Abstract
CMX001, an orally active lipid conjugate of cidofovir (CDV), is 50 times more active in vitro against herpes simplex virus (HSV) replication than acyclovir (ACV) or CDV. These studies compared the efficacy of CMX001 to ACV in BALB/c mice inoculated intranasally with HSV types 1 or 2. CMX001 was effective in reducing mortality using doses of 5 to 1.25 mg/kg administered orally once daily even when treatments were delayed 48-72 h post viral inoculation. Organ samples from mice treated with CMX001 had titers three to five log10 pfu/gram of tissue lower than samples from ACV treated mice, including five different regions of the brain. Detectable concentrations of drug-related radioactivity were documented in the central nervous system of mice following oral administration of 14C-CMX001. These studies indicated that CMX001 penetrates the blood brain barrier, is a potent inhibitor of HSV replication in disseminated and CNS infections and is superior to ACV.
Introduction
Herpes simplex virus (HSV) infects approximately 1500 neonates annually, many of whom will have central nervous system disease, and HSV encephalitis occurs in children over 6 months of age and in adults at 1 in 150,000 annually in the US [1-5]. Rapid diagnosis and early intervention with acyclovir (ACV) have improved survival and decreased long term neurological sequelae; however, mortality still exceeds 25% and over 50% of survivors have significant neurologic impairment [5-17]. Thus, a need exists for antiviral medications with improved distribution to the brain and greater efficacy. For the past 20 years ACV has been the only FDA-approved drug and is the standard of care for treatment of disseminated and CNS HSV infections in neonates and herpes encephalitis of older children and adults. Experimental studies in animals infected with HSV have demonstrated that ACV was effective in reducing mortality and viral replication in CNS tissues, as well as, in visceral organs and that ACV was superior to what had been previously reported for vidarabine [18-21]. These results in animal models were highly predictive for the outcome in clinical studies. Treatment with either vidarabine or ACV resulted in efficacy as evidenced by decreased mortality, but many survivors had permanent neurological sequelae suggesting that improved therapies for these diseases were needed [5, 15, 17, 22-23].
CMX001 (hexadecyloxypropyl cidofovir, HDP-CDV) was developed as an orally active, lipophilic form of cidofovir (CDV). It has enhanced activity in vitro and in vivo compared to CDV against certain herpesviruses, adenoviruses and orthopoxviruses [24-30]. CMX001 was studied previously in animal models of cytomegaloviruses (CMV) and orthopoxviruses and shown to be effective against murine [27] and human CMV, [31], vaccinia, cowpox,[32] and ectromelia virus infections in mice [33-34]and in primate models using monkeypox virus [35]. It was also synergistic when co-administered in vitro or in vivo with ST-246, a compound under development for treatment of orthopoxviruses[36]. CMX001 is currently in Phase II clinical studies for development as a therapeutic agent for human CMV, adenovirus and BK virus infections, as well as, for adverse events following smallpox vaccinations.
The purpose of the current studies was to determine the efficacy of CMX001 compared with ACV in two models of herpes encephalitis and disseminated neonatal herpes. These data were then correlated with the results from a quantitative whole body distribution study in uninfected mice using orally administered radiolabeled CMX001.
Materials and Methods
Experimental animals
Female BALB/c mice were purchased from Charles River Laboratories (Raleigh, North Carolina) at 3 - 4 weeks of age. Animals were quarantined and acclimated for 3 days prior to use. Mice were group housed in microisolator cages and utilized at a quantity of 15 mice per treatment group. They were obtained, housed, utilized and euthanized according to USDA and AAALAC regulatory policies. All animal procedures were approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee prior to initiation of studies.
Viruses and cells
The strain of HSV-1 utilized was E-377and the strain of HSV- 2 was MS. The origin of these viruses has been reported previously [29]. HFF cells were prepared as primary cultures from freshly obtained newborn human foreskins. Virus pools were prepared and quantified in primary rabbit kidney cells for use in vitro and in vivo. Culture medium for both cell lines was MEM with Earle’s salts containing 10% FBS and 2μM L-glutamine, 100 units/ml penicillin and 25 mM gentamicin.
Antiviral compounds
CMX001 was kindly provided by Chimerix, Inc. (Durham, North Carolina) and was suspended in 0.4% carboxymethylcellulose (CMC) for oral delivery to mice. ACV (Sigma Co., St. Louis, Missouri) was weighed and suspended in sterile water for oral treatment of mice. Compounds were prepared in a 0.2 ml volume which was administered once daily (CMX001) or twice daily (ACV). Treatments for efficacy evaluations were administered to mice for 7 consecutive days beginning 24-72 h post viral inoculation by oral gavage using doses of 5, 2.5 and 1.25 mg/kg of CMX001 administered once daily or doses of 120, 60 or 30 mg/kg of ACV given twice daily at approximately 12 h intervals. For pathogenesis experiments, treatments began 24 h post viral inoculation and were administered for 7 consecutive days using 5 mg/kg of CMX001 or 100 mg/kg of ACV. These doses were selected based upon the results obtained in the mortality experiments.
