Abstract
Liver diseases have still a high mortality even though liver transplantation has become a standard treatment. Currently, hepatocyte transplantation has been proposed as another promising strategy. One limitation is the availability of human livers as a source of hepatocytes. Because of an unlimited supply, the use of porcine hepatocytes might address this problem. Regardless of the source, hepatocytes once isolated lose specific functionality due to the loss of the natural microenvironment. For this reason, we tested the ability of a self-assembling peptide nanofiber (SAPNF) to provide a provisional three-dimensional (3-D) support to interact with cells to control their function in vivo. Isolated porcine hepatocytes were embedded in SAPNF, or collagen Type I and transplanted by direct injection into the splenic pulp of SCID mice suffering from acute liver failure (ALF) by 90% hepatectomy. SAPNF-porcine hepatocyte transplantation produced engraftment that was far superior to that obtained using collagen and prolonged the survival of mice with ALF, in contrast with controls. An ultrastructural evaluation using transmission electron microscopy indicated extensive cell-cell communication and preservation of hepatocyte architecture. The transplanted SAPNF-hepatocytes showed higher expression of albumin and PAS and lower apoptotic events assessed by TUNEL staining. Hepatocytes culture in a truly three-dimensional network allows in vivo maintaining of differentiated functions, and once transplanted between widely divergent species can function to correct acute liver failure in mice and prolong their survival.
Keywords: hepatocytes, acute liver failure, hepatocyte transplantation, xenotransplantation, self-assembling peptide nanofiber
INTRODUCTION
Currently, acute liver failure is a catastrophic illness associated with the death of many patients while waiting for transplantation (21). Considering the vigorous regenerative capacity of the liver (22), some forms of acute liver failure (ALF) can be managed with several bridging techniques during the waiting time (13,14,34,35). However, the lack of livers to used as a whole for orthotopic liver transplantation (OLT) (3) or to isolate hepatocytes to use as a temporal support while its own liver recovers is one of the major drawbacks (16). The used of xenogeneic hepatocytes, from animals such as pigs, might be advantageous for treating hepatic failure (5,25,27). Hepatocytes comprise the main metabolically active cells of the liver. However, when removed from the complex architecture of the liver, loss of cell anchorage occurs. It exerts major stress on hepatocytes by the disruption of cell-cell and cell-matrix interactions, resulting in loss of differentiated functions such as liver-specific gene expression, because they are anchorage-dependent and sensitive to environmental factors (29). The use of an adequate substratum may allow isolated hepatocytes to recover from the stress of the isolation procedure, to engraft in a better manner and therefore maintaining liver-specific functions in vivo. To this end, researchers have focused on the design of three-dimensional (3D) scaffolds synthesized from synthetic polymers (23,24,28,31) or from natural ECM components (2,9,17). A major limitation of ECM-derived systems is their potential inflammatory responses and cell cytotoxicity when the material is implanted into a host (4). In addition, these biomaterials are often made of microfibers with diameters drastically different in size, surface interaction, porosity and concentration relative to the native ECM and cells interacting with it. Therefore, cells attached on microfibers are in fact in 2D despite the various curvatures associated with the large diameter microfibers. Since the topography of 3-D influences cellular polarization and cytoskeletal arrangement, there is a close relationship between the 3-D architecture of the cells and their differentiated function (1). Understanding the conditions necessary for a normal hepatocyte to express its full functional repertoire is critically important for the selection of an appropriate scaffold to improve the success of hepatocytes transplantation. Efforts are increasing to recreate a functional liver outside its own niche. Thus it is important to know the need of the hepatocytes, and try to cover them in order to establish a real atmosphere where the hepatocytes can be placed and continue its functions. We have previously demonstrated the use of a 3D biomaterial through molecular self-assembling in hepatocyte culture (26), resulting in better preservation of hepatocyte functionality. In this study, we have paid close attention to this self-assembling peptide nanofiber (SAPNF) and tested its ability to function in vivo. SAPNF hydrogel is formed by 4 repeats of alternating hydrophilic and hydrophobic amino acids (36) [COCH3]-RADARADARADARADA-[CONH2]. Its amphyphilic peptides, charge density, and water-structuring abilities mimic the in vivo ECM. SAPNF forms nanofibers that are significantly smaller than cells in comparison with the synthetic polymers PLLA, PGA, PLGA. Thus, they are surrounded by the scaffold, providing a temporary template with the biomechanical structural characteristics of the native ECM until the cells produce their own (30,36). Here we have demonstrated that SAPNF has an excellent ability to promote hepatocyte engraftment, maintaining tremendous hepatocyte functions capable of rescuing mice from ALF in a xenotransplantation model.
