Abstract
The purpose of the present study was to develop tooth-binding micelle formulations and evaluate their ability to both inhibit initial biofilm formation as well as decrease the viability of preformed biofilm using an in vitro dental biofilm model. Alendronate (ALN, a bisphosphonate) was covalently attached to the ends of different Pluronic copolymers to confer tooth-binding ability to the micelles, and triclosan was used as a model drug. Based on different micelle preparation methods, Pluronic copolymers and ALN-terminated Pluronic copolymers were used to prepare triclosan-loaded tooth-binding micelles. The formulation was optimized for triclosan solubility, particle size, hydroxyapatite (HA) binding capacity and kinetics, and in vitro drug release behavior. In vitro biofilm treatment studies demonstrated that the triclosan-loaded tooth-binding micelles were able to inhibit initial biofilm growth of Streptococcus mutans UA159 by 6-log CFU/HA disc compared to the untreated control. These tooth-binding micelles were also capable of reducing the viability of preformed biofilm by 4-log CFU/HA disc compared to untreated control biofilm. In summary, triclosan-loaded tooth-binding micelles have been successfully developed and optimized in this study. These micelles demonstrated promising anti-biofilm capabilities that have the potential for use in the future treatment and prevention of dental diseases.
INTRODUCTION
Streptococcus mutans is well known in the field of oral microbiology and clinical dentistry as the etiologic agent of dental caries in humans (1). It was first found in human caries lesions by Clarke in 1924 and later its relationship with dental caries formation had been identified by Keyes in 1960 (2). It is now considered a primary etiological agent of dental caries in both humans and animals (3). S. mutans possesses several virulence factors that contribute to its ability to both initiate caries development and to facilitate progression of the disease. Among them, three vital factors have been highlighted in the literature, including the ability 1) to metabolize dietary carbohydrates and produce lactic acid (acidogenicity), 2) to grow and survive in a low pH environment (aciduricity), and 3) to efficiently utilize dietary sugars to produce glucan polymers that contribute to plaque biofilm formation (1, 4, 5). The ability of forming plaque on the tooth surface gives S. mutans a unique microenvironment for its growth, metabolism and survival (6).
Mature dental plaque is a complex multispecies biofilm that grows on the tooth surface, embedded in a protective matrix of host and bacterial polymers (7). Biofilm growth confers a survival mechanism to oral bacteria against many adverse factors such as variation in pH, nutrient and oxygen deprivation, and most importantly antimicrobial agents. It is well-known that bacterial biofilms are inherently more resistant to antimicrobial agents compared to planktonic cultures. This increased resistance has been reported to range from 2- to 1000-fold greater than corresponding planktonic cultures (8, 9). It is easy to envision that successful antimicrobial therapy against dental plaque biofilm is contingent on establishing an effective drug concentration at the tooth surface for a prolonged period of time (10).
Triclosan is a broad-spectrum antimicrobial agent that is very active against Gram-positive species, including S. mutans. It is the most commonly used and most potent example of the chlorinated diphenyl ether class of antibacterial compounds (11), which was approved by the US FDA in oral care products in 1997 (12). However, poor water solubility (11 μg/ml, which is lower than its MIC of 20 μg/ml) (13) and low retention (half-life for clearance is only 20 min) (14) in the oral cavity have limited its effectiveness and application for prevention and treatment of dental caries. Recent studies have shown that a concentration greater than the MIC of triclosan is required for effective killing of S. mutans in biofilm compared to killing in liquid cultures (15). When given as a pulse (a high initial inhibitor concentration which decreased with time), triclosan showed a reduced inhibitory effect, as bacterial numbers were restored within a few hours (16). Thus, a delivery system that can increase triclosan solubility in water and improve its retention in the oral cavity (especially tooth surface) is highly desirable.
To enhance the anti-caries efficacy of triclosan, we have successfully developed a mineral-binding micellar drug delivery system that quickly binds to hydroxyapatite (HA), and releases encapsulated drug over a prolonged period of time. This micellar delivery system is composed of pluronic copolymer and a mineral binding moiety alendronate (ALN, a bisphosphonate) that was covalently conjugated to the ends of Pluronic copolymer using “click chemistry” (17). The hydrophobic core of the pluronic micelle forms a host for hydrophobic drugs which should greatly increase drug solubility. ALN, which is known to have a high affinity for HA crystals (the main component of tooth enamel), should also dramatically enhance the retention of the drug on the tooth surface.
In this current study, we explored the feasibility of using this system to deliver triclosan to the tooth surface. The micelle formulation was optimized to achieve higher triclosan solubility, smaller particle size and higher binding capacity by employing different micelle preparation methods, different Pluronic copolymers and different ALN-terminated Pluronic copolymers (ALN-Ps). The optimized formulations were then tested in an in vitro biofilm model to evaluate their ability to inhibit initial biofilm formation and, for the first time, their ability to treat preformed biofilm.
MATERIALS AND METHODS
Chemicals
Alendronate (ALN) was purchased from Ultratech India Ltd. (New Mumbai, India). Triclosan was obtained from TCI America (Portland, OR). Hydroxyapatite particles (HA, DNA grade Bio-Gel HTP gel) were purchased from Bio-Rad (Hercules, CA). HA discs (0.5′ diameter × 0.04–0.06′ thick) were purchased from Clarkson Chromatography Products, Inc. (South Williamsport, PA). LH-20 resin was purchased from GE Healthcare (Piscataway, NJ). Pluronic® copolymers (P123, F127) were generously provided by BASF Corporation (Florham Park, NJ). All other reagents and solvents if not specified were purchased from either Fisher Scientific (Pittsburgh, PA) or Acros Organics (Morris Plains, NJ).