Experimental infections
Mice were manually restrained for intranasal inoculations using a total volume of 0.04 ml/mouse containing an approximate LD90 of either HSV-1, E-377 or HSV-2, strain MS. For these studies, the inoculum contained 4.4 × 104 pfu/mouse for HSV-1 or 1.1 × 105 pfu/mouse for HSV-2. For mortality experiments, animals were evaluated at least once daily for 21 days and four times daily during peak occurrence of clinical neurological signs so that mice could be humanely euthanized prior to death. Pathogenesis studies were performed for both HSV-1 and HSV-2 in order to compare the effect of CMX001 and ACV on viral replication of both viral types in target organs of mice. Three mice each from vehicle- and drug-treated groups were euthanized on days 1, 3, 5, 7 or 10 post inoculation for collection of lung, liver, spleen, kidney, olfactory bulbs, cerebral cortex, pons/medulla, diencephalon, cerebellum and trigeminal ganglia. Organ samples were pooled by tissue type and homogenized in a 10% w/v suspension, and frozen until assayed for virus. Virus titers were determined by plating of tissue homogenates on HFF cells and plaques were enumerated after three days incubation. The trigeminal ganglia were collected individually and co-cultured directly on primary rabbit kidney (RK) cells with N’ N’ dimethylbisacetamide for detection of latent virus as described previously [37]. Briefly, the ganglia were minced, placed onto tissue culture cell monolayers and monitored for viral cytopathic effects for 3 weeks. Ganglia were transferred weekly onto fresh RK cells with new media.
Statistical evaluation
Mortality rates were analyzed by Fisher’s exact test and the mean day of death (MDD) results were evaluated using the Mann-Whitney U rank sum test. A p value of ≤0.05 was considered significant when comparing each treated group to the vehicle treated group. There was no adjustment made for multiple group analysis.
Quantitative drug distribution study
This study was conducted in compliance with USDA Animal Welfare Act regulations, 9CFR 1-4. The procedures in the study were reviewed and approved by the Institutional Animal Care and Use Committee at QPS, LLC (Newark, DE). (C2-14C)-CMX001, monosodium salt (Moravek Biochemicals, Inc., Brea, CA; radiochemical purity >98%) was administered orally by gavage as a solution (10 mL /kg) to fed-state, male CD-1 albino mice at doses of 2.5, 5 and 10 mg/kg (220 to 890 μCi/kg). The disposition of radioactivity was quantified using whole body autoradiography at selected time points up to 72 h (n=1 animal per time point). After inducing deep anesthesia, each animal was euthanized at the selected time point. The mouse carcass was imbedded in 2% carboxymethylcellulose, frozen and sectioned in a Leica CM 3600 cryo-microtome maintained at approximately −20 °C. Sagittal animal sections containing all major organs were exposed to Molecular Dynamics phosphor imaging screens along with 14C-blood autoradiographic standards (in triplicate) for subsequent calibration by the image analysis software. Exposed screens were scanned using a Molecular Dynamics Typhoon phosphor imager. The co-exposed autoradiographic standards provided a calibration reference which linked levels of measured density counts to radioactivity per unit mass. Radioactive concentrations in each tissue were converted to μg-equivalents / g of tissue, using the known specific activity of the 14C-CMX001 that was administered. When possible, tissues were sampled in representative areas and at several sectioning levels to reduce sampling variance. In these cases, average values were determined and reported. The Lower Limit of Quantitation (LLOQ) was determined for each imaging plate and ranged between 0.013 to 0.016 μg equiv /g and the mean LLOQ was 0.015 μg equiv /g. Reported values below the LLOQ are the mean results obtained using > 1 sampling value of which some were above and below the LLOQ. Values that were below the LLOQ were treated as zero and were included in the mean.
Results
Efficacy studies in vivo
When CMX001 was administered orally to mice infected with HSV-1, mortality was reduced significantly (p≤0.001) with all three dose levels when treatments were initiated 24 h post viral inoculation (Table 1). When treatments were started 48 h post viral inoculation, 5 and 2.5 mg/kg significantly reduced mortality (p≤ 0.001). If treatments were delayed until 72 h post viral inoculation, CMX001 did not reduce mortality or increase the mean day to death. Although ACV was effective at all 3 times of initiation of therapy (p≤0.001), approximately ten-fold more drug was required.
Table 1. Effect of Treatment with CMX-001 or ACV on Mortality of BALB/c Mice Inoculated Intranasally with HSV-1 strain E-377.
| Mortality | |||||
|---|---|---|---|---|---|
| Treatmenta | Number | Percent | p-Value | MDD±SDb | p-Value |
| + 24 h | |||||
| Untreated | 15/15 | 100 | --- | 7.0 ± 14 | --- |
| Vehicle | 15/15 | 100 | --- | 5.2 ± 0.8 | --- |
| ACV 120 mg/kg | 0/15 | 0 | <0.001 | ||
| 60 mg/kg | 1/15 | 7 | <0.001 | 11.0 | 0.001 |
| 30 mg/kg | 7/15 | 47 | <0.01 | 9.9 ± 1.1 | NSc |
| CMX001 5 mg/kg | 0/15 | 0 | <0.001 | --- | |
| 2.5 mg/kg | 2/15 | 13 | <0.001 | 8.5 ± 2.1 | 0.001 |
| 1.25 mg/kg | 5/15 | 33 | <0.001 | 8.8 ± 1.1 | NS |
| + 48 h | |||||
| Vehicle | 15/15 | 100 | --- | 7.4 ± 2.0 | --- |
| ACV 120 mg/kg | 0/15 | 0 | <0.001 | --- | |
| 60 mg/kg | 1/15 | 7 | <0.001 | 9.0 | <0.05 |
| 30 mg/kg | 6/15 | 40 | 0.001 | 8.0 ± 1.5 | NS |
| CMX001 5 mg/kg | 4/15 | 27 | <0.001 | 8.5 ± 1.3 | NS |
| 2.5 mg/kg | 3/15 | 20 | <0.001 | 8.0 ± 0.0 | NS |
| 1.25 mg/kg | 15/15 | 100 | NS | 6.5 ± 1.1 | NS |
| + 72 h | |||||
| Vehicle | 15/15 | 100 | --- | 7.7 ± 0.9 | --- |
| ACV 120 mg/kg | 5/15 | 33 | <0.001 | 6.8 ± 1.8 | 0.08 |
| 60 mg/kg | 5/15 | 33 | <0.001 | 8.0 ± 2.0 | NS |
| 30 mg/kg | 9/15 | 60 | <0.05 | 9.9 ± 2.3 | NS |
| CMX001 5 mg/kg | 14/15 | 93 | NS | 7.7 ± 2.6 | <0.001 |
| 2.5 mg/kg | 11/15 | 73 | NS | 8.4 ± 0.8 | NS |
| 1.25 mg/kg | 12/15 | 80 | NS | 8.1 ± 1.2 | 0.001 |
CMX001 was suspended in 0.4% CMC and given orally in 0.2 ml doses. ACV was prepared in sterile water and given orally in 0.2 ml doses. Animals were treated with CMX001 once daily or with ACV twice daily for seven days beginning 24, 48, or 72 h after viral inoculation.
