Abstract
Objective
To determine the effects of reducing overnight basal insulin or a bedtime dose of terbutaline on nocturnal blood glucose (BG) nadir and hypoglycemia following exercise in children with type 1 diabetes mellitus (T1DM).
Study design
Sixteen youth (mean age 13.3 yrs) on insulin pumps were studied overnight on three occasions after a 60 minute exercise session with BG measurements every 30 minutes. Admissions were randomized to bedtime treatment with 2.5 mg oral terbutaline, 20% basal rate insulin reduction for six hours, or no treatment.
Results
Mean overnight nadir BG was 188 mg/dL after terbutaline and 172 mg/dL with basal rate reduction compared with 127 mg/dL on the control night (p = 0.002 and 0.042, respectively). Terbutaline eliminated nocturnal hypoglycemia but resulted in significantly more hyperglycemia (≥ 250 mg/dL) when compared with the control visit (p < 0.0001). The basal rate reduction resulted in fewer BG readings < 80 and < 70 mg/dL but more readings ≥ 250 mg/dL when compared with the control visit.
Conclusions
A basal insulin rate reduction was safe and effective in raising post-exercise nocturnal BG nadir and in reducing hypoglycemia in children with T1DM. Although effective at preventing hypoglycemia, a 2.5 mg terbutaline dose was associated with hyperglycemia.
Both the American Diabetes Association (ADA) (6) and the International Society for Pediatric and Adolescent Diabetes (1) recommend regular physical activity and have published exercise guidelines for children and adolescents with type 1 diabetes mellitus (T1DM) because of its beneficial effects in T1DM, including improved insulin sensitivity, improved body composition, improved lipid profile, and possible improved self-esteem (1-5)..
An important goal of diabetes management is to achieve as near normal HbA1c as can be attainable without excessive hypoglycemia. However hypoglycemia, and the fear of hypoglycemia (7;8), particularly for parents of children with T1DM (9), remain barriers to achieving glycemic control targets. Because hypoglycemia during or after exercise is the most frequently identified specific cause of severe hypoglycemia (10) and a majority of severe episodes occur at night (11), identification of methods to reduce the risk of hypoglycemia following exercise is important. The majority of episodes occur immediately after excerise or later that night, usually when the child is asleep. The DirecNet consortium found that following one hour of afternoon exercise, 48% of youth experienced hypoglycemia (blood glucose [BG] <60 mg/dL) during the ensuing night, and only 28% of the same children experienced nocturnal hypoglycemia following a sedentary day (12). The DirecNet group found the BG nadir to occur between midnight and 2 a.m. after an afternoon exercise session between 4 p.m. and 6 p.m. (12), a common time for children to participate in exercise activities. McMahon et al (13) replicated this finding in children and teenagers with T1DM by looking at the variation in the glucose infusion rates required to maintain euglycemia overnight following afternoon bicycling when the insulin dose was kept constant. They found higher glucose infusion rates were required 7-11 hours after exercise, or between approximately midnight and 4 a.m. Therefore, the child or adolescent is vulnerable to post-exercise hypoglycemia in the middle of the night, a time when blood glucose monitoring is often minimal.
Although ceasing basal insulin infusion during exercise has been shown to reduce hypoglycemia during exercise (14), we are unaware of trials to prevent delayed nocturnal hypoglycemia in children with T1DM. Terbutaline, a ß-agonist, has been studied in adults in an attempt to prevent nocturnal hypoglycemia (15). The purpose of this study was to determine the effects of a bedtime dose of terbutaline or a reduction in night-time basal insulin on both the nocturnal blood glucose nadir and the incidence of nocturnal hypoglycemia following exercise in children and adolescents with T1DM.