MATERIALS AND METHODS
Animals
White pigs weighing around 20 kg were used as donors of hepatocytes for transplantation experiments. Severe Combined Immunodeficient (SCID) mice (10–12 weeks old) were maintained at the Animal Center of Okayama University and used as the recipient mice for the hepatocyte transplantation. Mice were placed in cages within a temperature-controlled room with a 12-hour light/dark cycle and ad libitum access to food and water. All animal procedures were approved by the Okayama University Institutional Animal Care and Use Committee.
Hepatocyte Isolation
Under general anesthesia, pigs (JA West, Okayama, Japan) weighing 20 kg underwent upper middle incision and the left lateral lobes, weighing 80 g, were surgically removed for hepatocyte isolation. Hepatocytes were isolated with a four-step retrograde dispase/collagenase perfusion method as previously described (20). After isolation, the cells were resuspended with DMEM medium (Sigma), and the cell viability was determined by trypan blue exclusion test. In our experiments, we only used isolated hepatocytes if the viability of the cells exceeded 90%.
Hepatocyte Transplantation
90% hepatectomy was performed on SCID mice (Nippon CLEA Co. Tokyo, Japan) to induce ALF and simultaneously, SAPNF- or collagen- suspended hepatocytes were transplanted into the spleen of the mice by direct inoculation into the pulp. In brief, porcine hepatocytes were re-suspended with 50μl of 10% sucrose solution (Supelco. Bellefonte, PA), and 100μl of SAPNF 1% was added, mixed well by gentle pipetting, immediately after, 50μl of PBS was added, mixed gently and the mixture was pulled by a syringe with 20G needle to avoid bubble formation. For the intrasplenic injection, a 30G was used. On the other hand, porcine hepatocytes were re-suspended in 200μl of 9μg collagen type I diluted with saline solution mixed gently, with previous pH adjustment. A total of 1×106 hepatocytes were transplanted. All the surgical procedures were performed under inhalation anesthesia using Diethyl ether (Nacalai Tesque).
Transplantation Experiments
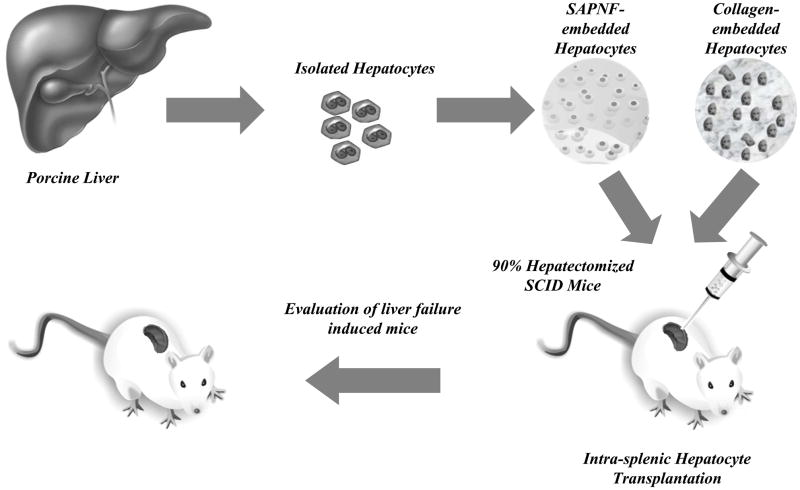
As shown in Fig. 1, the experimental groups were divided into the following five groups: Group 1: transplantation of 200μl of 0.5% SAPNF-suspended porcine hepatocytes (1×106) (n=10). Group 2: transplantation of 200 μl of collagen-suspended porcine hepatocytes (1×106) (n=10). Group 3: transplantation of porcine hepatocytes alone resuspended in 200μl of medium (1×106) (n=10). Group 4: transplantation of cellular homogenates (1×106) (n=10). Group 5: transplantation of 200μl of 0.5% SAPNF alone without hepatocytes (n=10).
Fig. 1. Schematic representation of the present study.
Porcine hepatocytes were isolated and embedded in Self-assembling peptide nanofiber (SAPNF) or Collagen Type I (Coll I). Such porcine hepatocytes were transplanted in the spleen of 90 % hepatectomized-mice by direct injection into the splenic pulp. The animals were followed for 30 days to evaluate their survival.
Histological Examination
One week after hepatocyte transplantation (HTX), splenectomy was performed in some animals for histological examinations. The rest of the animals were followed up post-transplantation for up to 30 days. To prevent dehydration of the experimental animals, subcutaneous injection of 2ml of saline was conducted on day 1 and on day 2 after 90% hepatectomy.
The liver and spleen specimens obtained from the mice were harvested and fixed with 10% buffered formalin, embedded in paraffin and processed for staining with hematoxylin and eosin (H&E).
