments on function. Journal of Comparitive Neurology. 1982a;212:38–52. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson E. Insula of the old world monkey. I: Architectonics in the insulo-orbito-temporal component of the paralimbic brain. Journal of Comparitive Neurology. 1982b;212:1–22. doi: 10.1002/cne.902120102. [DOI] [PubMed] [Google Scholar]
- Meyer M, Alter K, Friederici AD, Lohmann G, von Cramon DY. fMRI Reveals Brain Regions Mediating Slow Prosodic Modulations in Spoken Sentences. Human Brain Mapping. 2002;17:73–88. doi: 10.1002/hbm.10042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalopoulou PG, Surguladze S, Morley LA, Giampietro VP, Murray RM, Shergill SS. Facial Fear Processing and Psychotic Symptoms in Schizophrenia: Functional Magnetic Resonance Imaging Study. The British Journal of Psychiatry. 2008;192:191–196. doi: 10.1192/bjp.bp.106.032649. [DOI] [PubMed] [Google Scholar]
- Mitchell RLC, Elliot R, Barry M, Cruttenden A, Woodruff PWR. Neural response to emotional prosody in schizophrenia and bipolar affective disorder. British Journal of Psychiatry. 2004;184:223–230. doi: 10.1192/bjp.184.3.223. [DOI] [PubMed] [Google Scholar]
- Modinos G, Vercammen A, Mechelli A, Knegtering H, McGuire PK, Aleman A. Structural Covariance in the Hallucinating Brain: A voxel-based morphometry study. Journal of Psychiatry and Neuroscience. 2009;34(6):465–469. [PMC free article] [PubMed] [Google Scholar]
- Morgan K, Dazzan P, Orr K, Hutchinson G, Chitnis X, Suckling J, Lythgoe D, et al. Grey matter abnormalities in first-episode schizophrenia and affective psychosis. British Journal of Psychiatry. 2007;191(suppl. 51):s111–s116. doi: 10.1192/bjp.191.51.s111. doi:10.1192/bjp.191.51.s111. [DOI] [PubMed] [Google Scholar]
- Morris JS, Scott SK, Dolan RJ. Saying It with Feeling: Neural Responses to Emotional Vocalizations. Neuropsychologia. 1999;37:1155–1163. doi: 10.1016/s0028-3932(99)00015-9. [DOI] [PubMed] [Google Scholar]
- Morris JS, Friston KJ, Buchel C, Frith CD, Young AW, Calder AJ, Dolan RJ. A neuromodulatory role fo the human amygdala in processing emotional facial expressions. Brain. 1998;121:47–57. doi: 10.1093/brain/121.1.47. [DOI] [PubMed] [Google Scholar]
- Murphy D, Cutting J. Prosodic Comprehension and Expression in Schizophrenia. Journal of Neurology, Neurosurgery, and Psychiatry. 1990;53:727–730. doi: 10.1136/jnnp.53.9.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai M, Kishi K, Kato S. Insular cortex and neuropsychiatric disorders: A review of recent literature. European Psychiatry. 2007;22:387–394. doi: 10.1016/j.eurpsy.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesvag R, Lawyer G, Varnas K, Fjell AM, Walhovd KB, Frigessi A, Jonsson EG, et al. Regional thinning of the cerebral cortex in schizophrenia: Effects of diagnosis, age and antipsychotic medication. Schizophrenia Research. 2008;98:16–28. doi: 10.1016/j.schres.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Nyback H, Nordberg A, Langstrom B, Halldin C, Hartvig P, Ahlin A, Swahn CG, et al. Attempts to visualize nicotinic receptors in the brain of monkey and man by positron emission tomography. Progress in Brain Research. 1989;79:313–319. doi: 10.1016/s0079-6123(08)62490-5. [DOI] [PubMed] [Google Scholar]
- Olausson H, Lamarre Y, Backlund H, Morin C, Wallin BG, Starck G, Ekholm S, et al. Unmyelinated Tactile Afferents Signal Touch and Project to Insular Cortex. Nature Neuroscience. 2002;5(9):900–904. doi: 10.1038/nn896. [DOI] [PubMed] [Google Scholar]
