Abstract
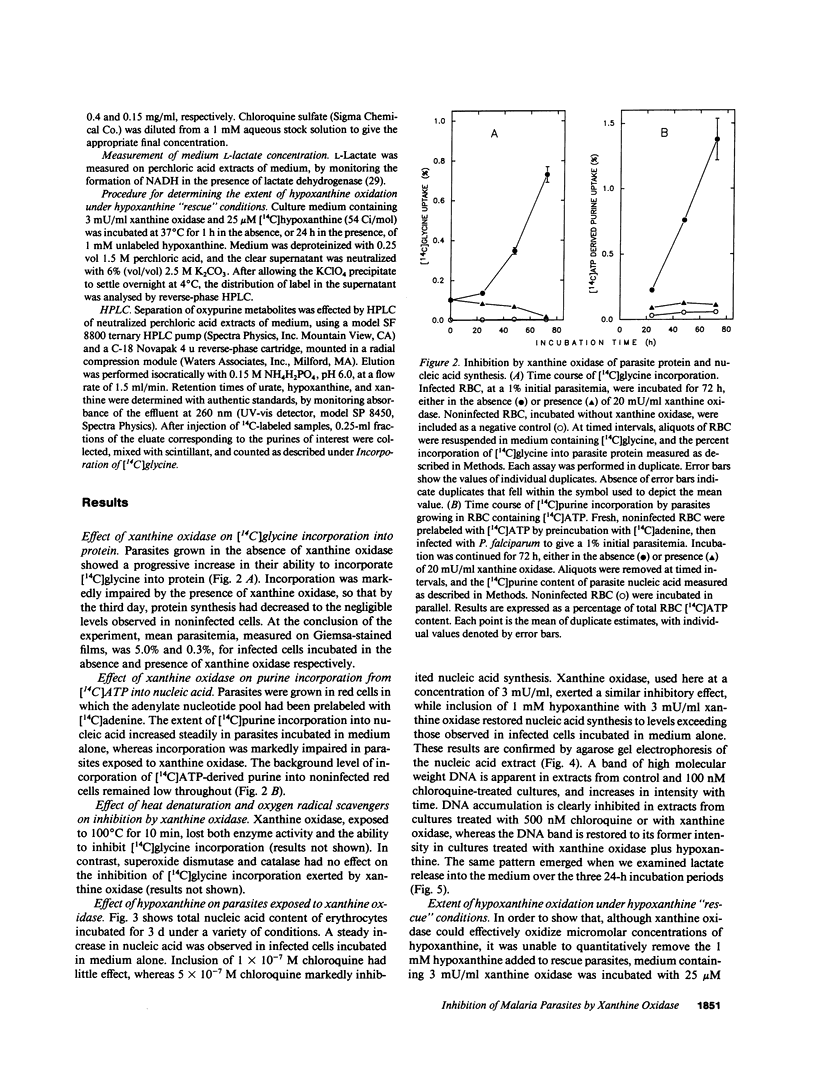
Malaria parasites, unable to synthesize purine de novo, use host-derived hypoxanthine preferentially as purine source. In a previous study (1990. J. Biol. Chem. 265:6562-6568), we noted that xanthine oxidase rapidly and completely depleted hypoxanthine in human erythrocytes, not by crossing the erythrocyte membrane, but rather by creating a concentration gradient which facilitated hypoxanthine efflux. We therefore investigated the ability of xanthine oxidase to inhibit growth of FCR-3, a chloroquine-resistant strain of Plasmodium falciparum in human erythrocytes in vitro. Parasites were cultured in human group O+ erythrocytes in medium supplemented, as required, with xanthine oxidase or chloroquine. Parasite viability was assessed by uptake of radiolabeled glycine and adenosine triphosphate-derived purine into protein and nucleic acid, respectively, by nucleic acid accumulation, by L-lactate production, and by microscopic appearance. On average, a 90% inhibition of growth was observed after 72 h of incubation in 20 mU/ml xanthine oxidase. Inhibition was notably greater than that exerted by 10(-7) M chloroquine (less than 10%) over a comparable period. The IC50 for xanthine oxidase was estimated at 0.2 mU/ml, compared to 1.5 x 10(-7) M for chloroquine. Inhibition was completely reversed by excess hypoxanthine, but was unaffected by oxygen radical scavengers, including superoxide dismutase and catalase. The data confirms that a supply of host-derived hypoxanthine is critical for nucleic acid synthesis in P. falciparum, and that depletion of erythrocyte hypoxanthine pools of chloroquine-resistant malaria infection in humans. of chloroquine-resistant malaria infection in humans.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abuchowski A., McCoy J. R., Palczuk N. C., van Es T., Davis F. F. Effect of covalent attachment of polyethylene glycol on immunogenicity and circulating life of bovine liver catalase. J Biol Chem. 1977 Jun 10;252(11):3582–3586. [PubMed] [Google Scholar]
- Adriaenssens K., Karcher D., Lowenthal A., Terheggen H. G. Use of enzyme-loaded erythrocytes in in-vitro correction of arginase-deficient erythrocytes in familial hyperargininemia. Clin Chem. 1976 Mar;22(3):323–326. [PubMed] [Google Scholar]
- Al-Khalidi U. A., Chaglassian T. H. The species distribution of xanthine oxidase. Biochem J. 1965 Oct;97(1):318–320. doi: 10.1042/bj0970318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaraf Y. G., Golenser J., Spira D. T., Bachrach U. Plasmodium falciparum: synchronization of cultures with DL-alpha-difluoromethylornithine, an inhibitor of polyamine biosynthesis. Exp Parasitol. 1986 Apr;61(2):229–235. doi: 10.1016/0014-4894(86)90156-6. [DOI] [PubMed] [Google Scholar]
- Berman P. A., Black D. A., Human L., Harley E. H. Oxypurine cycle in human erythrocytes regulated by pH, inorganic phosphate, and oxygen. J Clin Invest. 1988 Sep;82(3):980–986. doi: 10.1172/JCI113707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman P. A., Human L. Regulation of 5-phosphoribosyl 1-pyrophosphate and of hypoxanthine uptake and release in human erythrocytes by oxypurine cycling. J Biol Chem. 1990 Apr 25;265(12):6562–6568. [PubMed] [Google Scholar]