MDD = Mean Day of Death. SD=Standard Deviation.
NS = Not significant when compared to the vehicle control group.
When CMX001 was administered orally to mice infected with HSV-2, mortality was significantly reduced (p≤0.001) with all concentrations when treatments were initiated 24 or 48 h post viral inoculation (Table 2). When treatments were started 72 h post viral inoculation, the 2.5 and 1.25 mg/kg doses significantly reduced mortality (p≤0.001). ACV was effective at all 3 dosages and time points (p≤0.05) but again required higher dosage levels.
Table 2. Effect of Treatment with CMX001 or ACV on Mortality of BALB/c Mice Inoculated Intranasally with HSV-2 strain MS.
| Mortality | |||||
|---|---|---|---|---|---|
| Treatmenta | Number | Percent | p-Value | MDD±SDb | p-Value |
| + 24 h | |||||
| Untreated | 14/15 | 93 | --- | 9.4 ± 2.3 | --- |
| Vehicle | 15/15 | 100 | --- | 7.9 ± 1.1 | --- |
| ACV 120 mg/kg | 0/15 | 0 | <0.001 | --- | --- |
| 60 mg/kg | 1/15 | 7 | <0.001 | 9.0 | NSc |
| 30 mg/kg | 3/15 | 20 | <0.001 | 11.3 ± 1.2 | <0.01 |
| CMX001 5 mg/kg | 0/15 | 0 | <0.001 | --- | --- |
| 2.5 mg/kg | 2/15 | 13 | <0.001 | 10.5 ± 0.7 | <0.05 |
| 1.25 mg/kg | 7/15 | 47 | <0.001 | 9.4 ± 1.4 | <0.05 |
| + 48 h | |||||
| Vehicle | 12/15 | 80 | --- | 9.8 ± 2.7 | --- |
| ACV 120 mg/kg | 0/15 | 0 | <0.001 | --- | --- |
| 60 mg/kg | 1/15 | 7 | <0.001 | 14.0 | NS |
| 30 mg/kg | 1/15 | 7 | <0.001 | 9.0 | NS |
| CMX001 5 mg/kg | 1/15 | 7 | <0.001 | 11.0 | NS |
| 2.5 mg/kg | 4/15 | 27 | <0.001 | 10.5 ± 2.5 | NS |
| 1.25 mg/kg | 3/15 | 20 | <0.001 | 10.3 ± 2.1 | NS |
| + 72 h | |||||
| Vehicle | 13/15 | 87 | --- | 8.9 ± 1.1 | --- |
| ACV 120 mg/kg | 9/15 | 60 | <0.05 | 9.0 ± 1.4 | NS |
| 60 mg/kg | 4/15 | 27 | <0.001 | 11.5 ± 2.6 | 0.07 |
| 30 mg/kg | 5/15 | 33 | <0.001 | 11.0 ± 3.1 | NS |
| CMX001 5 mg/kg | 11/15 | 73 | NS | 9.4 ± 1.6 | NS |
| 2.5 mg/kg | 6/15 | 40 | 0.001 | 11.8 ± 3.7 | <0.05 |
| 1.25 mg/kg | 4/15 | 27 | <0.001 | 10.0 ± 1.8 | NS |
CMX001 was suspended in 0.4% CMC and given orally in 0.2 ml doses. ACV was prepared in sterile water and given orally in 0.2 ml doses. Animals were treated with CMX001 once daily or with ACV twice daily for seven days beginning 24, 48, or 72 h after viral inoculation.
MDD = Mean Day of Death. SD=Standard Deviation.
NS = Not significant when compared to the vehicle control group.