- Orbach J, Traub AC, Olson RM. Psychophysical Studies of Body-Image II: Normative data on the adjustable body-distorting mirror. Archives of General Psychiatry. 1966;14:41–47. doi: 10.1001/archpsyc.1964.01720250055007. [DOI] [PubMed] [Google Scholar]
- Oschner KN, Zaki J, Hanelin J, Ludlow DH, Knierim K, Ramachandran T, Glover GH, et al. Your Pain or Mine? Common and Distinct Neural Systems Supporting the Perception of Pain in Self and Other. Social Cognitive and Affective Neuroscience. 2008;3:144–160. doi: 10.1093/scan/nsn006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An Insular View of Anxiety. Biological Psychiatry. 2006;60:383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Pennington K, Dicker P, Hudson L, Cotter DR. Evidence for reduced neuronal somal size within the insular cortex in schizophrenia, but not in affective disorders. Schizophrenia Research. 2008a;106:164–171. doi: 10.1016/j.schres.2008.08.022. [DOI] [PubMed] [Google Scholar]
- Pennington K, Dicker P, Dunn MJ, Cotter DR. Proteomic Analysis Reveals Protein Changes Within Layer 2 of the Insular Cortex in Schizophrenia. Proteomics. 2008b;8:5097–5107. doi: 10.1002/pmic.200800415. [DOI] [PubMed] [Google Scholar]
- Perry EK, Perry RH. Acetylcholine and Hallucinations: Disease-Related Compared to Drug-Induced Alterations in Human Consciousness. Brain and Cognition. 1995;28:240–258. doi: 10.1006/brcg.1995.1255. [DOI] [PubMed] [Google Scholar]
- Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain: A review and meta-analysis. Neurophysiologie clinique. 2000;30:263–288. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Senior C, Brammer M, Andrews C, Calder AJ, Bullmore ET, et al. A specific neural substrate for perceiving facial expressions of disgust. Nature. 1997;389:495–498. doi: 10.1038/39051. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Gregory LJ, Cullen S, Cohen S, Ng V, Andrew C, Giampietro V, et al. The Effect of Negative Emotional Context on Neural and Behavioral Responses to Oesophageal Stimulation. Brain. 2003;126:669–684. doi: 10.1093/brain/awg065. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Williams L, Senior C, Bullmore ET, Brammer MJ, Andrew C, Williams SCR, et al. A differential neural response to threatening and non-threatening negative facial expressions in paranoid and non-paranoid schizophrenics. Psychiatry Research: Neuroimaging. 1999;92:11–31. doi: 10.1016/s0925-4927(99)00031-1. [DOI] [PubMed] [Google Scholar]
- Pijnenborg GHM, Withaar FK, van den Bosch RJ, Brouwer WH. Impaired Perception of Negative Emotional Prosody in Schizophrenia. The Clinical Neuropsychologist. 2007;21:762–775. doi: 10.1080/13854040600788166. [DOI] [PubMed] [Google Scholar]
- Postma P, Gray JA, Sharma T, Geyer M, Mehrotra R, Das M, Zachariah E, et al. A behavioral and functional neuroimaging investigation into the effects of nicotine on sensorimotor gating in healthy subjects and persons with schizophrenia. Psychopharmacology. 2006;184:589–599. doi: 10.1007/s00213-006-0307-5. [DOI] [PubMed] [Google Scholar]
