Abstract
The activation of neutral sphingomyelinase-2 (nSMase2) and consequent ceramide production are implicated in many stress-induced signaling pathways. Trafficking of nSMase2 from the Golgi compartment to the plasma membrane (PM) in response to signaling stimuli has been described. However, the precise mechanisms of transport remain unknown. This study aimed to investigate the trafficking of nSMase2 between the Golgi and the PM. We show here that V5-nSMase2 localizes at the PM and Golgi in MCF-7 cells and confirm relocalization of nSMase2 to the PM at confluence. Although cycloheximide (CHX) treatment partially inhibited the Golgi localization of GFP-nSMase2, recovery of GFP-nSMase2 to an intracellular compartment was still observed after photobleaching. Moreover, in the presence of CHX, GFP- and V5-nSMase2 co-localized with endosomal/recycling markers. In HEK293 cells, activation of either protein kinase C-alpha or betaII, with the phorbol ester PMA led to relocalization of both wild-type and inactive nSMase2 to the pericentrion, a PKC-dependent subset of recycling endosomes. Finally, inhibition of nSMase2 endocytosis by K + depletion reduced the intracellular pool of nSMase2 and increased nSMase2 activity resulting in elevated ceramide levels. Altogether, these results suggest that nSMase2 traffics from the Golgi to the PM as a membrane protein en route to the cell surface and recycles back to the Golgi through the endosomal/recycling compartment. Moreover, the recycling of nSMase2 from the PM is important for its catalytic regulation.
Keywords: Neutral sphingomyelinase, Plasma membrane, Golgi, Recycling endosome
1. Introduction
Ceramide is a bioactive sphingolipid involved in many biological functions such as proliferation, apoptosis, differentiation and inflammation [1]. Several enzymes of sphingolipid metabolism contribute to ceramide generation and this can occur in different subcellular compartments. For example, de novo ceramide synthesis takes place in the endoplasmic reticulum [2]. The sphingomyelinases (SMases) mediate the hydrolysis of sphingomyelin into ceramide and phosphocholine. Currently, at least six sphingomyelinases have been identified in mammalian cells; a lysosomal acid SMase, a secreted acid SMase, three neutral SMases (nSMases) and an alkaline SMase. The SMases have distinct localizations within the cell, including the plasma membrane (PM), lysosomes, Golgi, and endoplasmic reticulum (ER), suggesting they may be important for compartmentalized ceramide production [3].
The three mammalian nSMases termed nSMase1, 2, and 3, have been implicated in various signaling pathways [4]. Of the three enzymes, nSMase1 is reported to localize to the ER and nucleus [5]. But despite its in vitro activity, it does not appear to function as an in vivo nSMase [6]. In contrast, nSMase3 was reported to possess both in vitro and in vivo nSMase activity and is localized to the Golgi and ER [7]. More recently, nSMase3 was implicated in apoptosis induced by genotoxic agents [8]. Among the three enzymes, nSMase2 is the most studied. First purified from mammalian brain, nSMase2 is a membrane-associated enzyme of 71 kDa that is activated by anionic phospholipids such as phosphatidylserine (PS) and phosphatidic acid (PA) [9]. Recently, two palmitoylation sites on nSMase2 have been identified, one between the two hydrophobic segments near the N-terminus and another within the C-terminal catalytic domain [10]. Although some studies have found nSMase2 in the Golgi compartment, a PM localization of nSMase2 has also been described. Moreover, nSMase2 localization is affected by certain stimuli such as TNF-α, PMA, H2O2 and cell confluence, all of which have been reported to induce nSMase2 translocation from the Golgi compartment to the PM in lung epithelial cells A549, human airway epithelial cells and MCF-7 cells, respectively [11–13]. Subsequently, the novel PKCα and p38 MAPK were both implicated in the TNF-α-induced translocation of nSMase2 in A549 cells [14]. Taken together, this suggests that nSMase2 localization is a highly dynamic and regulated process. However, the precise mechanisms by which nSMase2 transport occurs are unknown.
Previous research has implicated nSMase2 in cell death, cell cycle arrest, and inflammation mediated by TNF-α and Il-1β [12,14,15]. Notably, more recent studies have suggested a role for nSMase2 in regulation of membranes and membrane trafficking [16]. In hippo-campal neuronal cells, nSMase2 was reported to participate in TNF-α–induced clustering of NMDA receptors in lipid rafts in accordance with studies showing nSMase activity in PM microdomains [17]. Additionally, a recent study implicated nSMase2 in the formation of exosomes occurring in the multivesicular endosomes [18]. In that study, ceramide generation was suggested to participate in the invagination of the endosomal membranes giving rise to exosomes.
