Abstract
Many studies have suggested that moderate alcohol consumption reduces mortality. There is also substantial evidence that lifespan is extended with suppression of TOR (target of rapamycin). It was reported recently that rapamycin is able to extend the lifespan of a mammal—implicating the mammalian TOR (mTOR). mTOR has a requirement for the lipid second messenger phosphatidic acid (PA), which is generated by phospholipase D (PLD). Therefore, in principle, suppression of PLD would be similar to treatment with rapamycin. Significantly, PLD utilizes ethanol preferentially over water in the hydrolysis reaction that ordinarily generates PA. In the presence of ethanol, phosphatidyl-ethanol is generated at the expense of PA leading to the suppression of mTOR. This reaction, known as the transphosphatidylation reaction, provides a mechanistic basis for the reduced mortality observed with moderate consumption of alcohol—that being the suppression of mTOR.
Keywords: mTOR, phospholipase D, lifespan, alcohol
Introduction
It has been widely accepted that there is a correlation between moderate consumption of alcohol and reduced mortality.1–3 While this correlation has been observed in many studies, there is very little mechanistic insight as to how alcohol might reduce mortality. There are many studies revealing that suppression of signaling by TOR (target of rapamycin) extends lifespan in yeast, C. elegans and Drosophila (reviewed in ref. 4), and a recent study has revealed that suppression of the mammalian target of rapamycin (mTOR) extends the lifespan of mice.5 This latter finding was the first report indicating that suppression of mTOR could extend lifespan in a mammal. This study is consistent with the emerging theme that mTOR, which is a key regulator of nutrient and energy sensing6 may play a central role in longevity and mortality. It has been suggested that mTOR may be a common denominator for determining lifespan and aging.4 The common effects of rapamycin and moderate alcohol consumption on mortality raise the question as to whether alcohol, like rapamycin, impacts on mTOR. This review highlights several studies during the past year that link moderate alcohol consumption with suppression of mTOR, and as a consequence, with reduced mortality.
Phospholipase D—The Link between Alcohol and mTOR
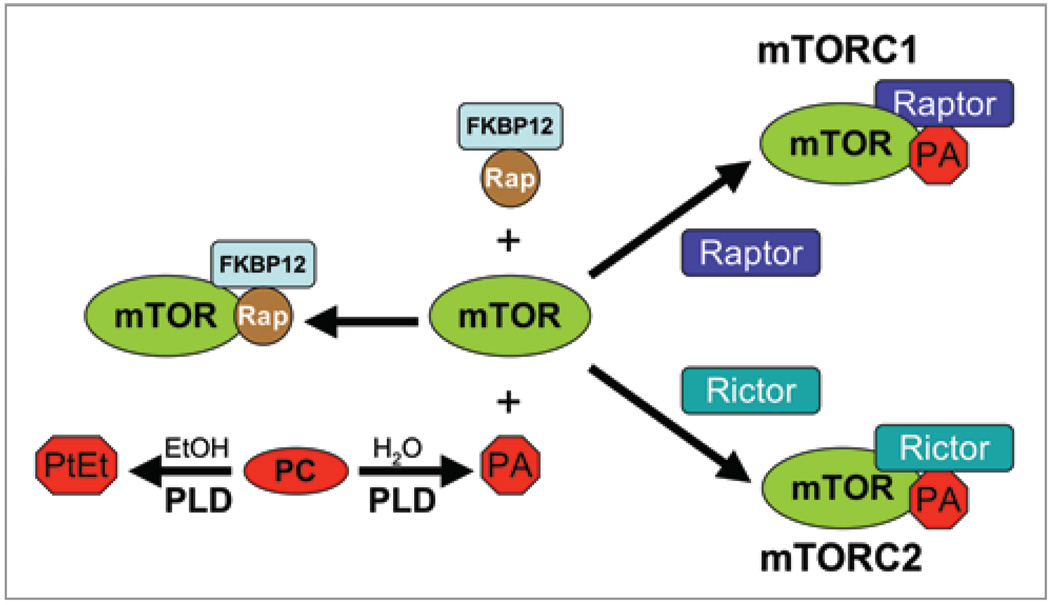
Rapamycin is a highly specific natural product inhibitor of mTOR.7 Rapamycin interacts with mTOR in a manner that is competitive with phosphatidic acid (PA).8–10 PA was recently shown to be required for the stability of the mTOR complexes—mTORC1 and mTORC2.10 In the absence of PA, mTOR does not form a complex with the companion proteins Raptor and Rictor that are part of mTORC1 and mTORC2 respectively. The PA required for mTOR complex formation and activity is generated by the phospholipase D (PLD)-catalyzed hydrolysis of phosphatidylcholine.11 The competition between PA and rapamycin for mTOR