Methods
This study was performed using a crossover design in which each participant underwent a total of three overnight study visits which included a 60 minute exercise session. For all visits, basal insulin was discontinued during the exercise followed by a 50% decrease in basal rate for 45 minutes after the exercise. In random sequence, each participant completed three intervention conditions: one visit with a bedtime dose of oral terbutaline, one visit with an overnight reduction in basal insulin rate, and one visit with no overnight intervention. The study was approved by both the Colorado Multiple Institutional Review Board and the Clinical and Translational Research Center (CTRC) at The Children’s Hospital in Aurora, Colorado. The trial is registered at (#NCT00974051). All study visits occurred at the CTRC.
Patients were eligible for inclusion in the study if they were aged 10-17 years at enrollment with a duration of diabetes greater than 12 months, on an insulin pump for greater than six months and with a HbA1c level < 10%. Patients were excluded if they had a severe hypoglycemic event in the last six months, a BMI > 95th percentile or < 5th percentile, a history of hypertension, recent use of ß-agonist or ß-blockade therapy, a known history of cardiovascular disease, a structural heart defect, or an inability to perform exercise due to physical limitation.
Eighteen patients with T1DM who had previously expressed interest in research studies were approached by study staff and enrolled after written informed consent was obtained. One patient came for the first study visit but then withdrew from the study due to acute illness at the time of the scheduled second visit, and another patient was ill at the time of the first study visit, withdrew as a result and thus did not complete any study visits. Thus 16 patients completed the study. At screening, all participants had a complete history and physical exam including direct Tanner staging, and blood for HbA1c analyzed using a DCA 2000+ Analyzer (Bayer, Tarrytown, NY). Eligible patients underwent randomization by computer program to determine the sequence of the three crossover arms.
Study Visits
Each of the three overnight visits in the CTRC had to be at least 72 hours apart and consisted of the same protocol. Patients were asked to abstain from exercise for the 24 hours prior to the study visit, and to perform an infusion set change the day before. Admission to the CTRC occurred between 11 a.m. and noon. Lunch occurred at noon and consisted of a frozen meal with known carbohydrate content. A peripheral intravenous catheter for sampling was inserted and a BG level was checked. An insulin bolus via the pump was given according to the patient’s home insulin to carbohydrate ratio and correction factor. At each subsequent visit, the lunch meal was exactly the same as the first visit.
The BG level was then checked hourly between 2 and 4 p.m. BG levels above 200 mg/dL were corrected with an intravenous regular insulin bolus of 0.05 units/kg to achieve a target glucose between 120-200 mg/dL at the beginning of the exercise session at 4 pm. BG levels were rechecked 15 and 30 minutes after treatment. If necessary the dose of insulin was repeated until the BG was in range. If BG was less than 120 mg/dL, 15-30 grams of oral, fast-acting carbohydrate was given at the discretion of the investigators to achieve the target BG.
Exercise Session
The exercise protocol was specifically chosen to be similar to previous exercise studies (12) and was performed in the same way for all three study visits for each participant. At 4 p.m., with the BG in range, the insulin pump was disconnected to minimize the risk of hypoglycemia during exercise, and a heart rate monitor was attached (Polar USA Inc). After an adequate heart rate signal was confirmed, the patient began exercising on a running treadmill. To standardize the exercise for individual variations in cardiovascular fitness, participants exercised to a target heart rate of 140 beats per minute, with study staff continuously monitoring the heart rate and adjusting the speed and incline of the treadmill to achieve this heart rate. Each session consisted of four 15-minute intervals on the treadmill for a total of one hour of aerobic exercise. A five minute rest occurred between each interval, at which time a BG was checked and hypoglycemia treated with 15-30 grams of juice if the BG was < 70 mg/dL.
At the conclusion of the fourth 15-minute interval, each patient had a BG checked, reconnected their pump under supervision and restarted their basal insulin rate at 50% of their usual rate for 45 minutes. After this period, the usual basal rate was recommenced and dinner was ordered. Upon arrival of dinner at 7 p.m., a BG was performed and an insulin bolus given for carbohydrates and any necessary correction as per their usual home regimen using the pump’s bolus calculator.