The degradation and recycling pathways are believed to connect the endosomal compartments with the Golgi and the PM [19]. The reported localizations of nSMase2 imply the trafficking of nSMase2 from the Golgi compartment or the PM to the endosomal compartment. However, the localization of nSMase2 in the endosomal system has not been determined.
In this study, we have investigated nSMase2 trafficking. We find that the Golgi localization of nSMase2 represents two different pools of protein; a pool of newly synthesized protein en route to the cell surface and a pool of recycled protein endocytosed from the PM. Our results further suggest that clathrin-dependent endocytosis of nSMase2 is followed by its traffic through the early and recycling endosomes. Finally, we show that preventing nSMase2 recycling results in increased activity and ceramide generation. Taken together, these results underscore the importance of nSMase2 recycling for the regulation of its catalytic activity and suggest that the PM is a major site for nSMase2-dependent ceramide generation.
2. Materials and methods
2.1. Reagents
PMA and Gö 6976 were from Calbiochem (San Diego, CA). Ruboxistaurin (Eli Lilly, Indianapolis, Indiana) was a generous gift from Dr. M. Buse (Medical University of South Carolina, SC). FIPI (5-Fluoro-2-indolyl des-chlorohalopemide) was a generous gift from Dr. M. Frohman (Stony Brook University, NY). Anti-Giantin antibody was from Covance (Emeryville, CA), anti-TGN46 was from Novus (Littleton, CO), anti-V5 was from Invitrogen (Carlsbad, CA), anti-EEA-1 and anti-transferrin-Receptor were from BD (Franklin Lakes, NJ). Transferrin-Alexa Fluor 555, cholera toxin β subunit Alexa Fluor 594, anti-mouse and anti-rabbit-Alexa-Fluor 555 secondary antibodies were from Molecular Probes/Invitrogen (Carlsbad, CA). [choline-methyl-14C] sphingomyelin was provided by Dr. Alicja Bielawska (Medical University of South Carolina, Charleston, SC). All lipids were purchased from Avanti Polar Lipids (Alabaster, AL). All other reagents were from Sigma (St. Louis, MO).
2.2. Cell culture
MCF-7 cells were maintained in RPMI 1640 (Invitrogen, Carlsbad, CA) media supplemented with 10% fetal bovine serum in a 5% CO2 incubator at 37 °C. A549 cells were maintained in DMEM (high glucose) media (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum in a 5% CO2 incubator at 37 °C. HEK293 cells were maintained in MEM media (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum in a 5% CO2 incubator at 37 °C.
2.3. Plasmid construction
The wild type V5-nSMase2 and GFP-nSMase2 constructs were previously described [10–12]. The catalytically inactive V5-nSMase2 was generated by site-directed mutagenesis of D428, D638 and H639 to alanine using the Quikchange kit from Stratagene (La Jolla, CA). The wild type p-BK-CMV-PKCβ-II was previously described [20]. The wild type PKCα was cloned between XhoI and KpnI sited of pEGFP-N3 vector. GFP tagged Rab-5 and Rab11 constructs were a generous gift from Dr. Marino Zerial (Max Planck Institute CBG, Dresden, Germany).
2.4. cDNA transfection
Cells were plated in 35-mm confocal dishes (MatTek, Ashland, MA) at different cell densities (0.05 to 0.5 × 106 cells/dish) and cultured for 24 h. MCF-7 and A549 cells were transfected with Effectene® transfection reagent (Qiagen, Turnberry Lane, Valencia, CA) using 0.25 µg of DNA/dish. HEK293 cells were transfected with Lipofectamine 2000 (Invitrogen, Carlsbad, CA) using 1 µg of DNA/dish. Cells were grown for 20–24 h after transfection and used for immunofluorescence analysis. To obtain stable cell lines, MCF-7, HEK293 and A549 cells were transfected as described above and selected in media containing 1 mg/ml Blasticidin.