- Potvin S, Marchand S. Hypoalgesia in Schizophrenia is Independent of Antipsychotic Drugs: A Systematic Quantitative Review of Experimental Studies. Pain. 2008;138:70–78. doi: 10.1016/j.pain.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Pressler M, Nopoulos P, Ho B, Andreasen NC. Insular cortex abnormalities in schizophrenia: Relationship to symptoms and typical neuroleptic exposure. Biological Psychiatry. 2005a;57:394–398. doi: 10.1016/j.biopsych.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Pressler M, Nopoulos P, Ho B, Andreasen NC. Insular cortex abnormalities in schizophrenia: Relationship to symptoms and typical neuroleptic exposure. Biological Psychiatry. 2005b;57:394–398. doi: 10.1016/j.biopsych.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Quadflieg S, Mohr A, Mentzel H, Miltner WHR, Straube T. Modulation of the neural work involved in the processing of anger prosody: The role of task-relevance and social phobia. Biological Psychiatry. 2008;78:129–137. doi: 10.1016/j.biopsycho.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Rabiner EA, Messa C, Sargent PA, Husted-Kjaer K, Montgomery A, Lawrence AD, Bench CJ, et al. A database of [11C]WAY-100635 binding to 5-HT1a receptors in normal male volunteers: Normative data and relationship to methodological, demographic, physiological, and behavioral variables. Neuroimage. 2002;15:620–632. doi: 10.1006/nimg.2001.0984. [DOI] [PubMed] [Google Scholar]
- Roiz-Santianez R, Perez-Iglesias R, Quintero C, Tordesillas-Gutierrez D, Mata I, Ayesa R, Rodriguez-Sanchez JM, et al. Insular cortex thinning in first episode schizophrenia patients. Psychiatry Research: Neuroimaging. 2010;182:216–222. doi: 10.1016/j.pscychresns.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Ross ED, Orbelo DM, Cartwright J, Hansel S, Burgard M, Testa JA, Buck R. Affective-prosodic Deficits in Schizophrenia: Profiles of Patients with Brain Damage and Comparison with Relation to Schizophrenic Symptoms. Journal of Neurology, Neurosurgery, and Psychiatry. 2001;70:597–604. doi: 10.1136/jnnp.70.5.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossell SL, Boundy CL. Are auditory-verbal hallucinations associated with auditory affective processing deficits? Schizophrenia Research. 2005;78:95–106. doi: 10.1016/j.schres.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Royet J, Plailly J, Delon-Martin C, Kareken DA, Segebarth C. fMRI of emotional responses to odors: Influence of hedonic valence and judgement, handedness, and gender. Neuroimage. 2003;20:713–728. doi: 10.1016/S1053-8119(03)00388-4. [DOI] [PubMed] [Google Scholar]
- Rubboli F, Court JA, Sala C, Morris C, Perry E, Clementi F. Distribution of neuronal nicotinic receptor subunits in human brain. Neurochemistry International. 1994;25(1):69–71. doi: 10.1016/0197-0186(94)90055-8. [DOI] [PubMed] [Google Scholar]
- Ruby P, Decety J. Effect of subjective perspective taking during simulation of action: A PET investigation of agency. Nature Neuroscience. 2001;4(5):546–550. doi: 10.1038/87510. [DOI] [PubMed] [Google Scholar]
- Sachs G, Steger-Wuchse D, Kryspin-Exner I, Gur RC, Katshnig H. Facial recognition deficits and cognition in schizophrenia. Schizophrenia Research. 2004;68:27–35. doi: 10.1016/S0920-9964(03)00131-2. [DOI] [PubMed] [Google Scholar]
- Sato M, Ago Y, Koda K, Nakamura S, Kawasaki T, Baba A, Matsuda T. Role of postsynaptic serotonin1A receptors in risperidone-induced increase in acetylcholine release in rat prefrontal cortex. European Journal of Pharmacology. 2007;(559):155–160. doi: 10.1016/j.ejphar.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Saze T, Hirao K, Namiki C, Fukuyama H, Hayashi T, Murai T. Insular volume reduction in schizophrenia. European Archives of Psychiatry and Clinical Neurosience. 2007;257:473–479. doi: 10.1007/s00406-007-0750-2. [DOI] [PubMed] [Google Scholar]
