Abstract
Background
Hepatitis B vaccine provides a model for improving uptake and completion of multi-dose vaccinations in the drug-using community.
Methods
DASH project conducted randomized controlled trial among not-in-treatment current drug users in two urban neighborhoods. Neighborhoods were cluster-randomized to receive a standard (HIV information) or enhanced (HBV vaccine acceptance/adherence) behavioral intervention; participants within clusters were randomized to a standard (0, 1, 6 mo) or accelerated (0, 1, 2 mo) vaccination schedule. Outcomes were completion of three-dose vaccine and HBV seroprotection.
Results
Of those screening negative for HIV/HBV, 77% accepted HB vaccination and 75% of those received all 3 doses. Injecting drug users (IDUs) on the accelerated schedule were significantly more likely to receive 3 doses (76%) than those on the standard schedule (66%, p=.04), although for drug users as a whole the adherence was 77% and 73%. No difference in adherence was observed between behavioral intervention groups. Predictors of adherence were older age, African American race, stable housing, and alcohol use. Cumulative HBV seroprotection (≥10 mIU/mL) was gained by 12 months by 65% of those completing. Seroprotection at 6 months was greater for the accelerated schedule group.
Conclusions
The accelerated vaccine schedule improves hepatitis B vaccination adherence among IDU.
Keywords: HIV infections, Hepatitis B, prevention & control, drug users, Hepatitis B vaccines, AIDS vaccines, behavioral intervention, vaccine schedules
INTRODUCTION
Hepatitis B is one of the most frequently reported preventable diseases in the U.S., with 78,000 new infections annually [1–3]. The most frequently reported risk factors for contracting hepatitis B virus (HBV) are multiple heterosexual sex partners, male-to-male sex, and injecting drug use. Individuals with at least one of these risk factors make up 75% of new HBV infections [4]. At least 20% of HBV infections occur in injecting drug users (IDUs) [5, 6]. Vaccine-preventable, HBV may result in persistent lifelong infection. The asymptomatic nature of chronic hepatitis B presents a public health threat because of its highly infectious nature. Long-term health consequences can develop in 15%–40% of chronically infected individuals, including cirrhosis, liver failure, and hepatocellular carcinoma [7]. Because the risk of developing clinical hepatitis after acute infection is greater in adults, vaccination will prevent more cases of clinical hepatitis and reduce future health-care costs [8].
Immunization strategies in the United States targeting healthcare workers, high-risk adults, and infants/children have been instrumental in reducing overall hepatitis B transmission and incidence. However, drug users have one of the lowest immunization rates in the nation [9, 10], with a continued high prevalence of HBV infection and chronic carrier status [4, 11–14]. We began the Drugs, AIDS, STDs, and Hepatitis (DASH) project to target drug users for AIDS, STDs, and hepatitis prevention research [15].
Effective HBV vaccination in drug users requires their adherence to a multi-dose vaccine schedule, which is needed for an adequate immune response to the vaccine. Few studies have focused on the behaviors that may affect vaccine acceptance and adherence in drug users. Instead, vaccination programs have sought to identify better ways to administer all three doses of the vaccine [16, 17], without addressing the behaviors and behavioral cognitions (e.g., attitudes towards vaccines) that could contribute to non-acceptance of or non-adherence to the HBV vaccine schedule [18–20].
An individual’s immune response to a multi-dose vaccine may be compromised by characteristics or behaviors specific to drug-using populations; identification of these factors is necessary to design effective vaccination initiatives. Previous research indicates that altering hepatitis B vaccine schedules may increase adherence and also elicit an earlier adequate protective immune response [21, 22]. Little is known of the durability of immune protection in drug users with shorter vaccination protocols.
HBV and hepatitis C virus (HCV) were endemic among IDU even before HIV. Common risk factors for these blood-borne viral agents, such as multi-person use of injecting equipment and risky sexual behaviors, have resulted in a high prevalence of infection of all three viruses among drug users. However, a significant proportion of this population remains at risk for these infections, and should be targeted for vaccination [15, 23]. The objectives of this study was to evaluate an HBV vaccination program as a model for future HIV or HCV vaccine efficacy trials in drug-using populations. Two components were analyzed to determine if they increased vaccine three-dose adherence: a behavioral intervention and an accelerated vaccine schedule. The latter was also evaluated to see if it had any significant effect on immune response.
METHODS
Study Design and Population
A randomized controlled trial was conducted among not-in-treatment current drug users in urban neighborhoods in Houston, Texas. This study was approved by the appropriate Institutional Review Board and followed USDHHS human experimentation guidelines.
From February 2004 through October 2007 we screened 2827 not-in-treatment drug users for HIV and HBV, HCV infections. Study participants were recruited by outreach workers and chain-referral methods from drug distribution areas, street corners, and crack houses in two neighborhoods. All screening took place at a designated community field site. Eligibility criteria were: (1) age 18 years or over, (2) local residence with valid contact information for follow-up, (3) self-report of illicit drug use (e.g., cocaine, heroin, methamphetamine, marijuana) in the 48 hours prior to screening, and (4) willingness and competency to give informed consent. Drug use was confirmed by urine screen using OnTrack Teststik (Varian Inc., Palo Alto, CA).
After verbal screening and informed consent, a medical assistant or nurse performed a blood draw of 10 ml for the preliminary susceptibility screening test, the Core Combo HIV-HBsAg-HCV rapid test (Core Diagnostics, UK), to detect antibodies to hepatitis B surface antigen (HBsAg), HIV 1/2 (anti-HIV), and HCV (anti-HCV). If the blood tested both HIV and HBsAg negative, the sample underwent testing for antibody to HBsAg (anti-HBs) by microparticle enzyme immunoassay (AxSYM, Abbott Laboratories, Chicago, IL). Screened participants negative for HBsAg, anti-HIV, and anti-HBs were qualified for enrollment into the randomized acceptance/adherence study. The hepatitis B core antibody (anti-HBc) was not tested at screening because a positive anti-HBc test alone does not indicate protective immunity, and it was deemed ethically necessary to revaccinate these participants. Anti-HBc positive participants were excluded from the immune response subgroup analysis.
