Abstract
Objective
To assess if birth at less than 26 weeks gestation is an important predictor of brain microstructure maturation as determined by using diffusion tensor imaging.
Study design
We performed serial MRI and diffusion tensor imaging in 176 infants born at < 33 weeks gestation. Diffusion parameters were calculated for white and gray matter regions. Linear regression for repeated measures was used to assess the effect of extremely premature birth on brain maturation.
Results
In white matter, fractional anisotropy increased by 0.008 per week (95% CI 0.007-0.009, p=<0.0001) and mean diffusivity decreased by 0.021 mm2/sec per week, (95% CI -0.24 to -0.018, p=<0.0001). Birth at < 26 weeks was associated with lower white matter fractional anisotropy (-0.01, 95% CI -0.018 to -0.003, p=0.008) but this effect was eliminated when co-morbid conditions were added to the model. Moderate-severe brain injury was associated with decreased mean white matter fractional anisotropy (-0.012, 95% CI -0.02 to -0.004, p=0.002).
Conclusion
Brain microstructure maturation as measured serially in premature infants is independent of extremely premature birth. Brain injury and co-morbid conditions may be the important determinants of microstructure maturation.
Keywords (MeSh): Infant, premature; Magnetic resonance imaging; Diffusion tensor imaging
Advances in perinatal care have led to an increase in the survival of extremely low birthweight infants, as well as an increase in infants born at the limits of viability (1, 2). These infants are at significant risk for motor and neurodevelopmental impairments and have the highest risk for adverse outcome (2–4). Infants born at the limits of viability are known to be at risk for early neonatal morbidity and long-term adverse neurodevelopmental outcomes (3–6). Although white matter injury is thought to be associated with these impairments, not all extremely premature infants have this type of injury identified on magnetic resonance imaging (MRI) (7, 8). Advanced MRI techniques that can be used to study regional and global brain development include diffusion tensor imaging, tractography, spectroscopy, volumetrics and deformation-based morphometry; these techniques may help to clarify the factors that lead to neurodevelopmental impairments (9–13). Outcomes of these neonates are most often attributed to premature birth, with the youngest having the worst outcomes. It remains unclear, however, whether birth at an extremely young age by itself leads to abnormal brain development, white matter injury, and poor neurodevelopmental outcome or whether the perinatal complications associated with extremely premature birth are the cause (6).
Diffusion tensor imaging is an imaging technique in which the microscopic random motion of water molecules can be used to study brain maturation in premature infants (13). As the brain develops, brain water content decreases, extracellular spaces diminish in size, and the intra- and intercellular microstructures become more complex and organized. The mean diffusivity of water decreases in gray and white matter structures and the directional coherence of water diffusion, represented by fractional anisotropy, increases (14) in the developing white matter, as the premature newborn develops to term-equivalent age (9, 15–17). In our previous work we showed that the presence of white matter injury is associated with impaired microstructural development in preterm newborns scanned with diffusion tensor imaging early in life and again at term-equivalent age (15). Abnormal white matter microstructure at term-equivalent age is associated with adverse neurodevelopmental (cognitive and motor) outcome (18–21). Premature newborns without focal white matter abnormalities at term-corrected age may have microstructural abnormalities evident at two years of age that correlate with neurodevelopmental assessment scores (22).
A major question in neonatal medicine remains whether the adverse outcomes of extremely premature neonates relate to the degree of prematurity itself or to adversities encountered by this group of newborns during their neonatal course, such as hypoxia-ischemia, infections, necrotizing enterocolitis (23), or chronic lung disease (CLD). The impact of premature birth has been addressed in MRI measures of brain volume at term-equivalent age (10). The purpose of this study is to determine the independent effect of extremely premature birth (24 to 25 6/7 weeks gestation) on the development of brain microstructure as assessed by diffusion tensor imaging in a large multi-center cohort of premature neonates studied serially.
METHODS
The study population includes infants born between 24 and 33 weeks gestation, admitted to the intensive care nurseries at University of California, San Francisco (UCSF) and the Children’s & Women’s Health Center of British Columbia (UBC). Exclusion criteria included evidence of congenital infections, malformations or chromosomal anomalies, and ultrasound evidence of large (>2cm) parenchymal hemorrhagic infarction. The study subjects were imaged twice according to study protocol, the first scan occurring as soon as the infant was deemed clinically stable by the attending neonatologist and the second prior to transfer to a referring institution, discharge home, or at term-equivalent age. The study was approved by the Committee on Human Research at UCSF and by the UBC Clinical Research Ethics Board. Informed consent was obtained from the parents or legal caregiver of each infant.
At both institutions infants were transported in an MR compatible incubator and accompanied by a physician and/or a team of dedicated neonatal research nurses. As described previously, the majority of scans at UCSF were obtained without the use of sedating medications (24). No sedation was used in the cohort from UBC. Clinical risk factors previously related to the risk of brain injury or adverse neurodevelopmental outcome were obtained prospectively and included gestational age at birth, birthweight, infant sex, mode of delivery, exposure to antenatal steroids, need for exogenous surfactant at delivery, Apgar scores, days of mechanical ventilation, presence of a patent ductus arteriosus (PDA), NEC, exposure to postnatal infection, and diagnosis of CLD (defined as the need for supplemental oxygen at 36 weeks corrected gestational age) (2, 4–6, 24–27). The clinical condition of the newborns at term equivalent age was described using a neuromotor score (range 0–5) previously found to predict adverse neurodevelopmental outcomes (scores 3 or more considered abnormal) (24).
