Abstract
Importance of the field
Disrupted L-methionine (Met) metabolism can lead to hepatic, neurological, and cardiovascular dysfunction in humans. Aberrant methyl group flux likely contributes to the development of these pathologies, but when patients also become hypermethionemic, additional toxicological mechanisms may be relevant.
Areas covered in this review
Following a discussion of the causes of hypermethionemia in humans, evidence for the toxicological roles and clinical significance of the Met transmethylation (TM), transamination (TA) and sulfoxidation (SO) pathways will be presented.
What the reader will gain
Recent data from freshly-isolated mouse hepatocytes (FIMHs) confirmed previous in vivo results in rodents that Met TM is a detoxification pathway while Met TA leads to toxicity. Gender-related differences in Met accumulation and metabolism in FIMHs correlated with gender differences in toxicity. Data obtained from FIMHs also implicated Met SO in Met metabolism and toxicity. Currently, little is known about the mechanisms and biological significance of Met sulfoxidation in humans.
Take home message
In hypermethionemic patients, clinical and dietary interventions should focus on increasing Met TM and decreasing Met TA and SO. Novel biomarkers of hypermethionemia in humans that correlate with pathological end points are needed to better understand the impact of the condition.
Keywords: hepatocytes, hypermethionemia, methionine, sulfoxidation, transmethylation, transamination
1. Introduction
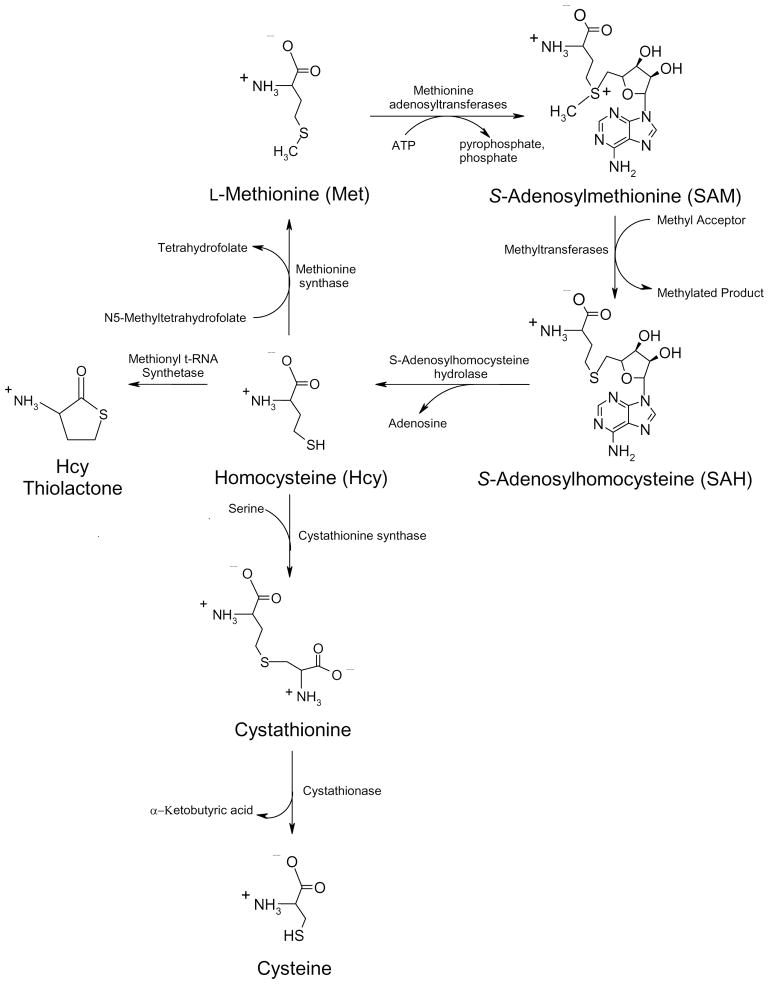
L-Methionine (Met) is an essential amino acid fundamentally involved in many biological processes [1]. Besides serving as a building block and start codon for protein synthesis, Met plays a pivotal role in methyl group metabolism and homeostasis through its conversion to S-adenosylmethionine (SAM). Among its many functions, SAM serves as the methyl group donor for all known mammalian DNA methyltransferases. The importance of DNA methylation and gene silencing in the development of cancer has now been established [2]. SAM formation and the Met transmethylation (TM) pathway as a whole (Fig. 1) are also important in the regulation of hepatocellular differentiation [3] and metabolism [4], and a dysfunctional Met TM is associated with many forms of chronic liver injury in animal models [5] as well as humans [6].
Figure 1.
The Met TM pathway, the major metabolic outlet for free Met. Adapted from Dever JT, Elfarra AA. Gender differences in methionine accumulation and metabolism in freshly isolated mouse hepatocytes: Potential roles in toxicity. Toxicol Appl Pharmacol 2009;236:358–65 with permission from Elsevier.
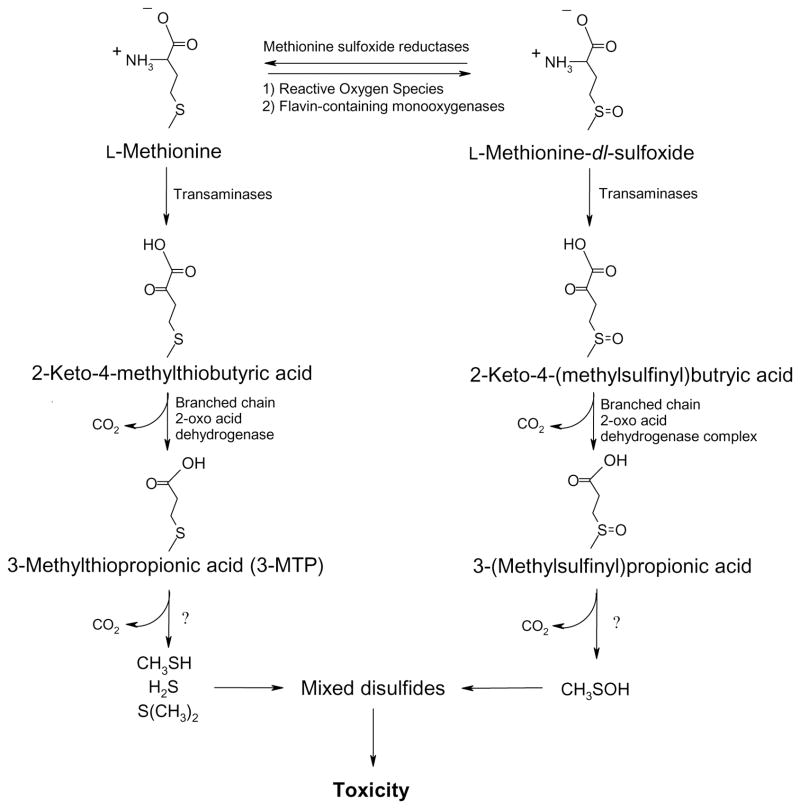
It is not clear how a blocked or deficient Met TM pathway leads to liver damage. Decreased or aberrant hepatic methyl group flux likely plays a major role [6], however, reduced Met TM may also lead to much higher tissue concentrations of Met which itself may be toxic [7–9]. The toxicological mechanisms of hypermethionemia have not been fully established, however, the excessive formation of reactive metabolites in the Met transamination (TA) pathway (Fig. 2) may be at least partially responsible [10,11]. In addition, recently obtained evidence also suggests a role for the Met sulfoxidation (SO) pathway (Fig. 2) in Met metabolism and toxicity [12,13].
Figure 2.
The Met SO and TA pathways, proposed Met bioactivation pathways. Adapted from Dever JT, Elfarra AA. Gender differences in methionine accumulation and metabolism in freshly isolated mouse hepatocytes: Potential roles in toxicity. Toxicol Appl Pharmacol 2009;236:358–65 with permission from Elsevier.
Thus, the purpose of this review is to highlight the potential clinical significance of hypermethionemia, particularly in human liver disease. After providing some relevant background on the causes of hypermethionemia in humans and its associated liver injury, the potential toxicological mechanisms of hypermethionemia will be examined. The functional roles of the Met TM, SO, and TA pathways in Met toxicity will be discussed since these pathways have garnered the greatest amount of research interest. Data acquired from both freshly isolated mouse hepatocytes (FIMHs) and hypermethionemic animal models will be discussed along with numerous studies in hypermethionemic humans to fully address the potential clinical impact of the condition. Finally, experimental strategies to improve our understanding of the toxicological role of hypermethionemia in liver injury will be presented.
