Abstract
Objective
The metabolic side effects of second-generation antipsychotics (SGA) are serious and have not been compared head to head in a meta-analysis. We conducted a meta-analysis of studies comparing the metabolic side effects of the following SGAs head-to-head: amisulpride, aripiprazole, clozapine, olanzapine, quetiapine, risperidone, sertindole, ziprasidone, zotepine.
Method
We searched the register of the Cochrane schizophrenia group (last search May 2007), supplemented by MEDLINE and EMBASE (last search January 2009) for randomized, blinded studies comparing the above mentioned SGA in the treatment of schizophrenia or related disorders. At least three reviewers extracted the data independently. The primary outcome was weight change. We also assessed changes of cholesterol and glucose. The results were combined in a meta-analysis.
Results
We included 48 studies with 105 relevant arms. Olanzapine produced more weight gain than all other second-generation antipsychotics except for clozapine where no difference was found. Clozapine produced more weight gain than risperidone, risperidone more than amisulpride, and sertindole more than risperidone. Olanzapine produced more cholesterol increase than aripiprazole, risperidone and ziprasidone. (No differences with amisulpride, clozapine and quetiapine were found). Quetiapine produced more cholesterol increase than risperidone and ziprasidone. Olanzapine produced more increase in glucose than amisulpride, aripiprazole, quetiapine, risperidone and ziprasidone; no difference was found with clozapine.
Conclusions
Some SGAs lead to substantially more metabolic side effects than other SGAs. When choosing an SGA for an individual patient these side effects with their potential cause of secondary diseases must be weighed against efficacy and characteristics of the individual patient.
Keywords: weight, cholesterol, glucose, individual treatment
1. Introduction
Second-generation antipsychotics (SGA) are commonly used in the treatment of patients with schizophrenia (Diabetes Expert Group, 2004; Leucht et al., 2009b) and have even become the drugs of choice in some countries, such as the United States. However, there are substantial concerns about the metabolic side effects of SGAs. In general, today there is significant agreement on the importance of the metabolic side effects, such as changes in body weight, glucose utilization, or lipid status, unrecognized or unknown at the beginning of the introduction of SGAs (Meyer, 2002). For some physicians these side effects are the most important as they might predispose to type 2 diabetes mellitus and cardiovascular disease (Meyer et al., 2008a; Daumit et al., 2008), while others may weigh them against other side effects such as extrapyramidal side effects or sexual problems. The metabolic side effects have become an issue in competitive advertising between pharmaceutical companies, thus causing a polarization. Randomized controlled trials (RCTs) are probably the most bias free way to compare such side effects. There is a substantial amount of research in this field, however, currently there is no meta-analysis comparing the metabolic side effects of the SGAs head-to-head.
We therefore conducted a meta-analysis of studies directly comparing the following SGAs to one another: amisulpride, aripiprazole, clozapine, olanzapine, quetiapine, risperidone, sertindole, ziprasidone, zotepine. This article is focusing on the metabolic side-effects of second-generation antipsychotics while the data on the efficacy of these medications have been published elsewhere (Leucht et al., 2009b).
2. Method
2.1. Search strategy
The register of the Cochrane Schizophrenia Group (CSG) was searched for randomized, blinded trials comparing orally administered second-generation antipsychotics (amisulpride, aripiprazole, clozapine, olanzapine, quetiapine, risperidone, sertindole, ziprasidone, zotepine) head-to-head in the treatment of schizophrenia or related disorder (schizoaffective, schizophreniform, or delusional disorder) without language restrictions. The search terms used were all possible 36 combinations of the names of the SGA including various trade names. The last search of the CSG register was done in May 2007; until January 2009, we searched MEDLINE and EMBASE for further randomized controlled studies fulfilling our inclusion criteria. The CSG register is compiled by regular methodical searches in numerous electronic databases (BIOSIS, CINAHL, Dissertation Abstracts, EMBASE, LILACS, MEDLINE, PSYNDEX, PsychINFO, RUSSMED, Sociofile), supplemented by hand searching of relevant journals and conference proceedings (for details see the description of the Cochrane Schizophrenia Group) (Adams et al., 2006). Only studies meeting the quality criteria A (adequate randomization) and B (primarily studies stated to be randomized without further details) according to the Cochrane handbook 2005 were included (Higgins and Green, 2005). All manufacturers of SGAs were contacted for further details of published studies and asked for unpublished trials; first authors of included studies were contacted for missing information. Detailed methodology is published in the Cochrane library in systematic reviews of individual SGAs in comparison to other SGAs (Komossa et al., 2007; Komossa et al., 2009a; Komossa et al., 2009c; Komossa et al., 2009b; Komossa et al., 2010c; Komossa et al., 2010a; Komossa et al., 2010d; Komossa et al., 2010b). Here we present a summary to show an overall picture of the metabolic side effects of the SGAs.