Methods
1H NMR spectra were recorded on a Varian Inova Unity 500 NMR Spectrometer. UV-visible spectra were measured on a Shimadzu UV-1601PC UV-Visible Spectrophotometer. Effective hydrodynamic diameters (Deff) of the micelles were measured by dynamic light scattering (DLS) using a “ZetaPlus” analyzer (Brookhaven Instrument Co.) equipped with a Multi Angle Sizing Option (BI-MAS). An Agilent 1100 HPLC system with a quaternary pump (with degasser), an autosampler, a fluorescence detector and a diode-array based UV detector was used for triclosan concentration analysis.
Synthesis of Alendronate-modified Pluronics (ALN-P)
Alendronate-modified Pluronics (ALN-P) were synthesized using “click chemistry” as described previously (23). Briefly, acetylene-alendronate was synthesized by 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride (EDC) coupling of 4-pentynoic acid and alendronate with N-hydroxysuccinimide (NHS) activation (Yield: 76%). P-toluenesulfonyl terminated Pluronics (Tos-P) were synthesized by the reaction of Pluronics with p-toluene sulfonyl chloride using 4-dimethylaminopyridine (DMAP) as a catalyst in dichloromethane (DCM) at room temperature (Yield: Tos-P85, 54%; Tos-P123, 60%; Tos-F127, 47%). Azide terminated Pluronics (Azido-P) were synthesized by the reaction of Tos-P with sodium azide at 110°C in DMF (Yield: Azide-P85, 94%; Azide-P123, 96%; Azide-F127, 90%). ALN-Ps were synthesized by the “click” reaction of acetylene-alendronate with Azido-P using sodium ascorbate and copper (II) sulfate pentahydrate as a catalyst in EtOH/H2O (1:1) at room temperature (Yield: ALN-P85, 65%; ALN-P123, 70%; ALN-F127, 73%). The products from each step were confirmed by 1H NMR. For all ALN-Ps, the percentage of Pluronic’s chain termini being modified by ALN is estimated to be > 90% by NMR spectra.
Triclosan-loaded Micelle Formulation
Three different methods were used to prepare triclosan-loaded micelles: film hydration, solvent evaporation and direct equilibrium. For the film hydration method, an excess amount of triclosan (100 mg) was dissolved in a methanol solution of Pluronic P123 (1mL, 100 mg/mL). Methanol was then removed by rotor evaporation at 60 °C to form a film of the drug and polymer. To completely remove the solvent residue, the film was placed in vacuum overnight. To prepare the micelle, the film was first heated at 60°C for 10 min and then hydrated by water at 60°C for 30 min. For the solvent evaporation method, Pluronic P123 and triclosan (100 mg each) were first dissolved in 1 mL of acetone and then added drop-wise to water (5mL) under stirring. The acetone was allowed to evaporate overnight. For the direct dissolution method, Pluronics (P123 or F127, containing different amounts of ALN-P85, ALN-P123, or ALN-F127; 100 mg polymer in total) were first dissolved in water (5 mL) and placed at 4°C overnight, and then the system was equilibrated at 24 °C for 24 h in order to allow micelle formation. Then an excess amount of triclosan (100 mg) was added to this solution and dissolution of the drug was allowed under stirring for 24 h. In all three methods, the undissolved drug was removed by centrifugation at 12,000 rpm for 0.5 min, followed by filtration of the supernatant through a 0.2 μm filter.
Characterization of Triclosan-loaded Micelles
To determine the triclosan solubility in each micelle formulation, an aliquot of each micelle solution was directly dissolved in acetonitrile to release all encapsulated drugs, and the drug concentration was measured by HPLC with the following conditions: Agilent C18 reverse-phase column (4.6 × 250 mm, 5 μm) was used with the mobile phase of acetonitrile/water (65:35, v/v) at a flow rate of 1 mL/min. The UV detection was set at 280 nm. Micelles were also characterized for their effective hydrodynamic diameters (Deff) using DLS.
In vitro HA Binding Assay of Triclosan-loaded Micelles
Micelle formulations (200 μL each) were mixed with HA particles (20 mg/tube) in Eppendorf centrifuge tubes. The tubes were placed on a Labquake® rotator to allow binding at room temperature. At each predetermined time points, 3 tubes were removed, centrifuged (12,000 rpm, 0.5 min), and 100 μL of the supernatant was collected. The concentration of triclosan in collected samples was then analyzed by HPLC with the conditions aforementioned. The amount of triclosan bound to HA particles via the micellar formulation was calculated by subtracting the amount of triclosan left in the supernatant from the initial amount of triclosan added.
In vitro Triclosan Release Study
Micelle formulations were sealed into a dialysis bag (MWCO 12,000) with or without HA particles (500 mg) and incubated in 20 mL of release medium (0.1 M PBS, pH = 7.4 containing 2% Pluronic P85) to release the encapsulated drug at 37 °C with gentle shaking. At predetermined time intervals, samples (0.5 mL) were removed from the release medium and replaced with fresh medium. The collected samples were then mixed with an equal volume of acetonitrile, filtered (0.2 μm) and analyzed with HPLC.