2.5. Immunofluorescence and confocal microscopy
Cells were fixed with 3.7% formaldehyde in phosphate-buffered saline (PBS) for 10 min and were permeabilized with 0.1% Triton X-100 in PBS for 10 min. Cells were then washed 3 times with PBS, blocked with 2% human serum (diluted in PBS) for 30 min and incubated with primary antibodies diluted in 2% human serum overnight at 4 °C or 1 h at room temperature before 1 h incubation with secondary antibodies at room temperature. Alternatively, for FRAP experiments, transfected cells were imaged live in media, 10% fetal bovine serum in an incubation chamber at 37 °C with 5% CO2. Photobleaching was done using a 488-nm laser, intensity 100%, 20 iterations. All confocal images were taken with a laser-scanning confocal microscope (LSM 510 Meta; Carl Zeiss, Thornwood, NY). Each microscopy image is representative of 20 fields and was taken in the equatorial plane of the cells. Raw data images were cropped in Adobe Photoshop® 7.0 for publication.
2.6. Western blotting
Total proteins were extracted from cells lysed in a buffer containing 20 mM Tris-HCl, pH 7.5, 1% TritonX-100, 1 mM EDTA, 150 mM NaCl, 1× protease inhibitors and phosphatase inhibitors mixture (Roche Diagnostics, Indianapolis, IN). Postnuclear supernatants were prepared after centrifugation at 2500 rpm for 10 min and protein content was determined by Bradford assay (Bio-Rad, Hercules, CA). Equal amounts of proteins were separated on 4–20% polyacrylamide gels.
2.7. Determination of in vitro nSMase activity
After the treatment, cells were washed 2 times with PBS, collected and lysed by 20 syringe passages in buffer containing 25 mM Tris-HCl, pH 7.4, 0.2% Triton X-100, 1 mM EDTA, 1× protease inhibitors and phosphatase inhibitors mixture (Roche Diagnostics, Indianapolis, IN). Postnuclear supernatants were prepared after centrifugation at 2500 rpm for 10 min and used for the enzyme activity assay. Cell lysates (100 µl) containing the appropriate amount of protein were incubated with 100 µl assay buffer containing 100 000 cpm choline-methyl 14C-sphingomyelin, 100 µM phosphatidylserine, 200 µM sphingomyelin, 200 mM Tris–HCl (pH 7.4), 10 mM MgCl2, 5 mM dithiotreitol, 0.2% Triton X-100 for 30 min at 37 °C. The reaction was stopped by adding 1.5 ml of chloroform/methanol (2:1, v/v) followed by addition of 0.4 ml water (Folch extraction). Phases were separated by centrifugation at 3000 rpm (2000g) for 5 min at room temperature. 800 µl of upper (aqueous) phase was counted by scintillation.
2.8. Inhibition of endocytosis
Cells were washed with K+free buffer containing 140 mM NaCl, 20 mM HEPES, 1 mM CaCl2, 1 mM MgCl2,1mg/ml glucose and submitted to a hypotonic shock in the presence of K+free buffer diluted with H2O (1:1, v/v) for 5 min. Cells were then washed 3 times with K+free buffer and incubated in K+free buffer or with control buffer containing 140 mM NaCl, 20 mM HEPES, 1 mM CaCl2, 1 mM MgCl2, 1 mg/ml glucose and 10 mM KCl for 2 or 4 h at 37 °C before microscopy analysis. Alternatively, cells were treated with 10 µM PAO for 2 and 4 h.
2.9. Measurement of ceramide levels by mass spectrometry
After the treatment, cells were washed 2 times with cold PBS, collected by centrifugation and the cell pellets were used for lipid extraction. Two ml of 70% isopropanol:ethyl acetate (2:3, v/v) was added directly to the cells. Internal standards for ceramide (50 pmol) were added to each sample. The samples were centrifuged at 2000g and the upper phase was transferred to a new glass tube, and the lower phase was re-extracted adding 2 ml of the same extraction solution. The two upper phases were combined in the same tube, and used to measure ceramide and inorganic phosphate. The identification of ceramide species was performed on a Thermo Finnigan TSQ 7000 triple quadrupole mass spectrometer (MS) operating in a multiple reaction monitoring positive ionization mode as described previously [21]. Results from MS analysis were normalized to total inorganic phosphate [22] present in the sample after a Bligh & Dyer extraction [23].
2.10. Statistical analysis
Results are expressed as means ± s.e.m. of at least three independent determinations per experiment. Mean values were compared using the Student's t-test. Differences were considered statistically significant when p<0.05 (*), p<0.01 (**) and p<0.001 (***).