Magnetic Resonance Imaging
Newborns at UCSF were scanned on a 1.5-Tesla Signa (GE Healthcare) using a MRI compatible incubator with a dedicated neonatal head coil. T1-weighted, T2-weighted and 3D spoiled gradient echo (SPGR) images were acquired using sequences optimized for this scanner (24). The diffusion tensor imaging data were acquired using a multi-repetition, single-shot echo planar sequence with 6 gradient directions (TR 7s/TE 100ms/slice thickness of 3mm), and 3 acquisition averages using b=600s/mm2, one b=0s/mm2 volume, and an in-plane resolution of 1.4 × 1.4mm. Newborns at UBC were scanned with a Siemens 1.5 Tesla Avanto using VB 13A software, an MRI-compatible isolette (Lammers Medical Technology) and specialized neonatal head coil (Advanced Imaging Research). Studies included: 3D coronal volumetric T1-weighted images (TR 36ms/TE 9.2ms/FOV 200mm/Slice thickness 1mm/No gap), and axial fast spin echo T2-weighted images. The diffusion tensor imaging data were acquired with a multi-repetition, single-shot echo planar sequence with 12 gradient directions (TR 4900ms/TE 104ms/FOV 160 mm/slice thickness 3mm/no gap), and three averages of two diffusion weightings of 600 and 700 sec/mm2 (b value) and an image without diffusion weighting, and an in-plane resolution of 1.3mm.
Two pediatric neuroradiologists at UCSF and one at UBC who were blinded to the infants’ neonatal course reviewed the images. The MRIs were scored for the presence and size of white matter injury (no white matter injury, minimal, moderate, and severe), using scores developed by our group with high reported inter- and intra-rater reliability (24, 28). Some infants were further categorized as having moderate to severe brain injury, defined a priori as the presence of moderate or severe white matter injury as described above, or ventriculomegaly (greater than 8mm measured at the level of the glomus of the choroid plexus) or the presence of grade III/IV intraventricular hemorrhage (24).
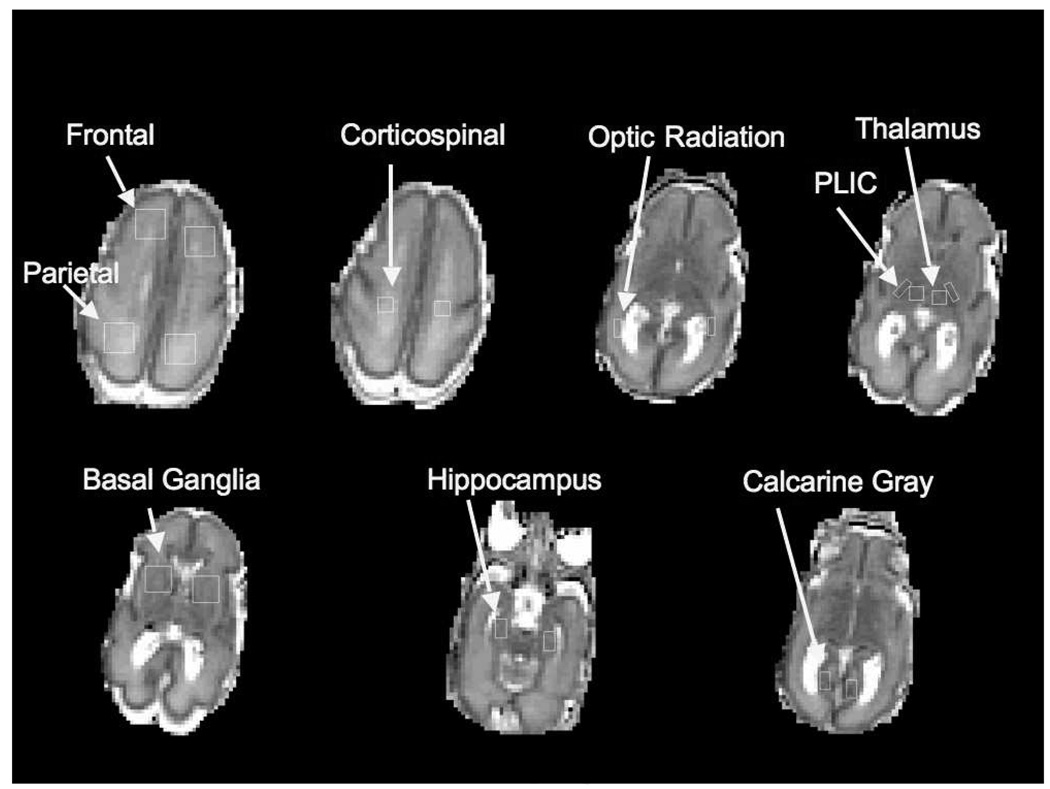
From the diffusion tensor imaging data, fractional anisotropy and mean diffusivity values were calculated from 5 white matter regions bilaterally and mean diffusivity values were also obtained from 4 gray matter regions bilaterally (Figure) (15).