2.2. Data extraction and outcome parameters
All data were extracted independently by at least three reviewers (KK, CR, HH, FS, SS, CAL, SL). Effects on the following clinically important metabolic side-effects were assessed: weight change as the primary outcome, glucose and cholesterol changes as secondary outcomes. Weight change was chosen as the primary outcome as it is the least sensitive for errors: neither fasting nor non-fasting will have a profound, immediate effect. Data on the Body Mass Index (BMI) – taking height into account - would have been preferred, but were rarely reported. Weight change was defined as the change in kilograms from baseline to endpoint; the changes in cholesterol and glucose were defined as the changes from baseline to endpoint in mg/dl.
The outcome ‘total cholesterol’ and not ‘triglycerides’ was chosen for the lipid status as the data on triglycerides were rarely reported. Furthermore, the total cholesterol would not be greatly influenced by the last meal, and therefore in contrast to triglycerides a potential non-fasting blood sample is not misleading (Herold, 2009). Intention-to-treat results were used whenever possible.
2.3. Meta-analytic calculations
Continuous outcomes were analyzed using differences of means (MD) and their 95% confidence interval, since this preserves the original units, which are intuitively interpreted (e.g. a MD of 5 means 5 kg difference in weight between the two groups). The studies were pooled with the random effects model of Der-Simonian and Laird (Der-Simonian and Laird, 1986), which takes into account certain differences between even if there is no statistically significant heterogeneity. In addition, a fixed effects model was used for the primary outcome to verify the results under this assumption. explored study heterogeneity by I-squared statistic assuming that I2 values greater 50% suggested considerable heterogeneity.
Addressing potential moderator variables
Unrestricted maximum likelihood random effects meta-regression assessed the effects o study duration, pharmaceutical company sponsorship, antipsychotic dose ratios, cholesterol, baseline glucose, baseline weight, washout period, and sex and distribution on the outcomes. Furthermore, the effects of study duration were assessed subgroup analyses including short term (≤ 12 weeks) and longer term studies separately.
Statistical analyses
The calculations were done with MetaView, the meta-analytic standard software used by the Cochrane Collaboration (Review Manager Version 4.2.2, Oxford, England; The Cochrane Collaboration, 2000) and with STATA version 7. All analyses were two-tailed with alpha set to 0.05, except for the heterogeneity test (alpha set to 0.1).
Presentation of the results
The pooled effect sizes of each SGA versus every other SGA are shown in Figures 2-4. It should be noted that all results are shown twice. For example, the comparison between amisulpride and olanzapine is described under “amisulpride versus other SGA” and under “olanzapine versus other SGA”. Despite the redundancy, the results are easier to understand. Otherwise, if the reader is interested in a given drug, he would have to look up the findings in different sections, making it difficult to see the gestalt. To save space in the text we present only: a) for significant results: the number of studies (N), the number of participants (n), the differences in means (MD) in weight change in kilograms, in cholesterol and glucose change in mg/dl, and the p-value, and b) for non-significant results: the number of studies and the number of participants. Minus values mean superiority of the first listed SGA throughout. All statistical details are presented in the Figures 2-4.
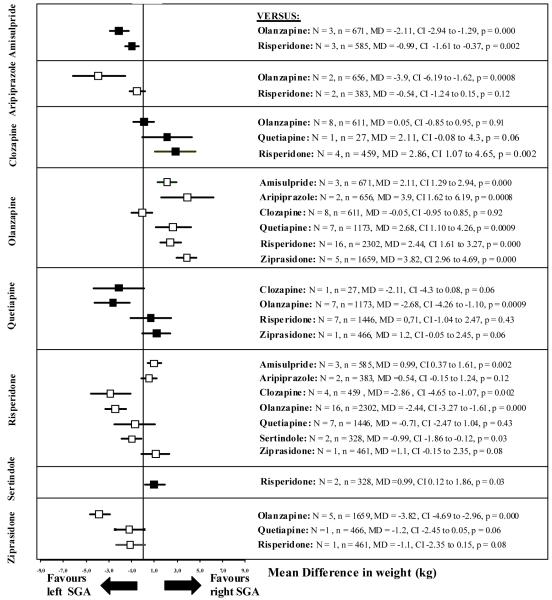
Figure 2.
Weight change
The left SGA is the one written vertically on the left side, the right SGA is the one written horizontally on the right side of the graph. N = number of studies, n = number of participants, MD = Mean Difference, CI = 95% confidence intervals.