Enhanced Behavioral and Accelerated Vaccine Schedule Interventions
Randomization of the enhanced and standard behavioral interventions occurred at the neighborhood level. Study participants enrolled in odd-numbered months received the HB vaccine (Engerix B, 20ug/1ml, GlaxoSmithKline) on the standard schedule of 0, 1, and 6 months. Participants enrolled in even-numbered months followed the accelerated schedule of 0, 1 and 2 months.
The HBV vaccination behavioral intervention of four, 15–20 min sessions was based on brief self-efficacy interventions previously developed for community-based HIV prevention programs [24]. The purpose was to increase drug users’ acceptance and adherence to HBV vaccine protocols by increasing self-efficacy, positive outcome expectations, perceived peer group support, and the value attached to HBV vaccination. The intervention provided accurate and salient information about HBV and HBV vaccination, the benefits that could be gained and the losses avoided by being tested and vaccinated for HBV, vicarious experience (discussion, stories, modeling, graduated mastery learning processes), verbal persuasion by peer outreach workers, and positive emotional arousal. Sessions 1 and 2 were delivered at screening and enrollment, after obtaining written informed consent, and Session 3 at the one-month visit (2nd dose). Session 4 was delivered before the 3rd dose of vaccine: for the accelerated schedule, at a 6-week visit with the last dose at 2 months; for the standard schedule, at the 2-month visit with the last dose at 6 months. The standard behavioral intervention, given at the same times, delivered NIDA information on HIV awareness and prevention [25].
Enrollment and Follow-up
Study participants were enrolled into one of four arms: standard and enhanced behavioral interventions crossed with standard (0, 1, 6 month) and accelerated (0, 1, 2 month) vaccine schedules (see Figure 1). Those who were eligible, based on blood screening results and acceptance of HB vaccination, were further enrolled in a sub-study, with separate consent, for follow-up, to track vaccine adherence efficacy and durability. After enrollment, these sub-study participants underwent follow-up at 1, 2, 6, 12, 18, and 24 months for interview and blood draw for anti-HIV, anti-HCV, anti-HBs, anti-HBc, and HBsAg. A gratuity of $30 was paid at enrollment, and $20 for each subsequent follow-up visit, to all participants.
Figure 1.
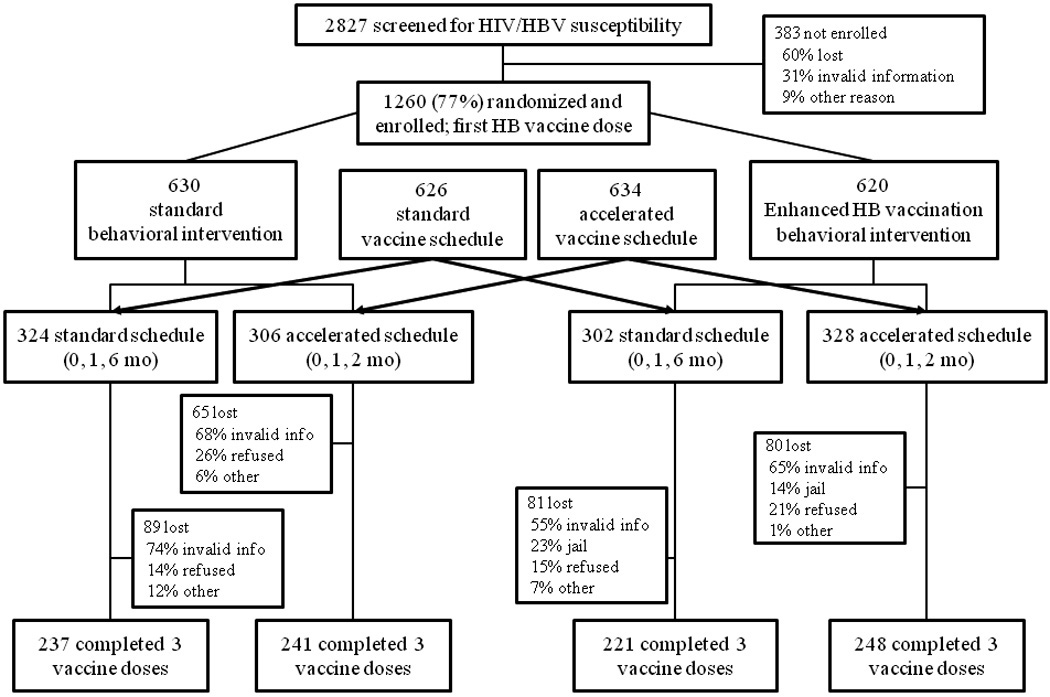
Participants’ Screening, Enrollment, and Follow Up in Hepatitis B Vaccine Intervention
Data Collection and Laboratory Methods
The screening, enrollment, and follow-up questionnaires were adapted from instruments used in previous studies. The enrollment baseline questionnaire included additional questions on drug bingeing (drug, places, sexual behaviors while bingeing), and HBV perception scales (strongly agree to strongly disagree) with regard to the transmission of HBV and to HBV vaccination. All interviews were verbally administered and recorded electronically via computer-assisted personal interview (CAPI, QDS, Bethesda, MD).
Blood specimens collected at enrollment and follow-up were tested for anti-HIV (Abbott PPC Commander system, Abbott Laboratories, Chicago, IL), and for anti-HCV, anti-HBs, HBsAg, anti-HBc (all Abbott’s AxSYM system). Repeatedly reactive HIV samples were confirmed by western blot (Cambridge Biotech).
Definitions
Western blots with an indeterminate or positive result were considered positive for HIV. Past or current HBV infection was defined as positive for HBsAg, anti-HBc, or both, with or without anti-HBs. HCV infection was defined as the detection of anti-HCV. Participants who were willing to receive at least one dose of HB vaccine and completed at least one dose were defined as acceptors and were compared to non-acceptors. Adherence was defined as completing all three doses of the HB vaccine, irrespective of schedule. An anti-HBs titer of ≥10 IU/mL was the cut point for seroprotection.