is shown schematically in Figure 1. There is an interesting property for the hydrolysis reaction catalyzed by PLD in that primary alcohols—including ethanol—are better substrates for PLD than water.12 In the presence of ethanol, PLD generates phosphatidyl-ethanol at the expense of PA (see Fig. 2A and B). It is believed that alcohols are better substrates than water because the aliphatic component of the alcohol can partition into the lipid bilayer where the OH group is better positioned to make a nucleophilic attack on the phosphate head group of phosphatidylcholine. Thus, in the presence of ethanol, you generate phosphatidylethanol instead of the PA needed for the formation of functional mTOR complexes. As with rapamycin, much higher concentrations of alcohol are needed to suppress mTORC2 than mTORC1,10 which implies that any effect of moderate alcohol consumption would impact mTORC1 preferentially. Consistent with an impact of alcohol on mTORC1, chronic alcohol treatment of rats resulted in a reduction in the phosphorylation of the mTORC1 substrate S6 kinase.13,14 Similarly, alcohol suppressed mTORC1 in mouse myocytes.15 Thus, the outcome from ethanol treatment is very similar to that of rapamycin treatment—mTORC1 is suppressed.
Figure 1.
The phospholipase D—mTOR connection. The interaction between PLDgenerated PA and mTOR allows the assembly of the mTOR complexes mTORC1 and mTORC2 by facilitating the association with the companion proteins Raptor and Rictor respectively. The model is an oversimplification in that there are several other components, but Raptor and Rictor have been the most widely used to distinguish mTORC1 from mTORC2. Rapamycin interacts with immunofilin FK506 binding protein 12 (FKBP12), which then interacts with mTOR competitively with PA to prevent the formation of the mTOR complexes. Phosphatidylcholine (PC) is hydrolyzed to PA by PLD, but in the presence of ethanol (ET OH), PLD catalyzes a transphosphatidylation reaction whereby phosphatidylcholine is converted to phosphatidyl-ethanol (PtEt) at the expense of PA production. mTORC2 is far more stable than mTORC1 and therefore mTORC1 is much more sensitive to rapamycin and PA levels.10
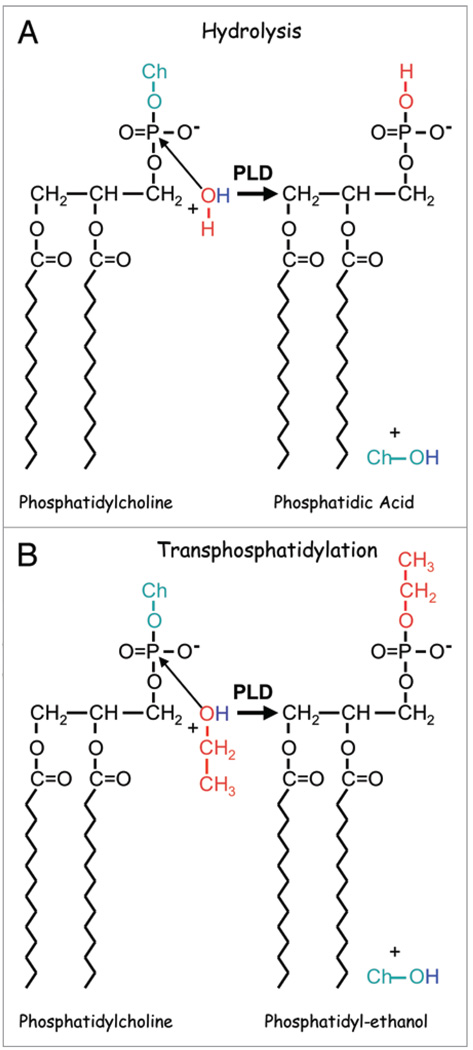
Figure 2.
Phospholipase D catalyzed reactions. (A) The hydrolysis of phosphatidylcholine catalyzed by PLD is shown whereby the oxygen of H2O makes a nucleophilic attack on the phosphate. The hydrolysis reaction results in the production of PA and free choline (Ch-OH). (B) The transphosphatidylation reaction occurs in the presence of a primary alcohol such as ethanol or 1-butanol (ethanol is shown in the figure). It is believed that the alcohols are better substrates than water because the aliphatic tails partition the alcohols into the membrane where the oxygen on the OH group is in a better position than water to make a nucleophilic attack on the phosphate to liberate the choline and generate phosphatidyl-ethanol instead of PA.