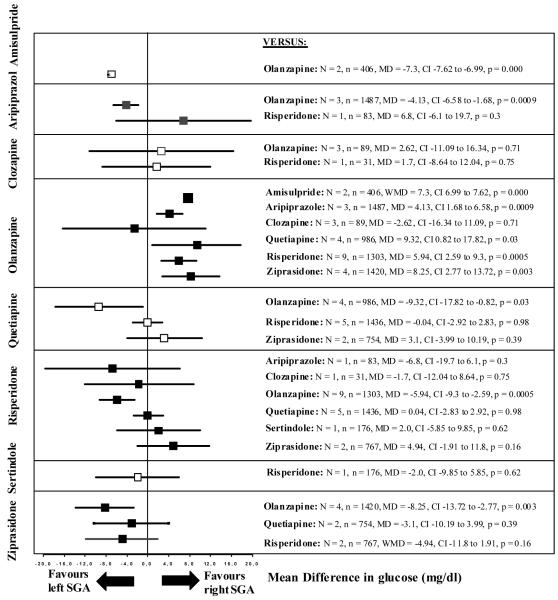
Figure 4.
Glucose change
The left SGA is the one written vertically on the left side, the right SGA is the one written horizontally on the right side of the graph. N = number of studies, n = number of participants, MD = Mean Difference, CI = 95% confidence intervals.
The results of the specific comparisons are always presented in alphabetical order to increase clarity; in the way that one antipsychotic is causing more side effect than the other antipsychotic, e.g. “olanzapine is causing more increase in cholesterol than aripiprazole”, as opposed to the reverse “aripiprazole is causing less cholesterol increase than olanzapine”. In this way, the results are easier to understand.
Further data, such as forest plots with the single studies, the standardized mean differences for each comparison, and the separate results for the short- and long-term studies for the primary outcome weight gain where most data were available, and the heterogeneity/meta-regressions results can be found on the journal’s website (supplemental Figures I-III, supplemental Tables I-VII).
3. Results
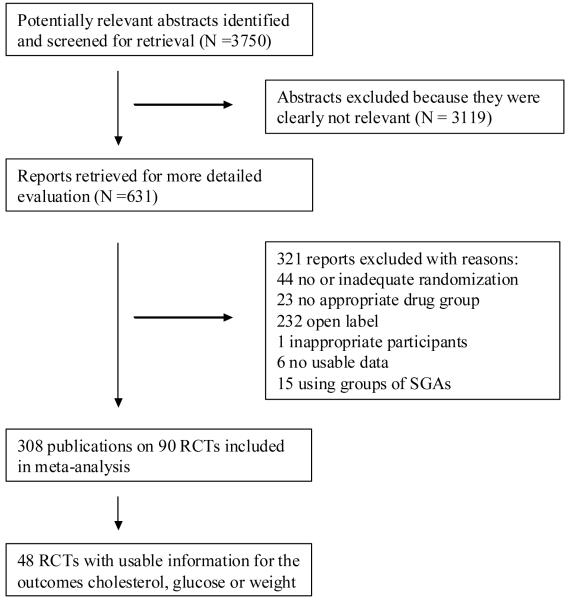
3.1. The search (for QUOROM flow diagram (Moher et al., 1999) see Figure 1)
Figure 1.
Quality of Reports of Meta-analysis (QUOROM) flow-diagram describing the search process
The search strategy identified 3750 citations. Of these, 3119 abstracts were excluded because they were clearly not relevant. 631 articles were ordered for more detailed evaluation and 321 of these were excluded. 310 publications on 90 studies were included; however, only 48 studies with 105 relevant arms reported usable data on at least one of the outcomes cholesterol, glucose or weight. Of these 48 studies, 6 studies included amisulpride, 5 aripiprazole, 11 clozapine, 37 olanzapine, 11 quetiapine, 28 risperidone, 1 sertindole and 6 ziprasidone.
The participants were relatively chronic with mean ages in the mid-thirties. The diagnostic criteria were mainly DSM-IV/DSM-III-R or ICD-10 (see detailed supplemental Table I on the journal’s website). Unpublished, industry-sponsored studies were not obtained.
3.2. Outcome results
3.2.1. Primary Outcome - Weight Change
Clozapine produced statistically significantly more weight gain (from baseline to endpoint in kg) than risperidone (N=4, n=459, MD 2.86 kg). Olanzapine produced statistically significantly more weight gain than amisulpride (N=3, n=671, MD 2.11 kg), aripiprazole (N=2, n=656, MD 3.9 kg), quetiapine (N=7, n=1173, MD 2.68 kg), risperidone (N=16, n=2302, MD 2.44 kg) and ziprasidone (N=5 n=1659, MD 3.82 kg). Risperidone produced significantly more weight gain than amisulpride (N=3, n=585, MD 0.99 kg). Sertindole produced significantly more weight gain than risperidone (N=2, n=328, MD 0.99 kg).