Statistical Analysis
Sample sizes were calculated by the methods of Dupont and Plummer [26] at the two-sided significance level of alpha=0.05. With a sample size of more than 300 in each group, the study had 80% power to detect a 10% difference between groups with α=.05.
Questionnaire data were exported into SAS 9.1 (Cary, NC). Data analysis was performed using STATA 9.1 (College Station, TX). For simple logistic regression analysis, odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for each risk factor and demographic variable. In the case of small cell sizes, the chi-square test and Fisher’s exact test were also used to determine significance of associations. Because of their small numbers, Asian and other races were combined with Hispanic race for analysis.
For multiple logistic regression analysis, risk factors with a p-value <0.2 in the simple logistic regression, together with age, gender, and race, were entered into the multiple logistic regression model. Independent variables in the multiple logistic regression models were eliminated based on backward stepwise regression [27]. Adjusted ORs and 95% CIs were calculated for the variables with a p-value ≤0.05 or which were biologically plausible in the final model.
Seroprotection rates were calculated and compared at 2, 6, and 12 months.
RESULTS
Study Population, Enrollment, and Acceptance of HB Vaccination
Of the participants who were screened, 58% (1643/2827) had negative results for anti-HIV, HBsAg, and anti-HBs, and 77% (1266/1643) of those were enrolled in the randomized intervention study. Of the 377 eligible but not enrolled, over 90% could not be re-contacted. Six participants had missing data in the intervention longitudinal analyses, for a total of 1260 for analysis (see Figure 1).
Significant differences existed among age groups between those receiving the enhanced and standard interventions (Table 1). Significant differences between the two vaccine groups existed in participants who traded sex for money or drugs in the last 30 days or used a combination of drugs in the past 30 days. The 2% who are listed as “No drug use in last 30 days” screened positive for drugs at the initial contact but denied current use at enrollment. The educational levels of all groups were higher than for the Houston population as a whole.
Table 1.
CHARACTERISTICS OF ENROLLED PARTICIPANTS
| TOTAL |
BEHAVIORAL INTERVENTION |
VACCINATION SCHEDULE |
|||
|---|---|---|---|---|---|
| CHARACTERISTIC | N=1260 | Standard Group n=630 |
Enhanced Group n=630 |
Standard Schedule (0, 1, 6 mo) n=626 |
Accelerated Schedule (0, 1, 2 mo) n=634 |
| Gender | |||||
| Male | 969 (78%) | 473 (75%) | 496 (79%) | 474 (76%) | 495 (78%) |
| Female | 291 (22%) | 157 (25%) | 133 (21%) | 152 (24%) | 139 (22%) |
| Age | |||||
| 18–29 | 119 (9%) | 46 (7%) | 73 (12%)* | 62 (10%) | 57 (9%) |
| 30–39 | 295 (23%) | 154 (24%) | 141 (22%) | 140 (23%) | 155 (24%) |
| 40–49 | 548 (44%) | 287 (46%) | 261 (42%) | 278 (44%) | 270 (43%) |
| ≥ 50 | 298 (24%) | 143 (23%) | 154 (24%) | 146 (23%) | 152 (24%) |
| Race | |||||
| Black | 1071 (85%) | 537 (85%) | 534 (85%) | 534 (85%) | 537 (85%) |
| White | 129 (10%) | 56 (9%) | 73 (12%) | 65 (10%) | 64 (10%) |
| Hispanic/Other | 60 (5%) | 37 (6%) | 22 (3%) | 27 (5%) | 33 (5%) |
| Education | |||||
| < High school | 70 (6%) | 36 (6%) | 34 (5%) | 30 (5%) | 40 (6%) |
| High school | 898 (71%) | 444 (70%) | 453 (72%) | 460 (73%) | 438 (69%) |
| Some college | 292 (23%) | 150 (24%) | 142 (23%) | 136 (22%) | 156 (25%) |
| Housing status | |||||
| Permanent | 52 (4%) | 27 (4%) | 25 (4%) | 23 (4%) | 29 (5%) |
| Temporary/streets | 1208 (96%) | 603 (97%) | 604 (96%) | 603 (96%) | 605 (95%) |
| Drug treatment (ever): yes | 763 (61%) | 369 (59%) | 393 (62%) | 373 (60%) | 390 (61%) |
| IDU ever: yes | 378 (30%) | 187 (30%) | 191 (30%) | 174 (28%) | 204 (32%) |
| HCV positive: yes | 423 (34%) | 99 (32%) | 223 (35%) | 200 (32%) | 223 (35%) |
| IDU in last 30 days: yes | 92 (7%) | 53 (8%) | 39 (6%) | 50 (8%) | 42 (7%) |
| Traded sex for money/drugs in last 30 days: yes |
217 (18%) | 108 (17%) | 109 (17%) | 93 (15%) | 124 (20%)* |
| Traded money/drugs for sex in last 30 days: yes |
200 (16%) | 97 (15%) | 103 (16%) | 87 (14%) | 113 (18%) |
| Crack in last 30 days: yes | 1151 (91%) | 575 (91%) | 576 (91%) | 560 (89%) | 591 (93%) |
| Cocaine in last 30 days: yes | 204 (16%) | 101 (16%) | 103 (16%) | 108 (17%) | 96 (15%) |
| Methamphetamines in last 30 days: yes |
50 (4%) | 26 (4%) | 24 (4%) | 25 (4%) | 25 (4%) |
| Fry in last 30 days: yes | 24 (2%) | 12 (2%) | 12 (2%) | 13 (2%) | 11 (2%) |
| Marijuana in last 30 days: yes | 620 (49%) | 306 (46%) | 314 (50%) | 306 (49%) | 314 (50%) |
| Alcohol in last 30 days: yes | 870 (69%) | 439 (70%) | 431 (68%) | 429 (69%) | 441 (70%) |
| Heroin in last 30 days: yes | 48 (4%) | 32 (5%) | 16 (3%) | 25 (4%) | 22 (4%) |
| Speedball in last 30 days: yes | 23 (2%) | 12 (2%) | 11 (2%) | 13 (2%) | 10 (2%) |
| Combination drug use in last 30 days |
|||||
| No drug use | 28 (2%) | 16 (3%) | 12 (2%) | 16 (3%) | 12 (2%)* |
| Used 1 drug | 21 (17%) | 99 (16%) | 116 (18%) | 92 (15%) | 123 (19%) |
| Used 2 drugs | 478 (38%) | 243 (39%) | 235 (37%) | 264 (42%) | 214 (34%) |
| Used ≥3 drugs | 539 (43%) | 272 (43%) | 267 (42%) | 254 (41%) | 285 (45%) |
p-value<0.05
Note: “Fry” is marijuana laced with PCP and embalming fluid; “speedball” is heroin and cocaine
When we compared the characteristics of participants who accepted the HB vaccine to participants who did not, after adjustment in the multivariable analysis (data not shown), females (OR=1.40, 95% CI=1.02–1.84), participants 50 years and older (reference, ≤29 years old; OR=2.2, 95% CI=1.43–3.30), Blacks (OR=1.51, 95% CI=1.04–2.20), and participants using drugs ≤10 times per week (OR=2.01, 95% CI=1.46–2.76) were significantly more likely to accept the vaccine.