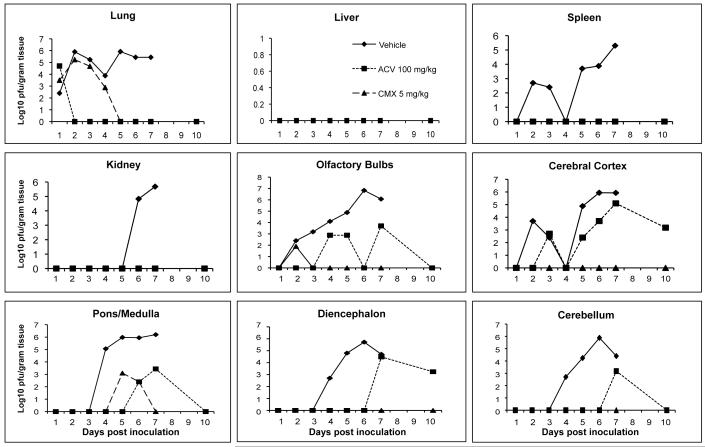
When mice were infected intranasally with HSV-1 and treatments initiated 24 h post viral inoculation using CMX001 at 5 mg/kg or ACV at 100 mg/kg, virus replication in target organs was reduced by both CMX001 and ACV when compared to vehicle treated mice (Figure 1). In lung, liver, kidney and spleen samples, both CMX001 and ACV reduced viral replication below the limits of detection by Day 5 post viral inoculation. A 6 log10 pfu/gram tissue reduction in virus titers in the lung, a critical target organ in disseminated disease, was seen when compared to vehicle treated controls. In all areas of the brain except the pons/medulla region, treatment with CMX001 reduced viral replication below the limits of detection by Day 3 post viral inoculation which indicated a 3-6 log10 pfu/gram tissue reduction when compared to vehicle treated controls. Samples taken on Day 10 after cessation of treatment also remained below the limits of detection for CMX001 treated mice. In contrast, however, samples from ACV treated mice still had detectable levels of virus present in olfactory bulbs, cerebral cortex, cerebellum, pons-medulla and diencephalon at various time points during treatment.
Figure 1.
Efficacy of CMX001 or ACV against HSV-1 replication in target organs of mice. Graphs depict mean viral titers shown as log10 pfu/gram of tissue over days 1 to 10 post viral infection of mice treated 24 hr post viral inoculation with vehicle, 5 mg/kg CMX001 or 100 mg/kg ACV. The limit of detection is 1.39 log10 pfu/gram of tissue.
There were no statistical differences in viral latency rates as determined by co-cultivation of trigeminal ganglia between groups treated with vehicle, ACV, or CMX001 in either HSV-1 or 2 infected mice (data not shown). Viral reactivation as evidenced by the appearance of typical cytopathic effect in RK cell monolayers was observed at an approximately equal rate among treatment groups.
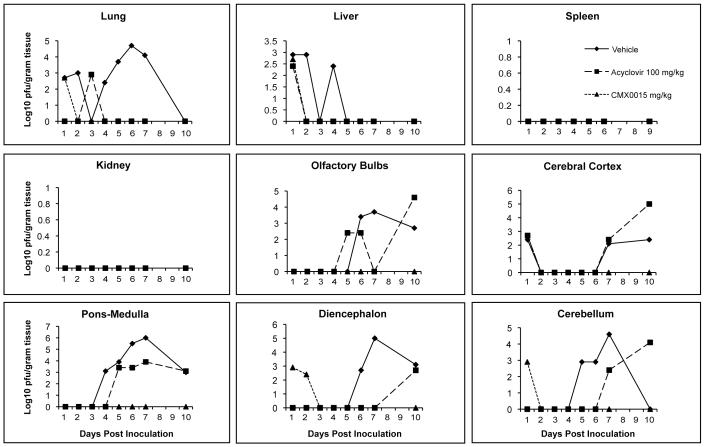
When mice were infected intranasally with HSV-2, and treatments initiated 24 h post viral inoculation using CMX001 at 5 mg/kg or ACV at 100 mg/kg, virus titers were reduced in all target organs by both CMX001 and ACV when compared to vehicle treated mice (Figure 2). In lung and liver samples, both CMX001 and ACV reduced viral replication below the limits of detection by Day 4 post viral inoculation which indicated a 3 log10 pfu/gram tissue reduction when compared to vehicle treated controls. In all areas of the brain, CMX001 reduced viral replication below the limits of detection by Day 3 post viral inoculation which indicated a 4-6 log10 pfu/gram tissue reduction when compared to vehicle treated controls. Samples taken on Day 10 after cessation of treatment also remained below the limits of detection for CMX001 treated mice. However, samples from ACV treated mice, again, showed detectable levels of virus present in olfactory bulbs, cerebral cortex, cerebellum, pons-medulla and diencephalon at various time points during treatment. In addition the samples taken on Day 10 after cessation of ACV treatment, had virus quantities that were equal to or exceeded vehicle treated mice in samples from all 5 regions of the CNS. This result suggested that there may have been a rebound of viral replication in these tissues after cessation of ACV treatment that was not observed in mice treated with CMX001.
Figure 2.
Efficacy of CMX001 or ACV against HSV-2 replication in target organs of mice. Graphs depict mean viral titers shown as log10 pfu/gram of tissue over days 1 to 10 post viral infection of mice treated 24 hr post viral inoculation with vehicle, 5 mg/kg CMX001 or 100 mg/kg ACV. The limit of detection is 1.39 log10 pfu/gram of tissue.