3. Results
3.1. nSMase2 localizes predominantly at the PM and the Golgi apparatus in MCF-7 cells
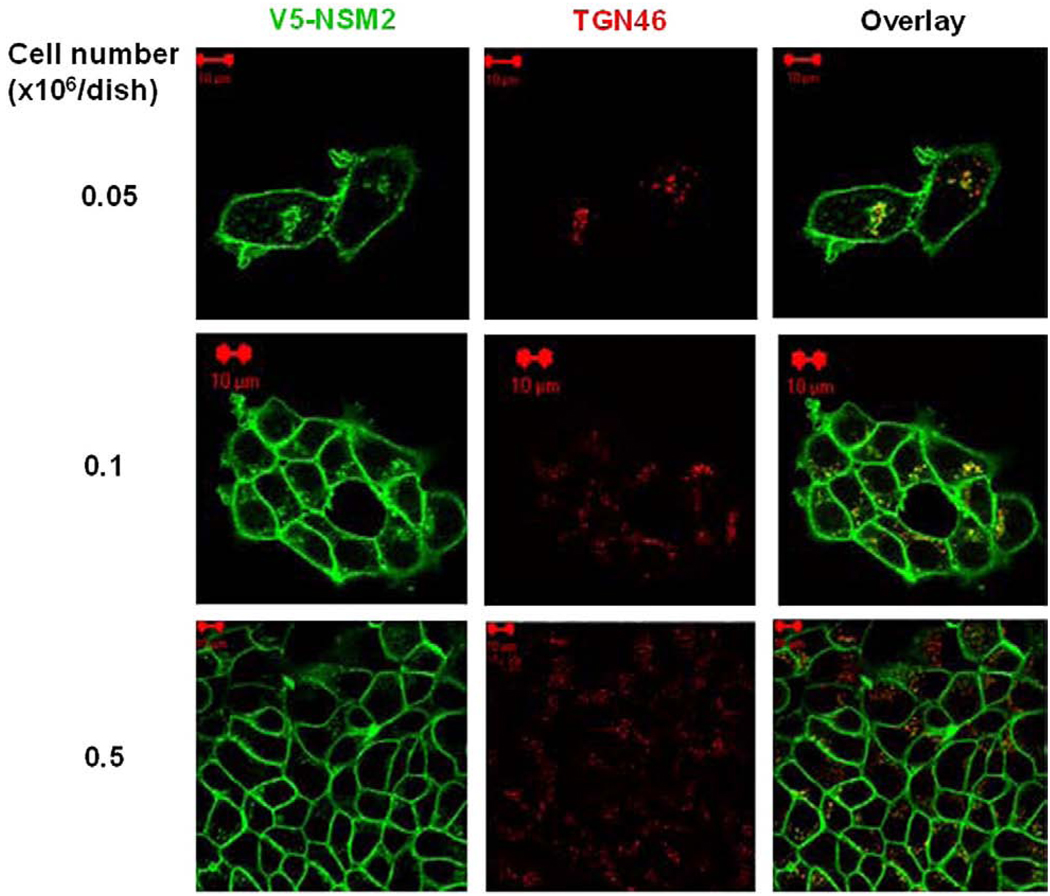
Previous studies using transiently transfected cells have indicated that nSMase2 localized predominantly to the Golgi compartment in subconfluent MCF-7 cells whereas at confluence, a plasma membrane (PM) localization of nSMase2 was observed [11,12]. To further investigate the mechanisms involved in the trafficking of nSMase2, MCF-7 cells were stably transfected with V5-tagged nSMase2 and visualized by immunofluorescence and confocal microscopy (Fig. 1). The effect of cell density on the co-localization of V5-nSMase2 with the Golgi markers TGN46 (Fig. 1) and giantin (data not shown) was analyzed. At low density, the majority of cells expressed V5-nSMase2 both in the Golgi and at the cell surface whereas at confluence, V5-nSMase2 localized exclusively to the PM. Thus, in stably expressing cells, nSMase2 localizes predominantly at the PM and in the Golgi and in accordance with previous studies in MCF-7 cells [12], confluence induces re-location/translocation of V5-nSMase2 from the Golgi to the PM.
Fig. 1.
Plasma membrane localization of stably overexpressed V5-nSMase2 increases with cell density. MCF-7 cells stably overexpressing V5-nSMase2 were seeded in 35-mm confocal dishes at different cell densities (0.05, 0.1 and 0.5 × 106/dish) and 24 h later were fixed and permeabilized. Cells were stained with anti-V5 (green) and anti-TGN46 (red) antibodies as described in Materials and methods for analysis by confocal microscopy. Pictures are representative of at least 4 fields taken from three independent experiments.