Bacterial Culturing Conditions
S. mutans UA159 (18) frozen stock cultures were maintained in 25% (v/v) glycerol at −80°C. For each experiment, S. mutans was streaked from a frozen stock onto Todd Hewitt-Yeast Extract (THYE; Todd Hewitt broth containing 0.3% w/v yeast extract) agar (1.5% w/v). After 48 h growth at 37°C and 5% CO2, a single colony of bacteria was inoculated into 3 mL of THYE broth (19) and allowed to grow statically overnight at 37°C and 5% CO2. The next day, the overnight culture was diluted to a density of 2×104 CFU/mL in chemically-defined media containing 0.25% w/v glucose and 0.25% w/v sucrose (CDM), prepared as previously described (19).
In vitro Inhibition of S. mutans Biofilm Formation on HA discs
Autoclaved HA discs (0.5′ diameter × 0.04–0.06′ thick) were incubated with different micelle solutions or CDM in a 24-well plate for 1 h and then washed in 40 mL of saline (under magnetic stirring at 200 rpm) for 5 s to remove unbound micelles. The HA discs were then transferred to wells containing 1 mL of S. mutans UA159 suspension (1×104 CFU/mL in CDM) and cultured statically for 48 h to allow biofilm growth at 37°C and 5% CO2, prior to quantification of bacterial growth.
In vitro Treatment of Preformed S. mutans Biofilm Growth on HA Discs
Autoclaved HA discs were incubated in 1 mL of S. mutans UA159 suspension (1×104 CFU/mL in CDM), and cultured statically for 48 h to allow biofilm growth at 37°C and 5% CO2. These 48 h HA disc biofilms were then either treated with different micelle formulations for 5 min, or were left as untreated controls. After treatment, the HA disc biofilms were washed in 40 mL of saline (under magnetic stirring at 200 rpm) for 5 s to remove loosely attached micelles before each being transferred to wells of a 24-well plate containing sterile CDM media, and grown at 37 °C, 5% CO2. This same procedure was repeated at 72 h and 96 h of growth, prior to quantification of biofilm growth.
Biofilm Analysis
At the end of each experiment, HA disc biofilms were individually transferred to a well containing 1 mL of THYE medium. The surface of each HA disc was gently scraped with a sterile spatula to harvest adherent cells. The removed biofilms were subjected to vortex mixing for 10 s and then serially diluted in THYE broth at a 1:10 ratio. The number of viable cells in each sample was quantified using the track dilution method (20). All plates were incubated for 48 h at 37°C with 5% CO2 and then the CFUs recovered per biofilm were determined. Specific differences between the log-CFU/biofilm of each experimental group were analyzed using the Student t-test. A p-value of < 0.05 was considered as statistically significant.
RESULTS
Influence of Formulation Method and Type of Pluronic on Triclosan Solubility and Micelle Size
Three different methods, including the film hydration (21), solvent evaporation (22) and direct dissolution (23), were used to prepare triclosan-loaded Pluronic micelles. The results presented in Table 1 showed that high triclosan solubility in the Pluronic P123 micelle was achieved when prepared by the direct dissolution method. This high solubility is in agreement with previously published data (23). To investigate the influence of different pluronic copolymers on triclosan solubility and micelle size, Pluronics P123 and F127, which are different in hydrophilicity and molecular weight (Table 2), were used to formulate triclosan-loaded micelles using the direct dissolution method. High and similar triclosan solubility was achieved using the two polymers, and they all have small but relatively widely distributed particle size. Based on these data, the direct dissolution method was chosen as the micelle formulation method in subsequent studies.
Table 1.
Influence of Preparation Method on Triclosan solubility and Particle Size of P85 Micelle. Data were expressed as mean ± SD, n=3.
| Preparation Method | Triclosan solubility (mg/ml) | Particle Size (nm) | PDI |
|---|---|---|---|
| Film Hydration | 0.4±0.04 | 135.6±7.9 | 0.23 |
| Solvent Evaporation | 0.2±0.02 | 117.9±2.8 | 0.24 |
| Direct Dissolution | 12.9±0.6 | 53.0±5.0 | 0.27 |
Table 2.
Influence of Pluronic Polymers on Triclosan Solubility and Particle Size. Micelle Formulations Were Prepared Using Direct Dissolution Method. Data were expressed as mean ± SD, n=3.
| Pluronic copolymers | Average Molecular Weight* | Average No. of EO/PO/EO units** | HLB* | Triclosan Solubility (mg/mL) | Particle Size (nm) | PDI |
|---|---|---|---|---|---|---|
| P123 | 5750 | 39/69/39 | 8 | 12.9±0.6 | 53.0±5.0 | 0.27 |
| F127 | 12600 | 200/65/200 | 24 | 11.5±1.3 | 43.6 ±8.4 | 0.25 |
The average molecular weights and HLB values were determined and provided by the manufacturer (BASF, Wyandotte, MI).
The average numbers of EO and PO units were calculated using the average molecular weights of the Pluronics.
Influence of the Addition of Different ALN-Ps on Triclosan Solubility and Micelle Size
ALN-P85, ALN-P123, and ALN-F127 were synthesized and added into the micelle structure to confer mineral binding ability to the micelles. As shown in Table 3, the triclosan solubility in P123 micelle formulations was improved by all ALN-Ps (all p<0.05). For the F127 micelle, none of the ALN-Ps were able to improve triclosan solubility. As for the particle size, the ALN-Ps did not show strong influence on particle size of the micelles.
Table 3.