Drug distribution studies
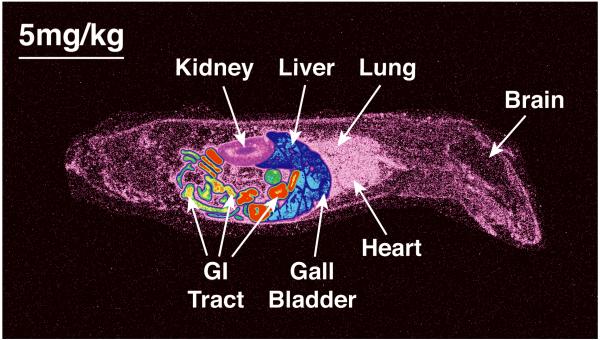
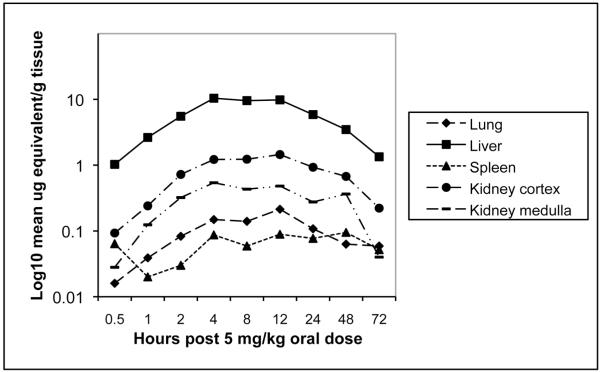
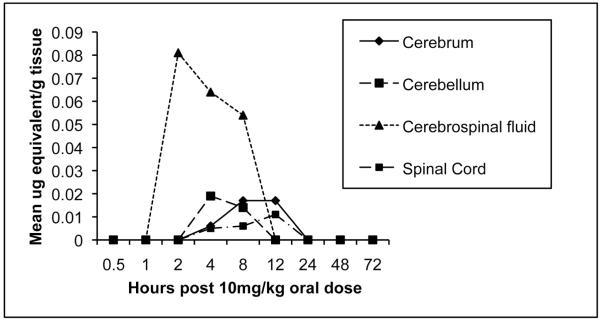
After a single oral administration of 5 mg of 14C-CMX001 /kg to male mice, drug-associated radioactivity was absorbed from the gastrointestinal tract and wide distribution to tissues was observed to occur by 2 h post dosing. Drug distribution was widespread at 4 h (Figure 3) and continued through 24 h. The highest levels of drug were detected in the small intestine and levels in the lung, liver, kidney and spleen ranged from 0.1 μg to 10 μg-equivalents per gm of tissue through 24 h post dosing (Figure 4). Moderate to low levels of drug were detected in remaining tissues and those in which levels were below the limits of quantitation included the brain and spinal cord. However, in mice that received 10 mg of 14C-CMX001 /kg, drug concentrations of 0.01-0.02 μg-eq/g were detected in the CSF, spinal cord and brain (cerebellum and cerebrum) (Figure 5).
Figure 3.
Whole body autoradiography showing biodistribution of [C2-14C] CMX001 4 h post oral gavage using 5 mg/kg in male CD mice. The highest concentrations of radioactivity are depicted by red color, and in decreasing magnitude in orange, yellow, green, blue and magenta colors.
Figure 4.
Concentration of radioactive-labeled drug in visceral organs at specified times post dose determined by whole-body autoradiography after a single oral administration of 5 mg of 14C-CMX001 /kg in male mice. Quantities depicted as log10 mean μg equivalents/gram tissue.
Figure 5.
Concentration of radioactive-labeled drug in CNS tissues at specified times post dose determined by whole-body autoradiography after a single oral administration of 10 mg of 14C-CMX001 /kg in male mice. Quantities depicted as mean μg equivalents/gram tissue.
Discussion
HSV infections of the CNS remain a significant cause of morbidity and mortality in humans, in spite of therapy with ACV. For example, with neonatal HSV-2 infections of the CNS the mortality is low at only 5% but well over 50% of survivors suffer from significant neurologic sequelea [12]. Similarly, with HSV-1 encephalitis of older children and adults the mortality increases to 25% and the morbidity to greater than 50%[5]. Thus, new therapeutic approaches are necessary to improve the outcome of these devastating infections. The current studies contained in this communication indicate that CMX001 may provide an alternative to ACV either by itself or in combination with ACV.
Historically, intranasal infections in mice utilizing HSV-1 have been considered to be an excellent model for human herpes encephalitis as it uses a natural route of infection and has been predictive for the efficacy of both vidarabine and ACV in treatment of human HSV infections [20-21, 38]. Viral replication first occurs in the nasopharynx, then spreads to the CNS by olfactory and trigeminal nerves and has served to model both adult encephalitis and neonatal CNS disease. Viral replication first is detected in the olfactory bulbs and spreads throughout the CNS by neural routes from the day after inoculation until death in untreated mice by day 7. Intranasal inoculation of BALB/c mice with HSV-2 has been used as a model of disseminated neonatal herpes with CNS involvement occurring later after infection and an extended mean day to death of approximately 9 days. These studies illustrate the importance of utilizing both HSV-1 and -2 in efficacy evaluations since HSV encephalitis in adults is predominantly due to HSV-1 and in neonates about 70% of cases are due to HSV-2. These models have been predictive for efficacy of vidarabine and ACV in treatment of both HSV type-1 and -2 infections in humans.
CDV despite being a potent inhibitor of HSV replication in vitro and in experimental animal infections has not been utilized for the treatment of HSV infections in humans due to its lack of oral activity [39] and its nephrotoxicity [40]. However, the drug has been used for the treatment of ACV-resistant HSV infections [41]. Several lipid conjugates of CDV have been synthesized that are active orally in animals, have reduced toxicity and have multi-fold enhanced activity against a variety of viruses including a number of the herpesviruses [24, 30]. The enhanced activity of the lipid conjugates appear to be due to their greater uptake into cells, possibly including the CNS, resulting in a significant increase in the amount of intracellular CDV [42]. The conjugate that has received the most interest is the hexadecyloxypropyl analog of CDV, originally termed HDP-CDV, which is currently under development as CMX001. The interest in this molecule has been in part due to its being highly active against orthopoxviruses, which are suspected agents of bioterror.