2. Methionine: Essential but hepatotoxic
The biological formation of Met occurs only in plants and some microorganisms via a seven-step synthesis requiring aspartate and cysteine [14]. Mammals must obtain their Met requirement from the diet in free or protein-bound form and thus, human Met-deficiency can be a clinical concern in areas where malnutrition is prevalent. Acute Met deficiency in animal models results in widespread physiological dysfunction. In a recent study, rats fed a diet devoid of Met lost weight, developed anemia, and suffered from liver dysfunction [15]. Other reported effects of Met deficiency include bladder and kidney lesions [16]. Recent experiments in calorically-restricted drosophila, which have an increased lifespan but decreased fecundity, also indicate a pivotal role for Met in reproduction. Increasing only the Met content of the calorically-restricted diet completely restored normal fecundity in flies while maintaining their extended lifespan [17].
2.1 Hypermethionemia in animal models
Despite its biological indispensability, decades of research have also demonstrated the negative effects of excessive dietary or endogenous Met. In fact, Met is one of the most acutely toxic amino acids of those normally involved in protein synthesis [18]. Early studies demonstrated that high levels of Met given orally or by i.p. injection to guinea pigs induced cholestatic liver damage with hepatic ATP depletion, inhibition of RNA synthesis, nuclear fragmentation as well as hypoglycemia, hypothermia, and eventual death [7–9]. High levels of dietary Met given to rats led to growth suppression and splenic abnormalities [19]. In another study, long-term consumption (6 months) of a Met-supplemented diet in rats, as well as rabbits, led to increased lipid peroxide levels in the liver [20,21]. In male mice, Met adenosyltransferase (MAT) 1A knockout strains have very high Met levels, low SAM levels, 40% lower GSH levels, and are much more prone to oxidative stress and tumor formation in the liver compared to wild type male mice [5,22].
Conversely, decreased Met consumption appears to have health benefits in rodents. Rats given a diet containing 0.17% Met lived 30% longer than rats fed a diet composed of 0.86% Met [23]. The effect was found to be independent of overall dietary energy intake. Average life-span increases were also noted in mice given a Met-restricted diet beginning at 6 weeks of age [24] and 12 months of age [25]. Met-restricted mice were also much more resistant to oxidative liver damage induced by a single acetaminophen challenge dose [24]. Other biochemical effects of Met restriction, including lower blood glucose levels, were also noted, however, the mechanisms by which Met restriction increases lifespan in rodents were not clear. The effects of Met-restricted diets in primates have not yet been examined, but the above described experiments in rodents suggest that the upper limit for optimal dietary Met intake in primates may be lower than previously thought.
2.2 Hypermethionemia in humans
Overall Met status of humans is typically assessed by measuring the plasma Met concentration. While plasma Met levels do appear to at least loosely correlate with liver Met levels in animal models [26], the underlying assumption that they are representative of overall Met status has not been definitively confirmed. In general, plasma Met concentrations of 10–40 μM are considered normal [27]. No clinical definition of hypermethionemia has been established, however, Met concentrations of greater than 500 μM have often been reported in hypermethionemic patients [28]. Indeed, one undiagnosed patient had a plasma Met concentration of over 2,500 μM [28]. Both genetic and environmental etiologies can result in hypermethionemia (Table 1). Regardless of the root cause, hypermethionemia in humans is almost always associated with a defective Met TM pathway, the major metabolic outlet for free Met.
Table 1.
Known causes of hypermethionemia in humans
| Causes | Plasma Methionine Concentrations (μM) | Major Clinical Symptoms | Prevalence |
|---|---|---|---|
| Genetic | |||
| Methionine adenosyltransferase I/III deficiency | 100–1270 [27,30,31] | Occasional neurological effects [32] | 1:28163 [29] |
| Glycine N-methyltransferase deficiency | 426–1049 [32,33] | Elevated serum liver enzymes [32,33] | Rare |
| S-adenosylhomocysteine hydrolase deficiency | 44–784 [37–39] | Elevated serum liver enzymes, myopathy, [37–39] | Rare |
| Cystathionine β-synthase deficiency | 58–1891 [40] | Mental retardation, cardiovascular disease [40–42] | 1:300000 [42] |
| Non-Genetic | |||
| Total Parenteral Nutrition (TPN) | 64–1005 [48,49] | TPN-associated cholestasis [48–52] | unknown |
| Liver Cirrhosis | 26–151 [55] | Liver failure | unknown |
2.2.1 Methionine adenosyltransferase I/III deficiency
The Met TM pathway (Figure 1) begins with the formation of SAM from Met and ATP via the Met adenosyltransferases (MAT I, II, and III). MAT I and MAT III are a tetramer and dimer, respectively, of the MAT1A subunit, and both are expressed primarily in the liver [3]. MAT II is expressed extrahepatically and is formed from the MAT2A gene. Over two dozen different mutations in the MAT1A gene, which leads to MAT I/III deficiency, have been described, and MAT1A mutations may be the most common cause of inherited hypermethionemia [6, 29,30]. While MAT I/III deficiency results in high plasma Met and low plasma SAM levels, it does not necessarily lead to overt clinical pathologies [28,31,32]. Neurological effects and associated deficiencies in cognitive functions have been reported [33]. Most patients with MAT I/III deficiency appear to have adequate liver function, however, the long-term effects of this deficiency remain in question since all of the available clinical information is from children and young adults. It is noteworthy that MAT1A knockout male mice are viable, but are much more susceptible to liver injury than their wild type counterparts [5].
2.2.2 Glycine N-methyltransferase deficiency
SAM is converted to S-adenosylhomocysteine (SAH) after methyl group donation via a wide range of methyltransferases. One of the most abundant is glycine N-methyltransferase (GNMT) which methylates glycine to sarcosine. Since sarcosine can be readily demethylated back to glycine, the function of this pathway may be to control the SAM/SAH ratio which is an important homeostatic regulator of methylation reactions [6]. Though rare, human GNMT deficiency has been described [34,35] and results in high levels of plasma Met and SAM, but normal levels of SAH. The primary pathology associated with the disorder appears to be mild liver damage including elevated serum aminotransferases and hepatomegaly. GNMT knockout mice also develop significant liver pathologies including steatosis, fibrosis, and hepatocellular carcinoma [36–38].
2.2.3 S-Adenosylhomocysteine hydrolase deficiency
The subsequent hydrolysis of SAH to form adenosine and homocysteine (Hcy) is catalyzed by SAH hydrolase (Fig. 1). Human SAH hydrolase deficiency also appears to be relatively rare having been described in only three patients [39–41]. Besides very high plasma Met, SAM, and SAH concentrations, the disorder leads to elevated serum liver enzymes, hepatitis, portal fibrosis, neurological pathologies, and developmental delay.
2.2.4 Cystathionine β-synthase deficiency
The last well-documented genetic cause of human hypermethionemia is cystathionine β-synthase (CBS) deficiency (Fig. 1). CBS irreversibly combines Hcy with serine to form cystathionine which is further metabolized to cysteine and α-ketobutyric acid. CBS deficiency not only results in hypermethionemia but also homocystinuria and is primarily associated with mental retardation and cardiovascular disease [42–44]. The vascular toxicity associated with this disorder is very likely related to the high levels of Hcy which has well-documented arteriosclerotic effects [45–47], possibly through formation of the reactive metabolite homocysteine thiolactone [48]. A major role for Hcy in Met-induced hepatotoxicity has yet to be established.
2.2.5 Non-genetically based causes of hypermethionemia
Human hypermethionemia may also develop from non-genetically based etiologies. Elevated Met levels have been detected in premature or postoperative infants given Met-containing total parenteral nutrition (TPN) [49–51], an intervention which can lead to TPN-associated cholestasis [52]. Rabbits infused with TPN solution or with Met alone at the same concentration present in TPN solution developed identical cholestatic liver disease characterized by balloon degeneration and portal inflammation suggesting that elevated Met levels may contribute to the pathology of this life-threatening condition in infants [53].
The increased toxicity of parenterally-administered Met suggests that Met metabolism in the gut is important for not only Met utilization, but also Met detoxification. Studies in piglets indicate that 20% of dietary Met is metabolized in the gastrointestinal tissues [54]. Furthermore, the Met requirement in parenterally-fed piglets was found to be ~69% that of enterally-fed piglets [55]. Further studies are needed to clarify the role of the gut in Met metabolism and toxicity, however, the above results should be considered when determining the appropriate Met concentrations of TPN solutions.