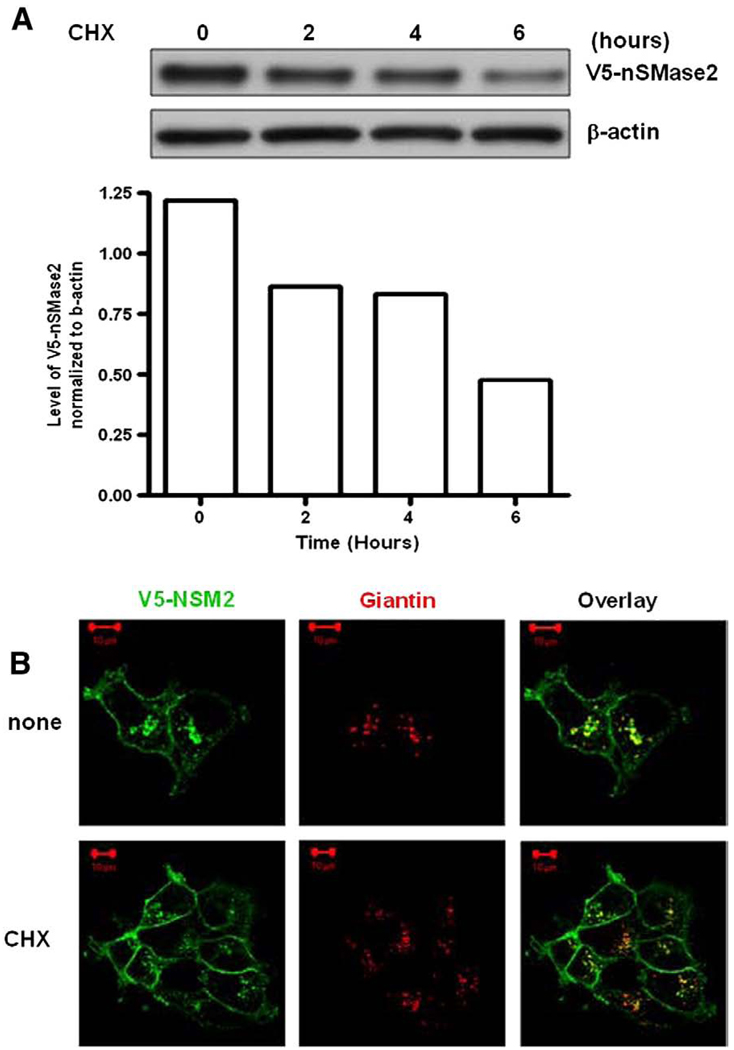
We speculated that the Golgi localization may represent an effect of overexpression and that the Golgi pool of nSMase2 represents a pool of newly synthesized protein en route to the cell surface. To assess this, the effects of the protein synthesis inhibitor cycloheximide (CHX) on the protein level and the localization of V5-nSMase2 were evaluated. Two hours after the treatment, a decrease in nSMase2 protein level was observed (Fig. 2A) whereas nSMase2 still localized in the Golgi (Fig. 2B). These results suggest that the Golgi pool of stably overexpressed nSMase2 does not represent newly synthesized protein.
Fig. 2.
V5-nSMase2 localizes in the Golgi and at the plasma membrane after inhibition of protein synthesis with cycloheximide. MCF-7 cells stably overexpressing V5-nSMase2 were seeded at 0.1 × 106/dish in 35-mm plates and 24 h later were treated with or without 50 µg/ml cycloheximide (CHX) for the indicated time (A) or for 2 h (B). (A) Total protein was extracted and immunoblotted with anti-V5 antibody as described in Materials and methods. The intensity of bands was evaluated with ImageJ software. Results are representative of three independent experiments. (B) Cells were fixed, permeabilized, and stained with anti-V5 (green) and anti-Giantin (red) antibodies as described in Materials and methods for analysis by confocal microscopy. Pictures are representative of at least 4 fields taken from three independent experiments.