In previous studies using cell cultures, CMX001 was about 100-fold more potent as an inhibitor of HSV replication than ACV[30]. In the experimental infections presented in these studies, CMX001 was highly effective in preventing mortality in mice infected with HSV-1 or HSV-2 when treatments were delayed until 48 to 72 h post viral inoculation, respectively by reducing viral replication in the CNS as well as in lung, liver, spleen and kidney. Importantly, CMX001 was more efficacious than ACV in reducing viral replication in CNS in either HSV-1 or HSV-2 infected mice. Additionally, no virus replication was detected in tissues after cessation of therapy with CMX001, whereas, a rebound in virus replication was observed after cessation of therapy with ACV. In these studies, CMX001 given at 5 mg/kg once daily was more efficacious than ACV at 100 mg/kg given twice daily and suggests that CMX001 may have potential for use in the treatment of herpes encephalitis, neonatal herpes or other severe HSV infections in humans.
When CMX001 is delivered orally, it has a very unique tissue distribution profile compared to parenterally administered CDV, which does not cross the blood brain barrier. High levels of drug were found very early in lung, liver and spleen but not kidney. CMX001 had little effect on the microtubules of the kidney and no apparent toxicity [43]. When mice were dosed with 5 mg of CMX001/kg in a quantitative drug distribution study the levels in the CNS were below the level of quantitation, however, detectable levels were observed in mice given 10 mg/kg of drug. In the antiviral studies reported in this paper the highest dose administered was 5 mg/kg once daily for 5 days. Given that the half-life of the activated antiviral form of CMX001, cidofovir-diphosphate, is 6.5 days (unpublished results), drug may accumulate in the CNS after seven daily doses, and reach levels in excess of those seen in the distribution study after a single 10 mg/kg dose. In these studies CMX001 had a significant effect on HSV replication in the CNS when therapy was delayed until 24 h post viral inoculation. This would suggest that only a small of amount of drug is needed in the CNS given its in vitro potency of about 0.03 μg/ml and/or that viral replication is also being inhibited in peripheral tissues and preventing spread to the CNS. The results obtained in these studies suggest that there is, in fact, virus already replicating in parts of the CNS when therapy was initiated and that deliverable drug can, in fact, inhibit replication in the CNS resulting in total clearance of virus from the CNS. The lack of viral persistence after treatment with CMX001 would be in contrast to previous studies showing that mice treated with valacyclovir had persistant virus detected in trigeminal ganglia and brain stem tissues [44]. It should also be pointed out that the drug distribution studies were carried out in uninfected mice and it is certainly feasible that in infected mice additional amounts of drug may have crossed compromised blood/brain or blood/cerebral spinal fluid barriers compared to those observed in uninfected mice. In previous human studies, ACV provided as oral valacyclovir using 1 gram three times daily provided 20% of the serum concentration entering the CSF with active transport out of the CSF compartment[45].
Considering the continuing mortality and long term sequelae of adults and infants infected with HSV and treated aggressively with ACV, CMX001 holds promise for improved outcomes. Its unique cellular uptake and biodistribution may prove more beneficial in particular with clinical cases of encephalitis. In addition, combination studies with CMX001 and ACV both in vitro and in vivo have been carried out and a synergistic interaction without additive toxicity has been demonstrated [46]. This combination may improve considerably the outcome and long term well being of patients infected with these and other herpesviruses and should be considered for use in these infections.
Acknowledgments
This work was supported by NIH, NIAID contracts NOI-AI-15439 and NOI-AI-30049 to the University of Alabama at Birmingham. ERK has an equity interest and serves as a consultant to Chimerix-Inc. The terms of this arrangement has been reviewed and approved by the University of Alabama at Birmingham in accordance with their conflict of interest policies.
The authors would like to thank Richard J. Whitley, M.D. for his expert review of the manuscript.
XV. References
- 1.Kramer AH, Bleck TP. Neurocritical care of patients with central nervous system infections. Curr Infect Dis Rep. 2007;9:308–14. doi: 10.1007/s11908-007-0048-6. [DOI] [PubMed] [Google Scholar]
- 2.Kramer AH, Bleck TP. Neurocritical care of patients with central nervous system infections. Curr Treat Options Neurol. 2008;10:201–11. doi: 10.1007/s11940-008-0022-0. [DOI] [PubMed] [Google Scholar]
- 3.Riera-Mestre A, Gubieras L, Martinez-Yelamos S, Cabellos C, Fernandez-Viladrich P. Adult herpes simplex encephalitis: fifteen years’ experience. Enferm Infecc Microbiol Clin. 2009;27:143–7. doi: 10.1016/j.eimc.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Tyler KL. Herpes simplex virus infections of the central nervous system: encephalitis and meningitis, including Mollaret’s. Herpes. 2004;11(Suppl 2):57A–64A. [PubMed] [Google Scholar]