Influence of different ALN-Ps on Triclosan Solubility and Micelle Size. Data were expressed as mean ± SD, n=3.
| Pluronic Copolymers | ALN-Ps | Percentage of ALN-Ps in Total Polymer (w/w) | Triclosan Solubility (mg/mL) | Particle Size (nm) |
|---|---|---|---|---|
| P123 Micelle | ALN-P85 | 10% | 15.4±0.8 | 36.2±4.2 |
| 20% | 15.5±1.1 | 33.0±6.7 | ||
| 40% | 14.0±1.1 | 43.3±19.2 | ||
| ALN-P123 | 10% | 15.0±1.4 | 23.8±3.0 | |
| 20% | 14.8±1.2 | 36.7±5.6 | ||
| 40% | 14.2±1.2 | 41.8±7.5 | ||
| ALN-F127 | 10% | 16.0±0.8 | 29.3±6.7 | |
| 20% | 16.0±1.2 | 29.7±4.8 | ||
| 40% | 15.2±0.6 | 36.7±5.0 | ||
| F127 Micelle | ALN-P85 | 10% | 10.8±1.3 | 38.5±10.7 |
| 20% | 10.6±1.2 | 37.4±8.0 | ||
| 40% | 10.3±1.4 | 43.7±8.7 | ||
| ALN-P123 | 10% | 10.2±0.8 | 38.6±5.5 | |
| 20% | 9.8±1.2 | 43.8±5.4 | ||
| 40% | 10.4±1.3 | 51.7±6.5 | ||
| ALN-F127 | 10% | 10.9±0.8 | 36.0±5.6 | |
| 20% | 10.5±1.0 | 43.9±8.1 | ||
| 40% | 10.8±0.5 | 51.4±12.1 | ||
In vitro HA Binding Assay of Triclosan-loaded Micelles
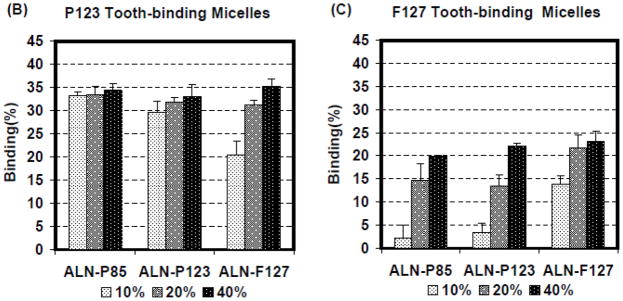
The HA binding assay was carried out using a previously-described method (24, 25). Without ALN-Ps, P123 and F127 micelles by itself showed very limited non-specific binding to HA powders (< 5%). The binding capacity (percentage of binding measured at 1h) of the F127 micelles was improved by increasing the amount of ALN-Ps from 10% to 40% in the micelle structures in all cases (all p<0.05), however, for P123 micelles except for the combination of P123 micelle with ALN-F127, no significant increase of binding capacity was observed (Fig. 1A&B). Results of the binding kinetic study (Fig. 2) showed that all three ALN-Ps were able to give the P123 micelle a very fast binding kinetics, where the majority of HA-binding occurred within 1 min.
Fig. 1.
Binding capacity of different micelle formulations (P123 or F127 micelle) containing various amounts (indicated as a percentage at the bottom of the figure) of different ALN-Ps or no ALN-P (non-binding micelles) on HA powers at 1h time point. Data are expressed as mean ± SD, n=3.
Fig. 2.
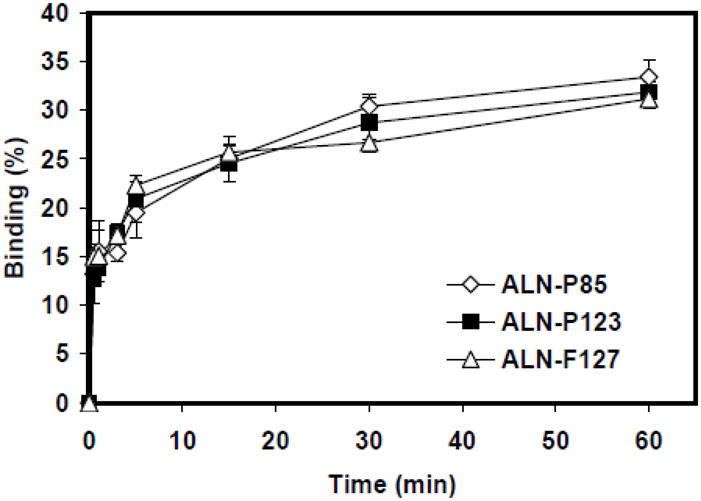
Binding kinetics of P123 micelles containing 20% of different ALN-Ps. Data are expressed as mean ± SD, n=3.