3.2. Catalytic activity is not essential for the plasma membrane localization of nSMase2
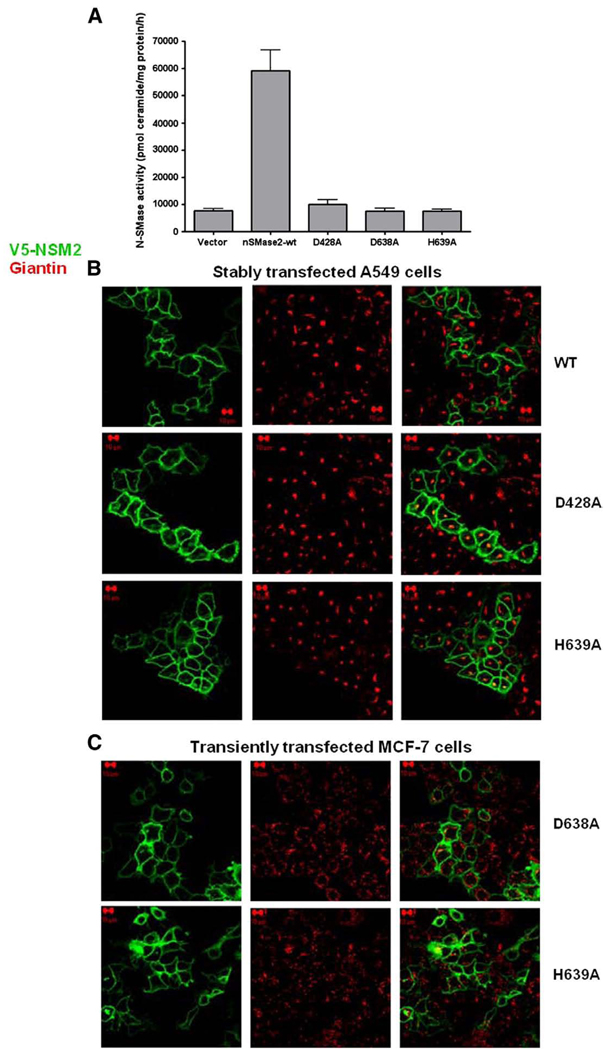
A previous study identified a role for nSMase2 in the trafficking of Golgi-derived secretory vesicles [24]. Thus, it became important to determine if enzymatic activity of nSMase2 was necessary for its PM localization. Accordingly, the localization of catalytically inactive mutants of nSMase2 compared to wild-type nSMase2 was evaluated in different cell types. A549 and MCF-7 cells were stably or transiently transfected with different constructs of V5-nSMase2 mutated in highly conserved, essential residues for hydrolytic activity (His-639, Asp-638) or substrate recognition (Asp-428) (reviewed in [4]). The absence of nSMase activity in A549 cells transiently overexpressing the catalytically inactive mutants of V5-nSMase2 was verified (Fig. 3A). In addition, the localization of stably overexpressed V5-nSMase2 to the PM at confluence was confirmed in A549 cells (Fig. 3B, upper panel). More importantly, all catalytically inactive mutants of V5-nSMase2 showed highly similar localization to wild-type nSMase2. As can be seen, catalytically inactive mutants predominantly localized to the PM in both confluent A549 cells (stably transfected) and MCF-7 (transiently transfected) (Fig. 3B and C). Taken together, these results suggest that the catalytic activity of nSMase2 is not essential for its PM localization and, by extension, is not required for the trafficking of nSMase2 from Golgi to the PM.
Fig. 3.
Catalytically inactive mutants of V5-nSMase2 localize at the plasma membrane in different cell types. (A) A549 cells were transiently transfected with wild type (WT) or catalytically inactive V5-nSMase2 (D428A, H639A, D638A) and in vitro nSMase activity in total cell lysate was measured as described in Materials and methods. (B) Localization of nSMase2 in confluent A549 cells stably overexpressing wild type (WT) or catalytically inactive V5-nSMase2 (D428A, H639A). Cells were plated for 48 h and fixed; (C) localization of catalytically inactive mutants of V5-nSMase2 (D638A, H639A) in confluent MCF-7 cells following transient overexpression. Cells were plated for 24 h and transiently transfected for 24 h prior to fixing. For (B) and (C), after fixation, cells were permeabilized and stained with anti-V5 (green) and anti-giantin (red) antibodies for analysis by confocal microscopy as described in Materials and methods. Pictures are representative of at least 4 fields taken from three independent experiments.