No statistically significant differences were found between aripiprazole and risperidone (N=2, n=383), clozapine and olanzapine (N=8, n=611), clozapine and quetiapine (N=1, n=27), quetiapine and risperidone (N=7, n=1446), quetiapine and ziprasidone (N=1, n=466), and risperidone and ziprasidone (N=1, n=461). Details are shown in Figure 2 and Supplemental Table II. The fixed-effects model found no important differences in results.
3.2.2. Secondary Outcome - Cholesterol Change
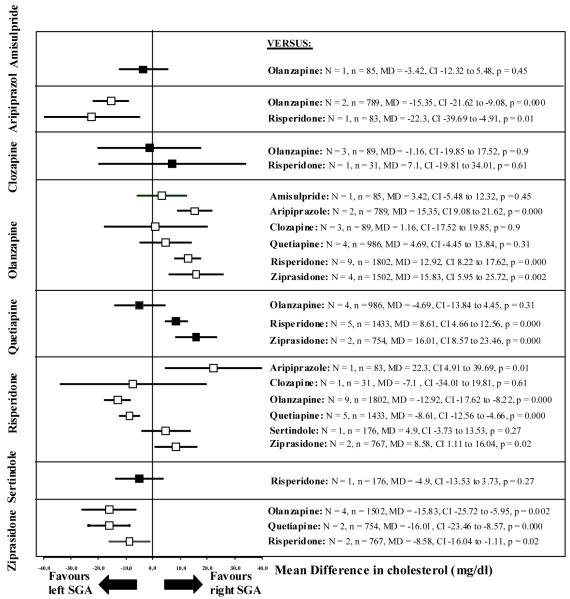
Olanzapine produced statistically significantly more increase in cholesterol than aripiprazole (N=2, n=789, MD=15.35 mg/dl), risperidone (N=9, n=1802, MD=12.92 mg/dl) and ziprasidone (N=4, n=1502, MD=15.83 mg/dl). Quetiapine produced significantly more increase in cholesterol than risperidone (N=5, n=1433, MD=8.61 mg/dl) and ziprasidone (N=2, n=754, MD=16.01 mg/dl).
Risperidone produced significantly more increase in cholesterol compared to aripiprazole (N=1, n=83, MD=22.3 mg/dl) and ziprasidone (N=2, n=767, MD=8.58 mg/dl).
There were no statistically significant differences in cholesterol changes between amisulpride and olanzapine (N=1, n=85), clozapine and olanzapine (N=3, n=89), clozapine and risperidone (N=1, n=31), olanzapine and quetiapine (N=4, n=986), and risperidone and sertindole (N=1, n=176). Further details are also shown in Figure 3 and Supplemental Table III.
Figure 3.
Cholesterol change
The left SGA is the one written vertically on the left side, the right SGA is the one written horizontally on the right side of the graph. N = number of studies, n = number of participants, MD = Mean Difference, CI = 95% confidence intervals.
3.2.3. Secondary Outcome - Glucose Change
Olanzapine produced statistically significantly more increase in glucose levels from baseline to endpoint than amisulpride (N=2, n=406, MD=7.3 mg/dl), aripiprazole (N=3, n=1487, MD=4.13 mg/dl), quetiapine (N=4, n=986, MD=9.32 mg/dl), risperidone (N=9, n=1303, MD=5.94 mg/dl) and ziprasidone (N=4, n=1420, MD=8.25 mg/dl).
There were no statistically significant differences in glucose changes between aripiprazole and risperidone (N=1, n=83), clozapine and olanzapine (N=3, n=89), clozapine and risperidone (N=1, n=31), quetiapine and risperidone (N=5, n=1436), quetiapine and ziprasidone (N=2, n=754), risperidone and sertindole (N=1, n=176), and risperidone and ziprasidone (N=2, n=767).
Further details are shown in Figure 4 and Supplemental Table IV.
3.3. Heterogeneity and Meta-regressions
There was some heterogeneity (see Supplemental Table VII); however, in most comparisons, the direction of the results was the same; i.e., the degree of weight gain was different, but not the direction of the weight gain. Our meta-regressions suggest that part of the heterogeneity was explained by study duration, dose of antipsychotics and sponsorship. The longer studies or higher doses of medication had greater differences in the outcomes, but the directions of the effects were the same and the results of the short- and long-term studies were consistent. In three comparisons sponsorship showed a significant effect; namely, that the sponsoring company showed lower and thus ‘better’ weight changes for its own antipsychotic medication than a neutral sponsor or another pharmaceutical company.