The spleen specimens obtained from the mice transplanted with porcine hepatocytes were also cryopreserved with optimal cutting temperature Tissue TECH compound (R&D Systems, Minneapolis, MN). Five-micrometer sections were prepared for immunofluorescence staining for porcine albumin expression using goat anti-pig albumin antibody (Bethyl, laboratories Inc.)and followed by FITC-conjugated mouse anti-goat IgG (Sigma). Localization of albumin was visualized by an Axiophot FL fluorescence microscope (CarlZeiss). Transferase-mediated dUTP nick end labeling (TUNEL) assays was used to examine apoptosis in the transplanted porcine hepatocytes using an in situ cell death detection system, TMR Red (Roche Diagnostics, Mannheim, Germany). The slides were stained in the presence of terminal deoxynucleotidyl transferase enzyme according to the manufacturer’s protocol (Roche Diagnostics, Indianapolis, IN). Red nuclei with nuclear condensation in stained cells were considered as TUNEL positive.
Ultrastuctural Analysis
For Transmission Electron Microscope (TEM), specimens were fixed first in 2.5% glutaraldehyde in 0.1 M PB, and then in 1.0% OsO4 in 0.1 M PB (pH 7.2). The samples were dehydrated through graded concentrations of ethanol and embedded in Epon, as previously reported (19).
Ultra thin sections of the samples were double-stained with uranyl, and observed under TEM. Ten different areas were randomly chosen and examined.
RNA isolation and quantitative RT-PCR analysis
To evaluate the capacity of porcine hepatocytes to maintain gene expression after in vivo transplantation, total RNA was extracted from the spleen containing the transplanted porcine hepatocytes under different conditions, using TRIzol (Invitrogen) reagent according to the manufacturer’s instruction. Porcine liver was used as a positive control. Reverse transcription (RT) was performed at 22°C for 10 minutes and then at 42°C for 20 minutes using 1.0 μg of RNA per reaction, to ensure that the amount of amplified DNAs was proportional to those of specific mRNAs in the original samples, as previously reported (18) using specific porcine primers. The following specific primers used were: albumin, sense 5′-cttattccaggggtctgtttc-3′, antisense 5′-tcgtttctctcaggctcttct-3′, G3PDH, sense 5′-catcatccctgcttctaccg-3′, and antisense 5′-cctgcttcaccactttcttg-3′. The PCR products were resolved on 1% agarose gels and visualized by ethidium bromide staining. Glyceraldehyde-3-phosphate dehydrogenase (G3PDH) served as an internal control for the efficiency of mRNA isolation and cDNA synthesis.
Statistical analyses
Mean values are presented with standard deviations (SDs). A two-tailed student’s t test was used to calculate the significance of difference in mean values. The Kaplan-Meier method was used to calculate the survival data, and their significance was determined by the Mann-Whitney U test. Bonferroni correction was conducted. A P value < 0.05 was considered statistically significant.
RESULTS
Transplantation of SAPNF- hepatocytes prolonged the survival of ALF mice
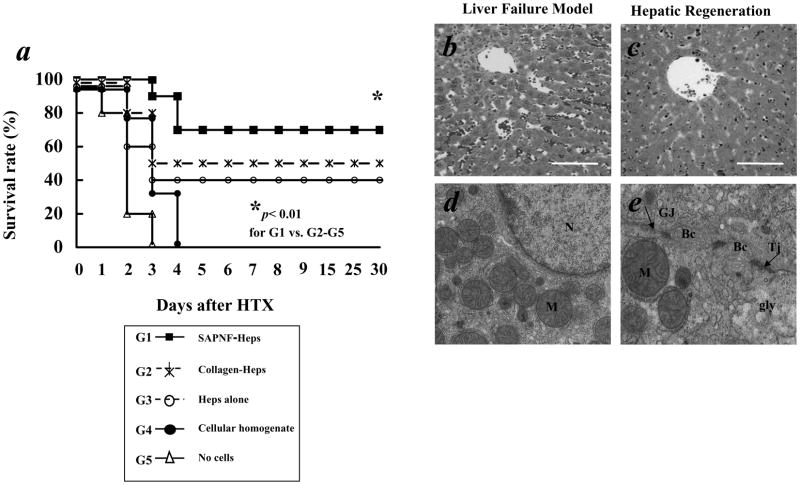
We evaluated the effect of transplantation of SAPNF-porcine hepatocytes on the survival of SCID mice suffering from ALF induced by 90% hepatectomy. After HTX, the mice receiving SAPNF-hepatocytes showed better general health than mice with transplantation of collagen-treated hepatocytes. The 30-day survival rate of the mice was 70% for SAPNF-hepatocyte transplantation, 50% for collagen-hepatocyte transplantation and 40% for hepatocyte transplantation alone (Fig. 2-A). Notably, all SCID mice in negative controls died of ALF within 4 days after 90% hepatectomy, which was confirmed by the histological examinations (Fig. 2-B,C).