Can Alcohol Suppress Cancer?
One of the consequences of ageing is an increased likelihood of cancer, which increases mortality and reduces lifespan. In addition to being a critical node for nutrition and energy sensing, mTOR has been widely implicated in signals that promote the survival of cancer cells.16 PLD has likewise been implicated cancer cell survival signals.17 Cancer is a disease of old age and therefore suppressing cancer cell survival signals with rapamycin could contribute to the reported increase in longevity in the mouse study where rapamycin extended lifespan.5 Similarly ethanol, by suppressing PA production could reduce some cancers and therefore suppress mortality. Recently Gartenhaus and colleagues performed a study that was based on the observation that lymphoma incidence is reduced amongst moderate drinkers. Lymphoma cells treated with chronic exposure to 0.1% ethanol blocked the association between mTOR and the companion protein Raptor, which is part of mTORC1.18 This effect was similar to that achieved with butanol where the formation of mTORC1 was blocked with a shorter-term treatment of 0.2% butanol.10 The Gartenhaus group extended their study to an animal model, and importantly, chronic exposure to modest levels of ethanol in the drinking water suppressed tumor formation by the lymphoma cells in a mouse xenograft tumor model.18 10% ethanol in the drinking water (less than alcohol content of wine) completely suppressed xenograft tumor growth. This study provided evidence that moderate levels of ethanol can suppress mTORC1 and block the growth of a tumor in an animal model. While it is intriguing to consider the cancer preventing potential of moderate alcohol consumption, it is important to note that excessive alcohol consumption is causative for some cancers—especially those of the liver and oral cavity.
Alcohol and Cardiovascular Disease
The beneficial effects of moderate alcohol consumption on cardiovascular disease has been widely reported (reviewed in refs. 19 and 20). Atherosclerosis is the result of increased proliferation and hypertrophy of arterial smooth muscle cells accompanied by the accumulation of lipids.21 Rapamycin was demonstrated to reduce established cardiac hypertrophy22 and to prevent hypertrophy in a mouse model for atherosclerosis.23,24 The cytostatic effect of rapamycin on smooth muscle cells has been exploited in the therapeutic application of rapamycin to drug-eluting stents for angioplasty. These stents significantly reduce the amount of arterial reblockage that results from proliferating vascular smooth muscle cells.25,26 Thus, inhibition of mTOR has profound effects on factors that impact on atherosclerosis, and it follows that alcohol, which suppresses the production of PA which is needed for mTOR activity should also suppress atherosclerosis. Epidemiological and experimental studies have revealed that a mild-to-moderate drinking of wine, particularly red wine, attenuates the cardiovascular, cerebrovascular and peripheral vascular risk.19,20 The similar effects of rapamycin and alcohol on the cardiovascular system indicate that the positive effect of alcohol on cardiovascular disease is due to the suppression of mTOR.
It has been widely suggested that the positive effects of alcohol consumption on cardiovascular disease are the result of an increase in the levels of high-density lipoproteins (HDLs) that is observed with moderate alcohol consumption.27 The mechanism for the elevated HDL levels seen with moderate drinking is not known, but it is of interest that the HDL in moderate and heavy drinkers contains substantial levels of phosphatidyl-ethanol—the product of the PLD-catalyzed transphosphatidylation reaction.
The presence of the phosphatidyl-ethanol in HDL might stabilize HDL such that it increases the level of HDL in circulation. It is not clear whether there is any dependence on mTOR for the elevated HDL levels, but the presence of phosphatidylethanol in the HDL of moderate drinkers clearly implicates PLD in this alteration to HDL. It remains to be established whether there is a role for mTOR and whether phosphatidyl-ethanol contributes to the elevated HDL seen with moderate alcohol consumption, but it has been reported that rapamycin treatment led to elevated levels of HDLs in a mouse model23 and in heart transplant patients who were on rapamycin to suppress immune rejection.28 Thus, suppression of mTOR in response to alcohol likely also contributes to elevated HDL with moderate alcohol consumption.
Is the Effect of Alcohol on Mortality Related to the Effects of Calorie Restriction?