Adherence to Three-dose HB Vaccine
Three-fourths of the enrollees (941/1260) completed all three vaccine doses (Figure 1). As seen in Table 2, two-arm comparison (vaccine schedule), the standard group had an adherence rate of 73% and the accelerated group’s rate was 77%, p=0.09. After stratification by IDU status, adherence rates were significantly different for those on the standard (66%) versus accelerated (76%) vaccine schedules (p=0.04), while no significant difference was observed in non-IDUs.
Table 2.
ADHERENCE TO THE THREE-DOSE HB VACCINE IN DRUG USERS BY INTERVENTION GROUPS AND STATUS OF INJECTING DRUG USE.
| TOTAL N=1260 |
IDUs N=378 |
NON-IDUs N=882 |
||||
|---|---|---|---|---|---|---|
| TWO ARM COMPARISON |
Adherence | OR (95% CI) P-value |
Adherence | OR (95% CI) P-value |
Adherence | OR (95% CI) P-value |
| Behavioral Intervention | ||||||
| Standard | 477/630 (76%) | Reference | 132/187 (71%) | Reference | 345/443 (78%) | Reference |
| Enhanced | 463/630 (74%) | 0.89 (0.69–1.15) P=0.37 |
137/191 (72%) |
1.06 (0.68–1.65) P=0.81 |
327/439 (75%) | 0.83 (0.61–1.13) P=0.24 |
| Vaccine Intervention | ||||||
| Standard Schedule | 454/626 (73%) | Reference | 115/174 (66%) | Reference | 339/452 (75%) | Reference |
| Accelerated Schedule | 486/634 (77%) | 1.24 (0.97–1.60) P=0.09 |
154/204 (76%) | 1.58 (1.01–2.47) P=0.04 |
333/430 (77%) | 1.14 (0.84–1.56) P=0.39 |
| FOUR ARM COMPARISON |
||||||
| Behavioral: Standard | ||||||
| Vaccine: Standard | 237/324 (73%) | Reference | 59/89 (66%) | Reference | 178/235 (76%) | Reference |
| Behavioral: Enhanced | ||||||
| Vaccine: Standard | 217/302 (72%) | 0.94 (0.66–1.33) P=0.18 |
56/85 (66%) | 0.98 (0.52–1.84) P=0.95 |
161/217 (74%) | 0.92 (0.60–1.41) P=0.70 |
| Behavioral: Standard | ||||||
| Vaccine: Accelerated | 240/306 (78%) | 1.34(0.92–1.93) P=0.08 |
73/98 (75%) | 1.48 (0.79–2.79) P=0.22 |
167/208 (80%) | 1.30 (0.83–2.05) P=0.25 |
| Behavioral: Enhanced | ||||||
| Vaccine: Accelerated | 247/328 (75%) | 1.12(0.78–1.59) P=0.80 |
81/106 (76%) | 1.65 (0.88–3.09) P=0.12 |
166/222 (75%) | 0.95 (0.62–1.45) P=0.81 |
In the four-arm comparison, the accelerated vaccine schedule may have improved adherence among those receiving the standard behavioral intervention (p=.08) but did not among those receiving the enhanced version (p=.80). In both the two-arm and four-arm comparisons, the enhanced behavioral intervention had no effect on improving adherence.
Among participants who did not complete the 3 doses of HB vaccine, about half received their first dose of vaccine only (data not shown). The major reason for non-adherence observed in this study was the inability to follow up with the individual due to invalid contact information; other reasons included jail and refusal.
When we compared the adherent and non-adherent subjects in the univariate analysis, African Americans, participants who traded sex for money or drugs in the past 30 days, participants currently using alcohol, and participants with stable housing were significantly more adherent to the HB vaccine. Participants who injected drugs, used crack cocaine, methamphetamines, or speedball were less adherent to the HB vaccine. No significant differences in adherence were found as the number of drugs used increased.
A multiple logistic regression was used to identify predictors of HB vaccine adherence (Table 3). Those on the accelerated vaccine schedule, older, African Americans, or alcohol users, were all significantly more likely to receive all three doses; participants who used speedball or who lived on the street were significantly less likely to do so. The enhanced behavioral intervention was not a significant predictor of receiving three HB vaccine doses.
Table 3.