Overnight Monitoring
A bedtime snack was allowed if part of the patient’s usual home regimen, and insulin was dosed according to the patient’s home bolus ratios. At 9 p.m., study conditions commenced. With the order of interventions randomized, each patient received either a 2.5 mg oral dose of terbutaline (“terbutaline visit”), had their pump basal insulin infusion rate decreased by 20% from 9 p.m. to 3 a.m. (“basal reduction visit”) or received no intervention with 100% of their home basal insulin infusion rate continued through the night (“control visit”). The BG was checked at the bedside via a free flowing sample from the IV every 30 minutes. Blood pressure and pulse rate were measured every 30 minutes.
If the patient experienced a BG < 70 mg/dL, treatment with 15 grams of juice was given and the BG was rechecked 15 minutes later. Repeat doses of juice were given until the BG was > 70 mg/dL, and then the protocol continued as above. At 6 a.m. study conditions ceased and breakfast was ordered for the patient. After a breakfast bolus using the patient’s standard home carbohydrate and correction ratios was given and breakfast was completed, the participant was discharged from the CTRC.
Statistical Analysis
We defined hypoglycemia as a blood glucose level < 70 mg/dL in accordance with the ADA definition.(16) Our sample size estimation was based on an expected 48% frequency of nocturnal hypoglycemia as observed by DirecNet following afternoon exercise (12).
Our pre-determined outcome measures were the overnight BG nadir and the reduction of nocturnal hypoglycemia. In secondary analysis, we also looked at frequency of BG levels < 80 and ≥ 250 mg/dL.
This crossover study was analyzed using a mixed effect model with subjects within sequence as a random effect, period and treatment as fixed effects for the continuous endpoints of BG nadir, BG max and BG over time. The Chi Square test of independence or Fisher’s Exact was used for the analysis of categorical variables. Data are presented as mean ± SD, least square mean ± SE or frequency and percent, as indicated.
Results
The study population consisted of seven females and nine males with a mean age of 13.3 ± 1.8 years, mean T1DM duration of 6.9 ± 3.5 years, mean weight of 52.3 ± 16 kg and mean HbA1c of 7.5 ± 0.7%. Total daily insulin dose was 0.76 ± 0.2 units/kg, with 0.40 ± 0.13 units/kg as basal insulin.
Glycemia During Exercise
Glucose levels < 70 mg/dL during exercise occurred in 4 of 16 subjects in the control visit, 5 of 16 in the terbutaline visit, and 2 of 16 in the basal reduction visit (p = 0.438)
Overnight Glycemia and BG Nadir
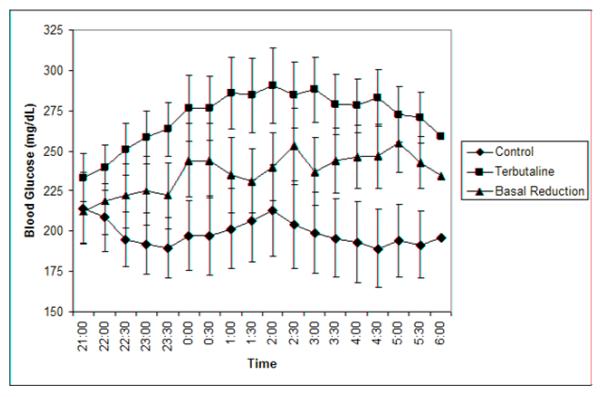
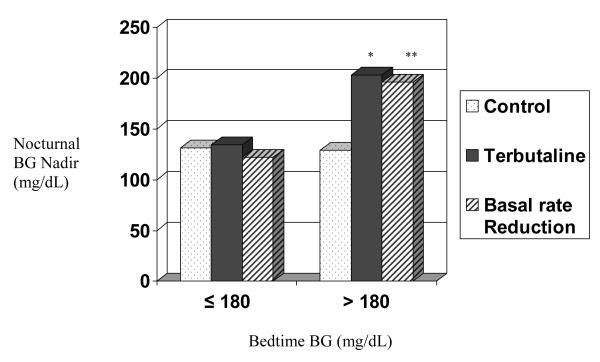
Figure 1 demonstrates the differences between BG curves among the three treatments (p < 0.0001). There was a significant difference (Table) in mean overnight nadir BG values between the terbutaline visit and the control visit (p = 0.001) and between the basal reduction visit and the control visit (p = 0.002). There was no difference in BG nadir between the terbutaline and the basal reduction visits. There were no significant differences between treatments in BGmax. Results for nadir BG values were higher for both the terbutaline (p = 0.003) and the basal reduction (p = 0.016) groups compared with the control group when the bedtime BG was > 180 mg/dL (Figure 2; available at www.jpeds.com). No participant experienced elevated blood ketones during the study.