In vitro Triclosan Release Study
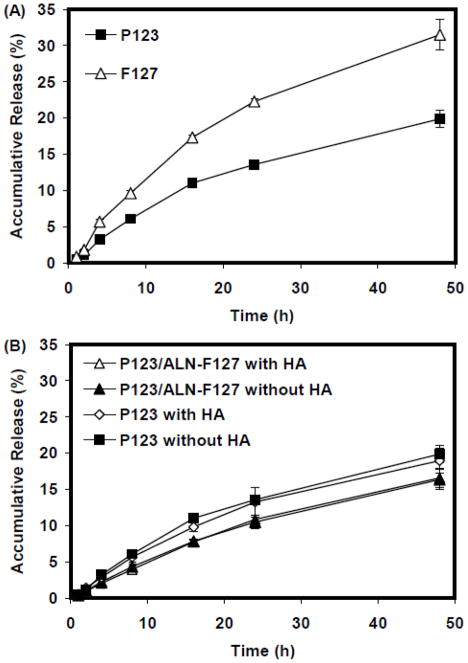
Triclosan release profiles from micelles prepared using P123 or F127 are shown in Fig. 3A. In both cases, triclosan was released from the micelles in a sustained manner within a 48 h period, and no burst release was observed. F127 micelle had a faster release rate than P123 micelles. Introduction of ALN-F127 into the micelle structure as well as its binding to HA particles seems to have very limited effect on triclosan release behavior (Fig. 3B). The combinations of P123 with 20% ALN-F127, which had high HA binding capacity and small particle size, were selected and further tested on an in vitro dental biofilm model.
Fig. 3.
In vitro triclosan release study. (A) Triclosan release from P123 or F127 micelles; (B) Triclosan release from P123 micelle or P123 micelle containing 20% F127 with or without the presence of HA particles. Data are expressed as mean ± SD, n=3.
In vitro Inhibition of S. mutans Biofilm Formation on HA Discs
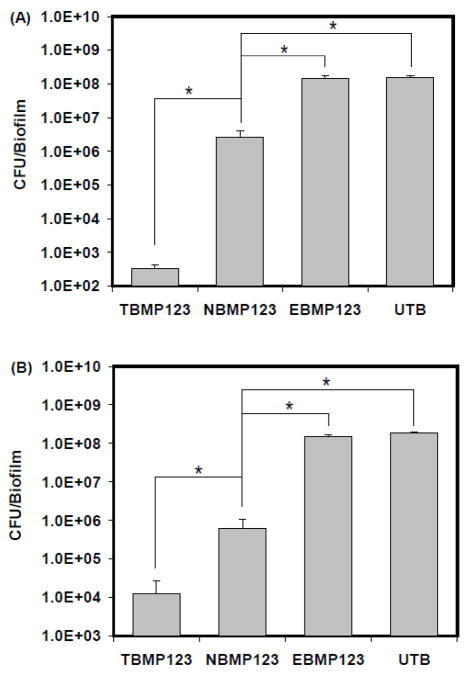
To study the effect of these micelles on initial biofilm formation, HA discs were first treated with different micelle formulations or untreated before they were washed with sterile saline and incubated with S. mutans to initiate biofilm formation. The results of these studies (Fig. 4A) demonstrate that the triclosan-containing tooth-binding P123 micelle (TBMP123, containing 20% ALN-F127) showed strong inhibition of biofilm formation on HA discs when compared to the untreated blank control (UTB). Interestingly, the triclosan-containing non-binding P123 micelles (NBMP123, containing 20% F127) also displayed a weak, albeit significant inhibitory effect when compared to the UTB. The vesicles themselves (empty tooth-binding P123 micelles, EBMP123, containing 20% ALN-F127,) did not show any inhibition on biofilm formation. Finally, the inhibitory effect of TBMP123 (6-log reduction in CFU/biofilm compared to UTB) was significantly higher than NBMP123 (2-log reduction in CFU/biofilm compared to UTB).
Figure 4.
(A) In vitro inhibition of S. mutans biofilm formation on HA discs. (B) In vitro treatment of preformed S. mutans biofilm on HA discs. TBMP123, tooth-binding P123 micelle containing 20% of ALN-F127; NBMP123, non-binding P123 micelle containing 20% of F127 instead of ALN-F127; EBMP123, empty (does not contain triclosan) tooth-binding micelle P123 containing 20% of ALN-F127; UTB, untreated blank control. Data are expressed as mean CFU/biofilm ±SD, n=3. The asterisks indicate statistically significant differences (p<0.05) between groups (indicated by connecting lines above the bars on the graphs).
In vitro Treatment of Preformed S. mutans Biofilm on HA Discs
To explore the feasibility of using tooth-binding micelle to treat preformed biofilm, HA discs were inoculated with S. mutans and incubated for 48 h to allow for biofilm development. Then, they were treated with different micelle formulations for 5 min per day at 2, 3, and 4 days post-inoculation. The results shown in Fig. 4B indicate that both the tooth-binding micelle and non-binding micelle were able to greatly reduce biofilm viability when compared to the UTB, whereas the vesicle itself (EBMP123) had no discernable effect on biofilm viability. Furthermore, the bacteria killing effect of TBMP123 was significantly stronger than that of NBMP123.
DISCUSSION
One critical factor that may predict the success of an anti-biofilm chemotherapy is how long it may remain on the tooth surface. Without adequate drug retention, any reduction in the amount of cariogenic bacteria caused by the application of an antimicrobial agent in the oral cavity can temporal, with the bacteria colonies easily re-established between doses. The delivery system we developed would provide a drug retention mechanism on the tooth surface, and allows for the constant release of drug for a prolonged period of time. This should keep an effective inhibitory concentration of the antimicrobial at the tooth surface and greatly reduce the potential of biofilm re-establishment. The goals of this current work are: (1) To prepare and optimize triclosan-loaded tooth-binding micelles with respect to triclosan solubility, particle size and HA binding kinetics; (2) To evaluate their ability to inhibit biofilm formation on HA surface; and (3) To explore the feasibility of using these micelles to treat preformed biofilms. To achieve these goals, different micelle preparation methods, different pluronic copolymers and different alendronate-terminated pluronic copolymers were compared.