3.3. Effects of fluorescence recovery after photobleaching on Golgi localization of GFP-nSMase2
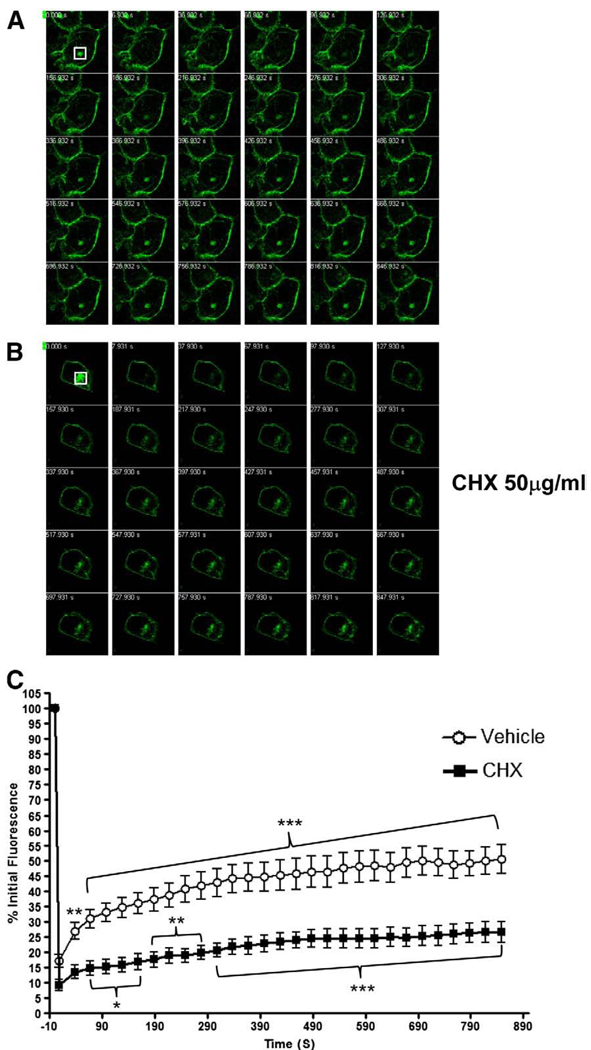
We next hypothesized that nSMase2 may recycle from the PM to the Golgi. Quantitative fluorescence microscopy approaches such as fluorescence recovery after photobleaching (FRAP) techniques are commonly used to study the trafficking of GFP-tagged proteins in living cells [25]. Accordingly, to address if nSMase2 traffics within the cell, FRAP experiments were performed on MCF-7 cells transiently transfected with GFP-nSMase2 and pretreated with or without CHX to distinguish newly synthesized nSMase2 from recycling nSMase2. In these experiments, the intracellular pool of GFP-nSMase2 was photobleached, and fluorescence recovery in the targeted region was monitored. As illustrated, the fluorescence reappeared a few minutes after photobleaching, indicating that the intracellular pool of GFP-nSMase2 is extremely dynamic (Fig. 4A). Notably, quantification of this process indicated a significant extent of fluorescence recovery (Fig. 4C). Treatment with 50 µg/ml CHX strongly attenuated recovery indicating that at least part of nSMase2 in the Golgi represents newly synthesized protein. Nonetheless, a significant recovery of fluorescence was still observed albeit to a lower extent (Fig. 4B and C). This suggests that the Golgi localization of nSMase2 represents two populations of GFP-nSMase2. One population represents newly synthesized protein en route to the cell surface that shows no recovery in the presence of CHX. The other population likely represents GFP-nSMase2 recycling from the PM, and this process is enhanced when protein synthesis is inhibited.
Fig. 4.
The recovery of an intracellular pool of GFP-nSMase2 after photobleaching is both dependent and independent of new protein synthesis. Subconfluent MCF-7 cells (0.3 × 106/dish) were transfected with 3′GFP-tagged nSMase2 for 18–20 h and treated with (B) or without (A) 50 µg/ml CHX for 2 h. After treatment, the intracellular pool of GFP-nSMase2 was photobleached (white box), and fluorescence recovery was analyzed every 30 s for 15 min. (A, B) Representative FRAP experiments are shown for each condition of treatment. (C) Results are represented as the percentage of initial fluorescence in the bleached area. Student's t-test was used to compare data at the first time point with data at the other time points.