Lifespan is extended by caloric restriction in many species29 including primates.30 mTOR is a central node for nutrient and energy sensing31 and therefore the lack of sufficient nutrients results in reduced mTOR activity. It has been proposed that the reduced mTOR activity is the common denominator for the observed increases in longevity.4,32 The recent study reporting prolonged lifespan in mice with rapamycin treatment5 supports the hypothesis that suppression of mTOR may be the key to increased longevity in mammals.
Critical mediators of the longevity observed with caloric restriction are sirtuins—a family of protein deacetylases that have wide ranging effects that influence ribosomal DNA recombination, gene silencing, DNA repair and chromosomal stability.33,34 It is therefore of significance that TOR has been linked to the sirtuin-mediated lifespan extension in Saccharomyces cerevisiae subjected to calorie restriction.35,36 Thus, caloric restriction and suppression may be doing essentially the same thing—that being the suppression of mTOR. Of interest is a report showing that sirtuins are upregulated in response to chronic ethanol treatment in mice.37 Therefore, alcohol, by suppressing mTOR and upregulating sirtuins may be acting in a manner similar to calorie restriction is reducing mortality.
How Does Resveratrol Enhance the Effect of Alcohol?
In addition to caloric restriction, rapamycin and moderate alcohol consumption, resveratrol, a polyphenolic compound found in red wine, has also been shown to extend lifespan.33 Significantly, resveratrol was recently reported to suppress both PLD activity38 and the mTOR/S6 kinase pathway. 39,40 Resveratrol, like caloric restriction, leads to the stimulation of the sirtuins that are critical for extended lifespan.41 There has been much discussion of the role that resveratrol in red wine might play in extending lifespan. The ability of resveratrol to suppress PLD activity could mean that it has an additive effect on top of the ethanol in wine and simply suppresses the production of PA by PLD more efficiently than ethanol by itself. The amount of ethanol needed to suppress mTOR over a short time frame in the laboratory is much higher than can be tolerated by humans—in the range of 1%. However, chronic exposure of lymphoma cells to 0.1% ethanol (slightly above the legal limit for operating a motor vehicle) for 10 days led to the inhibition of mTORC1.18 And moderate levels of alcohol in the drinking water of mice suppressed tumor growth.18 It has been widely noted that there are significant levels of phosphatidylethanol in the membranes of moderate drinkers. Phosphatidyl-ethanol has a half-life of about 4 days.27 Thus, continued exposure to moderate levels of ethanol may be able to substantially reduce PA production and suppress mTOR. The additional presence of resveratrol in red wine, which also suppresses PLD activity,38 may do the job even better.
Summary
To summarize, rapamycin, which targets mTORC1 with high specificity, suppresses mammalian cellular senescence42 and increases the lifespan of mice.5 Rapamycin interferes with the association of mTOR with PA, which is required for mTOR complex formation.10 The PA required for mTOR complex assembly is generated by the PLD mediated hydrolysis of phosphatidylcholine. 10 The ability of PLD to utilize ethanol preferentially over water as a substrate and generate phosphatidyl-ethanol at the expense of PA production12 offers mechanistic insight into the reduced mortality observed with moderate drinkers—that being the suppression of mTORC1. Therefore, by connecting the dots, there is compelling logic that much of the reduced mortality seen with moderate levels of alcohol consumption is due to reduced PA levels and the resulting suppression of mTOR.
Acknowledgements
The author is supported by National Cancer Institute grant CA046677.