- Schneider F, Gur R, Koch K, Backes V, Amunts K, Shah NJ, Bilker W, et al. Impairment in the specificity of emotion processing in schizophrenia. American Journal of Psychiatry. 2006;163:442–447. doi: 10.1176/appi.ajp.163.3.442. [DOI] [PubMed] [Google Scholar]
- Seiferth NY, Pauly K, Kellermann T, Shah NJ, Ott G, Herpetz-Dahlmann B, Kircher T, et al. Neuronal Correlates of Facial Emotion Discrimination in Early Onset Schizophrenia. Neuropsychopharmacology. 2009;34:477–487. doi: 10.1038/npp.2008.93. [DOI] [PubMed] [Google Scholar]
- Selden NR, Gitelman DR, Salamon-Murayama N, Parrish TB, Mesulam MM. Trajectories of cholinergic pathways within the cerebral hemispheres of the human brain. Brain. 1998;121:2249–2257. doi: 10.1093/brain/121.12.2249. [DOI] [PubMed] [Google Scholar]
- Shapleske J, Rossell SL, Chitnis XA, Suckling J, Simmons A, Bullmore ET, Woodruff PWR, et al. A computational morphometric MRI study of schizophrenia: Effects of hallucinations. Cerebral Cortex. 2002;12:1331–1341. doi: 10.1093/cercor/12.12.1331. [DOI] [PubMed] [Google Scholar]
- Shergill SS, Bullmore ET, Brammer MJ, Williams SCR, Murray RM, McGuire PK. A functional study of auditory verbal imagery. Psychological Medicine. 2001;31:241–253. doi: 10.1017/s003329170100335x. [DOI] [PubMed] [Google Scholar]
- Shergill SS, Cameron LA, Brammer MJ, Williams SCR, Murray RM, McGuire PK. Modality Specific Neural Correlates of Auditory and Somatic Hallucinations. Journal of Neurology, Neurosurgery, and Psychiatry. 2001;71:688–690. doi: 10.1136/jnnp.71.5.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shergill SS, Brammer MJ, Williams SCR, Murray RM, McGuire PK. Mapping auditory hallucinations in schizophrenia using functional magnetic resonance imaging. Archives of General Psychiatry. 2000;57:1033–1038. doi: 10.1001/archpsyc.57.11.1033. [DOI] [PubMed] [Google Scholar]
- Shergill SS, Brammer MJ, Williams SCR, Murray RM, McGuire PK. Temporal Course of Auditory Hallucinations. British Journal of Psychiatry. 2004;185:516–517. doi: 10.1192/bjp.185.6.516. [DOI] [PubMed] [Google Scholar]
- Shuren J. Insula and aphasia. Journal of Neurology. 1993;240:216–218. doi: 10.1007/BF00818707. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for Pain Involves the Affective but not Sensory Components of Pain. Science. 2004;303:1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Singh MK, Giles LL, Nasrallah HA. Pain Insensitivity in Schizophrenia: Trait or State Marker? Journal of Psychiatric Practice. 2006;12:90–102. doi: 10.1097/00131746-200603000-00004. [DOI] [PubMed] [Google Scholar]
- Sommer IEC, Diederen KMJ, Blom J, Willems A, Kushan L, Slotema K, Boks MPM, et al. Auditory Verbal Hallucinations Predominantly Activate the Right Inferior Frontal Area. Brain. 2008;131:3169–3177. doi: 10.1093/brain/awn251. [DOI] [PubMed] [Google Scholar]
- Sprengelmeyer R, Rausch M, Eysel UT, Przuntek H. Neural structures associated with recognition of facial expressions of basic emotions. Proceedings of the Royal Society of London B. 1998;265:1927–1931. doi: 10.1098/rspb.1998.0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein EA, Pankiewicz J, Harsch HH, Cho J, Fuller SA, Hoffmann RG, Hawkins M, et al. Nicotine-induced limbic cortical activation in the human brain: A functional MRI study. American Journal of Psychiatry. 1998;155(8):1009–1015. doi: 10.1176/ajp.155.8.1009. [DOI] [PubMed] [Google Scholar]
- Stephane M, Kuskowski M, McClannahan K, Surerus C, Nelson K. Evaluation of Speech Misattribution Bias in Schizophrenia. Psychological Medicine. 2009 doi: 10.1017/S003329170999081X. doi:10.1017/S003329170999081X. [DOI] [PubMed] [Google Scholar]