Figure 1.
Overnight mean blood glucose level curves for the three study visits p < 0.0001 for BGL’s across time between treatments. P < 0.0001 for terbutaline vs. control, basal reduction vs. control, and terbutaline vs. basal reduction.
Table.
Overnight blood glucose (BG) comparisons between treatments
| Control | Terbutaline | 20% Basal Reduction |
p value |
|
|---|---|---|---|---|
| BG Nadir | 128 ± 58 | 189 ± 60* | 172 ± 65** | 0.004 |
| BG Max | 285 ± 93 | 331 ± 70.4 | 305 ± 85 | 0.3 |
| BG 6am | 196 ± 90 | 259 ± 64*** | 234 ± 63 | |
| BG readings < 80 |
19 (6.6%); 4/16 patients |
0 | 14 (4.9%); 2/16 patients |
0.0001 |
| BG readings < 70 |
5 (1.7%); 2/16 patients |
0 | 1 (0.3%); 1/16 patients |
0.029 |
| BG readings ≥ 250 |
87 (30.2%); 9/16 patients |
183 (63.5%); 14/16 patients |
120 (41.7%); 12/16 patients |
<0.001 |
p = 0.001 for Control versus Terbutaline,
p = 0.002 for Control versus 20% reduction in Basal Insulin
p = 0.05 for 6am BG in control vs terbutaline (adjusted for multiple tests)
Footnote: none of the subjects experienced a β-hydroxybutyrate level > 0.6 during the study
Figure 2.
Nocturnal BG nadir by bedtime BG
*p = 0.003 terbutaline vs control
**p = 0.016 basal rate reduction vs. control
Overnight Hypoglycemic and Hyperglycemic Events
Although hypoglycemia is defined as a BG < 70 mg/dL, data is also provided for nocturnal BG < 80 mg/dL, which is a level of concern for many families following days with heavy exercise. Indeed the ADA working group suggests these events of relative hypoglycemia should be reported. (16)
During the control visit there were five separate episodes of a BG < 70 mg/dL for two participants (Table). The HbA1c for these individuals was 7.1% and 8.1% respectively, not significantly different than the overall study population. In addition, 25% of participants (4 of 16) had at least one overnight BG < 80 mg/dL, with a total of 17 separate events. In the basal reduction visit there was one episode of BG < 70 mg/dL in a participant who had a baseline HbA1c of 7.5% and a total of 14 events < 80 mg/dL in two participants. Treatment with basal reduction resulted in fewer BG readings < 80 and < 70 mg/dL than the control visit, but more readings ≥ 250 mg/dL. Treatment with terbutaline eliminated BG values < 80 mg/dL but there were significantly more readings ≥ 250 mg/dL than for the control visit (p < 0.001) or the basal reduction visit (p < 0.001). Six of the sixteen study participants had bedtime snacks. Those who chose to have bedtime snacks received a snack on each of the three visit as per protocol Neither of the two participants who experienced nocturnal hypoglycemia during the control visit had a bedtime snack. In contrast, the participant who became hypoglycemic during the basal rate reduction visit did have a bedtime snack. All three participants were treated for hypoglycemia during the preceding afternoon. The participant who became hypoglycemic during the basal reduced visit was treated for hypoglycemia at bedtime during the control visit.