References
- 1.de Groot LC, Zock PL. Moderate alcohol intake and mortality. Nutr Rev. 1998;56:25–26. doi: 10.1111/j.1753-4887.1998.tb01655.x. [DOI] [PubMed] [Google Scholar]
- 2.Yuan JM, Ross RK, Gao YT, Henderson BE, Yu MC. Follow up study of moderate alcohol intake and mortality among middle aged men in Shanghai, China. BMJ. 1997;314:18–23. doi: 10.1136/bmj.314.7073.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to alcohol consumption: a prospective study among male British doctors. Int J Epidemiol. 2005;34:199–204. doi: 10.1093/ije/dyh369. [DOI] [PubMed] [Google Scholar]
- 4.Blagosklonny MV. An anti-aging drug today: from senescence-promoting genes to antiaging pill. Drug Discov Today. 2007;12:218–224. doi: 10.1016/j.drudis.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polak P, Hall MN. mTOR and the control of whole body metabolism. Curr Opin Cell Biol. 2009;21:209–218. doi: 10.1016/j.ceb.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 7.Huang S, Bjornsti MA, Houghton PJ. Rapamycins: mechanism of action and cellular resistance. Cancer Biol Ther. 2003;2:222–232. doi: 10.4161/cbt.2.3.360. [DOI] [PubMed] [Google Scholar]
- 8.Fang Y, Vilella-Bach M, Bachmann R, Flanigan A, Chen J. Phosphatidic acid-mediated mitogenic activation of mTOR signaling. Science. 2001;294:1942–1945. doi: 10.1126/science.1066015. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Zheng Y, Foster DA. Phospholipase D confers rapamycin resistance in human breast cancer cells. Oncogene. 2003;22:3937–3942. doi: 10.1038/sj.onc.1206565. [DOI] [PubMed] [Google Scholar]
- 10.Toschi A, Lee E, Xu L, Garcia A, Gadir N, Foster DA. Regulation of mTORC1 and mTORC2 complex assembly by phosphatidic acid: competition with rapamycin. Mol Cell Biol. 2009;29:1411–1420. doi: 10.1128/MCB.00782-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foster DA, Toschi A. Targeting mTOR with rapamycin: one dose does not fit all. Cell Cycle. 2009;8:1026–1029. doi: 10.4161/cc.8.7.8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown HA, Henage LG, Preininger AM, Xiang Y, Exton JH. Biochemical analysis of phospholipase D. Methods Enzymol. 2007;434:49–87. doi: 10.1016/S0076-6879(07)34004-4. [DOI] [PubMed] [Google Scholar]
- 13.Vary TC, Deiter G, Goodman SA. Acute alcohol intoxication enhances myocardial eIF4G phosphorylation despite reducing mTOR signaling. Am J Physiol Heart Circ Physiol. 2005;288:121–128. doi: 10.1152/ajpheart.00440.2004. [DOI] [PubMed] [Google Scholar]
- 14.Vary TC, Deiter G, Lantry R. Chronic alcohol feeding impairs mTOR(Ser 2448) phosphorylation in rat hearts. Alcohol Clin Exp Res. 2008;32:43–51. doi: 10.1111/j.1530-0277.2007.00544.x. [DOI] [PubMed] [Google Scholar]
- 15.Hong-Brown LQ, Brown CR, Huber DS, Lang CH. Alcohol and indinavir adversely affect protein synthesis and phosphorylation of MAPK and mTOR signaling pathways in C2C12 myocytes. Alcohol Clin Exp Res. 2006;30:1297–1307. doi: 10.1111/j.1530-0277.2006.00157.x. [DOI] [PubMed] [Google Scholar]
- 16.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Foster DA. Phosphatidic acid signaling to mTOR: Signals for the survival of human cancer cells. Biochim Biophys Acta. 2009;1791:949–955. doi: 10.1016/j.bbalip.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hagner PR, Mazan-Mamczarz K, Dai B, Corl S, Zhao XF, Gartenhaus RB. Alcohol consumption and decreased risk of non-Hodgkin lymphoma: role of mTOR dysfunction. Blood. 2009;113:5526–5535. doi: 10.1182/blood-2008-11-191783. [DOI] [PubMed] [Google Scholar]
- 19.Imhof A, Koenig W. Alcohol inflammation and coronary heart disease. Addict Biol. 2003;8:271–277. doi: 10.1080/13556210310001602176. [DOI] [PubMed] [Google Scholar]
- 20.Li JM, Mukamal KJ. An update on alcohol and atherosclerosis. Curr Opin Lipidol. 2004;15:673–680. doi: 10.1097/00041433-200412000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Orlandi A, Bochaton-Piallat ML, Gabbiani G, Spagnoli LG. Aging, smooth muscle cells and vascular pathobiology: implications for atherosclerosis. Atherosclerosis. 2006;188:221–230. doi: 10.1016/j.atherosclerosis.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 22.McMullen JR, Sherwood MC, Tarnavski O, Zhang L, Dorfman AL, Shioi T, et al. Inhibition of mTOR signaling with rapamycin regresses established cardiac hypertrophy induced by pressure overload. Circulation. 2004;109:3050–3055. doi: 10.1161/01.CIR.0000130641.08705.45. [DOI] [PubMed] [Google Scholar]