FACTORS ASSOCIATED WITH ADHERENCE OF DRUG USERS TO THE 3-DOSE HB VACCINE BY MULTIPLE LOGISTIC REGRESSION
| 3-Doses Adherence |
Adjusted* Odds Ratio (95%CI) |
P-value | |
|---|---|---|---|
| Vaccine schedule intervention | |||
| Standard (0, 1, 6 mo) | 454/626 (73%) | Reference | |
| Accelerated (0, 1, 2 mo) | 486/634 (77%) | 1.26 (0.97–1.64) | 0.08 |
| Behavioral intervention | |||
| Standard | 477/630 (76%) | Reference | |
| Enhanced | 463/630 (74%) | 0.89 (0.48–1.16) | 0.40 |
| Age in years | |||
| ≤29 | 68/119 (57%) | Reference | |
| 30–39 | 210/295 (71%) | 1.73 (1.10–2.73) | 0.02 |
| 40–49 | 408/548 (74%) | 1.98 (1.30–3.03) | <0.01 |
| ≥50 | 254/297 (86%) | 3.96 (2.40–6.56) | <0.01 |
| Race | |||
| White | 78/129 (60%) | Reference | |
| Black | 824/1071 (77%) | 1.56 (1.04–2.34) | 0.03 |
| Hispanic/Other | 38/59 (64%) | 0.97 (0.50–1.88) | 0.92 |
| Housing status | |||
| Permanent/Temporary | 911/1207 (75%) | Reference | |
| Streets | 29/52 (66%) | 0.48 (0.26–0.87) | 0.02 |
| Current speedball use | |||
| No | 929/1236 (75%) | Reference | |
| Yes | 11/24 (46%) | 0.26 (0.11–0.63) | <0.01 |
| Current alcohol use | |||
| No | 190/451 (24%) | Reference | |
| Yes | 618/808 (76%) | 1.38 (1.05–1.80) | 0.02 |
Adjusted for interventions, age, race, housing status, speedball, alcohol, crack cocaine and methamphetamine use, injection drug use, trading sex for money or drugs
HB Seroprotection Rates among Susceptible Vaccinees
The substudy participants included 707 who were susceptible to HBV at enrollment, who completed three doses of the HB vaccine, and whose immune response to the vaccine could be assessed (Figure 2). Of the 308 participants not assessed, 33 participants had evidence of anti-HBs (≥10 mIU/mL) and 275 tested positive for anti-HBc. The characteristics of the 707 substudy participants resembled the total enrolled study participants (data not shown).
Figure 2.
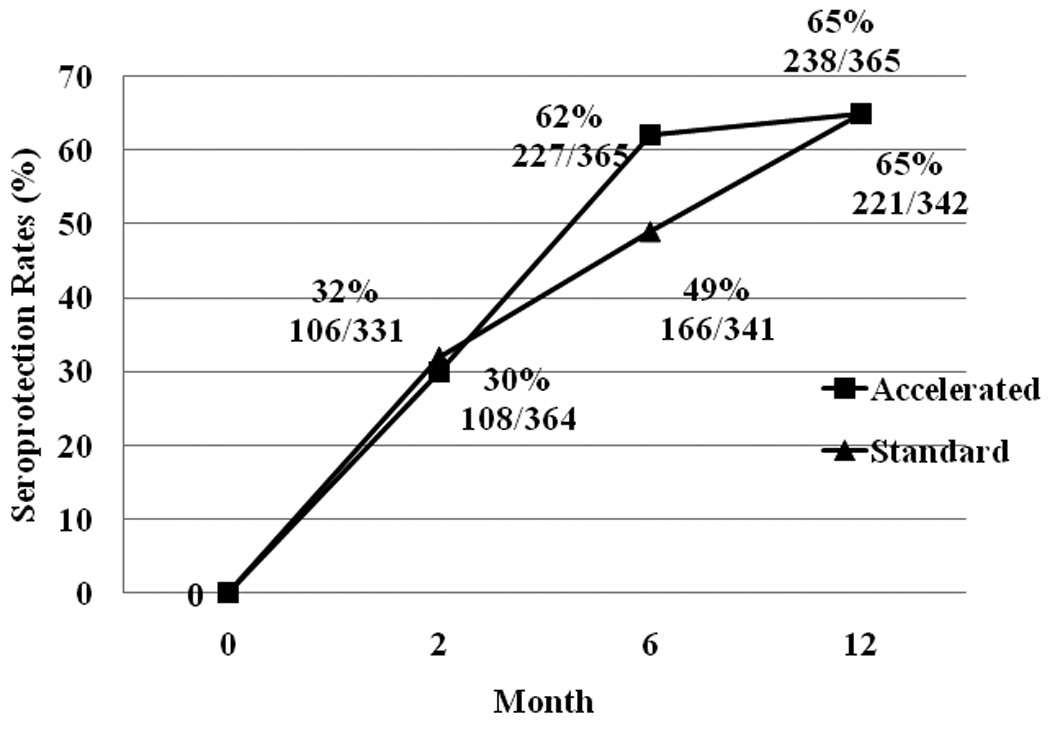
Cumulative Seroprotection Rates among Standard and Accelerated Schedule Groups at 2,6, and 12 Month Follow-up
For cumulative seroprotection, persons with measured anti-HBs titers were classified into seroprotection evident at the 2-, 6-, or 12-month visits, regardless of negative response at previous or subsequent visits. Overall, 459 of the 707 HBV-susceptible individuals (65%) developed the minimal adequate protective anti-HBs titer by 12 months after enrollment.
Because each vaccine group received their first two doses at 0 and 1 month, the rates of protection at 2 months were similar (Figure 2). Those on the accelerated schedule, who had received their third dose at 2 months, were significantly more likely (62% v 49%) to have effective seroprotection at 6 months than those on the standard schedule, who had yet to receive their third dose.
DISCUSSION
This is the first study to examine the effectiveness of an enhanced behavioral intervention as well as of an accelerated vaccine schedule in increasing acceptance and adherence to HB vaccination among not-in-treatment drug users. A slight difference was observed in the overall adherence rate between the standard (73%) and accelerated (77%) vaccine schedules, and those on the accelerated schedule were 26% more likely to achieve completion when controlling for factors including race and age (p=0.08). While this did not reach statistical significance, it is suggestive, and a study with a larger sample size might confirm it. However, the accelerated schedule made a significant difference (p=.04) for the IDU subgroup, raising completion rates by ten percentage points (66% to 76%). The behavioral intervention did not confound the association between the accelerated schedule intervention and completion of the series. The overall adherence rate of 75% is toward the higher end of those reported among many published studies (41%–88%), using different types of incentives [4, 13, 28–31]. The accelerated schedule adherence of 77% is higher than in other reported studies, 21%-70% [28, 30].