Elevated plasma Met levels have also been detected in alcoholics and other patients with hepatic encephalopathy and liver cirrhosis [56–58], possibly due to reduced activity of enzymes involved in Met TM [59]. Met toxicity may exacerbate hepatocellular necrosis and fibrogenesis in patients with chronic liver disease [60].
3. Methionine metabolism: Physiological roles and toxicological significance
Excessive Met is clearly toxic to laboratory animals. Humans who become hypermethionemic due to genetic defects or chronic liver disease may also develop cardiovascular, hepatic and neurological pathologies. Since human hypermethionemia generally arises as a secondary symptom of Met TM disruption, it is difficult to separate the clinical impact of disrupted methyl flux from the additional toxicological consequences of hypermethionemia itself. In both hypermethionemic animal models and humans, hepatotoxicity can be a clinical outcome possibly because the liver is also the primary organ involved in Met metabolism. Therefore hypermethionemia may lead to the formation of hepatotoxic Met metabolites via the Met TA and Met SO pathways in addition to the injury caused by methyl group imbalance.
3.1 Methionine transmethylation
The Met TM pathway (Fig. 1) is the most significant and physiologically important route of Met metabolism with as much as 50% of dietary Met being converted to SAM, much of it in the liver [61]. Methylation reactions are central for a broad range of biological processes including the regulation of gene expression and cellular growth. The Met TM pathway also leads to formation of the amino acid cysteine, a key precursor to glutathione. Alternatively, SAM decarboxylation is an important step in the synthesis of polyamines which are involved in cellular differentiation and growth. With the multiple biological functions of the Met TM pathway, it is not surprising that its disruption via genetic or environmental etiologies is associated with major biochemical imbalances, including hypermethionemia, and significant pathologies.
An efficient Met TM pathway not only prevents a buildup of excess Met, it appears to function as a Met detoxification pathway under hypermethionemic conditions. One line of evidence for this hypothesis is provided by observations that supplementation with Met TM cofactors decreases Met toxicity in animal models. For example, elevated glycine levels increase the rate of SAM utilization via glycine N-methyltransferase while elevated serine levels increase the favorability of cystathionine formation from Hcy [62]. Dietary stimulation of the Met TM pathway via glycine and serine supplementation in rats also fed excess Met prevented Met-induced growth inhibition and feed-intake reduction and also lowered hepatic SAM levels by 40% compared to rats fed only excess Met for two weeks [62]. Glycine and serine supplemented rats on the high Met diet also had dramatically lower plasma and liver Met concentrations compared with nonsupplemented rats.
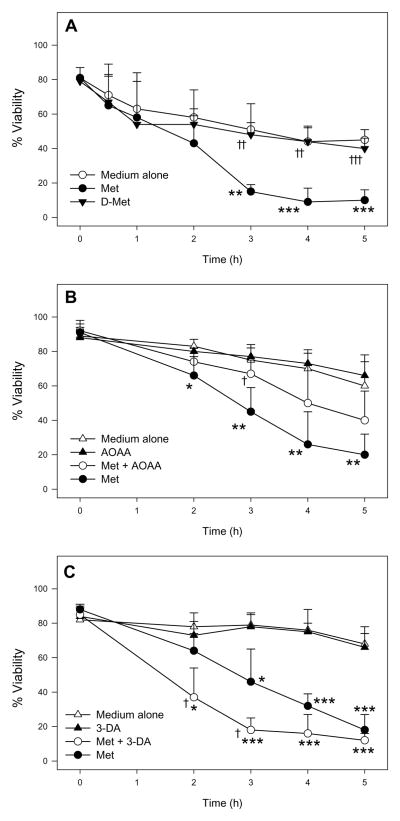
While stimulation of the Met TM pathway decreased Met toxicity, inhibition of the Met TM pathway increased Met cytotoxicity (Fig. 3) [11–13]. Addition of the SAH hydrolase and Met TM inhibitor 3-deazaadenosine (3-DA) [63] significantly potentiated Met-induced cytotoxicity in male FIMHs compared with those exposed to only Met [11] (Fig. 3C). Because 3-DA also inhibits formation of Hcy (Fig. 1), this finding suggested that Hcy was not responsible for eliciting Met toxicity in male FIMHs.
Figure 3.
Cell viability (as determined by LDH leakage) of freshly isolated male mouse hepatocytes (n=3–4) incubated at 37°C for 0–5 h in medium alone or medium spiked with Met (30 mM) compared with male hepatocytes exposed to medium spiked with D-Met (30 mM) (A), AOAA (0.2 mM) and Met + AOAA (B), and 3-DA (0.1 mM) or Met + 3-DA (C). The symbol * indicates values that were significantly lower than cells incubated with medium alone (*p<0.05, **p<0.01, ***p<0.001). The symbol † indicates values that were significantly different than cells incubated with only Met ( †p<0.05, † †p<0.01, † † †p<0.001). Adapted from Dever JT, Elfarra AA. L-methionine toxicity in freshly isolated mouse hepatocytes is gender-dependent and mediated in part by transamination. J Pharmacol Exp Ther 2008;326:809–817 with permission from the American Society for Pharmacology and Experimental Therapeutics.
In contrast to Hcy, several Met TM metabolites, most notably SAM and cysteine, have inherent cellular protective properties. Treatment with SAM has been shown to improve clinical outcomes of patients with various forms of liver disease by unestablished mechanisms [64]. In addition, SAM also has chemotherapeutic properties [65]. The protective properties of cysteine are well-known as it is central to the synthesis of glutathione. Administration of Met has been shown to result in higher glutathione levels in animal models [66]. Thus, besides serving as the primary metabolic outlet for the removal and, if necessary, the detoxification of free Met, the Met TM pathway converts Met into beneficial metabolites that may provide an additional level of cellular protection from the potentially toxic metabolites formed from Met TA and potentially Met SO.
3.2 Methionine transamination
Since the discovery and characterization of the Met TA pathway over 30 years ago, its metabolic and toxicological relevance, particularly in humans, has been the subject of much study [19,26,31,32]. Two major conclusions are well-supported by experimental data. First, the Met TA pathway results in the formation of toxic metabolites. Second, these metabolites are often formed at significant levels in hypermethionemic humans.
Multiple transaminases are likely involved in Met TA (Figure 2) with glutamine transaminase L and K having been confirmed to participate [13,67]. In rats and sheep, the highest tissue Met TA activity was detected in liver extracts with kidney extracts also demonstrating significant activity [67]. The in vitro Km of Met TA by glutamine transaminase K was determined to be 3.3 mM which is much higher than the Km for SAM formation (0.003–1.3 mM) [1,68]. This supports the observation that, in humans with normal physiological Met concentrations, Met TA metabolite levels are extremely low or not detectable [32].
Met TA results in formation of 2-keto-4-methylthiobutyric acid, the keto-acid of Met (Fig. 2). This may be further metabolized, primarily in mitochondria, by branched-chain 2-oxo acid dehydrogenase complex to 3-methylthiopropionic acid (3-MTP) [69–71]. The toxicity of 3-MTP has been investigated. Rats fed a diet spiked with 3-MTP (2.57%) or an equimolar amount of Met for two weeks had similar growth depression, lower levels of food intake, and splenic abnormalities [19]. Further metabolism of 3-MTP in rat liver homogenate or rat and human hepatocytes exposed to 3-MTP resulted in formation of methanethiol, dimethylsulfide, and methanethiol-mixed disulfides [72,73]. Short-term exposure of liver, spleenic and red blood cell extracts to methanethiol (10 min) resulted in depressed cytochrome c oxidase and catalase activities [10], and similar reductions in the activities of these enzymes were detected in rats fed a diet containing 3% Met [19]. These data suggested that hypermethionemic conditions may lead to the formation of toxic volatile sulfur molecules such as methanethiol that inhibit enzyme activity, likely via reactions with free sulfhydryl groups.