Of the three methods used to prepare triclosan-loaded micelles, the direct dissolution method achieved the highest triclosan solubility. In contrast, the film hydration and solvent evaporation methods showed much lower triclosan solubility, probably because in these two methods, the triclosan crystallization process was faster than the micelle formation process (22). Therefore, only a portion of the drug was able to incorporate into the micelle core during the micelle formation process while the rest of the drug formed drug crystals. On the other hand, a dynamic transferring process was established in the direct dissolution method. Drug transferred from drug crystals to the water phase, and then to the hydrophobic micelle core until equilibrium was reached (26). Although the direct dissolution method was more time consuming when compared to the other two methods, it was chosen for use in subsequent studies based on the high drug solubility and simplicity. In the core-shell structure of Pluronic micelles, the hydrophilic corona (formed by the ethylene oxide (EO) block of the polymer) provides dispersion stability to the micelle, while the hydrophobic propylene oxide (PO) core serves as a microenvironment for the incorporation of hydrophobic compounds (27). Studies have shown that hydrophobic Pluronics which have a higher PO/EO ratio exhibit higher solubilization capacity than hydrophilic Pluronics (28). This is consistent with the results in Table 2, where micelles prepared with P123 showed the higher triclosan solubility. Another finding was that all micelle preparations showed a high PDI value, which means that the preparations had wide size distributions (Table 2). However, only monomodal size distribution pattern was observed in all preparations (data not shown). This wide distribution may be attributed to the wide polydispersity of commercial Pluronic copolymers. The different drug content among micelles may contribute to this wide size distribution as well. We also tried to use Pluronic P85 to prepare the micelle, however, the resulting micelle solution was unstable and phase separation happened during high-speed centrifugation. The P85 micelle solutions were opalescent in appearance, whereas the P123 and F127 micelle solutions were clear and transparent. The particle size of P85 micelles was measured to be around 100 nm with a large PDI. Based on the molecular weights and PO/EO compositions of P85, P123, and F127, the P85 micelle should theoretically be the smallest amongst the three (28). Therefore, the observations described above seems to suggest that a secondary association has taken place in the P85 micelle solution (23, 29), and thus it was not selected for further investigation due to its instability.
The incorporation of ALN-Ps into the micelle structure did not significantly affect the triclosan solubility and particle size of F127 micelles. However, ALN-Ps were able to increase triclosan solubility in P123 micelles. ALN-Ps are more hydrophilic when compare to their precursors, due to the presence of the ALN head group. Therefore, for the hydrophobic Pluronic copolymer P123, the introduction of the relatively more hydrophilic ALN-Ps into the micelle structure likely led to the reduction of the micelle association number and reduced core size (30, 31). In our experiment, we did observe a slight decrease in particle size of P123 micelle when ALN-Ps were added into micelle structure, however, at current PDI value (0.2–0.3), it is difficult to make a comparison.
The influence of different ALN-Ps on micelle binding capacity to HA particles was also investigated (Fig. 1). For F127 micelles, the increase of ALN-Ps content from 10% to 40% was found to promote the micelle binding, but the effect was not observed in P123 micelles. This may suggest the presence of a small amount of ALN-P in P123 micelle structure is likely sufficient to induce strong HA binding and saturate the HA surface (Fig. 1). Further binding kinetic studies were then carried out (Fig. 2), and a swift binding curve was observed, similar to previous reports (17). It should be noted that due to the molecular weight difference among the three ALN-Ps, equivalent amount (weight) of different ALN-Ps contains different amounts of ALN.
Another potential advantage of polymeric micelles is their ability to slowly release a molecule over time (32). Sustained release profiles of triclosan from both Pluronic P123 and F127 micelles were observed in the in vitro release study (Fig. 3A). The F127 micelle showed a faster release of encapsulated triclosan compared to the P123 micelle. The long EO chain of F127 can increase the distribution of EO units and water molecules into the core of micelle, which may facilitate the release of triclosan molecules into the surrounding media (21, 33). However, this release-facilitating effect of F127 was not observed when P123 was mixed with ALN-F127 (Fig. 3B). Also, the binding of micelles to HA particles (Fig. 3B) did not significantly change the release profile, suggesting micelle can slowly release drug even after binding to the tooth surface.
The features of an ideal tooth-binding micelle formulation should include: (1) a high drug solubility in the solution and a high binding capacity of the micelle to HA surface, such that the maximal amount of drug can be immobilized onto the tooth surface; (2) a small particle size, so that the micelles can have access to narrow areas such as between caries-prone tooth-tooth contact areas (34) and potentially penetrate the biofilm; (3) a fast binding kinetic to increase patient compliance; and (4) a sustained release behavior to provide prolonged antimicrobial exposure to dental biofilm. From the results of the micelle formulation optimization and characterization in this study, we have chosen the combination of P123/ALN-F127, which has a small particle size and high binding capacity, to proceed with the next step of our study-in vitro biofilm evaluation.
The in vitro biofilm inhibition study (Fig. 4A) was designed to evaluate the ability of tooth-binding micelles to prevent biofilm accumulation on a clean fresh tooth surface (e.g. after tooth brushing). Results showed that the P123/ALN-F127 (TBMP123) tooth-binding micelle was able to efficiently prevent biofilm accumulation, and the effect was significantly stronger than the non-binding micelle P123/F127 (NBMP123). Another interesting observation in this experiment was that identifiable bacteria growth was observed at the bottom of wells around the P123/ALN-F127 (TBMP123) bound discs (data not shown). This observation may indicate that the killing effect of P123/ALN-F127 (TBMP123) micelle is more concentrated at the HA surface. In reality, targeting killing of plaque bacteria attached to tooth surface is desirable, as this may limit the unwanted interruption and suppression of the beneficial oral flora.