Figure. Regions of Interest.
These are the unsmoothed ADC maps that demonstrate the regions of interest in which mean diffusivity and fractional anisotropy were measured in white and gray matter regions. This infant was born at 24.5 weeks gestation and imaged at 29 weeks.
Data Analysis
Statistical analyses were performed using Stata 9.2 (Stata Corporation, College Station, Texas). The difference between clinical predictors in the two sites and in extremely premature newborns relative to the others was assessed using rank-sum for continuous variables and Fisher Exact test for categorical variables. In comparing the diffusion tensor imaging data of the extremely premature newborns to the other newborns in the cohort, the outcome variables were mean fractional anisotropy in the 5 white matter regions and mean mean diffusivity in the same white matter regions as well as in the 4 gray matter regions (Figure).
Linear regression for repeated measures (using generalized estimating equations) with robust standard errors was used in order to account for the hierarchical structure of the data. This type of analysis was necessary as each infant had two MRI exams with measures of fractional anisotropy and mean diffusivity in multiple brain regions. In order to determine the independent effect of extreme premature birth on the diffusion tensor imaging parameters, the following factors were included as covariates in the regression model: postmenstrual age at time of scan, the presence of moderate to severe brain injury on the first scan, and an interaction term for site (UCSF or UBC) by region of interest. The interaction term for site by region of interest allows for the diffusion tensor imaging values in each region to vary by site (UCSF or UBC) as study subjects were imaged in different MRI scanners at two medical centers. The neonatal co-morbidities most strongly associated with extreme preterm birth in our cohort (days of mechanical ventilation, PDA, NEC) were then added to the model to examine whether effects relate to the degree of prematurity or the severity of neonatal illness.
RESULTS
Serial diffusion tensor imaging data were available for a total of 176 infants, 97 enrolled between April 2001 and March 2008 at UCSF and 79 enrolled from April 2006 to December 2008 at UBC. During the study period, at UCSF there were an additional 54 infants enrolled who either had only 1 scan (36 infants) or the diffusion tensor imaging data were motion degraded (18 infants) and they were not included in this analysis. At UBC there were an additional 27 infants enrolled that were not imaged serially and so they were not included in the analysis. At UCSF, 27% of the subjects received sedation. There were no differences in the clinical characteristics such as gestational age at birth, birth weight, and neonatal morbidities at either institution between those with serial diffusion tensor imaging data and those without.
There were no important differences in preterm newborns at each site in terms of gestational age at birth, number of infants born at less than 26 weeks, perinatal variables such as mode of delivery, treatment with antenatal steroids, or surfactant administration, or exposure to postnatal infection (Table I). More infants in the UBC cohort had a diagnosis of chronic lung disease and patent ductus arteriosus. This may reflect differences in clinical practice, as at UCSF prophylactic indomethacin is administered to all newborns less than 28 weeks gestation. There was a difference between the sites in the timing of the second MRI, with those in the UBC cohort being imaged about 4 weeks later than those at UCSF. The presence of moderate to severe brain injury on the first scan was similar in neonates at both sites.
Table 1.
Clinical characteristics by site
| UCSF n=97 |
UBC n=79 |
*p-value | |
|---|---|---|---|
| Gestational Age | 28.43 | 27.3 | 0.73 |
| Birth weight in grams | 950 (750, 1220) | 995 (815, 1285) | 0.57 |
| Male | 47(48) | 35(44) | 0.65 |
| Cesarean Delivery | 61(62.9) | 45(60) | 0.44 |
| Antenatal Steroids | 81 (83.5) | 57 (74) | 0.14 |
| Surfactant | 60 (61.9) | 55 (71.4) | 0.20 |
| APGAR at 5 min | 7 (5,8) | 8 (6,9) | 0.06 |
| Days intubated | 4 (0,16) | 3 (0, 25.5) | 0.56 |
| Birth at <26 weeks gestation | 25 (25.8) | 22 (27.9) | 0.86 |
| CLD | 25 (25.8) | 36 (46.2) | 0.007 |
| PDA | 31 (32) | 39 (49.4) | 0.02 |
| NEC | 11 (11.3) | 15 (19) | 0.20 |
| Postnatal Infection | 43 (44) | 25 (32) | 0.09 |
| PMA Scan 1, in weeks | 32 (30.7, 33.1) | 32 (30.3, 33.6) | 0.93 |
| PMA Scan 2, in weeks | 36 (35.1, 37.3) | 40 (38.4, 42.6) | <0.001 |
| Moderate-Severe Brain Injury | 30 (31) | 28 (35.4) | 0.63 |
CLD – chronic lung disease, PDA – patent ductus arteriosus, NEC – necrotizing enterocolitis, PMA – postmenstrual age at time of MRI
Data presented as median (p25, p75) or number (%)
Fisher exact for categorical variables; Rank-sum for continuous variables
When comparing premature newborns born at less than 26 weeks gestation with those born at or greater than 26 weeks, there was a significant increase in the number of days intubated (median of 38 days vs 1 day, p < 0.001), presence of a PDA (70% vs 28%, p < 0.001), NEC (28% vs 10%, p < 0.007), postnatal infection (74% vs 26%, p < 0.001) and chronic lung disease (80% vs 19%, p < 0.001). The timing of the scans and the proportion of those with moderate to severe injury (36% vs 31%, p = 0.6) on the first MRI were not different between these groups. More extremely premature infants had an abnormal neuromotor assessment at time of the second scan (70% vs 41%, p = 0.003).