The cluster-randomized design for the enhanced behavioral intervention, with randomization at the neighborhood level, was necessary to prevent contamination between the two groups. We adjusted the difference of independent variables at baseline among intervention groups in the analysis to minimize bias and confounding factors.
The results of this study indicate that providing monetary incentives at each visit, free vaccinations, and a shorter vaccine schedule may encourage adherence, particularly among the highly at-risk group of IDUs. It also showed that enrollment and follow-up of drug users can be effectively achieved without the necessity of establishing an association with a healthcare/STD clinic, needle exchange program, or other services in contact with this population [17].
Adherence to multi-dose vaccine schedules by drug users may be affected primarily by obstacles that prevent repeated contact with healthcare services, such as lack of a permanent residence, involvement in illicit activities, incarceration, and treatment center visits. This is particularly true for IDUs, who are also less likely to accept or complete the HBV vaccine series than non-injection drug users [13]. In the current study, drug users living on the streets were twice as likely to not receive all three vaccine doses. Users of speedball (mixed heroin and cocaine, injected) were significantly less likely to be adherent.
Young drug users are a cause of concern because of poor compliance to preventive health behaviors and disparity in HBV infection. From 1982 to 1989, the majority of acute HBV infections occurred in individuals aged 20–29 years. Two studies in California showed that only 10% of drug users less than 30 years old completed the vaccine schedule [32, 33]. Addressing low vaccination is critical in young drug users because most incident transmission occurs soon after the initiation of injection drug use [11, 34]. Other studies have shown that non-traditional means to provide vaccination, such as flexible vaccine schedules and the use of outreach workers, worked to remove barriers to enrollment and adherence for young drug users [17, 21, 35]. The current study was not successful in enrolling a proportionate number of younger participants (18–29), perhaps because of the chain-referral recruiting methods, which will tend to skew to those who have been in an area longer (and are thus older). Results of this study showed that older drug users were more likely to complete the HB vaccine series.
The behavioral intervention used in this study aimed at increasing HB vaccine adherence by increasing drug users’ self-efficacy beliefs. The results suggest that this behavioral intervention had no significant effect on HB vaccine adherence, though other studies have shown that high self-efficacy increases it [9, 36, 37]. In our study, the standard behavioral intervention provided health-related and prevention information about HIV; a no-intervention control group might have provided more information about the effect of a brief intervention on drug users’ motivations to comply with a multi-dose schedule. Future qualitative studies of drug users may be needed to identify behavioral barriers preventing adherence to the HB vaccine and to develop and test behavioral interventions to increase such adherence.
While the educational component appears to have made no significant difference in the completion rates of the two different vaccination schedules, completion rates for both schedules were high. This suggests that the driving force for completion was financial rather than informational. The $20 per follow up visit appears to have been a primary motivation for return even up to a year later. This is consistent with findings that show that such moderate compensation for participation in public health research is part of an “informal economy” that is valued by people on the margins of the formal economy [38]. Rather than limiting the efficacy of the behavioral intervention, the payments appear to have compensated, as it were, for the behavioral intervention’s inefficacy.
The overall seroprotection rate of 65% among the HBV-susceptible subgroup is comparable to the findings from existing HB vaccine research on drug-using populations (66.4%–76%) [21, 29, 39–42]. The substantial difference in cumulative six-month seroprotection rates between the standard (49%) and accelerated schedules (62%) underscores the need to administer subsequent vaccine doses as rapidly as possible. Compressed schedules are particularly germane to IDUs, who were 58% more likely to receive 3 doses if they were on the accelerated rather than the standard dosing schedule. It is further worth noting that receiving the third dose at 2 months rather than at 6 months may provide additional months of protection [43], months during which an initiating drug user may migrate to injection drug use or partake in risky sexual behaviors (increasing the risk of transmission). This suggests that the focus of multi-dose vaccination programs for adult drug users should be on ensuring schedule completion using accelerated schedules, thus eliminating potential reservoirs of hepatitis B and transmission threats to their drug-using networks. Further follow-up of long-term immune protection in such populations is needed.
There remains an urgent need for better hepatitis B vaccines for at-risk populations, such as injecting drug users, those who are HIV-positive, other immunocompromised patients, dialysis patients, and those with end-stage liver disease. It should be emphasized that our study population was HIV negative; therefore, our results are valid for the immune-competent.
This study serves as a model for a future HIV or HCV vaccine trial and provides information on the effectiveness of accelerated vaccine schedules for increasing immunization in the drug user community. Creating a model for an HIV/HCV vaccine’s acceptance and adherence among drug users is an important public health goal. Drug users, especially minority drug users, are the largest group of newly diagnosed HIV and HCV cases. To effectively control HIV and HCV epidemics, once vaccines become available, the drug-using population will need to be targeted. Unless an effective model based upon empirical experience with drug users is developed, any attempt to implement an HIV or HCV vaccination program among drug users is likely to be thwarted. Our study indicated that straightforward payment for the receipt of immunizations of benefit to the recipient may not only be ethically sound but make economic sense for the use of limited public health resources.
Acknowledgements
We would like to thank the efforts of our field data collection staff, Sandra Timpson, Janel Dennison, Jay Johnson, Lawrence Duncan, Madelyn Randle, Janice Robinson, and Edward Johnson. We also thank Karyn Popham for editorial assistance.
Funding/Support: This study was funded by the National Institute of Drug Abuse, National Institutes of Health (NIDA# 1R01DA017505).