Fig. 2. Survival of hepatectomized mice after Hepatocyte transplantation and histological findings of ALF mice.
We evaluated the effect of SAPNF transplantation on the surface of the spleen containing porcine hepatocytes on SCID mice that were hepatectomized (90% liver removal). (A) survival was determined for 30 days. Seventy percent survival rate at 30 days was achieved in the mice with SAPNF-porcine hepatocyte transplantation (G1, n=10). On the other hand 50%, and 40% survival rate was achieved with Collagen-porcine hepatocytes (G2, n=10), and hepatocytes alone (G3, n=10), respectively. In contrast, control mice with transplantation of cellular homogenate (G4, n=10) and no hepatocytes (G5, n=10) died within 4 days due to liver failure. * indicates p < 0.05 for G1 vs G2, G3, G4 and G5.
(B) The liver specimen obtained from control mice that died of ALF demonstrated massive liver necrosis and hemorrhage (original magnification; x100). (C) The liver of the mice regenerated and showed the normal structure when sacrificed on the 30th day post-transplant (original magnification; x100). Bar= 100 micrometer.
(D) TEM showed that SAPNF-hepatocytes demonstrated well-preserved nuclei (N), active mitochondria (M). (E) Gap-junction (Gj) and Tight Junction (Tj) between the cells, well-developed bile caniculi (Bc) and glycogen rosettes (Gly); (original magnification; x 15, 000).
SAPNF-hepatocyte transplantation promoted hepatocyte regeneration, and maintained hepatocyte ultra-structure
All of 90% hepatectomized mice without hepatocyte transplantation (control mice) died of ALF, demonstrating jaundice and hemorrhage, confirmed by the histological examination of the liver of massive necrosis and hemorrhage (Fig. 2-B). In contrast, surviving mice with HTX showed almost normal structure of the liver on day 30 (Fig. 2-C) when they were sacrificed for histological examinations. TEM indicated good morphology among hepatocytes with cytoplasm and organelles well preserved, nucleus with normal chromatin and dense nucleolus, bile canaliculi and gap junction between the cells as well as glycogen particles, indicative of functional hepatocytes (Fig. 2-D,E).
Incorporation of transplanted hepatocytes into the splenic parenchyma (Figs 3 & 4)
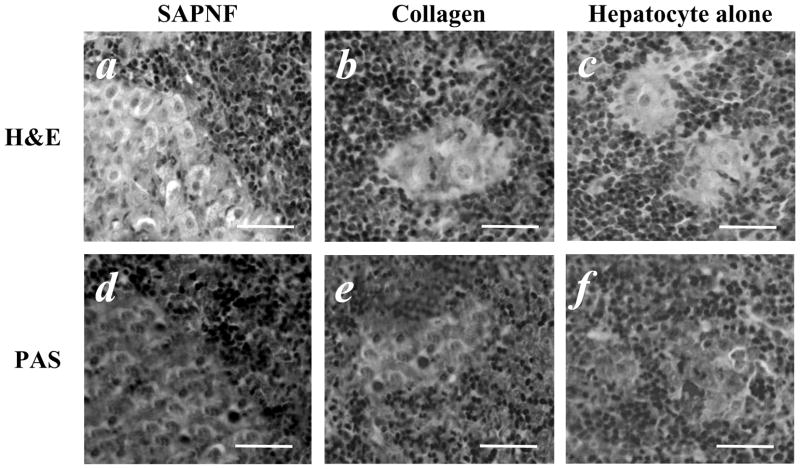
Fig. 3. Histological findings of the spleen after porcine hepatocyte transplantation.
(A) In the spleen removed 7 days after porcine hepatocyte transplantation, a considerable number of porcine hepatocytes were detected when SAPNF was used for transplantation, they had a trabecular arrangement, and demonstrated capacity for glycogen storage as demonstrated by PAS staining (D). (B, E) small clusters of porcine hepatocytes, weakly stained for PAS where observed when collagen was used. (C, F) Few hepatocytes were detected when transplanted alone (original magnification; x 40). Bars=50μm
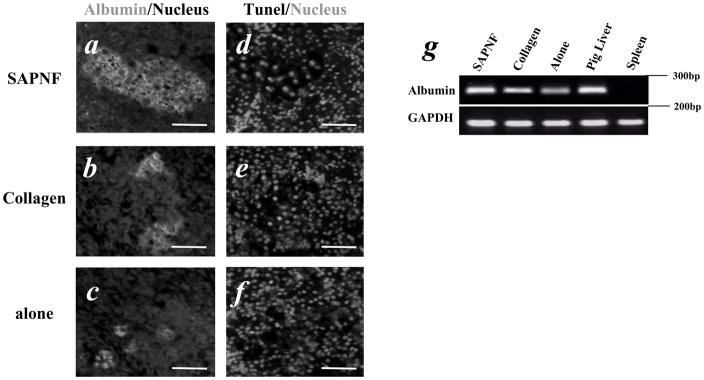
Fig. 4. Immunohistological, apoptotic and gene expression analysis in porcine hepatocytes after transplantation.