Effect of Gestational Age at Birth on diffusion tensor imaging parameters
The results of our analyses are presented in Tables II and III. To understand the effect of gestational age at birth and extreme premature birth on diffusion tensor imaging parameters we first demonstrated how the mean diffusivity and fractional anisotropy values change with each week increase in PMA. In white matter regions of interest, for each week increment in PMA at scan, mean diffusivity decreased by 0.021 mm2/sec per week, (95% CI -0.24 to -0.018, p=<0.0001) and fractional anisotropy increased by 0.008 per week (95%CI 0.007 to 0.009, p=<0.0001). Then we examined the effect of gestational age at the time of birth as a linear variable on the change in diffusion parameters in white and gray matter. In our primary model we accounted for the postmenstrual age at the time of scan, region of interest, an interaction term to account for scans at two sites, and for the presence of moderate to severe brain injury. There was no effect of gestational age at birth as a linear variable on the change in mean diffusivity in white matter or in gray matter mean diffusivity with increasing postmenstrual age (Table II). In these multivariate models, the presence of moderate to severe brain injury was significantly associated with higher mean diffusivity (0.03 mm2/sec, 95% CI 0.003 to 0.058, p=0.03) and lower fractional anisotropy (-0.012, 95% CI -0.19 to -0.005, p=0.002). In gray matter regions, for each week increment in the PMA at scan mean diffusivity decreased by 0.018 mm2/sec (95%CI -0.02 to -0.016, p<0.001). Neither gestational age at birth nor the presence of moderate to severe MRI abnormalities had a significant effect on gray matter mean diffusivity.
Table 2.
Association of gestational age (as a linear variable) at birth with the change in diffusion parameters in white and gray matter regions of interest in a multivariate regression model accounting for post-menstrual age (PMA) at scan, region of interest, and an interaction term to account for the two sites.
| White Matter | Mean diffusivity | Fractional anisotropy | ||||
|---|---|---|---|---|---|---|
| Mean Change | 95% CI | p-value | Mean Change | 95% CI | p-value | |
| Each week increment in PMA | − 0.021 mm2/sec | − 0.24 to −0.018 | <0.0001 | 0.008 | 0.007 to 0.009 | <0.0001 |
| GA as a Linear Variable | 0.002 mm2/sec | − 0.003 to 0.008 | 0.8 | 0.0009 | − 0.006 to 0.003 | 0.2 |
| Moderate-Severe Brain Injury | 0.03 mm2/sec | 0.003 to 0.058 | 0.03 | − 0.012 | − 0.19 to − 0.005 | 0.002 |
| Gray Matter | Mean diffusivity | |||||
| Mean Change | 95% CI | p-value | ||||
| Each week increment in PMA | −0.018 mm2/sec | −0.02 to −0.016 | 0.001 | |||
| GA as a Linear Variable | 0.003 mm2/sec | −0.0001 to 0.007 | 0.06 | |||
| Moderate-Severe Brain Injury | 0.012 mm2/sec | −0.009 to 0.033 | 0.28 | |||
All models include terms for post-menstrual age (PMA) at scan, region of interest, and an interaction term to account for the two sites.
Table 3.
Association of birth <26 weeks with the change in diffusion parameters in white and gray matter regions of interest in a multivariate model including terms for post-menstrual age (PMA) at scan, region of interest, and an interaction term to account for the two sites.
| White Matter | Mean diffusivity | Fractional anisotropy | ||||
|---|---|---|---|---|---|---|
| Mean Change | 95% CI | p-value | Mean Change | 95% CI | p-value | |
| Each week increment in PMA | − 0.021 mm2/sec | − 0.24 to −0.018 | <0.0001 | 0.008 | 0.007 to 0.009 | <0.0001 |
| Birth at < 26 weeks | 0.023 mm2/sec | − 0.006 to 0.051 | 0.12 | − 0.01 | − 0.018 to − 0.003 | 0.008 |
| Moderate-Severe Brain Injury | 0.03 mm2/sec | 0.003 to 0.058 | 0.03 | − 0.012 | − 0.19 to − 0.005 | 0.002 |
| Model including additional terms for days of mechanical ventilation, PDA, and NEC: | ||||||
| Each week increment in PMA | 0.008 | 0.007 to 0.009 | <0.0001 | |||
| Birth at < 26 weeks | − 0.002 | − 0.02 to 0.004 | 0.53 | |||
| Moderate-Severe Brain Injury | − 0.012 | − 0.19 to − 0.005 | 0.002 | |||
| Gray Matter | Mean diffusivity | |||||
| Mean Change | 95% CI | p-value | ||||
| Each week increment in PMA | −0.018 mm2/sec | −0.02 to −0.016 | 0.001 | |||
| Birth at < 26 weeks | −0.013 mm2/sec | −0.03 to 0.005 | 0.16 | |||
| Moderate-Severe Brain Injury | 0.012 mm2/sec | −0.009 to 0.033 | 0.28 | |||
All models include terms for post-menstrual age (PMA) at scan, region of interest, and an interaction term to account for the two sites.