Abbreviations
- anti-HBc
antibody to hepatitis B core antigen
- anti-HBs
antibody to HBsAg
- anti-HCV
antibody to hepatitis C virus
- CI
confidence interval
- DASH
Drugs AIDS STDs Hepatitis project
- HBsAg
hepatitis B surface antigen
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- IDU
injecting drug use, injecting drug users
- OR
odds ratio
Footnotes
The authors do not have a commercial or other association that might pose a conflict of interest.
References
- 1.Centers for Disease Control, Viral Hepatitis. [Accessed 03 October 2007];Hepatitis B: Vaccine Facts. 2007 Available at: http://www.cdc.gov/ncidod/diseases/hepatitis/b/factvax.htm.
- 2.San Francisco Department of Public Heatlh, California. [Accessed 09 October 2007];STD Basics: Hepatitis. 2007 Available at: http://www.dph.sf.ca.us/sfcityclinic/stdbasics/hepatitis.asp.
- 3.Rhodes SD, Hergenrather KC. Exploring hepatitis B vaccination acceptance among young men who have sex with men: facilitators and barriers. Prev Med. 2002;35:128–134. doi: 10.1006/pmed.2002.1047. [DOI] [PubMed] [Google Scholar]
- 4.Altice FL, Bruce RD, Walton MR, Buitrago MI. Adherence to hepatitis B virus vaccination at syringe exchange sites. J Urban Health. 2005;82:151–161. doi: 10.1093/jurban/jti016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Des Jarlais DC, Fisher DG, Newman JC, et al. Providing hepatitis B vaccination to injection drug users: referral to health clinics vs on-site vaccination at a syringe exchange program. Am J Public Health. 2001;91:1791–1792. doi: 10.2105/ajph.91.11.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuo I, Sherman SG, Thomas DL, Strathdee SA. Hepatitis B virus infection and vaccination among young injection and non-injection drug users: missed opportunities to prevent infection. Drug Alcohol Depend. 2004;73:69–78. doi: 10.1016/j.drugalcdep.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Lok AS. Chronic hepatitis B. N Engl J Med. 2002;346:1682–1683. doi: 10.1056/NEJM200205303462202. [DOI] [PubMed] [Google Scholar]
- 8.McMahon BJ, Alward WL, Hall DB, et al. Acute hepatitis B virus infection: relation of age to the clinical expression of disease and subsequent development of the carrier state. J Infect Dis. 1985;151:599–603. doi: 10.1093/infdis/151.4.599. [DOI] [PubMed] [Google Scholar]
- 9.Seal KH, Ochoa KC, Hahn JA, Tulsky JP, Edlin BR, Moss AR. Risk of hepatitis B infection among young injection drug users in San Francisco: opportunities for intervention. West J Med. 2000;172:16–20. doi: 10.1136/ewjm.172.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Center for Disease Control and Prevention. Hepatitis B vaccination among high-risk adolescents and adults--San Diego, California, 1998–2001. MMWR Morb Mortal Wkly Rep. 2002;51:618–621. [PubMed] [Google Scholar]
- 11.Goldstein ST, Alter MJ, Williams IT, et al. Incidence and risk factors for acute hepatitis B in the United States, 1982–1998: implications for vaccination programs. J Infect Dis. 2002;185:713–719. doi: 10.1086/339192. [DOI] [PubMed] [Google Scholar]
- 12.Hagan H, Thiede H, McGough JP, Alexander ER. Hepatitis B vaccination among research participants, Seattle, Washington. Am J Public Health. 2002;92:1756. doi: 10.2105/ajph.92.11.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ompad DC, Galea S, Wu Y, et al. Acceptance and completion of hepatitis B vaccination among drug users in New York City. Commun Dis Public Health. 2004;7:294–300. [PubMed] [Google Scholar]
- 14.Hutchinson SJ, Wadd S, Taylor A, et al. Sudden rise in uptake of hepatitis B vaccination among injecting drug users associated with a universal vaccine programme in prisons. Vaccine. 2004;23:210–214. doi: 10.1016/j.vaccine.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 15.Hwang LY, Ross MW, Zack C, Bull L, Rickman K, Holleman M. Prevalence of sexually transmitted infections and associated risk factors among populations of drug abusers. Clin Infect Dis. 2000;31:920–926. doi: 10.1086/318131. [DOI] [PubMed] [Google Scholar]
- 16.Budd J, Robertson R, Elton R. Hepatitis B vaccination and injecting drug users. Br J Gen Pract. 2004;54:444–447. [PMC free article] [PubMed] [Google Scholar]
- 17.Jain N, Yusuf H, Wortley PM, Euler GL, Walton S, Stokley S. Factors associated with receiving hepatitis B vaccination among high-risk adults in the United States: an analysis of the National Health Interview Survey, 2000. Fam Med. 2004;36:480–486. [PubMed] [Google Scholar]
- 18.de Wit JB, Vet R, Schutten M, van Steenbergen J. Social-cognitive determinants of vaccination behavior against hepatitis B: an assessment among men who have sex with men. Prev Med. 2005;40:795–802. doi: 10.1016/j.ypmed.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 19.Quaglio G, Lugoboni F, Mezzelani P, Des Jarlais DC, Lechi A. Hepatitis vaccination among drug users. Vaccine. 2006;24:2702–2709. doi: 10.1016/j.vaccine.2005.12.045. [DOI] [PubMed] [Google Scholar]
- 20.van Houdt R, Sonder GJ, Dukers NH, et al. Impact of a targeted hepatitis B vaccination program in Amsterdam, The Netherlands. Vaccine. 2007;25:2698–2705. doi: 10.1016/j.vaccine.2006.06.058. [DOI] [PubMed] [Google Scholar]