(A) Immunofluorescent study to detect albumin expression indicated by green signal indicated strong expression of albumin in hepatocytes transplanted with SAPNF, (B) collagen-hepatocyte transplantation, (C) hepatocytes alone. (D), less apoptotic events indicated by red signal in SAPNF hepatocytes, (E) collagen hepatocytes with more apoptotic cells and (F) hepatocytes alone. (Original magnification; x 20). Bars=50μm (G) Albumin gene expression in the spleen containing porcine hepatocytes. Note the higher expression when SAPNF was used as a substrate. (negative control = spleen without hepatocyte transplantation, positive control= porcine liver).
H&E study of the spleen removed on day 7 from the mice with SAPNF-porcine hepatocyte transplantation demonstrated viable porcine hepatocytes with trabecular arrangement (Fig. 3-A). The hepatocytes forming these clusters were strongly positive to PAS staining (Fig. 3-D). On the other hand, there were considerably smaller clusters of the transplanted hepatocytes when collagen was used for transplantation or when hepatocytes were transplanted alone (Fig. 3-B,C). These clusters were weakly stained with PAS (Fig. 3-E,F). Immunofluorescent study demonstrated a stronger expression of albumin, indicated by a green signal, in SAPNF-porcine hepatocytes (Fig. 4-A) than collagen-ones and hepatocytes transplanted alone (Fig. 4-B,C). In addition, TUNEL positive apoptotic transplanted hepatocytes, indicated by a red signal, were much less in SAPNF-porcine hepatocytes (Fig. 4-D) than hepatocytes transplanted with collagen (Fig. 4-E). Considerably smaller clusters of hepatocytes were observed in the spleen of the mice receiving HTX alone. Such transplanted cells showed higher apoptotic signals (Fig. 4-F).
Gene expression profile of the porcine hepatocytes
To determine the degree to which porcine hepatocytes were able to maintain the capacity of hepatic specific gene expression once transplanted between divergent species, albumin was analyzed. We used reverse transcriptase (RT)-PCR to analyze gene expression of albumin (Alb) from the spleen of the transplanted animals. Albumin gene expression was maintained with higher intensity in animals transplanted with SAPNF-porcine hepatocytes (Fig. 4-G). Albumin gene expression was also detected in animals transplanted with collagen-porcine hepatocytes, although to a lesser extent (Fig. 4-G). Similar results were observed when hepatocytes were transplanted alone.
DISCUSSION
Hepatocytes are highly differentiated cells in the normal liver. However, once isolated, the cells quickly lose their differentiated phenotype (12). The maintenance of the differentiated function of the isolated hepatocytes is a crucial issue in the development of liver-cell based therapies. The surrounding environment actively affects differentiated characteristics of the hepatocytes and maintenance of the liver-specific functions requires a basement membrane-like matrix as a scaffold. Focusing on the lack of available livers to performed therapies that help patients to bring them a temporal support while an organ becomes available or their own liver regenerate, we decided to test functions of porcine hepatocytes, because of their unlimited supply (25).
In this study, we have applied a 3-D scaffold, SAPNF, for transplantation of porcine hepatocytes in an ectopic site (the spleen) in order to improved the cell engraftment and maintain their function to help in the recovery of mice with liver failure. This material previously tested for hepatocyte culture by our group was able to provide a three-dimensional environment and a good attachment condition to the cells. Such atmosphere promoted cell-cell and cell-matrix interactions, which coordinately modulate liver transcription factors and allowed the cultured hepatocytes to perform sophisticated functions (26).