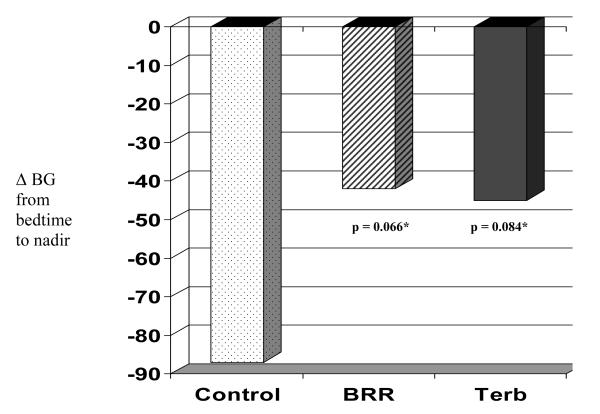
The delta BG from bedtime to nocturnal nadir was −87 ± 17 mg/dL for the control visit compared with −45 ± 17 mg/dL for terbutaline (p = 0.084) and −42 ± 17 mg/dL for basal reduction (p = 0.066) (Figure 3; available at www.jpeds.com).
Figure 3.
Drop in BGL from bedtime to nocturnal nadir by visit type
*pair-wise tests vs control
Effect of Afternoon Glycemia on Overnight Glycemia
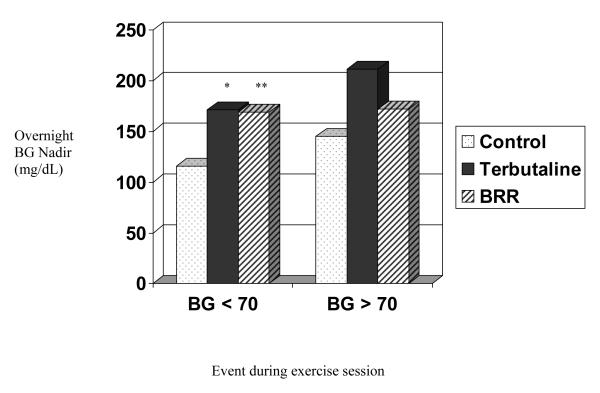
All participants who became hypoglycemic overnight during the study had been low on the preceding afternoon. Overall, for those who had BG values < 70 mg/dL during exercise, mean nocturnal BG nadir was significantly higher when they had either intervention compared with the control (Figure 4).
Figure 4.
Effect of Hypoglycemia During Exercise on Nocturnal BG Nadir, by treatment group
* p = 0.02
** p = 0.02
Side Effects of Terbutaline
Terbutaline did not result in any episodes of tachycardia or hypertension. No differences in BP or heart rate were found between groups.
Discussion
We assessed specific interventions directed at preventing the common problem of delayed nocturnal hypoglycemia after daytime exercise. In children and adolescents with T1DM treated with an insulin pump, both terbutaline and an overnight reduction in the basal insulin infusion rate were effective in raising the overnight BG nadir after exercise. The rate of nocturnal hypoglycemia in the control condition was lower than expected and, therefore, we were underpowered to detect a significant effect of our interventions on the rate of nocturnal hypoglycemia. Given that antecedent hypoglycemia is a well known risk factor for subsequent hypoglycemia (23), we speculate that by discontinuing the basal rate during exercise and using a reduced rate for 45 minutes post-exercise, the frequency of afternoon hypoglycemia was decreased resulting in a reduced risk for delayed hypoglycemia during the night.
Although discontinuation of basal insulin during exercise had been shown to lower concurrent hypoglycemia (14), its effect on nocturnal hypoglycemia had not been previously studied. In a study conducted by the DirecNet study group (12), 52% of subjects who performed an identical exercise protocol had a blood glucose below 70 mg/dl during the exercise session when their basal insulin was continued through the exercise. Furthermore, one-third of those subjects required more than one treatment to correct the hypoglycemia. In that same study, subjects who had exercised were found to have a 48% rate of nocturnal hypoglycemia (defined as a BG level below 60 mg/dL). As in our study, bedtime snacks were allowed in the DirecNet study if participants would normally have one at home. In contrast, discontinuation of insulin during exercise resulted in only 23% of participants with hypoglycemia during exercise, and 12.5% during the night in the control condition of our study. We did not observe any BG levels below 60 mg/dL during the night.