Meta-regressions on the influence of sex distribution, washout period and race distribution on the outcome weight change found only very few or no significant results: for the moderator ‘washout period’ there are significant results in two comparisons (clozapine vs risperidone and quetiapine versus risperidone, showing the longer the washout period, the greater the difference in weight gain to the disadvantage of clozapine or quetiapine, respectively). For the moderator ‘sex distribution’ we found two significant results: in the comparison clozapine vs risperidone the more females the greater the difference in weight gain to the disadvantage of clozapine and in the comparison quetiapine vs risperidone the same result, but to the disadvantage of quetiapine. For the moderator ‘race distribution’ there was no significant result. The main reason might be that only few data (<10 studies) were available here limiting the potential of meta-regression to find an effect (Higgins and Green, 2005).
4. Discussion
We present the first head-to-head meta-analysis of the metabolic side effects of second-generation antipsychotics in randomized controlled trials, showing three similar clusters for the three outcomes with olanzapine and clozapine showing the most elevation of weight, cholesterol, and glucose. Quetiapine, risperidone, and sertindole had intermediate elevations. Aripiprazole and amisulpride displayed intermediate or low elevations and ziprasidone the lowest elevations.
We found that olanzapine showed more weight gain, cholesterol and glucose elevation than all other SGA’s except for clozapine where no difference with olanzapine was demonstrated. To illustrate the results of our primary outcome weight change, olanzapine produced about two kilograms of weight gain more than amisulpride and four kilograms more than aripiprazole in 2 to 6 months study durations. Clozapine produced about three kilograms more than risperidone (MD 2.9 kg), risperidone produced more weight gain than amisulpride (MD 0.99 kg), and sertindole more than risperidone (MD 0.99). Weight gain is rapid in the first few weeks, and the rapid rate decreases gradually until the weight gain plateaus after several months, after 4-9 months for olanzapine and after 42-46 months for clozapine (Bai et al., 2009; Henderson et al., 2000; Kinon et al., 2005). Our meta-regressions ‘duration of study’ could explain some of the heterogeneity found, as longer studies produced more weight gain than shorter studies; e.g. in short-term studies (≤12 weeks) olanzapine produced 2.5 kg more weight gain than ziprasidone, whereas in long-term studies the difference was about 4 kg. The number of studies for individual drug comparisons was too small for a meta-regression of each. Over the long term the absolute changes will grow, and therefore this is not an absolute estimate of these outcomes, but a relative one. Other factors potentially explaining parts of the heterogeneity were the washout periods, the race distribution, sponsorship, and the dose of medication: short washout periods, often only 24 hours in clinical reality, make the differences in the outcome smaller than they actually are and women seem to be more vulnerable for weight gain with clozapine and quetiapine than men. Sponsorship was found to be some factor in three comparisons in our meta-regressions for sponsorship, but not in the majority of studies as the studied outcomes are rather “hard” outcomes that are difficult to be influenced. The dose of antipsychotic medication was shown in some of our meta-regressions to influence the results: e.g. the higher the olanzapine dose, the higher the difference in the outcome in favor of the comparator antipsychotic. Furthermore, the prior antipsychotic medication taken before the currently tested agent might have also influenced the results on the metabolic outcomes; however, such data were not available and therefore could not be analyzed.
Olanzapine caused the most elevation in cholesterol, clearly more than aripiprazole, risperidone, and ziprasidone. No differences were found in comparison with amisulpride, clozapine and quetiapine. However, quetiapine showed more cholesterol increase than risperidone and was close to that observed with olanzapine. Interestingly, CATIE also found quetiapine had more elevation of cholesterol than risperidone (Lieberman et al., 2005) as well as elevations in triglycerides (Lieberman et al., 2005; Meyer et al., 2008b). This suggests that quetiapine has a greater metabolic effect than the conventional wisdom that it was the same as risperidone (Diabetes Expert Group, 2004). Risperidone showed more cholesterol increase than aripiprazole and ziprasidone. Our results demonstrate that aripiprazole and ziprasidone have the least effects on the lipid status (Greenberg and Citrome, 2007; Casey, 2004).
The major finding in our third outcome – changes in glucose utilisation – is similar to the general pattern of the first two outcomes: olanzapine showed significantly more increase in glucose levels compared with the other SGAs. Again, no difference was found between olanzapine and clozapine. The ‘change in glucose levels’ may be somewhat ‘weaker’ than the two other metabolic outcomes, e.g. because glucose changes will be more affected in the long-term (Diabetes Expert Group, 2004), and because of the fact that patients might not always be fasting when the blood samples are taken. Nevertheless, these findings clearly show that changes in glucose utilisation significantly differ between the SGAs, and that olanzapine is producing more increase in the glucose metabolism than the other SGAs except for clozapine.