- 5.Whitley RJ. Herpes simplex encephalitis: adolescents and adults. Antiviral Res. 2006;71:141–8. doi: 10.1016/j.antiviral.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Markoula S, Giannopoulos S, Pelidou SH, Argyropoulou M, Lagos G, Kyritsis AP. MRI deterioration in herpes simplex encephalitis despite clinical recovery. Neurologist. 2009;15:223–6. doi: 10.1097/NRL.0b013e3181921abc. [DOI] [PubMed] [Google Scholar]
- 7.Poissy J, Wolff M, Dewilde A, et al. Factors associated with delay to acyclovir administration in 184 patients with herpes simplex virus encephalitis. Clin Microbiol Infect. 2009;15:560–4. doi: 10.1111/j.1469-0691.2009.02735.x. [DOI] [PubMed] [Google Scholar]
- 8.Chaudhuri A, Kennedy PG. Diagnosis and treatment of viral encephalitis. Postgrad Med J. 2002;78:575–83. doi: 10.1136/pmj.78.924.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.James SH, Kimberlin DW, Whitley RJ. Antiviral therapy for herpesvirus central nervous system infections: neonatal herpes simplex virus infection, herpes simplex encephalitis, and congenital cytomegalovirus infection. Antiviral Res. 2009;83:207–13. doi: 10.1016/j.antiviral.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones CA, Walker KS, Badawi N. Antiviral agents for treatment of herpes simplex virus infection in neonates. Cochrane Database Syst Rev. 2009 doi: 10.1002/14651858.CD004206.pub2. CD004206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kesson AM. Management of neonatal herpes simplex virus infection. Paediatr Drugs. 2001;3:81–90. doi: 10.2165/00128072-200103020-00001. [DOI] [PubMed] [Google Scholar]
- 12.Kimberlin D. Herpes simplex virus, meningitis and encephalitis in neonates. Herpes. 2004;11(Suppl 2):65A–76A. [PubMed] [Google Scholar]
- 13.Schloss L, Falk KI, Skoog E, Brytting M, Linde A, Aurelius E. Monitoring of herpes simplex virus DNA types 1 and 2 viral load in cerebrospinal fluid by real-time PCR in patients with herpes simplex encephalitis. J Med Virol. 2009;81:1432–7. doi: 10.1002/jmv.21563. [DOI] [PubMed] [Google Scholar]
- 14.Skoldenberg B, Aurelius E, Hjalmarsson A, et al. Incidence and pathogenesis of clinical relapse after herpes simplex encephalitis in adults. J Neurol. 2006;253:163–70. doi: 10.1007/s00415-005-0941-6. [DOI] [PubMed] [Google Scholar]
- 15.Whitley R. Diagnosis and treatment of herpes simplex encephalitis. Annu Rev Med. 1981;32:335–40. doi: 10.1146/annurev.me.32.020181.002003. [DOI] [PubMed] [Google Scholar]
- 16.Whitley RJ. Interim summary of mortality in herpes simplex encephalitis and neonatal herpes simplex virus infections: vidarabine versus acyclovir. J Antimicrob Chemother. 1983;12(Suppl B):105–12. doi: 10.1093/jac/12.suppl_b.105. [DOI] [PubMed] [Google Scholar]
- 17.Whitley RJ, Alford CA, Hirsch MS, et al. Vidarabine versus acyclovir therapy in herpes simplex encephalitis. N Engl J Med. 1986;314:144–9. doi: 10.1056/NEJM198601163140303. [DOI] [PubMed] [Google Scholar]
- 18.Kern ER. In: Animal models as assay systems for the development of antivirals. Development AD, DeClercq E, Walker RT, editors. Plenum Press; New York: 1988. [Google Scholar]
- 19.Kern ER. Preclinical evaluation of antiviral agents: in vitro and animal model testing. In: Galasso GJ, Whitley RJ, Merigan TC, editors. Antiviral agents and viral diseases of man. Raven Press; New York: 1990. [Google Scholar]
- 20.Kern ER, Richards JT, Glasgow LA, Overall JC, Jr., de Miranda P. Optimal treatment of herpes simplex virus encephalitis in mice with oral acyclovir. Am J Med. 1982;73:125–31. doi: 10.1016/0002-9343(82)90077-8. [DOI] [PubMed] [Google Scholar]
- 21.Kern ER, Richards JT, Overall JC., Jr. Acyclovir treatment of disseminated herpes simplex virus type 2 infection in weanling mice: alteration of mortality and pathogenesis. Antiviral Res. 1986;6:189–95. doi: 10.1016/0166-3542(86)90001-x. [DOI] [PubMed] [Google Scholar]
- 22.Whitley RJ, Soong SJ, Dolin R, Galasso GJ, Ch’ien LT, Alford CA, National Institute of Allergy and Infectious Diseases collaborative antiviral study Adenine arabinoside therapy of biopsy-proved herpes simplex encephalitis. N Engl J Med. 1977;297:289–94. doi: 10.1056/NEJM197708112970601. [DOI] [PubMed] [Google Scholar]
- 23.Whitley RJ. Herpes simplex virus infections of the central nervous system. Encephalitis and neonatal herpes. Drugs. 1991;42:406–27. doi: 10.2165/00003495-199142030-00005. [DOI] [PubMed] [Google Scholar]