Routine dental hygiene procedures are unable to completely remove dental plaque in the oral cavity. Therefore, it is of great interest to investigate if tooth-binding micelles can treat existing dental biofilm. To test the feasibility of this application, an in vitro preformed biofilm treatment study was carried out on HA discs (Fig. 4B). In this study, biofilm was allowed to grow on HA discs for 48 h before micelle formulations were applied. After 3 days of treatment at only 5 min per day, the tooth-binding micelle formulation greatly reduced viability of the preformed biofilm when compared to untreated control, indicating that the tooth-binding micelle was able to target and be retained on the HA surface in the presence of preformed biofilm. Retention of the tooth-binding micelles in this scenario might be attributed to binding of the micelles to exposed HA surface between biofilm microcolonies or the access of the micelle to the HA disc surface via the water channels in the biofilm. The P123/ALN-F127 (TBMP123) micelle showed a significantly stronger treatment efficacy compared to non-binding micelle (NBMP123). Non-binding micelles also showed bacterial killing, perhaps due to a greatly increased drug solubility and non-specific interaction between the micelle and the HA surface and/or biofilm.
CONCLUSION
In this study, we have successfully prepared triclosan-loaded tooth-binding micelles and optimized their formulation by assessing different preparation methods, different Pluronic copolymers, and different ALN-terminated Pluronics. The tooth-binding formulation that displayed high triclosan solubility, small particle size, potent HA binding capacity, and sustained in vitro drug release behavior, was further evaluated using an in vitro dental biofilm model. This tooth-binding micelle showed strong inhibition of biofilm formation on clean HA surface and was also able to effectively reduce the viability of preformed biofilms. Such micelle formulations may have strong potential for the prevention and treatment of dental caries.
Acknowledgments
This work was supported in part by NIH grants R03 DE019179 (KCR), R01 AI038901 (KWB) and R01 AR053325 (DW).
References
- 1.Senadheeraand D, Cvitkovitch DG. Quorum sensing and biofilm formation by Streptococcus mutans. Adv Exp Med Biol. 2008;631:178–88. doi: 10.1007/978-0-387-78885-2_12. [DOI] [PubMed] [Google Scholar]
- 2.Keyes PH. The infectious and transmissible nature of experimental dental caries. Findings and implications. Arch Oral Biol. 1960;1:304–20. doi: 10.1016/0003-9969(60)90091-1. [DOI] [PubMed] [Google Scholar]
- 3.Loesche WJ. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986;50:353–80. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuramitsu HK. Virulence factors of mutans streptococci: role of molecular genetics. Crit Rev Oral Biol Med. 1993;4:159–76. doi: 10.1177/10454411930040020201. [DOI] [PubMed] [Google Scholar]
- 5.Kuramitsu HK. Virulence properties of oral bacteria: impact of molecular biology. Curr Issues Mol Biol. 2001;3:35–6. [PubMed] [Google Scholar]
- 6.Bowdenand GH, Hamilton IR. Survival of oral bacteria. Crit Rev Oral Biol Med. 1998;9:54–85. doi: 10.1177/10454411980090010401. [DOI] [PubMed] [Google Scholar]
- 7.Marshand PD, Bradshaw DJ. Dental plaque as a biofilm. J Ind Microbiol. 1995;15:169–75. doi: 10.1007/BF01569822. [DOI] [PubMed] [Google Scholar]
- 8.Gilbert P, Das J, Foley I. Biofilm susceptibility to antimicrobials. Adv Dent Res. 1997;11:160–7. doi: 10.1177/08959374970110010701. [DOI] [PubMed] [Google Scholar]
- 9.Ceri H, Olson ME, Stremick C, Read RR, Morck D, Buret A. The Calgary Biofilm Device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J Clin Microbiol. 1999;37:1771–6. doi: 10.1128/jcm.37.6.1771-1776.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Ouderaaand F, Cummins D. Delivery systems for agents in supra- and sub-gingival plaque control. J Dent Res. 1989;68:1617–24. [Google Scholar]
- 11.Furiaand TE, Schenkel AG. A new, broad spectrum bacteriostat. Soap Chem Specialties. 1968;44:47–50. 116–22. [Google Scholar]
- 12.Food and Drug Administration. FDA Talk paper. FDA approves first toothpaste for gum disease, FDA Talk paper, July 14th (1997) [Google Scholar]
- 13.Raghavan SL, Schuessel K, Davis A, Hadgraft J. Formation and stabilisation of triclosan colloidal suspensions using supersaturated systems. Int J Pharm. 2003;261:153–8. doi: 10.1016/s0378-5173(03)00299-0. [DOI] [PubMed] [Google Scholar]
- 14.Gilbertand RJ, Williams PE. The oral retention and antiplaque efficacy of triclosan in human volunteers. Br J Clin Pharmacol. 1987;23:579–83. doi: 10.1111/j.1365-2125.1987.tb03094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phanand TN, Marquis RE. Triclosan inhibition of membrane enzymes and glycolysis of Streptococcus mutans in suspensions and biofilms. Can J Microbiol. 2006;52:977–83. doi: 10.1139/w06-055. [DOI] [PubMed] [Google Scholar]