We found substantial differences in the metabolic side effects of weight, cholesterol and glucose changes of the available SGAs. At least in terms of standardized effect sizes these differences are generally larger than the differences in efficacy. To illustrate this, the standardized effect sizes of olanzapine for efficacy ranged from .11 to .29 in comparison to the other SGAs (Leucht et al., 2009b), similar to those of the glucose changes of olanzapine (.16 to .26). The effect sizes for cholesterol (.34, .46, 1.15) and weight changes (.39, .42. .47, .52, .72) were clearly larger. For perspective, as a rule of thumb, effect sizes of 0.2 represent a small effect, 0.5 a moderate effect, and ≥0.8 a large effect (Cohen, 1988). Even though, these effect sizes cannot be compared 1:1, as there are qualitative differences between efficacy and side effects (Leucht et al., 2009a), the differences in efficacy and the three metabolic outcomes need to be projected throughout the patients lifetime because even small differences are magnified over the years and impact both, on quality of life (Leucht et al., 2009a) and length of life.
Patients being treated with antipsychotics need to be informed about the differences in efficacy and side effects as different patients have different values, preferences and tolerability for side effects (Hamann et al., 2003): e.g., for some patients extrapyramidal side effects frequently caused by high-potency typical antipsychotics and by the atypical antipsychotic risperidone are extremely critical, for others elevation in prolactin serum levels with associated sexual side effects - often seen during the treatment with e.g. risperidone or amisulpride – are difficult. Sedation, common with e.g. clozapine and low-potency typical antipsychotics, is problematic for other patients. However, differences in metabolic side effects as shown in this meta-analysis are especially important in this context as they have significant long-term consequences, such as cardiovascular disease (CVD). Overweight, especially abdominal obesity and dyslipidemia seem to play an important role as an early marker for the metabolic syndrome (Schorr et al., 2009). Schorr et al. showed that the metabolic syndrome is dynamic, i.e. can be reversed, but chances of reversing are smaller for overweight patients.
In line with our finding that olanzapine showed the most changes on weight, cholesterol and glucose, Daumit et al. found significant differences in the antipsychotic effects on the estimated 10-year coronary heart disease risk in the CATIE study with olanzapine and quetiapine being associated with increased risks, while risks decreased with perphenazine, risperidone and ziprasidone (Daumit et al., 2008). The largest differences between these antipsychotics were seen for older patients and patient with baseline coronary risk factors.
Risk factors for CVD such as obesity, smoking, diabetes, hypertension and dyslipidemia are more common in patients with schizophrenia than in the general population (Fleischhacker et al., 2008), and, in addition, antipsychotic medication can induce weight gain or worsen CVD risk factors (De Hert et al., 2009). As a result, people with schizophrenia have a reduced life expectancy compared to the general population (Fleischhacker et al., 2008). It therefore seems essential to focus on reducing the risk factors, as the beneficial effects of interventions to reduce the risks of CVD have been well studied: if blood cholesterol is reduced by 10%, the risk of coronary heart disease is reduced by 20 - 30% and an ideal body weight (BMI 18.5 -25) is reducing the risk by 35 – 60% (Rich-Edwards et al., 1995); a reduction of 6 mmHg in diastolic blood pressure reduces the risk by 16% (Hennekens, 1998) and an active lifestyle (at least 30 minutes walk daily) by 30 -50% (Bassuk and Manson, 2005).