We have established a simple and functional transplantation procedure that can be used as a step for cell-based therapies. This method using SAPNF supported not only the engraftment of transplanted hepatocytes but also the viability, resulting in a better survival rate of animals suffering from ALF. In addition, these hepatocytes had the ability to expressed albumin similar to the native liver, as evaluated by immunostaining and gene expression analysis. This clearly demonstrated that SAPNF-porcine hepatocytes integrated into the splenic pulp and interacted with surrounding cells and the scaffold to build their own niche for a better survival. Although the survival in mice treated with SAPNF-porcine hepatocyte transplantation was significantly better than the mice transplanted with collagen-porcine hepatocytes or hepatocytes alone, we lost however some animals. To overcome this problem, would be advantageous, to improved the conditions in which hepatocytes are placed, by combination of growth factors such as HGF (dHGF) (6,33), because HGF is one of the most important key factors to facilitate liver regeneration after experimental hepatectomy (11,22), and to promote hepatocyte differentiation (33). Additional factors that might induce the decrease of hepatocyte functions in vivo would be the lack of liver non-parenchymal cells for cell-cell interactions. Our group has already demonstrated the importance of liver non-parenchymal cells in the differentiation of Mouse Embryonic Stem cells into hepatocytes (32). Experiments are now on-going to establish the functional in vitro hepatocyte culture system using co-culture of hepatocytes with liver non-parenchymal cells and the supplementation of anti-apoptotic molecules in conjunction with SAPNF.
The spleen is one of the most attractive sites for cell transplantation since it is readily reachable, allocates big numbers of cells and a splenectomy is a quite safe procedure if complications or extraction of transplanted cells is required. However, the main problem in transplanting cells into the spleen has been the insufficient engraftment of hepatocytes and low survival (7). The present results, clearly demonstrate that hepatocytes could be efficiently engrafted into the spleen when SAPNF was used as a substratum. The engrafted cells, demonstrated an efficient capacity to storage glycogen, and to express albumin. Moreover, a lesser extends of apoptotic events were found in these hepatocytes. The implications of these findings include the possibility that SAPNF works as a true three-dimensional extracellular matrix helping hepatocytes to maintain normal cell-matrix and cell interactions, thus preventing apoptosis events causing by deprivation of ECM. It is well known that interactions between cells and their surrounding environment, such as extracellular matrices, and growth and nutrition factors play a pivotal role in tissue morphogenesis, cell migration, survival and differentiation of a variety of cell types. In the case of hepatocytes, sustained expression of liver-specific function requires a basement membrane-like matrix as a substratum, which in this case, SAPNF played that role.
SAPNF is an appropriate scaffold, which has been shown to be biocompatible, is nontoxic and nonimmunogenic (8,15). In our study, we agreed with previous in vivo results, since we did not find adverse effects or cytotoxicity in porcine hepatocytes or in the host.
An additional advantage is that the breakdown products of SAPNF are amino acids, that can be easily degraded over time and work as tissue building blocks by the cells which helps to avoid occurrences of unwanted side effects (10).
In summary, the present study demonstrates an efficient transplantation system to use as a platform for hepatocyte transplantation. Because of the simplicity of our approach, such an intervention could represent a possibility for future application in tissue-basedtherapies.
Footnotes
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
The authors declare that there are no potential conflicts of interest relevant to this publication.
References
- 1.Arterburn LM, Zurlo J, Yager JD, Overton RM, Heifetz AH. A morphological study of differentiated hepatocytes in vitro. Hepatology. 1995;22(1):175–187. [PubMed] [Google Scholar]
- 2.Bissell DM, Caron JM, Babiss LE, Friedman JM. Transcriptional regulation of the albumin gene in cultured rat hepatocytes. Role of basement-membrane matrix. Mol Biol Med. 1990;7(2):187–197. [PubMed] [Google Scholar]