Effect of Extreme Premature Birth on diffusion tensor imaging Parameters
Next we examined the effect of extremely premature birth (birth at < 26 weeks gestation) on the diffusion parameters in the same primary model. In the model adjusting for postmenstrual age at time of scan, region of interest, an interaction term for site (UCSF or UBC) by region of interest, and the presence of moderate to severe brain injury on the first scan, birth at < 26 weeks gestation was significantly associated with lower white matter fractional anisotropy (-0.01, 95% CI -0.018 to -0.003, p=0.008) (Table III). The effect of extremely premature birth on fractional anisotropy did not differ significantly by white matter regions of interest (p=0.2). In contrast, extremely premature birth had no effect on mean diffusivity values in the white or gray matter regions.
In order to determine the independent effect of extreme premature birth, we added the important markers of early neonatal illness most strongly associated with extreme premature birth to the model: days of mechanical ventilation, presence of a PDA and NEC (Table III). Adding these variables to the model resulted in a loss of effect of extreme premature birth on the change in mean fractional anisotropy (-0.002, 95% CI - 0.02 to 0.004, p=0.53). Moderate to severe brain injury on the first scan continued to have a significant effect, resulting in a decrease in mean fractional anisotropy (-0.012, 95% CI -0.02 to -0.004, p=0.002). Adding post-natal infection and CLD to this model did not affect these associations of extremely premature birth or moderate to severe brain injury on fractional anisotropy values. In addition, there was no interaction between sex and extreme premature birth, p=0.6.
We then examined whether extremely premature birth was associated with different fractional anisotropy and mean diffusivity values at term-equivalent age. We repeated the multivariate regressions, limiting the analysis to scans occurring at >36 weeks postmenstrual age (N=120). In these analyses, extremely premature birth was not associated with significantly different white matter fractional anisotropy (-0.008, 95% CI -0.03 to 0.01, p=0.4) or mean diffusivity values (-0.002 mm2/sec, 95% CI -0.06 to 0.05 p=0.9).
DISCUSSION
The effect of gestational age at birth on developing white and gray matter microstructure can be assessed by diffusion parameter (fractional anisotropy or mean diffusivity) changes over time, and we were unable to demonstrate an effect. Extremely premature birth, at less than 26 weeks, had a detrimental effect on the change in white matter fractional anisotropy. However, this effect was eliminated by neonatal morbidities that commonly accompany extreme premature birth, such as the presence of a PDA, need for mechanical ventilation, and NEC. These findings suggest that younger gestational age at birth is not in itself a strong determinant of brain development as measured serially by diffusion tensor imaging parameters.
With a large sample size we were not able to demonstrate an independent effect of extremely premature birth on white or gray matter microstructure maturation. Our results suggest that infants born extremely prematurely have a normal capacity for brain development, as measured at the microstructural level by diffusion tensor imaging, that is similar to premature newborns delivered later in gestation. These findings are also consistent with those of Boardman et al. who showed that premature infants without focal brain injury and imaged at term had similar brain volumes as term born infants, although those with prolonged oxygen exposure had slightly decreased volumes (10). As the primary objective of this study was to compare the diffusion tensor imaging measures of maturation of those born extremely prematurely to those born later in gestation, we did not perform a comparison with diffusion tensor imaging parameters of term born infants. Others have looked at hippocampal volumes at term and found no independent effect of prematurity, but that white matter injury and postnatal factors such as steroid and indomethacin exposure negatively affect hippocampal volumes (27). We did find that the presence of brain injury had a significant effect on brain microstructure maturation. Moderate to severe brain injury, evident on conventional MRI, resulted in a reduction of the expected maturation in the diffusion tensor imaging parameters and the magnitude of the effect was equivalent to the expected change in fractional anisotropy or mean diffusivity for each week increment of postmenstrual age. The negative effect of focal injury in the white matter on surrounding microstructure and remote regions of grey matter (cortex, thalamus, hippocampus, cerebellum) have been demonstrated in both imaging and neuro-histopathologic studies (29–31). The exact mechanisms remain to be determined and may include the roles of the late oligodendrocyte progenitors, the subplate neurons, failure of myelination, arrested oligodendrocyte maturation and axonal damage (32).