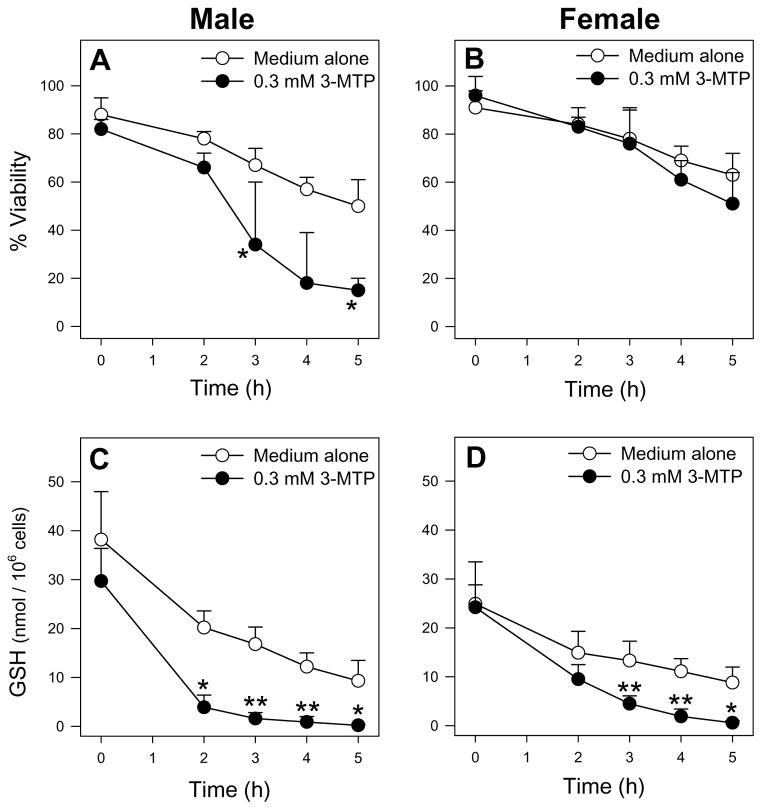
Recent Met metabolism and toxicity studies in FIMHs also support a prominent bioactivating role for Met TA. Whereas L-Met was cytotoxic to male FIMHs, equimolar doses of D-Met did not cause cytotoxicity (Fig. 3A) [11] consistent with the finding that D-amino-acid oxidase is present in mouse kidney but not liver [74]. Addition of the Met transaminase inhibitor aminooxyacetic acid (AOAA) [75] to Met-exposed male FIMHs significantly decreased both Met-induced cytotoxicity and GSH depletion compared with FIMHs exposed to Met alone [11]. Furthermore, exposure to 3-MTP elicited a similar degree of cytotoxicity and GSH depletion without glutathione disulfide (GSSG) formation in male hepatocytes at 100-fold lower concentrations than Met (Fig. 4).
Figure 4.
Cell viability (as determined by LDH leakage) and cellular GSH levels of freshly isolated male (A, C) and female (B, D) hepatocytes (n=3–4) incubated with medium alone or medium spiked with 3-MTP (0.3 mM) for 0–5 h at 37°C. The symbol * indicates values that were significantly lower than cells incubated with medium alone (*p<0.05, **p<0.01). Adapted from Dever JT, Elfarra AA. L-methionine toxicity in freshly isolated mouse hepatocytes is gender-dependent and mediated in part by transamination. J Pharmacol Exp Ther 2008;326:809–817 with permission from the American Society for Pharmacology and Experimental Therapeutics.
In the FIMH model, male, but not female, hepatocytes were sensitive to Met toxicity and also obtained much higher intracellular Met levels following Met exposure (Table 2) [11]. Addition of AOAA further increased cellular Met levels in Met-exposed male, but not female, hepatocytes. This suggested that Met TA was only significant in the male hepatoctyes, consistent with the increased sensitivity to Met of that gender. Supportively, no gender differences were detected in Met TA activity by glutamine transaminase K (GTK) in male and female mouse liver cytosol, and AOAA completely inhibited Met TA in the cytosol of both genders [11].
Table 2. Area under the curve (AUC) analysis for intracellular Met, SAM, Met-d-O, and Met-l-O in male or female FIMHs incubated with 30 mM Met for 1.5 h with or without 0.2 mM AOAA.
(Adapted from JT, Elfarra AA. Gender differences in methionine accumulation and metabolism in freshly isolated mouse hepatocytes: Potential roles in toxicity. Toxicol Appl Pharmacol 2009;236:358–65 with permission from Elsevier).
| Treatment | AUC0–1.5h (nmol · h/106 cells)a | |||
|---|---|---|---|---|
| Met | SAM | Met-d-O | Met-l-O | |
| Male | ||||
| Met | 40.3±11.4b | 2.9±1.1 | 5.2±1.2 | 5.2±2.7 |
| Met + AOAA | 50.6±11.3b | 3.1±1.4 | 9.2±2.6c | 6.6±4.0 |
| Female | ||||
| Met | 15.8±1.4 | 7.4±3.3 | 6.8±0.8 | 3.7±0.2 |
| Met + AOAA | 18.7±4.4 | 8.0±4.8 | 6.6±2.1 | 4.0±0.4 |
Data are expressed as mean ± SD (n = 3–4).
Values are significantly higher than the corresponding values obtained in FIMHs of the opposite gender (p<0.05).
Values are significantly higher than the corresponding values obtained in FIMHs of the same gender incubated with Met only (p<0.05).
Met TA metabolites have been detected in hypermethionemic humans. Increased levels of 2-keto-4-methylthiobutyric acid, 3-MTP, methanethiol, and protein and non-protein methanethiol-mixed disulfides were observed in the plasma of patients with MAT [31] and CBS [42] deficiency. The increases were most notable in patients with plasma Met levels greater than 350 μM [28]. Increases in Met TA metabolites were also detected in the plasma of healthy human volunteers given 0.1 g/kg Met [76]. Thus, the formation of methanethiol as well as methanethiol mixed-disulfides from the Met TA pathway does occur in humans, but their clinical impact, particularly in patients with chronic hypermethionemia, has been difficult to distinguish from the effects of altered or disrupted Met TM. Nevertheless, reduction of Met TA in hypermethionemic humans, perhaps using competitive amino acid inhibitors of transaminases, could be beneficial. The use of dietary glycine and serine for this purpose might also work to simultaneously stimulate Met TM [62]. In addition, treatment of hypermethionemic patients with N-acetyl cysteine could help restore GSH levels depleted by reactive Met TA metabolites.
3.3 Methionine sulfoxidation
The metabolic and toxicological role of the Met SO pathway has received less attention than the Met TM and TA pathways, however, emerging evidence suggests that its significance in both physiological and hypermethionemic conditions may have been overlooked.
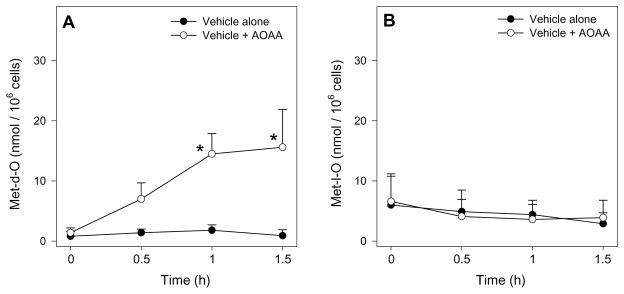
Biological Met S-oxidation to methionine-d-sulfoxide (Met-d-O) or methionine-l-sulfoxide (Met-l-O) is likely accomplished through multiple mechanisms including chemical oxidation by reactive oxygen species (ROS) [77] and enzymatic oxidation by flavin-containing monooxygenases and/or other unidentified enzymes [78–81]. Experiments in FIMHs have provided the most recent evidence that Met SO may be enzymatically catalyzed and physiologically significant [12,13]. In one set of studies, male, but not female, FIMHs exposed only to the transaminase inhibitor AOAA showed an unexpected, time-dependent increase in cellular Met-d-O levels compared with control FIMHs (Fig. 5) [12]. This result led to the identification of methionine-dl-sulphoxide (MetO) TA as a novel metabolic pathway of interest and suggested that Met S-oxidation and MetO TA may occur in vivo at physiological Met concentrations and have some unidentified physiological role.
Figure 5.
Cellular Met-d-O (A) and Met-l-O (B) levels in freshly isolated male hepatocytes (n=3–4) incubated with medium alone or medium spiked with AOAA (0.2 mM) for 0–1.5 h at 37°C. The symbol * indicates values that were significantly higher than cells incubated with medium alone (*p<0.05). Adapted from Dever JT, Elfarra AA. L-Methionine-dl-sulfoxide metabolism and toxicity in freshly isolated mouse hepatocytes: Gender differences and inhibition with aminooxyacetic acid. Drug Metab Dispos 2008;36:2252–2260 with permission from the American Society for Pharmacology and Experimental Therapeutics.