- 3.Cameron AM, Ghobrial RM, Yersiz H, Farmer DG, Lipshutz GS, Gordon SA, Zimmerman M, Hong J, Collins TE, Gornbein J. Optimal utilization of donor grafts with extended criteria: a single-center experience in over 1000 liver transplants. Ann Surg. 2006;243(6):748–753. doi: 10.1097/01.sla.0000219669.84192.b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canaple L, Nurdin N, Angelova N, Saugy D, Hunkeler D, Desvergne B. Maintenance of primary murine hepatocyte functions in multicomponent polymer capsules--in vitro cryopreservation studies. J Hepatol. 2001;34(1):11–18. doi: 10.1016/s0168-8278(00)00086-6. [DOI] [PubMed] [Google Scholar]
- 5.Chari RS, Collins BH, Magee JC, DiMaio JM, Kirk AD, Harland RC, McCann RL, Platt JL, Meyers WC. Brief report: treatment of hepatic failure with ex vivo pig-liver perfusion followed by liver transplantation. N Engl J Med. 1994;331(4):234–237. doi: 10.1056/NEJM199407283310404. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Kobayashi N, Suzuki S, Soto-Gutierrez A, Rivas-Carrillo JD, Tanaka K, Navarro-Alvarez N, Fukazawa T, Narushima M, Miki A. Transplantation of human hepatocytes cultured with deleted variant of hepatocyte growth factor prolongs the survival of mice with acute liver failure. Transplantation. 2005;79(10):1378–1385. doi: 10.1097/01.tp.0000160813.37515.97. [DOI] [PubMed] [Google Scholar]
- 7.David P, Alexandre E, Audet M, Chenard-Neu MP, Wolf P, Jaeck D, Azimzadeh A, Richert L. Engraftment and albumin production of intrasplenically transplanted rat hepatocytes (Sprague-Dawley), freshly isolated versus cryopreserved, into Nagase analbuminemic rats (NAR) Cell Transplant. 2001;10(1):67–80. [PubMed] [Google Scholar]
- 8.Davis ME, Motion JP, Narmoneva DA, Takahashi T, Hakuno D, Kamm RD, Zhang S, Lee RT. Injectable self-assembling peptide nanofibers create intramyocardial microenvironments for endothelial cells. Circulation. 2005;111(4):442–450. doi: 10.1161/01.CIR.0000153847.47301.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunn JC, Tompkins RG, Yarmush ML. Long-term in vitro function of adult hepatocytes in a collagen sandwich configuration. Biotechnol Prog. 1991;7(3):237–245. doi: 10.1021/bp00009a007. [DOI] [PubMed] [Google Scholar]
- 10.Ellis-Behnke RG, Liang YX, Tay DK, Kau PW, Schneider GE, Zhang S, Wu W, So KF. Nano hemostat solution: immediate hemostasis at the nanoscale. Nanomedicine. 2006;2(4):207–215. doi: 10.1016/j.nano.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43(2 Suppl 1):S45–53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- 12.Fisher RA, Bu D, Thompson M, Wolfe L, Ritter JK. Optimization of conditions for clinical human hepatocyte infusion. Cell Transplant. 2004;13(6):677–689. doi: 10.3727/000000004783983576. [DOI] [PubMed] [Google Scholar]
- 13.Fisher RA, Strom SC. Human hepatocyte transplantation: worldwide results. Transplantation. 2006;82(4):441–449. doi: 10.1097/01.tp.0000231689.44266.ac. [DOI] [PubMed] [Google Scholar]
- 14.Fox IJ, Chowdhury JR, Kaufman SS, Goertzen TC, Chowdhury NR, Warkentin PI, Dorko K, Sauter BV, Strom SC. Treatment of the Crigler-Najjar syndrome type I with hepatocyte transplantation. N Engl J Med. 1998;338(20):1422–1426. doi: 10.1056/NEJM199805143382004. [DOI] [PubMed] [Google Scholar]
- 15.Horii A, Wang X, Gelain F, Zhang S. Biological designer self-assembling Peptide nanofiber scaffolds significantly enhance osteoblast proliferation, differentiation and 3-d migration. PLoS ONE. 2007;2:e190. doi: 10.1371/journal.pone.0000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horslen SP, Fox IJ. Hepatocyte transplantation. Transplantation. 2004;77(10):1481–1486. doi: 10.1097/01.tp.0000113809.53415.c2. [DOI] [PubMed] [Google Scholar]
- 17.Kimata T, Nagaki M, Ogiso T, Naiki T, Kato T, Moriwaki H. Actin organization and hepatocyte differentiation are regulated by extracellular matrix via PI-4,5-bisphosphate in the rat. Hepatology. 2006;44(1):140–151. doi: 10.1002/hep.21215. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi N, Fujiwara T, Westerman KA, Inoue Y, Sakaguchi M, Noguchi H, Miyazaki M, Cai J, Tanaka N, Fox IJ. Prevention of acute liver failure in rats with reversibly immortalized human hepatocytes. Science. 2000;287(5456):1258–1262. doi: 10.1126/science.287.5456.1258. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi N, Taguchi T, Noguchi H, Okitsu T, Totsugawa T, Watanabe T, Matsumura T, Fujiwara T, Urata H, Kishimoto N. Rapidly functional immobilization of immortalized human hepatocytes using cell adhesive GRGDS peptide-carrying cellulose microspheres. Cell Transplant. 2001;10(4–5):387–392. [PubMed] [Google Scholar]