Abnormal fractional anisotropy and mean diffusivity values at term corrected age have been associated with focal white matter injury, diffuse white matter injury, and adverse neurodevelopmental outcome (18–20, 33, 34). We performed a separate analysis in those infants who had their second imaging study at > 36 weeks but did not find an independent effect of extremely premature birth on these “term-equivalent” scans. These findings are important as they emphasize the significance of the hospital course and imply that exposure to systemic illness and the manner in which we care for these infants may influence their brain development. For example, postnatal infections are increasingly recognized as a risk factor for white matter injury in the premature newborn (35–37), potentially affecting brain microstructure maturation. Tyson et al showed in a large Neonatal Research Network study of extremely premature infants that other factors combined with gestational age were a better predictor of outcome than gestational age at birth alone (6). Our findings together with those of Tyson et al suggest that gestational age alone should not be used as the most important factor in counseling families regarding neurodevelopmental outcome. Prior studies utilizing diffusion tensor imaging have primarily used scans at term-equivalent age and so could not address the issue of risk of abnormal change in fractional anisotropy or mean diffusivity in white and gray matter development due to age at birth (19, 20, 33, 34). We believe that the use of serial scans strengthens our results.
There are several limitations to this study. First, by performing the second scan at a mean post-menstrual age of 36 weeks, we may have missed an effect of extreme prematurity, as it is possible that the effect of extreme prematurity on brain micro- and macrostructure may take more time to become detectable, although this was done in order to maximize the number of infants with serial scans and allows us to evaluate change over time. We did repeat our analysis looking solely at data obtained at greater than 36 weeks post-menstrual age. This analysis included 36 of the 47 infants born extremely prematurely and still we found no independent effect. Second, the use of specific regions of interest for measurements may have caused us to overlook an effect outside these regions, but these regions of interest represented fairly comprehensive white and gray matter regions. Indeed, the effect of extremely premature birth on fractional anisotropy did not differ significantly by white matter regions of interest. We believe these limitations are compensated for by our relatively large study population, which enabled us to examine the effects of extremely premature birth and measures of clinical illness in newborns exposed to clinical practice in two different tertiary level intensive care nurseries. An additional limitation is the lack of association with long-term neurodevelopmental follow-up. In the future we hope to study the association between the change in microstructure during the hospital course and the neuromotor and cognitive outcomes of these infants.
In conclusion extremely premature birth was not in itself a strong determinant of adverse brain development, as measured by serial diffusion tensor imaging, particularly when accounting for common, early, neonatal co-morbidities. In contrast, the effects of brain injury on brain development are important and appear to have a greater effect than extreme prematurity itself. In addition, the co-morbid adverse events that occur during intensive care may play a larger role in brain development after birth than the degree of prematurity, suggesting a possible window of opportunity to improve neurodevelopmental outcome.
Abbreviations
- MRI
magnetic resonance imaging
- PDA
patent ductus arteriosus
- NEC
necrotizing enterocolitis
- CLD
chronic lung disease
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Funding and conflict of interest information is available at www.jpeds.com (Appendix).
REFERENCES
- 1.Wilson-Costello D, Friedman H, Minich N, Fanaroff AA, Hack M. Improved survival rates with increased neurodevelopmental disability for extremely low birth weight infants in the 1990s. Pediatrics. 2005 Apr;115:997–1003. doi: 10.1542/peds.2004-0221. [DOI] [PubMed] [Google Scholar]
- 2.Eichenwald EC, Stark AR. Management and outcomes of very low birth weight. N Engl J Med. 2008 Apr 17;358:1700–1711. doi: 10.1056/NEJMra0707601. [DOI] [PubMed] [Google Scholar]
- 3.Hack M, Fanaroff AA. Outcomes of children of extremely low birthweight and gestational age in the 1990s. Semin Neonatol. 2000 May;5:89–106. doi: 10.1053/siny.1999.0001. [DOI] [PubMed] [Google Scholar]