In healthy individuals, MetO is present in the plasma at low but detectable concentrations (4 ± 1 μM) [77] indicating that it is present in humans with normal Met levels. MetO levels were higher in the serum and urine of hypermethionemic infants compared with physiologically normal infants [82]. In a human with MAT deficiency, high concentrations (460 μM) of plasma Met-d-O were observed [31]. Two-fold increases in Met-d-O were also detected in the liver and plasma of male and female mice given a single, high Met dose [26]. The detection of only Met-d-O suggested either stereoselective S-oxidation of Met to Met-d-O or selective subsequent metabolism of Met-l-O to yield further metabolites. Most importantly, these findings provide compelling evidence for the metabolic significance of the Met S-oxidation.
To investigate the potential toxicological role of Met SO, the direct toxicity of MetO was examined using FIMHs [12]. As with Met, male, but not female FIMHs were sensitive to MetO toxicity. Also similar to Met, MetO toxicity was characterized by cytotoxicity preceded by GSH depletion without GSSG formation and was strongly inhibited by AOAA. Thus, along with its potential physiological significance, MetO TA also appeared to be toxicologically relevant. It was further hypothesized that MetO TA could lead to the formation of methanesulfenic acid (Fig. 2) which, like methanethiol, should react rapidly with free sulfhydryl groups. Because MetO toxicity was elicited at similar concentrations as Met (>20 mM) [12], the role of Met sulfoxidation in Met toxicity is likely additive to that of the Met TA metabolites.
Cellular levels of Met-d-O and Met-l-O were assessed in FIMHs exposed to toxic levels of Met [13]. Consistent with previous in vivo results, increases in Met-d-O, but not Met-l-O, were detected in FIMHs of both genders. Interestingly, addition of AOAA resulted in significant increases in Met-d-O levels in Met-exposed male, but not female, hepatocytes (Table 2). This result suggested that Met-d-O TA was more active in male hepatocytes. Because MetO TA activity by GTK in mouse liver cytosol of both genders was similar, the exact reason for the increased MetO TA in male hepatocytes was not clear, but it correlated with the higher accumulation of Met in the male mouse hepatocytes (Table 2). It was also consistent with the increased sensitivity of male hepatocytes to Met toxicity.
The Met and MetO metabolism and toxicity experiments in FIMHs confirmed the significance of Met SO at both low and high biological Met concentrations. They also led to the identification of MetO TA as a pathway of potential metabolic and toxicological interest. Currently, these studies constitute the only detailed studies of the potential metabolic and toxicological significance of the Met SO pathway. Further experiments are now required in humans as well as in vivo animal models to elucidate the biological significance of MetO formation.
4. Assessing the clinical impact of hypermethionemia: Future studies
Excessive bodily Met loads have established harmful effects in a variety of animal models. Under hypermethionemic conditions, Met TM appears to play a detoxification role while Met TA is bioactivating. In addition, the Met SO pathway may have a significant but as yet undetermined role in Met metabolism and toxicity. In humans, hypermethionemia is typically caused by genetic or environmental Met TM disruption. Thus, it is difficult to distinguish the clinical impact of Met toxicity from that of disrupted methyl group homeostasis. Innovative approaches are now required to decisively determine whether human hypermethionemia is a benign biochemical anomaly or a cause for clinical concern.
One established technique that may be useful in accurately measuring the rate of formation rather than just the presence of toxicologically-relevant Met metabolites is Met multiple stable isotope tracing. Through the infusion of dual labeled [1-13C; methyl-2H3] Met, the rates of Met TM, transsulfuration, and incorporation into protein can be simultaneously measured. This approach has been used with great success in animal models [83] as well as humans [84,85] to study how Met metabolism is regulated under particular dietary and environmental conditions. Met isotope tracing could potentially be used to measure the rate of formation of potentially toxic Met TA metabolites in hypermethionemic humans. For example, [1-13C; methyl-2H3] Met TA should result in the formation of labeled methanethiol and methanethiol mixed disulfides which can be measured by GC-MS [31]. Determining the rate of [1-13C; methyl-2H3] Met S-oxidation to [1-13C; methyl-2H3] MetO would also be of interest to determine the quantitative significance of the Met SO pathway in hypermethionemic humans.
The use of Met stable isotope tracing has the potential to greatly improve our understanding of the metabolic significance of Met TA and Met SO, however, research efforts should also focus on establishing direct links between hypermethionemia and its associated clinical pathologies. A bottom up metabolomic and/or proteomic search for toxicologically-relevant serum or urinary biomarkers of hypermethionemia in humans would be a first step toward this goal. Candidate biomarkers could then be examined for their correlation to any observed pathologies. This approach has already been piloted in rats given excessive dietary Met where serum levels of Hcy, cystathionine, and one unidentified metabolite successfully discriminated between subtoxic and toxic Met levels [86]. Because hypermethionemia in humans is usually due to a dysfunctional Met TM rather than Met overconsumption, human biomarkers of hypermethionemia may be different than those identified in the rat study.
5. Conclusions
The precise clinical impact of human hypermethionemia remains unclear, but much is known regarding the metabolic mechanisms that could elicit Met toxicity in humans. Disruption of the Met TM pathway, the major cause of human hypermethionemia, not only leads to greatly elevated Met levels, it marginalizes Met detoxification. The Met TA pathway appears to elicit Met toxicity in animal and cellular models. Met TA metabolites can be detected in hypermethionemic humans, but their toxicological significance remains unclear. The Met SO pathway and subsequent MetO TA may also have a role in toxicity that warrants further investigation for its human physiological relevance. Gender-related differences in Met uptake and cytotoxicity in human hepatocytes should be investigated as significant gender-related differences in Met uptake and toxicity have been observed in FIMHs. In addition, next-generation metabolomic and proteomic technologies will be important for further clarification of the relationship between hypermethionemia and its associated clinical pathologies.
6. Expert Opinion
Extensive strides have been made over the past thirty years to clarify the biochemical mechanisms that elicit the toxicity of Met in various in vitro and in vivo models. Despite these advances, we still have a poor understanding of the toxicological impact of hypermethionemia in humans. Tackling this problem will require more sophisticated approaches than simply measuring serum Met metabolite levels. To a large degree, the toxicological impact of Met probably depends on its metabolic partitioning between Met TM, the detoxification route, and Met and MetO TA, the bioactivation routes. Under normal physiological conditions, Met TM is heavily favored while Met TA is not significant. A blocked TM pathway leads to a dramatic reversal of this metabolic equilibrium resulting in higher rates of Met and MetO TA and the potential for increased toxicity. Determining Met metabolic partitioning rates between Met TM, TA and SO, perhaps through the use of stable isotope tracer techniques, may provide more useful data from which to make extrapolations regarding the potential toxicity of Met in individuals.
Many scientists have concluded that the toxicological effects of disrupted Met TM probably outweigh those of direct Met toxicity. This hypothesis is well-founded due to the ubiquitous cellular impact of methylation reactions. Nevertheless, it would be rash to clinically dismiss the additional toxicological impact of hypermethionemia as only minor without more robust evidence supporting that claim. The presence of even small amounts of Met TA metabolites in hypermethionemic humans is a cause for concern considering that these metabolites have proven toxicity in animal models. The lack of any detectable acute toxicological effects of hypermethionemia in healthy individuals does suggest that, in most cases, hypermethionemic humans are able to effectively detoxify reactive Met and MetO TA metabolites. The more compelling question resides in the long-term health consequences of chronically excessive Met and MetO TA.
From a research standpoint, the Met SO pathway currently represents the most intriguing Met metabolic pathway. Several mysteries, including the apparent stereoselective formation of Met-d-O at physiological and high Met states and the physiological significance of MetO TA, await experimental insights. The long standing assumption that MetO formation is primarily carried out by ROS may need to be revisited. Most importantly, the Met SO pathway must be characterized in healthy and hypermethionemic humans to determine its significance. These studies represent an exciting frontier in the field of Met metabolism and toxicity.
Article Highlights Box.
L-Methionine (Met) is toxic in animal models, but hypermethionemic humans do not always develop overt pathologies.
Hypermethionemia in humans usually develops secondary to a defective Met transmethylation pathway, the major metabolic outlet for free Met.
The Met transamination pathway appears to elicit Met toxicity in animal models exposed to high levels of Met, but its toxicological significance in humans remains unclear.
Met sulfoxidation (SO) to form L-methionine-d-sulfoxide (Met-d-O) and subsequent Met-d-O transamination may have both physiological and toxicological importance.
Next-generation metabolomic and proteomic technologies will be important to further clarify the relationship between hypermethionemia and clinical pathologies.
Acknowledgments
Declaration of interest
This work was supported by grants from the National Institute of Health: R01 NIDDK 044295, T32 ES 007015, and T32 DK007665.