In order to prevent well studied long-term consequences of the metabolic risk factors such as hypertension, type 2 diabetes, strokes and heart attacks (Anderson et al., 2003; Casey, 2004; Colton and Manderscheid, 2006) a careful individual decision for an antipsychotic, a close monitoring for metabolic side effects of the chosen antipsychotic, switching the antipsychotic therapy if appropriate (Arango et al., 2008) and focusing on how to improve physical activity of the individual patient (Vancampfort et al., 2010) are essential.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Adams CE, Coutinho E, Davis JM, Duggan L, Lee C, Leucht S, Tharyan P. Cochrane Schizophrenia Group in The Cochrane Library Chichester. John Wiley & Sons Ltd; UK: 2006. [Google Scholar]
- Anderson JW, Kendall CW, Jenkins DJ. Importance of weight management in type 2 diabetes: review with meta-analysis of clinical studies. J Am Coll Nutr. 2003;22:331–339. doi: 10.1080/07315724.2003.10719316. [DOI] [PubMed] [Google Scholar]
- Arango C, Bobes J, Aranda P, Carmena R, Garcia-Garcia M, Rejas J. A comparison of schizophrenia outpatients treated with antipsychotics with and without metabolic syndrome: findings from the CLAMORS study. Schizophr Res. 2008;104:1–12. doi: 10.1016/j.schres.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Bai YM, Chen JY, Chen TT, Lin CY, Chou P, Su TP, Lin CC. Weight gain with clozapine: 8-year cohort naturalistic study among hospitalized Chinese schizophrenia patients. Schizophr Res. 2009;108:122–126. doi: 10.1016/j.schres.2008.10.023. [DOI] [PubMed] [Google Scholar]
- Bassuk SS, Manson JE. Epidemiological evidence for the role of physical activity in reducing risk of type 2 diabetes and cardiovascular disease. J Appl Physiol. 2005;99:1193–1204. doi: 10.1152/japplphysiol.00160.2005. [DOI] [PubMed] [Google Scholar]
- Casey DE. Dyslipidemia and atypical antipsychotic drugs. J Clin Psychiatry. 2004;65(Suppl 18):27–35. [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2 edn. Lawrence Erlbaum Associates; Hillsdale, New Jersey: 1988. [Google Scholar]
- Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3:A42. [PMC free article] [PubMed] [Google Scholar]
- Daumit GL, Goff DC, Meyer JM, Davis VG, Nasrallah HA, McEvoy JP, Rosenheck R, Davis SM, Hsiao JK, Stroup TS, Lieberman JA. Antipsychotic effects on estimated 10-year coronary heart disease risk in the CATIE schizophrenia study. Schizophr Res. 2008;105:175–187. doi: 10.1016/j.schres.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Hert M, Dekker JM, Wood D, Kahl KG, Holt RI, Moller HJ. Cardiovascular disease and diabetes in people with severe mental illness position statement from the European Psychiatric Association (EPA), supported by the European Association for the Study of Diabetes (EASD) and the European Society of Cardiology (ESC) Eur Psychiatry. 2009;24:412–424. doi: 10.1016/j.eurpsy.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Der-Simonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Diabetes Expert Group Consensus development conference on antipsychotic drugs and obesity and diabetes. J Clin Psychiatry. 2004;65:267–272. doi: 10.4088/jcp.v65n0219. [DOI] [PubMed] [Google Scholar]
- Fleischhacker WW, Cetkovich-Bakmas M, De HM, Hennekens CH, Lambert M, Leucht S, Maj M, McIntyre RS, Naber D, Newcomer JW, Olfson M, Osby U, Sartorius N, Lieberman JA. Comorbid somatic illnesses in patients with severe mental disorders: clinical, policy, and research challenges. J Clin Psychiatry. 2008;69:514–519. doi: 10.4088/jcp.v69n0401. [DOI] [PubMed] [Google Scholar]
- Greenberg WM, Citrome L. Ziprasidone for schizophrenia and bipolar disorder: a review of the clinical trials. CNS Drug Rev. 2007;13:137–177. doi: 10.1111/j.1527-3458.2007.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann J, Leucht S, Kissling W. Shared decision making in psychiatry. Acta Psychiatrica Scandinavica. 2003;107:403–409. doi: 10.1034/j.1600-0447.2003.00130.x. [DOI] [PubMed] [Google Scholar]
- Henderson DC, Cagliero E, Gray C, Nasrallah RA, Hayden DL, Schoenfeld DA, Goff DC. Clozapine, diabetes mellitus, weight gain, and lipid abnormalities: A five-year naturalistic study. Am J Psychiatry. 2000;157:975–981. doi: 10.1176/appi.ajp.157.6.975. [DOI] [PubMed] [Google Scholar]
- Hennekens CH. Increasing burden of cardiovascular disease: current knowledge and future directions for research on risk factors. Circulation. 1998;97:1095–1102. doi: 10.1161/01.cir.97.11.1095. [DOI] [PubMed] [Google Scholar]
- Herold G. Innere Medizin Herold. Köln, Germany: 2009. [Google Scholar]
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5 Chichester. 2005. [Google Scholar]