We interpret our results with caution because there were some differences between our study and the DirecNet study. Although the demographic characteristics of our study participants were similar to those in the DirecNet study (mean age 14.8 ± 1.7 years, duration of diabetes 7.0 ± 3.7 years and mean A1c 7.8 % ± 0.7), only 54% of DirecNet subjects used pumps to deliver insulin (46% used injections). In addition, the mean bedtime BG level of DirecNet participants was significantly lower than in this study (141 vs. 228 mg/dl). It is also noteworthy that 6% of DirecNet study participants had experienced a severe hypoglycemic episode during the 6 months preceding their participation in the study, and we excluded those individuals.
Secondary analysis showed both interventions in our study to be effective in reducing the rate of post-exercise nocturnal BG levels < 80 mg/dL. In fact, treatment with a single dose of 2.5mg of terbutaline at bedtime completely eliminated overnight BG readings <80 mg/dl, but at the cost of significant hyperglycemia.
Overnight counterregulatory hormone responses to spontaneous hypoglycemia are impaired in children with T1DM (17-20), and thus a pharmacological intervention such as the ß-agonist terbutaline is attractive. In adults with T1DM, Cryer et al examined bedtime administration of terbutaline in an attempt to prevent nocturnal hypoglycemia (15). A dose of 5 mg of terbutaline was found to be effective, but at the expense of morning hyperglycemia. A follow-up study found that a lower dose of 2.5 mg was safe and moderately effective (21). Importantly, however, these studies were not performed in children, and not directed specifically at post-exercise nocturnal hypoglycemia. There is currently little data to inform recommendations for intervention in children.
The terbutaline dose of 2.5 mg at 9 p.m. in the current study effectively raised the nocturnal BG nadir and eliminated hypoglycemia. However, similar to the findings of Cryer et al in adults, this resulted in significant hyperglycemia and is, thus, not a practical dose for hypoglycemia prevention. Potentially, a dose based on weight and age may be more effective; trials are needed to look at smaller doses. It is reassuring that we saw no episodes of hypertension or tachycardia. It also is possible that an additional reduction in basal insulin during the night would be beneficial for some individuals.
In summary, this study suggests specific interventions to prevent the common problem of post-exercise nocturnal hypoglycemia in children and adolescents. Suspending the basal insulin rate during exercise may have an impact on reducing the subsequent risk of nocturnal hypoglycemia. The basal rate reduction between 9 p.m. and 3 a.m. was effective in raising the nocturnal BG nadir and reducing the number of low BG levels. The flexibility to adjust basal rates by the hour remains one of the most attractive features of an insulin pump and is thus particularly useful for the active person with T1DM (22;23). Although terbutaline prevented nocturnal hypoglycemia, significant hyperglycemia occurred, and further dosing studies are necessary. For those patients in whom basal insulin reduction is not an option (for those using multiple daily injections of insulin), further research is required.
Acknowledgments
We thank all the study patients and their families for their participation, the Pediatric Clinical Translational Research Center at The University of Colorado and Children’s Hospital, Denver, Casey Weimer for recruitment of subjects and IRB review preparation, and Brandon VanderWel for assistance with exercise supervision during study visits.
Sponsored by The National Institutes of Health General Clinical Research Center (grant RR00069). C.T. was supported by NIH T32 grant 5T32 DK63687.
List of abbreviations
- BG
blood glucose
- T1DM
type 1 diabetes mellitus
- ADA
American Diabetes Association
- HbA1c
Hemoglobin A1c
- CTRC
Clinical and Translational Research Center
- BMI
body mass index
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
Registered Clinical Trial Number #NCT00974051
Contributor Information
Craig E. Taplin, University of Colorado.