Abbreviations
- AOAA
aminooxyacetic acid
- AUC
area under the curve
- CBS
cystathionine β-synthase
- 3-DA
3-deazaadenosine
- D-Met
D-methionine
- FIMHs
freshly-isolated mouse hepatocytes
- FMO
Flavin-containing monooxygenase
- GNMT
glycine N-methyltransferase
- GSH
glutathione
- GSSG
glutathione disulfide
- GTK
glutamine transaminase K
- Hcy
homocysteine
- LDH
lactate dehydrogenase
- MAT
methionine adenosyltransferase
- Met
L-methionine
- MetO
L-methionine-dl-sulfoxide
- Met-d-O
L-methionine-d-sulfoxide
- Met-l-O
L-methionine-l-sulfoxide
- 3-MTP
3-methylthiopropionic acid
- ROS
reactive oxygen species
- SAH
S-adenosyl-L-homocysteine
- SAM
S-adenosyl-L-methionine
- SO
sulfoxidation
- TA
transamination
- TB
trypan blue
- TM
transmethylation
- TPN
total parenteral nutrition
Contributor Information
Dr Joseph Dever, Email: jtdever@wisc.edu, University of Wisconsin, Madison, WI, USA.
Dr Adnan Elfarra, Email: elfarra@svm.vetmed.wisc.edu, University of Wisconsin, Madison, WI, USA.
Bibliography
- 1.Finkelstein JD. Methionine metabolism in mammals. J Nutr Biochem. 1990;1:228–237. doi: 10.1016/0955-2863(90)90070-2. [DOI] [PubMed] [Google Scholar]
- 2.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3:415–426. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Trevijano ER, Latasa MU, Carretero MV, et al. S-Adenosylmethionine regulates MAT1A and MAT2A gene expression in cultured rat hepatocytes: a new role for S-adenosylmethionine in the maintenance of the differentiated status of the liver. FASEB J. 2000;14:2511–2518. doi: 10.1096/fj.00-0121com. [DOI] [PubMed] [Google Scholar]
- 4.Finkelstein JD. Metabolic regulatory properties of S-Adenosylmethionine and S-adenosylhomocysteine. Clin Chem Lab Med. 2007;45:1694–1699. doi: 10.1515/CCLM.2007.341. [DOI] [PubMed] [Google Scholar]
- *5.Lu SC, Alvarez L, Huang ZZ, et al. Methionine adenosyltransferase 1A knockout mice are predisposed to liver injury and exhibit increased expression of genes involved in proliferation. Proc Natl Acad Sci. 2001;98:5560–5565. doi: 10.1073/pnas.091016398. Characterizes the biochemical, physiological, and molecular impact of MAT1A deficiency in mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mato JM, Martinez-Chantar ML, Lu SC. Methionine metabolism and liver disease. Annu Rev Nutr. 2008;28:273–293. doi: 10.1146/annurev.nutr.28.061807.155438. [DOI] [PubMed] [Google Scholar]
- 7.Hardwick DF, Applegarth DA, Cockcroft DM, et al. Pathogenesis of methionine-induced toxicity. Metabolism. 1970;19:381–391. doi: 10.1016/0026-0495(70)90135-6. [DOI] [PubMed] [Google Scholar]
- 8.Shinozuka H, Estes LW, Farber E. Studies on acute methionine toxicity. I. Nucleolar disaggregation in guinea pig hepatic cells with methionine or ethionine and its reversal with adenine. Amer J Path. 1971;64:241–249. [PMC free article] [PubMed] [Google Scholar]
- 9.Cox R, Martin JT, Shinozuka H. Studies on acute methionine toxicity. II. Inhibition of ribonucleic acid synthesis in guinea pig liver by methionine and ethionine. Lab Invest. 1973;29:54–59. [PubMed] [Google Scholar]
- 10.Finkelstein A, Benevenga NJ. The effect of methanethiol and methionine toxicity on the activities of cytochrome c oxidase and enzymes involved in protection from peroxidative damage. J Nutr. 1986;116:204–215. doi: 10.1093/jn/116.2.204. [DOI] [PubMed] [Google Scholar]
- **11.Dever JT, Elfarra AA. L-methionine toxicity in freshly isolated mouse hepatocytes is gender-dependent and mediated in part by transamination. J Pharmacol Exp Ther. 2008;326:809–817. doi: 10.1124/jpet.108.141044. Demonstrates gender differences in Met toxicity in FIMHs and evidence for the detoxification and bioactivating role of Met TM and TA, respectively. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **12.Dever JT, Elfarra AA. L-Methionine-dl-sulfoxide metabolism and toxicity in freshly isolated mouse hepatocytes: Gender differences and inhibition with aminooxyacetic acid. Drug Metab Dispos. 2008;36:2252–2260. doi: 10.1124/dmd.108.023390. Demonstrates gender differences in MetO toxicity in FIMHs and provides evidence for Met-d-O TA at low and high Met concentrations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *13.Dever JT, Elfarra AA. Gender differences in methionine accumulation and metabolism in freshly isolated mouse hepatocytes: Potential roles in toxicity. Toxicol Appl Pharmacol. 2009;236:358–65. doi: 10.1016/j.taap.2009.02.009. Correlates increased Met toxicity in male compared with female FIMHs to increased cellular Met concentrations, decreased Met TA, increased Met TM, and increased Met-d-O TA in male versus female FIMHs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hesse H, Kreft O, Maimann S, et al. Current understanding of the regulation of methionine biosynthesis in plants. J Exp Bot. 2004;55:1799–1808. doi: 10.1093/jxb/erh139. [DOI] [PubMed] [Google Scholar]
- 15.Oz HS, Chen TS, Neuman M. Methionine deficiency and hepatic injury in a dietary steatohepatitis model. Dig Dis Sci. 2008;53:767–776. doi: 10.1007/s10620-007-9900-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newberne PM, Young VR. Effect of diets marginal in methionine and choline with and without vitamin B12 on rat liver and kidney. J Nutr. 1966;89:69–79. doi: 10.1093/jn/89.1.69. [DOI] [PubMed] [Google Scholar]
- 17.Grandison RC, Piper MDW, Partridge L. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature. 2009;462:1061–1065. doi: 10.1038/nature08619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benevenga NJ. Toxicities of methionine and other amino acids. J Agr Food Chem. 1974;22:2–9. doi: 10.1021/jf60191a036. [DOI] [PubMed] [Google Scholar]
- 19.Steele RD, Barber TA, Lalich J, et al. Effects of dietary 3-methylthiopropionate on metabolism, growth, and hematopoiesis in the rat. J Nutr. 1979;109:1739–1751. doi: 10.1093/jn/109.10.1739. [DOI] [PubMed] [Google Scholar]
- 20.Toborek M, Kopieczna-Grzebieniak E, Drozdz M, et al. Increased lipid peroxidation and antioxidant activity in methionine-induced hepatitis in rabbits. Nutrition. 1996;12:534–537. doi: 10.1016/s0899-9007(96)00108-6. [DOI] [PubMed] [Google Scholar]
- 21.Mori N, Hirayama K. Long-term consumption of methionine-supplemented diet increases iron and lipid peroxide levels in rat liver. J Nutr. 2000;130:2349–2355. doi: 10.1093/jn/130.9.2349. [DOI] [PubMed] [Google Scholar]
- 22.Martinez-chantar ML, Corrales FJ, Martinez-Cruz LA, et al. Spontaneous oxidative stress and liver tumors in mice lacking methionine adenosyltransferase 1A. FASEB J. 2002;16:1292–1294. doi: 10.1096/fj.02-0078fje. [DOI] [PubMed] [Google Scholar]
- 23.Orentriech N, Matias JR, DeFelice A, et al. Low methionine ingestion by rats extends life span. J Nutr. 1993;123:269–274. doi: 10.1093/jn/123.2.269. [DOI] [PubMed] [Google Scholar]
- 24.Miller RA, Buehner G, Chang Yayi, et al. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-1 and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging cell. 2005;4:119–125. doi: 10.1111/j.1474-9726.2005.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun L, Sadighi Akha AA, Miller RA, et al. Life-span extension in mice by preweaning food restriction and by methionine restriction in middle age. J Gerontol A Biol Sci Med Sci. 2009;64A:711–722. doi: 10.1093/gerona/glp051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dever JT, Elfarra AA. In vivo metabolism of L-methionine in mice: Evidence for stereoselective formation of methionine-d-sulfoxide and quantitation of other major metabolites. Drug Metab Dispos. 2006;34:2036–2043. doi: 10.1124/dmd.106.012104. [DOI] [PubMed] [Google Scholar]