- Bitonti A. J., Dumont J. A., Bush T. L., Edwards M. L., Stemerick D. M., McCann P. P., Sjoerdsma A. Bis(benzyl)polyamine analogs inhibit the growth of chloroquine-resistant human malaria parasites (Plasmodium falciparum) in vitro and in combination with alpha-difluoromethylornithine cure murine malaria. Proc Natl Acad Sci U S A. 1989 Jan;86(2):651–655. doi: 10.1073/pnas.86.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontemps F., Van den Berghe G., Hers H. G. Pathways of adenine nucleotide catabolism in erythrocytes. J Clin Invest. 1986 Mar;77(3):824–830. doi: 10.1172/JCI112379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büngener W. Einfluss von Allopurinol auf Zyklusdauer und Vermehrungsrate von Plasmodium vinckei in der Ratte. Tropenmed Parasitol. 1974 Dec;25(4):464–468. [PubMed] [Google Scholar]
- Chen R. H., Abuchowski A., Van Es T., Palczuk N. C., Davis F. F. Properties of two urate oxidases modified by the covalent attachment of poly(ethylene glycol). Biochim Biophys Acta. 1981 Aug 13;660(2):293–298. doi: 10.1016/0005-2744(81)90173-x. [DOI] [PubMed] [Google Scholar]
- Chulay J. D., Haynes J. D., Diggs C. L. Plasmodium falciparum: assessment of in vitro growth by [3H]hypoxanthine incorporation. Exp Parasitol. 1983 Feb;55(1):138–146. doi: 10.1016/0014-4894(83)90007-3. [DOI] [PubMed] [Google Scholar]
- Cox F. E. Malaria. Variation and vaccination. Nature. 1991 Jan 17;349(6306):193–193. doi: 10.1038/349193a0. [DOI] [PubMed] [Google Scholar]
- Daddona P. E., Wiesmann W. P., Lambros C., Kelley W. N., Webster H. K. Human malaria parasite adenosine deaminase. Characterization in host enzyme-deficient erythrocyte culture. J Biol Chem. 1984 Feb 10;259(3):1472–1475. [PubMed] [Google Scholar]
- Daddona P. E., Wiesmann W. P., Milhouse W., Chern J. W., Townsend L. B., Hershfield M. S., Webster H. K. Expression of human malaria parasite purine nucleoside phosphorylase in host enzyme-deficient erythrocyte culture. Enzyme characterization and identification of novel inhibitors. J Biol Chem. 1986 Sep 5;261(25):11667–11673. [PubMed] [Google Scholar]
- Divo A. A., Jensen J. B. Studies on serum requirements for the cultivation of Plasmodium falciparum. 2. Medium enrichment. Bull World Health Organ. 1982;60(4):571–575. [PMC free article] [PubMed] [Google Scholar]
- Freese J. A., Sharp B. L., Ridl F. C., Markus M. B. In vitro cultivation of southern African strains of Plasmodium falciparum and gametocytogenesis. S Afr Med J. 1988 Jun 18;73(12):720–722. [PubMed] [Google Scholar]
- Henderson J. F., Brox L. W., Kelley W. N., Rosenbloom F. M., Seegmiller J. E. Kinetic studies of hypoxanthine-guanine phosphoribosyltransferase. J Biol Chem. 1968 May 25;243(10):2514–2522. [PubMed] [Google Scholar]
- Hough-Evans B. R., Howard J. Genome size and DNA complexity of Plasmodium falciparum. Biochim Biophys Acta. 1982 Jul 30;698(1):56–61. doi: 10.1016/0167-4781(82)90184-1. [DOI] [PubMed] [Google Scholar]