- 4.Wood NS, Marlow N, Costeloe K, Gibson AT, Wilkinson AR. Neurologic and developmental disability after extremely preterm birth. EPICure Study Group. N Engl J Med. 2000 Aug 10;343:378–384. doi: 10.1056/NEJM200008103430601. [DOI] [PubMed] [Google Scholar]
- 5.Wood NS, Costeloe K, Gibson AT, Hennessy EM, Marlow N, Wilkinson AR. The EPICure study: associations and antecedents of neurological and developmental disability at 30 months of age following extremely preterm birth. Arch Dis Child Fetal Neonatal Ed. 2005 Mar;90:F134–F140. doi: 10.1136/adc.2004.052407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tyson JE, Parikh NA, Langer J, Green C, Higgins RD. Intensive care for extreme prematurity--moving beyond gestational age. N Engl J Med. 2008 Apr 17;358:1672–1681. doi: 10.1056/NEJMoa073059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inder TE, Wells SJ, Mogridge NB, Spencer C, Volpe JJ. Defining the nature of the cerebral abnormalities in the premature infant: a qualitative magnetic resonance imaging study. J Pediatr. 2003 Aug;143:171–179. doi: 10.1067/S0022-3476(03)00357-3. [DOI] [PubMed] [Google Scholar]
- 8.Dyet LE, Kennea N, Counsell SJ, Maalouf EF, Ajayi-Obe M, Duggan PJ, et al. Natural history of brain lesions in extremely preterm infants studied with serial magnetic resonance imaging from birth and neurodevelopmental assessment. Pediatrics. 2006 Aug;118:536–548. doi: 10.1542/peds.2005-1866. [DOI] [PubMed] [Google Scholar]
- 9.Berman JI, Mukherjee P, Partridge SC, Miller SP, Ferriero DM, Barkovich AJ, et al. Quantitative diffusion tensor MRI fiber tractography of sensorimotor white matter development in premature infants. Neuroimage. 2005 Oct 1;27:862–871. doi: 10.1016/j.neuroimage.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 10.Boardman JP, Counsell SJ, Rueckert D, Hajnal JV, Bhatia KK, Srinivasan L, et al. Early growth in brain volume is preserved in the majority of preterm infants. Ann Neurol. 2007 Aug;62:185–192. doi: 10.1002/ana.21171. [DOI] [PubMed] [Google Scholar]
- 11.Boardman JP, Counsell SJ, Rueckert D, Kapellou O, Bhatia KK, Aljabar P, et al. Abnormal deep grey matter development following preterm birth detected using deformation-based morphometry. Neuroimage. 2006 Aug 1;32:70–78. doi: 10.1016/j.neuroimage.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 12.Deipolyi AR, Mukherjee P, Gill K, Henry RG, Partridge SC, Veeraraghavan S, et al. Comparing microstructural and macrostructural development of the cerebral cortex in premature newborns: diffusion tensor imaging versus cortical gyration. Neuroimage. 2005 Sep;27:579–586. doi: 10.1016/j.neuroimage.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 13.Huppi PS, Inder TE. Magnetic resonance techniques in the evaluation of the perinatal brain: recent advances and future directions. Semin Neonatol. 2001 Apr;6:195–210. doi: 10.1053/siny.2001.0039. [DOI] [PubMed] [Google Scholar]
- 14.Mukherjee P, Miller JH, Shimony JS, Philip JV, Nehra D, Snyder AZ, et al. Diffusion-tensor MR imaging of gray and white matter development during normal human brain maturation. AJNR Am J Neuroradiol. 2002 Oct;23:1445–1456. [PMC free article] [PubMed] [Google Scholar]
- 15.Miller SP, Vigneron DB, Henry RG, Bohland MA, Ceppi-Cozzio C, Hoffman C, et al. Serial quantitative diffusion tensor MRI of the premature brain: development in newborns with and without injury. J Magn Reson Imaging. 2002 Dec;16:621–632. doi: 10.1002/jmri.10205. [DOI] [PubMed] [Google Scholar]
- 16.Partridge SC, Mukherjee P, Berman JI, Henry RG, Miller SP, Lu Y, et al. Tractography-based quantitation of diffusion tensor imaging parameters in white matter tracts of preterm newborns. J Magn Reson Imaging. 2005 Oct;22:467–474. doi: 10.1002/jmri.20410. [DOI] [PubMed] [Google Scholar]
- 17.Partridge SC, Mukherjee P, Henry RG, Miller SP, Berman JI, Jin H, et al. Diffusion tensor imaging: serial quantitation of white matter tract maturity in premature newborns. Neuroimage. 2004 Jul;22:1302–1314. doi: 10.1016/j.neuroimage.2004.02.038. [DOI] [PubMed] [Google Scholar]
- 18.Rose J, Mirmiran M, Butler EE, Lin CY, Barnes PD, Kermoian R, et al. Neonatal microstructural development of the internal capsule on diffusion tensor imaging correlates with severity of gait and motor deficits. Dev Med Child Neurol. 2007 Oct;49:745–750. doi: 10.1111/j.1469-8749.2007.00745.x. [DOI] [PubMed] [Google Scholar]
- 19.Arzoumanian Y, Mirmiran M, Barnes PD, Woolley K, Ariagno RL, Moseley ME, et al. Diffusion tensor brain imaging findings at term-equivalent age may predict neurologic abnormalities in low birth weight preterm infants. AJNR Am J Neuroradiol. 2003 Sep;24:1646–1653. [PMC free article] [PubMed] [Google Scholar]
- 20.Krishnan ML, Dyet LE, Boardman JP, Kapellou O, Allsop JM, Cowan F, et al. Relationship between white matter apparent diffusion coefficients in preterm infants at term-equivalent age and developmental outcome at 2 years. Pediatrics. 2007 Sep;120:e604–e609. doi: 10.1542/peds.2006-3054. [DOI] [PubMed] [Google Scholar]