- 24.Beadle JR, Hartline C, Aldern KA, et al. Alkoxyalkyl esters of cidofovir and cyclic cidofovir exhibit multiple-log enhancement of antiviral activity against cytomegalovirus and herpesvirus replication in vitro. Antimicrob Agents Chemother. 2002;46:2381–6. doi: 10.1128/AAC.46.8.2381-2386.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keith KA, Wan WB, Ciesla SL, Beadle JR, Hostetler KY, Kern ER. Inhibitory activity of alkoxyalkyl and alkyl esters of cidofovir and cyclic cidofovir against orthopoxvirus replication in vitro. Antimicrob Agents Chemother. 2004;48:1869–71. doi: 10.1128/AAC.48.5.1869-1871.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kern ER. In vitro activity of potential anti-poxvirus agents. Antiviral Res. 2003;57:35–40. doi: 10.1016/S0166-3542(02)00198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kern ER, Collins DJ, Wan WB, Beadle JR, Hostetler KY, Quenelle DC. Oral treatment of murine cytomegalovirus infections with ether lipid esters of cidofovir. Antimicrob Agents Chemother. 2004;48:3516–22. doi: 10.1128/AAC.48.9.3516-3522.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kern ER, Hartline C, Harden E, et al. Enhanced inhibition of orthopoxvirus replication in vitro by alkoxyalkyl esters of cidofovir and cyclic cidofovir. Antimicrob Agents Chemother. 2002;46:991–5. doi: 10.1128/AAC.46.4.991-995.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prichard MN, Quenelle DC, Hartline CB, et al. Inhibition of herpesvirus replication by 5-substituted 4′-thiopyrimidine nucleosides. Antimicrob Agents Chemother. 2009;53:5251–8. doi: 10.1128/AAC.00417-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams-Aziz SL, Hartline CB, Harden EA, et al. Comparative activities of lipid esters of cidofovir and cyclic cidofovir against replication of herpesviruses in vitro. Antimicrob Agents Chemother. 2005;49:3724–33. doi: 10.1128/AAC.49.9.3724-3733.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bidanset DJ, Beadle JR, Wan WB, Hostetler KY, Kern ER. Oral activity of ether lipid ester prodrugs of cidofovir against experimental human cytomegalovirus infection. J Infect Dis. 2004;190:499–503. doi: 10.1086/421912. [DOI] [PubMed] [Google Scholar]
- 32.Quenelle DC, Collins DJ, Wan WB, Beadle JR, Hostetler KY, Kern ER. Oral treatment of cowpox and vaccinia virus infections in mice with ether lipid esters of cidofovir. Antimicrob Agents Chemother. 2004;48:404–12. doi: 10.1128/AAC.48.2.404-412.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buller RM, Owens G, Schriewer J, Melman L, Beadle JR, Hostetler KY. Efficacy of oral active ether lipid analogs of cidofovir in a lethal mousepox model. Virology. 2004;318:474–81. doi: 10.1016/j.virol.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 34.Parker S, Touchette E, Oberle C, et al. Efficacy of therapeutic intervention with an oral ether-lipid analogue of cidofovir (CMX001) in a lethal mousepox model. Antiviral Res. 2008;77:39–49. doi: 10.1016/j.antiviral.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huggins JW, Baker RO, Beadle JR, Hostetler KY. Orally active ether lipid prodrugs of cidofovir for treatment of smallpox. Antiviral Research. 2002;53:A66. [Google Scholar]
- 36.Quenelle DC, Prichard MN, Keith KA, et al. Synergistic efficacy of the combination of ST-246 with CMX001 against orthopoxviruses. Antimicrob Agents Chemother. 2007;51:4118–24. doi: 10.1128/AAC.00762-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bernstein DI, Kappes JC. Enhanced in vitro reactivation of latent herpes simplex virus from neural and peripheral tissues with hexamethylenebisacetamide. Arch Virol. 1988;99:57–65. doi: 10.1007/BF01311023. [DOI] [PubMed] [Google Scholar]
- 38.Kern ER. Animal Models for central nervous system and disseminated infections with herpes simplex virus. In: Zak O, Sande MA, editors. Handbook of animal models of infection. Academic Press; San Diego: 1999. [Google Scholar]
- 39.Cundy KC, Bidgood AM, Lynch G, Shaw JP, Griffin L, Lee WA. Pharmacokinetics, bioavailability, metabolism, and tissue distribution of cidofovir (HPMPC) and cyclic HPMPC in rats. Drug Metab Dispos. 1996;24:745–52. [PubMed] [Google Scholar]
- 40.Safrin S, Cherrington J, Jaffe HS. Clinical uses of cidofovir. Rev Med Virol. 1997;7:145–156. doi: 10.1002/(sici)1099-1654(199709)7:3<145::aid-rmv196>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 41.Andrei G, Fiten P, Goubau P, et al. Dual infection with polyomavirus BK and acyclovir-resistant herpes simplex virus successfully treated with cidofovir in a bone marrow transplant recipient. Transpl Infect Dis. 2007;9:126–31. doi: 10.1111/j.1399-3062.2006.00186.x. [DOI] [PubMed] [Google Scholar]
- 42.Aldern KA, Ciesla SL, Winegarden KL, Hostetler KY. Increased antiviral activity of 1-O-hexadecyloxypropyl-[2-(14)C]cidofovir in MRC-5 human lung fibroblasts is explained by unique cellular uptake and metabolism. Mol Pharmacol. 2003;63:678–81. doi: 10.1124/mol.63.3.678. [DOI] [PubMed] [Google Scholar]
- 43.Painter GR, Trost LC, Lampert BM, et al. CMX001. Drugs of the Future. 2008;33:1–7. [Google Scholar]
- 44.Thackray AM, Field HJ. Persistence of infectious herpes simplex virus type 2 in the nervous system in mice after antiviral chemotherapy. Antimicrob Agents Chemother. 2000;44:97–102. doi: 10.1128/aac.44.1.97-102.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lycke J, Malmestrom C, Stahle L. Acyclovir levels in serum and cerebrospinal fluid after oral administration of valacyclovir. Antimicrob Agents Chemother. 2003;47:2438–41. doi: 10.1128/AAC.47.8.2438-2441.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quenelle DC, Prichard MN, Hartline CB, et al. Enhanced efficacy using suboptimal concentrations of CMX001 with acyclovir against herpes simplex virus infections in vitro and in mice. Antiviral Research. 2010 In Press. [Google Scholar]