- 16.Bradshaw DJ, Marsh PD, Watson GK, Cummins D. The effects of triclosan and zinc citrate, alone and in combination, on a community of oral bacteria grown in vitro. J Dent Res. 1993;72:25–30. doi: 10.1177/00220345930720010301. [DOI] [PubMed] [Google Scholar]
- 17.Chen F, Liu XM, Rice KC, Li X, Yu F, Reinhardt RA, Bayles KW, Wang D. Tooth-binding micelles for dental caries prevention. Antimicrob Agents Chemother. 2009;53:4898–902. doi: 10.1128/AAC.00387-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murchison HH, Barrett JF, Cardineau GA, Curtiss R., 3rd Transformation of Streptococcus mutans with chromosomal and shuttle plasmid (pYA629) DNAs. Infect Immun. 1986;54:273–82. doi: 10.1128/iai.54.2.273-282.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biswas I, Drake L, Biswas S. Regulation of gbpC expression in Streptococcus mutans. J Bacteriol. 2007;189:6521–31. doi: 10.1128/JB.00825-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jett BD, Hatter KL, Huycke MM, Gilmore MS. Simplified agar plate method for quantifying viable bacteria. Biotechniques. 1997;23:648–50. doi: 10.2144/97234bm22. [DOI] [PubMed] [Google Scholar]
- 21.Wei Z, Hao J, Yuan S, Li Y, Juan W, Sha X, Fang X. Paclitaxel-loaded Pluronic P123/F127 mixed polymeric micelles: formulation, optimization and in vitro characterization. Int J Pharm. 2009;376:176–85. doi: 10.1016/j.ijpharm.2009.04.030. [DOI] [PubMed] [Google Scholar]
- 22.Blanco E, Bey EA, Dong Y, Weinberg BD, Sutton DM, Boothman DA, Gao J. Beta-lapachone-containing PEG-PLA polymer micelles as novel nanotherapeutics against NQO1-overexpressing tumor cells. J Control Release. 2007;122:365–74. doi: 10.1016/j.jconrel.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiappetta DA, Degrossi J, Teves S, D’Aquino M, Bregni C, Sosnik A. Triclosan-loaded poloxamine micelles for enhanced topical antibacterial activity against biofilm. Eur J Pharm Biopharm. 2008;69:535–45. doi: 10.1016/j.ejpb.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 24.Shinoda H, Adamek G, Felix R, Fleisch H, Schenk R, Hagan P. Structure-activity relationships of various bisphosphonates. Calcif Tissue Int. 1983;35:87–99. doi: 10.1007/BF02405012. [DOI] [PubMed] [Google Scholar]
- 25.Fujisawa R, Wada Y, Nodasaka Y, Kuboki Y. Acidic amino acid-rich sequences as binding sites of osteonectin to hydroxyapatite crystals. Biochim Biophys Acta. 1996;1292:53–60. doi: 10.1016/0167-4838(95)00190-5. [DOI] [PubMed] [Google Scholar]
- 26.Yang L, Wu X, Liu F, Duan Y, Li S. Novel biodegradable polylactide/poly(ethylene glycol) micelles prepared by direct dissolution method for controlled delivery of anticancer drugs. Pharm Res. 2009;26:2332–42. doi: 10.1007/s11095-009-9949-4. [DOI] [PubMed] [Google Scholar]
- 27.Oh KT, Bronich TK, Kabanov AV. Micellar formulations for drug delivery based on mixtures of hydrophobic and hydrophilic Pluronic block copolymers. J Control Release. 2004;94:411–22. doi: 10.1016/j.jconrel.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 28.Nagarajan R. Solubilization of hydrocarbons and resulting aggregate shape transitions in aqueous solutions of Pluronic® (PEO–PPO–PEO) block copolymers. Coll Surf B: Biointerf. 1999:55–72. [Google Scholar]
- 29.Xu RL, Winnik MA, Hallett FR, Riess G, Croucher MD. Light-scattering study of the association behavior of styrene-ethylene oxide block copolymers in aqueous solution. Macromolecules. 1991;24:87–93. [Google Scholar]
- 30.Chuand B, Zhou Z. Nonionic Surfactants: polyoxyalkylene block copolymers. Marcel Dekker, Inc; New York: 1996. [Google Scholar]
- 31.Kabanovand AV, Alakhov VY. Pluronic block copolymers in drug delivery: from micellar nanocontainers to biological response modifiers. Crit Rev Ther Drug Carrier Syst. 2002;19:1–72. doi: 10.1615/critrevtherdrugcarriersyst.v19.i1.10. [DOI] [PubMed] [Google Scholar]
- 32.Croyand SR, Kwon GS. Polymeric micelles for drug delivery. Curr Pharm Des. 2006;12:4669–84. doi: 10.2174/138161206779026245. [DOI] [PubMed] [Google Scholar]
- 33.Kozlov MY, Melik-Nubarov NS, Batrakova EV, Kabanov AV. Relationship between Pluronic Block Copolymer Structure, Critical Micellization Concentration and Partitioning Coefficients of Low Molecular Mass Solutes. Macromolecules. 2000;33:3305–13. [Google Scholar]
- 34.Sjogren K, Lundberg AB, Birkhed D, Dudgeon DJ, Johnson MR. Interproximal plaque mass and fluoride retention after brushing and flossing--a comparative study of powered toothbrushing, manual toothbrushing and flossing. Oral Health Prev Dent. 2004;2:119–24. [PubMed] [Google Scholar]