Erin Cobry, University of Colorado.
Laurel Messer, University of Colorado.
Kim McFann, University of Colorado.
H. Peter Chase, University of Colorado.
Rosanna Fiallo-Scharer, University of Colorado.
Reference List
- 1.Robertson K, Adolfsson P, Scheiner G, Hanas R, Riddell MC. Exercise in Children and Adolescents With Diabetes. Pediatric Diabetes. 2009;10(suppl 12):154–68. doi: 10.1111/j.1399-5448.2009.00567.x. [DOI] [PubMed] [Google Scholar]
- 2.Wolfsdorf JI. Children With Diabetes Benefit From Exercise. Archives of Disease in Childhood. 2005;90:1215–7. doi: 10.1136/adc.2005.082446. [Comment] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riddell MC, Iscoe KE. Physical Activity, Sport, and Pediatric Diabetes. Pediatr.Diabetes. 2006;7:60–70. doi: 10.1111/j.1399-543X.2006.00146.x. [DOI] [PubMed] [Google Scholar]
- 4.Herbst A, Bachran R, Kapellen T, Holl RW. Effects of Regular Physical Activity on Control of Glycemia in Pediatric Patients With Type 1 Diabetes Mellitus. Arch.Pediatr.Adolesc.Med. 2006;160:573–7. doi: 10.1001/archpedi.160.6.573. [DOI] [PubMed] [Google Scholar]
- 5.Herbst A, Kordonouri O, Schwab KO, Schmidt F, Holl RW. Impact of Physical Activity on Cardiovascular Risk Factors in Children With Type 1 Diabetes: a Multicenter Study of 23,251 Patients. Diabetes Care. 2007;30:2098–100. doi: 10.2337/dc06-2636. [DOI] [PubMed] [Google Scholar]
- 6.Zinman B, Ruderman N, Campaigne BN, Devlin JT, Schneider SH, American Diabetes Association Physical Activity/Exercise and Diabetes. Diabetes Care. 2004;27(Suppl 1):S58–62. doi: 10.2337/diacare.26.2007.s73. [DOI] [PubMed] [Google Scholar]
- 7.Brazeau A, Rabasa-Lhoret R, Strychar I, Mircescu H. Barriers to Physical Activity Among Patients With Type 1 Diabetes. Diabetes Care. 2008;31:2108–9. doi: 10.2337/dc08-0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonder-Frederick LA, Fisher CD, Ritterband LM, Cox DJ, Hou L, DasGupta AA, Clarke WL. Predictors of Fear of Hypoglycemia in Adolescents With Type 1 Diabetes and Their Parents. Pediatric Diabetes. 2006;7:215–22. doi: 10.1111/j.1399-5448.2006.00182.x. [DOI] [PubMed] [Google Scholar]
- 9.Patton SR, Dolan LM, Henry R, Powers SW. Parental Fear of Hypoglycemia: Young Children Treated With Continuous Subcutaneous Insulin Infusion. Pediatric Diabetes. 2007;8:362–8. doi: 10.1111/j.1399-5448.2007.00242.x. [DOI] [PubMed] [Google Scholar]
- 10.Bhatia V, Wolfsdorf JI. Severe Hypoglycemia in Youth With Insulin-Dependent Diabetes Mellitus: Frequency and Causative Factors. Pediatrics. 1991;88:1187–93. [PubMed] [Google Scholar]
- 11.Davis EA, Keating B, Byrne GC, Russell M, Jones TW. Hypoglycemia: Incidence and Clinical Predictors in a Large Population-Based Sample of Children and Adolescents With IDDM. Diabetes Care. 1997;20:22–5. doi: 10.2337/diacare.20.1.22. [DOI] [PubMed] [Google Scholar]