- 27.Garlick PJ. Toxicity of methionine in humans. J Nutr. 2006;136:1722S–1725S. doi: 10.1093/jn/136.6.1722S. [DOI] [PubMed] [Google Scholar]
- 28.Mudd SH, Levy HL, Tangerman A, et al. Isolated persistent hypermethionemia. Am J Hum Genet. 1995;57:882–892. [PMC free article] [PubMed] [Google Scholar]
- 29.Baric I. Inherited disorders in the conversion of methionine to homocysteine. J Inherit Metab Dis. 2009;32:459–471. doi: 10.1007/s10545-009-1146-4. [DOI] [PubMed] [Google Scholar]
- 30.Couce ML, Boveda MD, Castineiras DE, et al. Hypermethionemia due to methionine adenosyltransferase I/III (MAT I/III) deficiency. Diagnosis in an expanded neonatal screening programme. J Inherit Metab Dis. 2008;31:467–468. doi: 10.1007/s10545-008-0811-3. [DOI] [PubMed] [Google Scholar]
- 31.Gahl WA, Bernardini I, Finkelstein JD, et al. Transsulfuration in an adult with hepatic methionine adenosyltransferase deficiency. J Clin Invest. 1988;81:390–397. doi: 10.1172/JCI113331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blom HJ, Boers GHJ, van den Elzen JPAM, et al. Transamination of methionine in humans. Clin Sci (Colch) 1989;76:43–49. doi: 10.1042/cs0760043. [DOI] [PubMed] [Google Scholar]
- 33.Chamberlin ME, Ubagai T, Mudd SH, et al. Demyelination of the brain is associated with methionine adenosyltransferase I/III deficiency. J Clin Invest. 1996;98:1021–1027. doi: 10.1172/JCI118862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mudd SH, Cerone R, Schiaffino MC, et al. Glycine N-methyltransferase deficiency: A novel inborn error causing persistent isolated hypermethionaemia. J Inhert Metab Dis. 2001;24:448–464. doi: 10.1023/a:1010577512912. [DOI] [PubMed] [Google Scholar]
- 35.Augoustides-Savvopoulou P, Luka Z, Karyda S, et al. Glycine N-methyltransferase deficiency: A new patient with a novel mutation. J Inhert Metab Dis. 2003;26:745–759. doi: 10.1023/B:BOLI.0000009978.17777.33. [DOI] [PubMed] [Google Scholar]
- 36.Luka Z, Capdevila A, Mato JM, et al. A glycine N-methyltransferase knockout mouse model for humans with deficiency of this enzyme. Transgenic Res. 2006;15:393–397. doi: 10.1007/s11248-006-0008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu SP, Li YS, Chen YJ, et al. Glycine N-methyltransferase −/− mice develop chronic hepatitis and glycogen storage disease in the liver. Hepatology. 2007;46:1413–1425. doi: 10.1002/hep.21863. [DOI] [PubMed] [Google Scholar]
- 38.Martinez-Chantar ML, Vazquez-Chantada M, Ariz U, et al. Loss of the glycine N-methyltransferase gene leads to steatosis and hepatocellular carcinoma in mice. Hepatology. 2008;47:1191–1199. doi: 10.1002/hep.22159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baric I, Fumic K, Glenn B, et al. S-adenosylhomocysteine hydrolase deficiency in a human: a genetic disorder of methionine metabolism. Proc Natl Acad Sci. 2004;101:4234–4239. doi: 10.1073/pnas.0400658101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baric I, Cuk M, Fumic K, et al. S-Adenosylhomocysteine hydrolase deficiency: a second patient, the younger brother of the index patient, and outcomes during therapy. J Inhert Metab Dis. 2005;28:885–902. doi: 10.1007/s10545-005-0192-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buist NRM, Glenn B, Vugrek O, et al. S-Adenosylhomocysteine hydrolase deficiency in a 26-year-old man. J Inherit Metab Dis. 2006;29:538–545. doi: 10.1007/s10545-006-0240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *42.Tangerman A, Wilcken B, Levy HL, et al. Methionine transamination in patients with homocystinuria due to cystathionine β-synthase deficiency. Metabolism. 2000;49:1071–1077. doi: 10.1053/meta.2000.7709. Demonstrates that Met TA becomes especially significant in humans with plasma Met concentrations of >350 μM. [DOI] [PubMed] [Google Scholar]
- 43.Blom HJ, Boers G, Tribels J, et al. Cystathionine-synthase-deficient patients do not use the transamination pathway of methionine to reduce hypermethionemia and homocystineria. Metabolism. 1989;38:577–582. doi: 10.1016/0026-0495(89)90220-5. [DOI] [PubMed] [Google Scholar]
- 44.Naughten E, Yap S, Mayne PD. Newborn screening for homocystinuria: Irish and world experience. Eur J Pediatr. 1998;157(Suppl 2):S84–S87. doi: 10.1007/pl00014310. [DOI] [PubMed] [Google Scholar]
- 45.Clarke R. Homocysteine studies collaboration. Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. JAMA. 2002;288:2015–2022. doi: 10.1001/jama.288.16.2015. [DOI] [PubMed] [Google Scholar]
- 46.Anderson JL, Muhlestein JB, Horne BD, et al. Plasma homocysteine predicts mortality independently of traditional risk factors and C-reactive protein in patients with angiographically defined coronary artery disease. Circulation. 2000;102:1227–1232. doi: 10.1161/01.cir.102.11.1227. [DOI] [PubMed] [Google Scholar]
- 47.Seshadri S, Beiser A, Selhub J, et al. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N Engl J Med. 2002;346:476–83. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- 48.Jakubowski H. Molecular basis of homocysteine toxicity in humans. Cell Mol Life Sci. 2004;61:470–487. doi: 10.1007/s00018-003-3204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brown MR, Putnam TC. Cholestasis associated with central intravenous nutrition in infants. N Y State J Med. 1978;78:27–30. [PubMed] [Google Scholar]
- 50.Coran AG, Drongowski RA. Studies on the toxicity and efficacy of a new amino acid solution in pediatric parenteral nutrition. J Parenteral Enteral Nutr. 1987;11:368. doi: 10.1177/0148607187011004368. [DOI] [PubMed] [Google Scholar]
- 51.Moss RL, das JB, Ansari G, et al. Hepatobiliary dysfunction during total parenteral nutrition is caused by the infusate, not the route of administration. J Pediatr Surg. 1993;28:391–397. doi: 10.1016/0022-3468(93)90238-g. [DOI] [PubMed] [Google Scholar]
- 52.Ekema G, Falchetti D, Boroni G, et al. Reversal of severe parenteral nutrition-associated liver disease in an infant with short bowel syndrome using parenteral fish oil (Omega-3 fatty acids) J Pediatr Surg. 2008;43:1191–1195. doi: 10.1016/j.jpedsurg.2008.01.005. [DOI] [PubMed] [Google Scholar]
- *53.Moss LR, Haynes AL, Pastuszyn A, et al. Methionine infusion reproduces liver injury of parenteral nutrition cholestasis. Pediatr Res. 1999;45:664–668. doi: 10.1203/00006450-199905010-00009. Provides evidence that Met toxicity may contribute to TPN-cholestasis in TPN-infused rabbits. [DOI] [PubMed] [Google Scholar]
- 54.Riedijk MA, Stoll B, Chacko S, et al. Methionine transmethylation and transsulfuration in the piglet gastrointestinal tract. Proc Natl Acad Sci. 2007;104:3408–3413. doi: 10.1073/pnas.0607965104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shoveller AK, Brunton JA, Pencharz PB, et al. The methionine requirement is lower in neonatal piglets fed parenterally than in those fed enterally. J Nutr. 2003;133:1390–1397. doi: 10.1093/jn/133.5.1390. [DOI] [PubMed] [Google Scholar]