- Krogstad D. J., Gluzman I. Y., Kyle D. E., Oduola A. M., Martin S. K., Milhous W. K., Schlesinger P. H. Efflux of chloroquine from Plasmodium falciparum: mechanism of chloroquine resistance. Science. 1987 Nov 27;238(4831):1283–1285. doi: 10.1126/science.3317830. [DOI] [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985 Jan 17;312(3):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- Newbold C. Malaria: the path of drug resistance. Nature. 1990 May 17;345(6272):202–203. doi: 10.1038/345202a0. [DOI] [PubMed] [Google Scholar]
- Pyatak P. S., Abuchowski A., Davis F. F. Preparation of a polyethylene glycol: superoxide dismutase adduct, and an examination of its blood circulation life and anti-inflammatory activity. Res Commun Chem Pathol Pharmacol. 1980 Jul;29(1):113–127. [PubMed] [Google Scholar]
- Roth E. F., Jr, Ruprecht R. M., Schulman S., Vanderberg J., Olson J. A. Ribose metabolism and nucleic acid synthesis in normal and glucose-6-phosphate dehydrogenase-deficient human erythrocytes infected with Plasmodium falciparum. J Clin Invest. 1986 Apr;77(4):1129–1135. doi: 10.1172/JCI112412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth E., Jr, Ogasawara N., Schulman S. The deamination of adenosine and adenosine monophosphate in Plasmodium falciparum-infected human erythrocytes: in vitro use of 2'deoxycoformycin and AMP deaminase-deficient red cells. Blood. 1989 Aug 15;74(3):1121–1125. [PubMed] [Google Scholar]
- Simmonds H. A., Fairbanks L. D., Morris G. S., Webster D. R., Harley E. H. Altered erythrocyte nucleotide patterns are characteristic of inherited disorders of purine or pyrimidine metabolism. Clin Chim Acta. 1988 Feb 15;171(2-3):197–210. doi: 10.1016/0009-8981(88)90145-3. [DOI] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Webster H. K., Whaun J. M. Purine metabolism during continuous erythrocyte culture of human malaria parasites (P. falciparum). Prog Clin Biol Res. 1981;55:557–573. [PubMed] [Google Scholar]
- Webster H. K., Wiesmann W. P., Pavia C. S. Adenosine deaminase in malaria infection: effect of 2'-deoxycoformycin in vivo. Adv Exp Med Biol. 1984;165(Pt A):225–229. doi: 10.1007/978-1-4684-4553-4_44. [DOI] [PubMed] [Google Scholar]
- Webster H. K., Wiesmann W. P., Walker M. D., Bean T., Whaun J. M. Hypoxanthine metabolism by human malaria infected erythrocytes: focus for the design of new antimalarial drugs. Adv Exp Med Biol. 1984;165(Pt A):219–223. doi: 10.1007/978-1-4684-4553-4_43. [DOI] [PubMed] [Google Scholar]
- Wyler D. J. Malaria--resurgence, resistance, and research. (First of two parts). N Engl J Med. 1983 Apr 14;308(15):875–878. doi: 10.1056/NEJM198304143081505. [DOI] [PubMed] [Google Scholar]
- Yayon A., Cabantchik Z. I., Ginsburg H. Identification of the acidic compartment of Plasmodium falciparum-infected human erythrocytes as the target of the antimalarial drug chloroquine. EMBO J. 1984 Nov;3(11):2695–2700. doi: 10.1002/j.1460-2075.1984.tb02195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolg J. W., MacLeod A. J., Dickson I. H., Scaife J. G. Plasmodium falciparum: modifications of the in vitro culture conditions improving parasitic yields. J Parasitol. 1982 Dec;68(6):1072–1080. [PubMed] [Google Scholar]