- 12.Tsalikian E, Mauras N, Beck RW, Tamborlane WV, Janz KF, Chase HP, Wysocki T, Weinzimer SA, Buckingham BA, Kollman C, Xing D, Ruedy KJ, Diabetes Research In Children Network Direcnet Study Group Impact of Exercise on Overnight Glycemic Control in Children With Type 1 Diabetes Mellitus. Journal of Pediatrics. 2005;147:528–34. doi: 10.1016/j.jpeds.2005.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McMahon SK, Ferreira LD, Ratnam N, Davey RJ, Youngs LM, Davis EA, Fournier PA, Jones TW. Glucose Requirements to Maintain Euglycemia After Moderate-Intensity Afternoon Exercise in Adolescents With Type 1 Diabetes Are Increased in a Biphasic Manner. Journal of Clinical Endocrinology & Metabolism. 2007;92:963–8. doi: 10.1210/jc.2006-2263. [See Comment] [DOI] [PubMed] [Google Scholar]
- 14.Tsalikian E, Kollman C, Tamborlane WB, Beck RW, Fiallo-Scharer R, Fox L, Janz KF, Ruedy KJ, Wilson D, Xing D, Weinzimer SA. Prevention of Hypoglycemia During Exercise in Children With Type 1 Diabetes by Suspending Basal Insulin. Diabetes Care. 2006;29:2200–4. doi: 10.2337/dc06-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raju B, Arbelaez AM, Breckenridge SM, Cryer PE. Nocturnal Hypoglycemia in Type 1 Diabetes: an Assessment of Preventive Bedtime Treatments. Journal of Clinical Endocrinology & Metabolism. 2006;91:2087–92. doi: 10.1210/jc.2005-2798. [DOI] [PubMed] [Google Scholar]
- 16.Defining and Reporting Hypoglycemia in Diabetes: a Report From the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care. 2005;28:1245–9. doi: 10.2337/diacare.28.5.1245. [DOI] [PubMed] [Google Scholar]
- 17.Buckingham B, Wilson DM, Lecher T, Hanas R, Kaiserman K, Cameron F. Duration of Nocturnal Hypoglycemia Before Seizures. Diabetes Care. 2008;31:2110–2. doi: 10.2337/dc08-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cryer PE. Exercise-Related Hypoglycemia-Associated Autonomic Failure in Diabetes. Diabetes. 2009;58:1951–2. doi: 10.2337/db09-0834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diabetes Research in Children Network (DirecNet) Study Group Impaired Overnight Counterregulatory Hormone Responses to Spontaneous Hypoglycemia in Children With Type 1 Diabetes. Pediatric Diabetes. 2007;8:199–205. doi: 10.1111/j.1399-5448.2007.00248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arbelaez AM, Powers WJ, Videen TO, Price JL, Cryer PE. Attenuation of Counterregulatory Responses to Recurrent Hypoglycemia by Active Thalamic Inhibition: a Mechanism for Hypoglycemia-Associated Autonomic Failure. Diabetes. 2008;57:470–5. doi: 10.2337/db07-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cooperberg BA, Breckenridge SM, Arbelaez AM, Cryer PE. Terbutaline and the Prevention of Nocturnal Hypoglycemia in Type 1 Diabetes. Diabetes Care. 2009;31:2271–2. doi: 10.2337/dc08-0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scrimgeour L, Cobry E, McFann K, Burdick P, Weimer C, Slover R, Chase HP. Improved Glycemic Control After Long-Term Insulin Pump Use in Pediatric Patients With Type 1 Diabetes. Diabetes Technology & Therapeutics. 2007;9:421–8. doi: 10.1089/dia.2007.0214. [DOI] [PubMed] [Google Scholar]
- 23.Buckingham B, Cobry E, Clinton P, Gage V, Caswell K, Kunselman E, Cameron F, Chase HP. Preventing Hypoglycemia Using Predictive Alarm Algorithms and Insulin Pump Suspension. Diabetes Technology & Therapeutics. 2009;11:93–7. doi: 10.1089/dia.2008.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]