- 21.Drobyshevsky A, Bregman J, Storey P, Meyer J, Prasad PV, Derrick M, et al. Serial diffusion tensor imaging detects white matter changes that correlate with motor outcome in premature infants. Dev Neurosci. 2007;29:289–301. doi: 10.1159/000105470. [DOI] [PubMed] [Google Scholar]
- 22.Counsell SJ, Edwards AD, Chew AT, Anjari M, Dyet LE, Srinivasan L, et al. Specific relations between neurodevelopmental abilities and white matter microstructure in children born preterm. Brain. 2008 Dec;131:3201–3208. doi: 10.1093/brain/awn268. [DOI] [PubMed] [Google Scholar]
- 23.Bodeau-Livinec F, Marlow N, Ancel PY, Kurinczuk JJ, Costeloe K, Kaminski M. Impact of intensive care practices on short-term and long-term outcomes for extremely preterm infants: comparison between the British Isles and France. Pediatrics. 2008 Nov;122:e1014–e1021. doi: 10.1542/peds.2007-2976. [DOI] [PubMed] [Google Scholar]
- 24.Miller SP, Ferriero DM, Leonard C, Piecuch R, Glidden DV, Partridge JC, et al. Early brain injury in premature newborns detected with magnetic resonance imaging is associated with adverse early neurodevelopmental outcome. J Pediatr. 2005 Nov;147:609–616. doi: 10.1016/j.jpeds.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 25.Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med. 2006 Aug 17;355:685–694. doi: 10.1056/NEJMoa053792. [DOI] [PubMed] [Google Scholar]
- 26.Thompson DK, Warfield SK, Carlin JB, Pavlovic M, Wang HX, Bear M, et al. Perinatal risk factors altering regional brain structure in the preterm infant. Brain. 2007 Mar;130:667–677. doi: 10.1093/brain/awl277. [DOI] [PubMed] [Google Scholar]
- 27.Thompson DK, Wood SJ, Doyle LW, Warfield SK, Lodygensky GA, Anderson PJ, et al. Neonate hippocampal volumes: prematurity, perinatal predictors, and 2-year outcome. Ann Neurol. 2008 May;63:642–651. doi: 10.1002/ana.21367. [DOI] [PubMed] [Google Scholar]
- 28.Miller JH, McKinstry RC, Philip JV, Mukherjee P, Neil JJ. Diffusion-tensor MR imaging of normal brain maturation: a guide to structural development and myelination. AJR Am J Roentgenol. 2003 Mar;180:851–859. doi: 10.2214/ajr.180.3.1800851. [DOI] [PubMed] [Google Scholar]
- 29.Pierson CR, Folkerth RD, Billiards SS, Trachtenberg FL, Drinkwater ME, Volpe JJ, et al. Gray matter injury associated with periventricular leukomalacia in the premature infant. Acta Neuropathol. 2007 Dec;114:619–631. doi: 10.1007/s00401-007-0295-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haynes RL, Billiards SS, Borenstein NS, Volpe JJ, Kinney HC. Diffuse axonal injury in periventricular leukomalacia as determined by apoptotic marker fractin. Pediatr Res. 2008 Jun;63:656–661. doi: 10.1203/PDR.0b013e31816c825c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tam EW, Ferriero DM, Xu D, Berman JI, Vigneron DB, Barkovich AJ, et al. Cerebellar development in the preterm neonate: effect of supratentorial brain injury. Pediatr Res. 2009 Mar 12; doi: 10.1203/PDR.0b013e3181a1fb3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller SP, Ferriero DM. From selective vulnerability to connectivity: insights from newborn brain imaging. Trends Neurosci. 2009 Sep;32:496–505. doi: 10.1016/j.tins.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Counsell SJ, Allsop JM, Harrison MC, Larkman DJ, Kennea NL, Kapellou O, et al. Diffusion-weighted imaging of the brain in preterm infants with focal and diffuse white matter abnormal. Pediatrics. 2003 Jul;112:1–7. doi: 10.1542/peds.112.1.1. [DOI] [PubMed] [Google Scholar]
- 34.Huppi PS, Murphy B, Maier SE, Zientara GP, Inder TE, Barnes PD, et al. Microstructural brain development after perinatal cerebral white matter injury assessed by diffusion tensor magnetic resonance imaging. Pediatrics. 2001 Mar;107:455–460. doi: 10.1542/peds.107.3.455. [DOI] [PubMed] [Google Scholar]
- 35.Glass HC, Bonifacio SL, Chau V, Glidden D, Poskitt K, Barkovich AJ, et al. Recurrent postnatal infections are associated with progressive white matter injury in premature infants. Pediatrics. 2008 Aug;122:299–305. doi: 10.1542/peds.2007-2184. [DOI] [PubMed] [Google Scholar]
- 36.Chau V, Poskitt KJ, Mcfadden DE, Bowen-Roberts T, Synnes A, Brant R, et al. Effect of Chorioamnionitis on Brain Development and Injury in Premature Newborns. Ann Neurol. 2009 doi: 10.1002/ana.21713. In Press. [DOI] [PubMed] [Google Scholar]
- 37.Shah DK, Doyle LW, Anderson PJ, Bear M, Daley AJ, Hunt RW, et al. Adverse neurodevelopment in preterm infants with postnatal sepsis or necrotizing enterocolitis is mediated by white matter abnormalities on magnetic resonance imaging at term. J Pediatr. 2008 Aug;153:170–175. doi: 10.1016/j.jpeds.2008.02.033. 5 e1. [DOI] [PubMed] [Google Scholar]