- 56.Higashi T. Impaired metabolism of methionine in severe liver diseases I. Clinical and pathophysiological significance of elevated serum methionine levels. Gastroenterol Jpn. 1982;17:117–24. doi: 10.1007/BF02774550. [DOI] [PubMed] [Google Scholar]
- 57.Tietge UJF, Bahr MJ, Manns MP, et al. Hepatic amino-acid metabolism in liver cirrhosis and in the long-term course after liver transplantation. Transplant Int. 2003;18:1–8. doi: 10.1007/s00147-002-0484-z. [DOI] [PubMed] [Google Scholar]
- 58.Marchesini G, Bugianesi E, Bianchi G, et al. Defective methionine metabolism in cirrhosis: Relation to severity of liver disease. Hepatology. 1992;16:149–155. doi: 10.1002/hep.1840160125. [DOI] [PubMed] [Google Scholar]
- 59.Avila MA, Berasain C, Torres L, et al. Reduced mRNA abundance of the main enzymes involved in methionine metabolism in human liver cirrhosis and hepatocellular carcinoma. J Hepatol. 2000;33:907–914. doi: 10.1016/s0168-8278(00)80122-1. [DOI] [PubMed] [Google Scholar]
- 60.Finkelstein JD. Methionine metabolism in liver diseases. Am J Clin Nutr. 2003;77:1094–1095. doi: 10.1093/ajcn/77.5.1094. [DOI] [PubMed] [Google Scholar]
- 61.Lu SC. S-Adenosylmethionine. Int J Biochem Cell B. 2000;32:391–395. doi: 10.1016/s1357-2725(99)00139-9. [DOI] [PubMed] [Google Scholar]
- *62.Regina M, Korhonen V, Smith T, et al. Methionine toxicity in the rat in relation to hepatic accumulation of S-adenosylmethionine: Prevention by dietary stimulation of the hepatic transsulfuration pathway. Arch Biochem Biophys. 1993;300:598–607. doi: 10.1006/abbi.1993.1083. Demonstrates that dietary stimulation of Met TM reduces Met toxicity. [DOI] [PubMed] [Google Scholar]
- 63.Duerre JA. Effect of 3-deazaadenosine on methionine metabolism in isolated perfused livers. Biochem Cell Biol. 1988;66:1032–1039. doi: 10.1139/o88-119. [DOI] [PubMed] [Google Scholar]
- 64.Mato JM, Lu SC. Role of S-Adenosyl-L-methionine in liver health and injury. Hepatology. 2007;45:1306–1312. doi: 10.1002/hep.21650. [DOI] [PubMed] [Google Scholar]
- 65.Lu SC, Ramani K, Ou X, et al. S-Adenosylmethionine in the chemoprevention and treatment of hepatocellular carcinoma in a rat model. Hepatology. 2009;50:462–471. doi: 10.1002/hep.22990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang S, Chen H, Sheen L, et al. Methionine and cysteine affect glutathione level, glutathione-related enzyme activities and the expression of glutathione S-transferase isozymes in rat hepatocytes. J Nutr. 1997;127:2135–2141. doi: 10.1093/jn/127.11.2135. [DOI] [PubMed] [Google Scholar]
- 67.Scislowski PWD, Pickard K. Methionine transamination – metabolic function and subcellular compartmentation. Mol Cell Biochem. 1993;129:39–45. doi: 10.1007/BF00926574. [DOI] [PubMed] [Google Scholar]
- 68.Cooper AJL, Meister A. Isolation and properties of a new glutamine transaminase from rat kidney. J Biol Chem. 1974;249:2554–2561. [PubMed] [Google Scholar]
- 69.Steele RD, Benevenga NJ. Identification of 3-methylthiopropionic acid as an intermediate in mammalian methionine metabolism in vitro. J Biol Chem. 1978;253:7844–7850. [PubMed] [Google Scholar]
- 70.Dixon JL, Benevenga NJ. The decarboxylation of α-keto-γ-methiolbutryate in rat liver mitochondria. Biochem Biophys Res Commun. 1980;97:939–946. doi: 10.1016/0006-291x(80)91467-9. [DOI] [PubMed] [Google Scholar]
- 71.Jones SMA, Yeaman SJ. Oxidative decarboxylation of 4-methylthio-2-oxobutyrate by branched-chain 2-oxo acid dehydrogenase complex. Biochem J. 1986;237:621–623. doi: 10.1042/bj2370621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Steele RD, Benevenga NJ. The metabolism of 3-methylthiopropionate in rat liver homogenates. J Biol Chem. 1979;254:8885–8890. [PubMed] [Google Scholar]
- 73.Blom HJ, van den Elzen JP, Yap SH, et al. Methanethiol and dimethylsulfide formation from 3-methylthiopropionate in human and rat hepatocytes. Biochim Biophys Acta. 1988;972:131–136. doi: 10.1016/0167-4889(88)90111-5. [DOI] [PubMed] [Google Scholar]
- 74.Konno R, Sasaki M, Asakura S, et al. D-amino-acd oxidase is not present in the mouse liver. Biochem Biophys Acta. 1997;1335:173–181. doi: 10.1016/s0304-4165(96)00136-5. [DOI] [PubMed] [Google Scholar]
- 75.Mitchell AD, Benevenga NJ. The role of transamination in methionine oxidation in the rat. J Nutr. 1978;108:67–78. doi: 10.1093/jn/108.1.67. [DOI] [PubMed] [Google Scholar]
- 76.Bugianesi E, Tangerman A, Ronchi M, et al. Transamination of methionine after loading in patients with cirrhosis. J Hepatol. 1996;24:95–100. doi: 10.1016/s0168-8278(96)80192-9. [DOI] [PubMed] [Google Scholar]
- 77.Mashima R, Nakanishi-Ueda T, Yamamoto Y. Simultaneous determination of methionine sulphoxide and methionine in blood plasma using gas chromatography-mass spectrometry. Anal Biochem. 2003;313:28–33. doi: 10.1016/s0003-2697(02)00537-7. [DOI] [PubMed] [Google Scholar]
- **78.Duescher RJ, Lawton MP, Philpot RM, et al. Flavin-containing monooxygenase (FMO)-dependent metabolism of methionine and evidence for FMO3 being the major FMO involved in methionine sulfoxidation in rabbit liver and kidney microsomes. J Biol Chem. 1994;269:17525–17530. First demonstration of enzymatically-mediated Met S-oxidation in vitro by flavin-containing monooxygenases. [PubMed] [Google Scholar]
- 79.Krause RJ, Ripp SL, Sausen PJ, et al. Characterization of the methionine-S-oxidase activity of rat liver and kidney microsomes: Immunochemical and kinetic evidence for FMO3 being the major catalyst. Arch Biochem Biophy. 1996;333:109–116. doi: 10.1006/abbi.1996.0370. [DOI] [PubMed] [Google Scholar]
- 80.Ripp SL, Kiyoshi I, Philpot RM, et al. Species and sex differences in expression of flavin-containing monooxygenase form 3 in liver and kidney microsomes. Drug Metab Dispos. 1999;27:46–52. [PubMed] [Google Scholar]
- *81.Ripp SL, Itagaki K, Philpot RM, et al. Methionine S-oxidation in human and rabbit liver microsomes: Evidence for a high-affinity methionine S-oxidase activity that is distinct from flavin-containing monooxygenase 3. Arch Biochem Biophys. 1999;367:322–332. doi: 10.1006/abbi.1999.1247. Characterization of an unidentified, high-affinity Met S-oxidase activity in human and rabbit liver microsomes. [DOI] [PubMed] [Google Scholar]
- 82.Perry TL, Hansen S, MacDougall L. Sulfur-containing amino acids in plasma and urine of homocystinurics. Clin Chim Acta. 1965;15:409–420. doi: 10.1016/0009-8981(67)90005-8. [DOI] [PubMed] [Google Scholar]
- 83.Bauchart-Thevret C, Stoll B, Chacko S, et al. Sulfur amino acid deficiency upregulates intestinal methionine cycle activity and suppresses epithelial growth in neonatal pigs. Am J Physiol Endocrinol Metab. 2009;296:E1239–1250. doi: 10.1152/ajpendo.91021.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Storch KJ, Wagner DA, Burke JF, et al. Quantitative study in vivo of methionine cycle in humans using [methyl-2H3]- and [1-13C]methionine. Am J Physiol Endocrinol Metab. 1988;255:E322–E331. doi: 10.1152/ajpendo.1988.255.3.E322. [DOI] [PubMed] [Google Scholar]
- 85.Mercier S, Breuille D, Buffiere C, et al. Methionine kinetics are altered in the elderly both in the basal state and after vaccination. Am J Clin Nutr. 2006;83:291–298. doi: 10.1093/ajcn/83.2.291. [DOI] [PubMed] [Google Scholar]
- 86.Toue S, Kodama R, Amao M, et al. Screening of toxicity biomarkers for methionine excess in rats. J Nutr. 2006;136:1716S–1721. doi: 10.1093/jn/136.6.1716S. [DOI] [PubMed] [Google Scholar]