- 21.Lum PJ, Ochoa KC, Hahn JA, Page Shafer K, Evans JL, Moss AR. Hepatitis B virus immunization among young injection drug users in San Francisco, Calif: the UFO Study. Am J Public Health. 2003;93:919–923. doi: 10.2105/ajph.93.6.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marchou B, Excler JL, Bourderioux C, et al. A 3-week hepatitis B vaccination schedule provides rapid and persistent protective immunity: a multicenter, randomized trial comparing accelerated and classic vaccination schedules. J Infect Dis. 1995;172:258–260. doi: 10.1093/infdis/172.1.258. [DOI] [PubMed] [Google Scholar]
- 23.Ross MW, Hwang LY, Leonard L, Teng M, Duncan L. Sexual behaviour, STDs and drug use in a crack house population. Int J STD AIDS. 1999;10:224–230. doi: 10.1258/0956462991913989. [DOI] [PubMed] [Google Scholar]
- 24.Bandura A. Self-efficacy mechanism in human agency. Am Psychol. 1982;37:122–147. [Google Scholar]
- 25.NIDA. The NIDA Community-Based Outreach Model: A Manual To Reduce the Risk of HIV and Other Blood-Borne Infections in Drug Users. 2002 [Google Scholar]
- 26.Dupont WD, Plummer WD. Power and sample size calculations: a review and computer program. Control Clin Trials. 1990;11:116–128. doi: 10.1016/0197-2456(90)90005-m. [DOI] [PubMed] [Google Scholar]
- 27.Hosmer DW, Lemeshow S. Applied logistic regression. 2nd ed. New York: Wiley; 2000. Wiley series in probability and statistics. Texts and references section. [Google Scholar]
- 28.Dal-Re R, Gonzalez A, Ramirez V, Ballesteros J, del Romero J, Bru F. Compliance with immunization against hepatitis B. A pragmatic study in sexually transmitted disease clinics. Vaccine. 1995;13:163–167. doi: 10.1016/0264-410x(95)93130-2. [DOI] [PubMed] [Google Scholar]
- 29.Quaglio G, Talamini G, Lugoboni F, et al. Compliance with hepatitis B vaccination in 1175 heroin users and risk factors associated with lack of vaccine response. Addiction. 2002;97:985–992. doi: 10.1046/j.1360-0443.2002.00147.x. [DOI] [PubMed] [Google Scholar]
- 30.Rodrigo JM, Serra MA, Aparisi L, et al. Immune response to hepatitis B vaccine in parenteral drug abusers. Vaccine. 1992;10:798–801. doi: 10.1016/0264-410x(92)90516-m. [DOI] [PubMed] [Google Scholar]
- 31.Samoff E, Dunn A, VanDevanter N, Blank S, Weisfuse IB. Predictors of acceptance of hepatitis B vaccination in an urban sexually transmitted diseases clinic. Sex Transm Dis. 2004;31:415–420. doi: 10.1097/01.olq.0000130533.53987.78. [DOI] [PubMed] [Google Scholar]
- 32.Seal KH, Kral AH, Lorvick J, McNees A, Gee L, Edlin BR. A randomized controlled trial of monetary incentives vs. outreach to enhance adherence to the hepatitis B vaccine series among injection drug users. Drug Alcohol Depend. 2003;71:127–131. doi: 10.1016/s0376-8716(03)00074-7. [DOI] [PubMed] [Google Scholar]
- 33.Cox J, De P, Morissette C, et al. Low perceived benefits and self-efficacy are associated with hepatitis C virus (HCV) infection-related risk among injection drug users. Soc Sci Med. 2008;66:211–220. doi: 10.1016/j.socscimed.2007.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rhodes SD, Hergenrather KC. Using an integrated approach to understand vaccination behavior among young men who have sex with men: stages of change, the health belief model, and self-efficacy. J Community Health. 2003;28:347–362. doi: 10.1023/a:1025444629753. [DOI] [PubMed] [Google Scholar]
- 35.van Steenbergen JE. Results of an enhanced-outreach programme of hepatitis B vaccination in the Netherlands (1998–2000) among men who have sex with men, hard drug users, sex workers and heterosexual persons with multiple partners. J Hepatol. 2002;37:507–513. doi: 10.1016/s0168-8278(02)00213-1. [DOI] [PubMed] [Google Scholar]
- 36.Zeldis JB, Jain S, Kuramoto IK, et al. Seroepidemiology of viral infections among intravenous drug users in northern California. West J Med. 1992;156:30–35. [PMC free article] [PubMed] [Google Scholar]
- 37.Garfein RS, Vlahov D, Galai N, Doherty MC, Nelson KE. Viral infections in short-term injection drug users: the prevalence of the hepatitis C, hepatitis B, human immunodeficiency, and human T-lymphotropic viruses. Am J Public Health. 1996;86:655–661. doi: 10.2105/ajph.86.5.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slomka J, McCurdy S, Ratliff EA, Timpson S, Williams ML. Perceptions of financial payment for research participation among African-American drug users in HIV studies. J Gen Intern Med. 2007;22:1403–1409. doi: 10.1007/s11606-007-0319-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lugoboni F, Migliozzi S, Schiesari F, et al. Immunoresponse to hepatitis B vaccination and adherence campaign among injecting drug users. Vaccine. 1997;15:1014–1016. doi: 10.1016/s0264-410x(96)00290-3. [DOI] [PubMed] [Google Scholar]
- 40.Minniti F, Baldo V, Trivello R, et al. Response to HBV vaccine in relation to anti-HCV and anti-HBc positivity: a study in intravenous drug addicts. Vaccine. 1999;17:3083–3085. doi: 10.1016/s0264-410x(99)00143-7. [DOI] [PubMed] [Google Scholar]
- 41.Rumi M, Colombo M, Romeo R, et al. Suboptimal response to hepatitis B vaccine in drug users. Arch Intern Med. 1991;151:574–578. [PubMed] [Google Scholar]
- 42.West DJ. Clinical experience with hepatitis B vaccines. Am J Infect Control. 1989;17:172–180. doi: 10.1016/0196-6553(89)90213-7. [DOI] [PubMed] [Google Scholar]
- 43.Jilg W, Schmidt M, Deinhardt F. Vaccination against hepatitis B: comparison of three different vaccination schedules. J Infect Dis. 1989;160:766–769. doi: 10.1093/infdis/160.5.766. [DOI] [PubMed] [Google Scholar]