- Sugiura M, Kawashima R, Nakamura K, Okada K, Kato T, Nakamura A, Hatano K, et al. Passive and Active Recognition of One’s Own Face. Neuroimage. 2000;11:36–48. doi: 10.1006/nimg.1999.0519. [DOI] [PubMed] [Google Scholar]
- Sutoo D, Akiyama K, Yabe K, Kohno K. Quantitative analysis of immunohistochemical distributions of cholinergic and catecholaminergic systems in the human brain. Neuroscience. 1994;58(1):227–234. doi: 10.1016/0306-4522(94)90170-8. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Suzuki M, Hagino H, Zhou S, Kawasaki Y, Nohara S, Nakamura K, et al. Bilateral volume reduction of the insular cortex in patients with schizophrenia: a volumetric MRI study. Psychiatry Research: Neuroimaging. 2004;131:185–194. doi: 10.1016/j.pscychresns.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Suzuki M, Zhou S, Hagino H, Tanino R, Kawasaki Y, Nohara S, et al. Volumetric MRI study of the short and long insular cortices in schizophrenia spectrum disorders. Psychiatry Research: Neuroimaging. 2005;138:209–220. doi: 10.1016/j.pscychresns.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Wood SJ, Yung AR, Phillips LJ, Soulsby B, McGorry PD, Tanino R, et al. Insular Cortex Grey Matter Changes in Individuals at Ultra-High-Risk of Developing Psychosis. Schizophrenia Research. 2009;111:94–102. doi: 10.1016/j.schres.2009.03.024. [DOI] [PubMed] [Google Scholar]
- Traub AC, Olson RM, Orbach J, Cardone SS. Psychophysical Studies of Body-Image III: Initial studies of disturbances in a chronic schizophrenia group. Archives of General Psychiatry. 1967;17:664–670. doi: 10.1001/archpsyc.1967.01730300024005. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Kohler CG, Indersmitten T, Bhati MT, Charbonnier D, Gur RC. Facial emotion recognition in schizophrenia: When and why does it go awry? Schizophrenia Research. 2007;94:253–263. doi: 10.1016/j.schres.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van’t Wout M, van Dijke A, Aleman A, Kessels RPC, Pijpers W, Kahn RS. Fearful faces in schizophrenia: The relationship between patient characteristics and facial affect recognition. The Journal of Nervous and Mental Disease. 2007;195(9):758–764. doi: 10.1097/NMD.0b013e318142cc31. [DOI] [PubMed] [Google Scholar]
- Wicker B, Keysers C, Plailly J, Gallese V, Rizzolatti G. Both of us disgusted in my insula: The common neural basis of seeing and feeling disgust. Neuron. 2003;40:655–664. doi: 10.1016/s0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]
- Williams LM, Das P, Liddell BJ, Olivieri G, Peduto AS, David AS, Gordon E, et al. Fronto-limbic and autonomic disjunctions to negative emotion distinguish schizophrenia subtypes. Psychiatry Research: Neuroimaging. 2007;155:29–44. doi: 10.1016/j.pscychresns.2006.12.018. [DOI] [PubMed] [Google Scholar]
- Woodward ND, Zald DH, Ding Z, Riccardi P, Ansari MS, Baldwin RM, Cowan RL, et al. Cerebral Morphology and Dopamine D2/D3 Receptor Distribution in Humans: A Combined [18F]fallypride and Voxel-Based Morphometry Study. Neuroimage. 2009;46:31–38. doi: 10.1016/j.neuroimage.2009.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita H, Okamoto Y, Morinobu S, Yamawaki S, Kahkonen S. Visual emotional stimuli modulation of auditory sensory gating studied by magnetic P50 suppression. European Archives of Psychiatry and Clinical Neurosience. 2005;255:99–103. doi: 10.1007/s00406-004-0538-6. [DOI] [PubMed] [Google Scholar]