- 23.Elloso MM, Azrolan N, Sehgal SN, Hsu PL, Phiel KL, Kopec CA, et al. Protective effect of the immunosuppressant sirolimus against aortic atherosclerosis in apo E-deficient mice. Am J Transplant. 2003;3:562–569. doi: 10.1034/j.1600-6143.2003.00094.x. [DOI] [PubMed] [Google Scholar]
- 24.Pakala R, Stabile E, Jang GJ, Clavijo L, Waksman R. Rapamycin attenuates atherosclerotic plaque progression in apolipoprotein E knockout mice: inhibitory effect on monocyte chemotaxis. J Cardiovasc Pharmacol. 2005;46:481–486. doi: 10.1097/01.fjc.0000177985.14305.15. [DOI] [PubMed] [Google Scholar]
- 25.Easton JB, Houghton PJ. Therapeutic potential of target of rapamycin inhibitors. Expert Opin Ther Targets. 2004;8:551–564. doi: 10.1517/14728222.8.6.551. [DOI] [PubMed] [Google Scholar]
- 26.Butt M, Connolly D, Lip GY. Drug-eluting stents: a comprehensive appraisal. Future Cardiol. 2009;5:141–157. doi: 10.2217/14796678.5.2.141. [DOI] [PubMed] [Google Scholar]
- 27.Hannuksela ML, Rämet ME, Nissinen AE, Liisanantti MK, Savolainen MJ. Effects of ethanol on lipids and atherosclerosis. Pathophysiology. 2004;10:93–103. doi: 10.1016/j.pathophys.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 28.Tenderich G, Fuchs U, Zittermann A, Muckelbauer R, Berthold HK, Koerfer R. Comparison of sirolimus and everolimus in their effects on blood lipid profiles and haematological parameters in heart transplant recipients. Clin Transplant. 2007;21:536–543. doi: 10.1111/j.1399-0012.2007.00686.x. [DOI] [PubMed] [Google Scholar]
- 29.Guarente L. Calorie restriction and SIR2 genes-towards a mechanism. Mech Ageing Dev. 2005;126:923–928. doi: 10.1016/j.mad.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 30.Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin DE, Hall MN. The expanding TOR signaling network. Curr Opin Cell Biol. 2005;17:158–166. doi: 10.1016/j.ceb.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 32.Blagosklonny MV. Aging-suppressants: cellular senescence (hyperactivation) and its pharmacologic deceleration. Cell Cycle. 2009;8:1883–1887. doi: 10.4161/cc.8.12.8815. [DOI] [PubMed] [Google Scholar]
- 33.Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J. 2007;404:1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finkel T, Deng CX, Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009;460:587–591. doi: 10.1038/nature08197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Powers RW, 3rd, Kaeberlein M, Caldwell SD, Kennedy BK, Fields S. Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes Dev. 2006;20:174–184. doi: 10.1101/gad.1381406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Medvedik O, Lamming DW, Kim KD, Sinclair DA. MSN2 and MSN4 link calorie restriction and TOR to sirtuin-mediated lifespan extension in Saccharomyces cerevisiae. PLoS Biol. 2007;5:261. doi: 10.1371/journal.pbio.0050261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oliva J, French BA, Li J, Bardag-Gorce F, Fu P, French SW. Sirt1 is involved in energy metabolism: the role of chronic ethanol feeding and resveratrol. Exp Mol Pathol. 2008;85:155–159. doi: 10.1016/j.yexmp.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Issuree PD, Pushparaj PN, Pervaiz S, Melendez AJ. Resveratrol attenuates C5a induced inflammatory responses in vitro and in vivo by inhibiting phospholipase D and sphingosine kinase activities. FASEB J. 2009;23:2412–2424. doi: 10.1096/fj.09-130542. [DOI] [PubMed] [Google Scholar]
- 39.Demidenko ZN, Blagosklonny MV. At concentrations that inhibit mTOR, resveratrol suppresses cellular senescence. Cell Cycle. 2009;8:1901–1904. doi: 10.4161/cc.8.12.8810. [DOI] [PubMed] [Google Scholar]
- 40.Armour SM, Baur JA, Hsieh SN, Land-Bracha A, Thomas SM, Sinclair DA. Inhibition of mammalian S6 kinase by resveratrol suppresses autophagy. Aging. 2009;1:515–528. doi: 10.18632/aging.100056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 42.Demidenko ZN, Zubova SG, Bukreeva EI, Pospelov VA, Pospelova TV, Blagosklonny MV. Rapamycin decelerates cellular senescence. Cell Cycle. 2009;8:1888–1895. doi: 10.4161/cc.8.12.8606. [DOI] [PubMed] [Google Scholar]