- Kinon BJ, Kaiser CJ, Ahmed S, Rotelli MD, Kollack-Walker S. Association between early and rapid weight gain and change in weight over one year of olanzapine therapy in patients with schizophrenia and related disorders. J Clin Psychopharmacol. 2005;25:255–258. doi: 10.1097/01.jcp.0000161501.65890.22. [DOI] [PubMed] [Google Scholar]
- Komossa K, Hunger H, Schmidt F, Schwarz S, Leucht S, Rummel-Kluge C. Risperidone versus other atypical antipsychotics for schizophrenia (protocol) The Cochrane Library. 2007 doi: 10.1002/14651858.CD006626.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komossa K, Rummel-Kluge C, Hunger H, Schmid F, Schwarz S, Duggan L, Kissling W, Leucht S. Olanzapine versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2010a;3:CD006654. doi: 10.1002/14651858.CD006654.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komossa K, Rummel-Kluge C, Hunger H, Schmid F, Schwarz S, Kissling W, Leucht S. Zotepine versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2010b:CD006628. doi: 10.1002/14651858.CD006628.pub2. [DOI] [PubMed] [Google Scholar]
- Komossa K, Rummel-Kluge C, Hunger H, Schmid F, Schwarz S, Silveira da Mota Neto JI, Kissling W, Leucht S. Amisulpride versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2010c:CD006624. doi: 10.1002/14651858.CD006624.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komossa K, Rummel-Kluge C, Hunger H, Schwarz S, Bhoopathi PS, Kissling W, Leucht S. Ziprasidone versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2009a:CD006627. doi: 10.1002/14651858.CD006627.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komossa K, Rummel-Kluge C, Hunger H, Schwarz S, Schmidt F, Lewis R, Kissling W, Leucht S. Sertindole versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2009b:CD006752. doi: 10.1002/14651858.CD006752.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komossa K, Rummel-Kluge C, Schmid F, Hunger H, Schwarz S, El-Sayeh HG, Kissling W, Leucht S. Aripiprazole versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2009c:CD006569. doi: 10.1002/14651858.CD006569.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komossa K, Rummel-Kluge C, Schmid F, Hunger H, Schwarz S, Srisurapanont M, Kissling W, Leucht S. Quetiapine versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2010d:CD006625. doi: 10.1002/14651858.CD006625.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leucht S, Kissling W, Davis JM. Second generation antipsychotics for schizophrenia: can we resolve the conflict? Psychol Med. 2009a doi: 10.1017/S0033291709005455. in press. [DOI] [PubMed] [Google Scholar]
- Leucht S, Komossa K, Rummel-Kluge C, Corves C, Hunger H, Schmid F, Asenjo LC, Schwarz S, Davis JM. A meta-analysis of head-to-head comparisons of second-generation antipsychotics in the treatment of schizophrenia. Am J Psychiatry. 2009b;166:152–163. doi: 10.1176/appi.ajp.2008.08030368. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RS, Davis SM, Davis CE, Lebowitz BD, Severe J, Hsiao JK. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- Meyer JM. A retrospective comparison of weight, lipid, and glucose changes between risperidone- and olanzapine-treated inpatients: metabolic outcomes after 1 year. J Clin Psychiatry. 2002;63:425–433. doi: 10.4088/jcp.v63n0509. [DOI] [PubMed] [Google Scholar]
- Meyer JM, Davis VG, Goff DC, McEvoy JP, Nasrallah HA, Davis SM, Rosenheck RA, Daumit GL, Hsiao J, Swartz MS, Stroup TS, Lieberman JA. Change in metabolic syndrome parameters with antipsychotic treatment in the CATIE Schizophrenia Trial: prospective data from phase 1. Schizophr Res. 2008a;101:273–286. doi: 10.1016/j.schres.2007.12.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JM, Davis VG, McEvoy JP, Goff DC, Nasrallah HA, Davis SM, Daumit GL, Hsiao J, Swartz MS, Stroup TS, Lieberman JA. Impact of antipsychotic treatment on nonfasting triglycerides in the CATIE Schizophrenia Trial phase 1. Schizophr Res. 2008b;103:104–109. doi: 10.1016/j.schres.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet. 1999;354:1896–1900. doi: 10.1016/s0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- Rich-Edwards JW, Manson JE, Hennekens CH, Buring JE. The primary prevention of coronary heart disease in women. N Engl J Med. 1995;332:1758–1766. doi: 10.1056/NEJM199506293322607. [DOI] [PubMed] [Google Scholar]
- Schorr SG, Slooff CJ, Bruggeman R, Taxis K. The incidence of metabolic syndrome and its reversal in a cohort of schizophrenic patients followed for one year. J Psychiatr Res. 2009;43:1106–1111. doi: 10.1016/j.jpsychires.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Vancampfort D, Knapen J, Probst M, van WR, Deckx S, Maurissen K, Peuskens J, De HM. Considering a frame of reference for physical activity research related to the cardiometabolic risk profile in schizophrenia. Psychiatry Res. 2010;177:271–279. doi: 10.1016/j.psychres.2010.03.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.