- 20.Kunieda T, Maruyama M, Okitsu T, Shibata N, Takesue M, Totsugawa T, Kosaka Y, Arata T, Kobayashi K, Ikeda H. Cryopreservation of primarily isolated porcine hepatocytes with UW solution. Cell Transplant. 2003;12(6):607–616. doi: 10.3727/000000003108747217. [DOI] [PubMed] [Google Scholar]
- 21.Lee WM. Acute liver failure. N Engl J Med. 1993;329(25):1862–1872. doi: 10.1056/NEJM199312163292508. [DOI] [PubMed] [Google Scholar]
- 22.Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276(5309):60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 23.Mikos AG, Bao Y, Cima LG, Ingber DE, Vacanti JP, Langer R. Preparation of poly(glycolic acid) bonded fiber structures for cell attachment and transplantation. J Biomed Mater Res. 1993;27(2):183–189. doi: 10.1002/jbm.820270207. [DOI] [PubMed] [Google Scholar]
- 24.Mikos AG, Sarakinos G, Leite SM, Vacanti JP, Langer R. Laminated three-dimensional biodegradable foams for use in tissue engineering. Biomaterials. 1993;14(5):323–330. doi: 10.1016/0142-9612(93)90049-8. [DOI] [PubMed] [Google Scholar]
- 25.Nagata H, Nishitai R, Shirota C, Zhang JL, Koch CA, Cai J, Awwad M, Schuurman HJ, Christians U, Abe M. Prolonged survival of porcine hepatocytes in cynomolgus monkeys. Gastroenterology. 2007;132(1):321–329. doi: 10.1053/j.gastro.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 26.Navarro-Alvarez N. Self-Assembling Peptide Nanofiber as a Novel Culture System for Isolated Porcine Hepatocytes. Cell Transplant. 2006;15(10):921–927. doi: 10.3727/000000006783981387. [DOI] [PubMed] [Google Scholar]
- 27.Nishitai R, Koch CA, Ogata K, Knudsen BE, Plummer TB, Butters KA, Platt JL. Toward the survival and function of xenogeneic hepatocyte grafts. Liver Transpl. 2005;11(1):39–50. doi: 10.1002/lt.20305. [DOI] [PubMed] [Google Scholar]
- 28.Saavedra YG, Mateescu MA, Averill-Bates DA, Denizeau F. Polyvinylalcohol three-dimensional matrices for improved long-term dynamic culture of hepatocytes. J Biomed Mater Res A. 2003;66(3):562–570. doi: 10.1002/jbm.a.10583. [DOI] [PubMed] [Google Scholar]
- 29.Selden C, Khalil M, Hodgson HJ. What keeps hepatocytes on the straight and narrow? Maintaining differentiated function in the liver. Gut. 1999;44(4):443–446. doi: 10.1136/gut.44.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Semino CE, Merok JR, Crane GG, Panagiotakos G, Zhang S. Functional differentiation of hepatocyte-like spheroid structures from putative liver progenitor cells in three-dimensional peptide scaffolds. Differentiation. 2003;71(4–5):262–270. doi: 10.1046/j.1432-0436.2003.7104503.x. [DOI] [PubMed] [Google Scholar]
- 31.Shapiro L, Cohen S. Novel alginate sponges for cell culture and transplantation. Biomaterials. 1997;18(8):583–590. doi: 10.1016/s0142-9612(96)00181-0. [DOI] [PubMed] [Google Scholar]
- 32.Soto-Gutierrez A, Kobayashi N, Rivas-Carrillo JD, Navarro-Alvarez N, Zhao D, Okitsu T, Noguchi H, Basma H, Tabata Y, Chen Y. Reversal of mouse hepatic failure using an implanted liver-assist device containing ES cell-derived hepatocytes. Nat Biotechnol. 2006;24(11):1412–1419. doi: 10.1038/nbt1257. [DOI] [PubMed] [Google Scholar]
- 33.Soto-Gutierrez A, Navarro-Alvarez N, Rivas-Carrillo JD, Chen Y, Yamatsuji T, Tanaka N, Kobayashi N. Differentiation of human embryonic stem cells to hepatocytes using deleted variant of HGF and poly-amino-urethane-coated nonwoven polytetrafluoroethylene fabric. Cell Transplant. 2006;15(4):335–341. doi: 10.3727/000000006783981945. [DOI] [PubMed] [Google Scholar]
- 34.Strain AJ, Neuberger JM. A bioartificial liver--state of the art. Science. 2002;295(5557):1005–1009. doi: 10.1126/science.1068660. [DOI] [PubMed] [Google Scholar]
- 35.Strom SC, Fisher RA, Thompson MT, Sanyal AJ, Cole PE, Ham JM, Posner MP. Hepatocyte transplantation as a bridge to orthotopic liver transplantation in terminal liver failure. Transplantation. 1997;63(4):559–569. doi: 10.1097/00007890-199702270-00014. [DOI] [PubMed] [Google Scholar]
- 36.Yokoi H, Kinoshita T, Zhang S. Dynamic reassembly of peptide RADA16 nanofiber scaffold. Proc Natl Acad Sci USA. 2005;102(24):8414–8419. doi: 10.1073/pnas.0407843102. [DOI] [PMC free article] [PubMed] [Google Scholar]