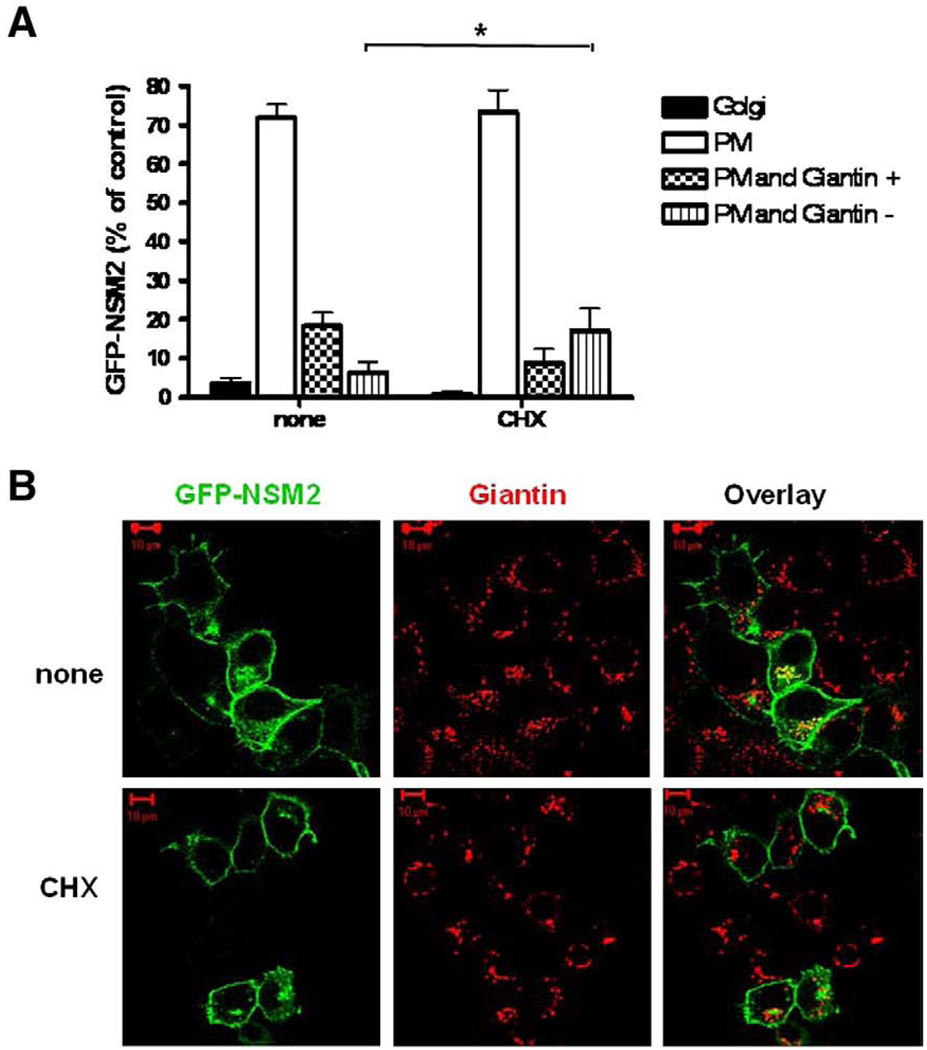
To further investigate the recycling of GFP-nSMase2, the effect of CHX on its co-localization with the Golgi marker giantin was quantified (Fig. 5). GFP-nSMase2 localized mainly at the PM at sub-confluence with 72 ± 4% of cells expressing nSMase2 exclusively at the PM and 18 ± 4% displaying dual localization in the Golgi and at the cell surface (PM and Giantin+). However, in some cells expressing GFP-nSMase2 at the PM, an intracellular pool of GFP-nSMase2 in the perinuclear region that did not co-localize with giantin was observed (PM and Giantin−). Moreover, treatment with CHX significantly increased the number of cells displaying this pattern whereas those with Golgi localization decreased (Fig. 5A and B). This indicated the existence of a non-Golgi associated pool of intracellular nSMase2 that may be recycled from the PM.
Fig. 5.
The intracellular pool of GFP-nSMase2 is not exclusively associated with the Golgi apparatus. MCF-7 cells were plated at low density (0.3 × 106/dish) and, 24 h later, were transfected with 3′-GFP-tagged nSMase2 (0.5 µg/dish). After 18–20 h, cells were treated with (CHX) or without (none) 50 µg/ml CHX for 2 h and co-localization of 3′-GFP-nSMase2 with giantin was analyzed by confocal microscopy. (A) Co-localization of GFP-nSMase2 with giantin and localization at cell surface was quantified. Results are represented as the percentage of cells displaying localization in the Golgi (Golgi), at the plasma membrane (PM), or showing both localization at the PM and co-localization with giantin (PM and Giantin+) or not (PM and Giantin−). For each condition, at least 100 cells were quantified under the microscope. Values are means±s.e.m. of three independent experiments. (B) Pictures are representative of at least 4 fields taken from four independent experiments.
3.4. The non-Golgi associated nSMase2 is localized to endosomal compartments
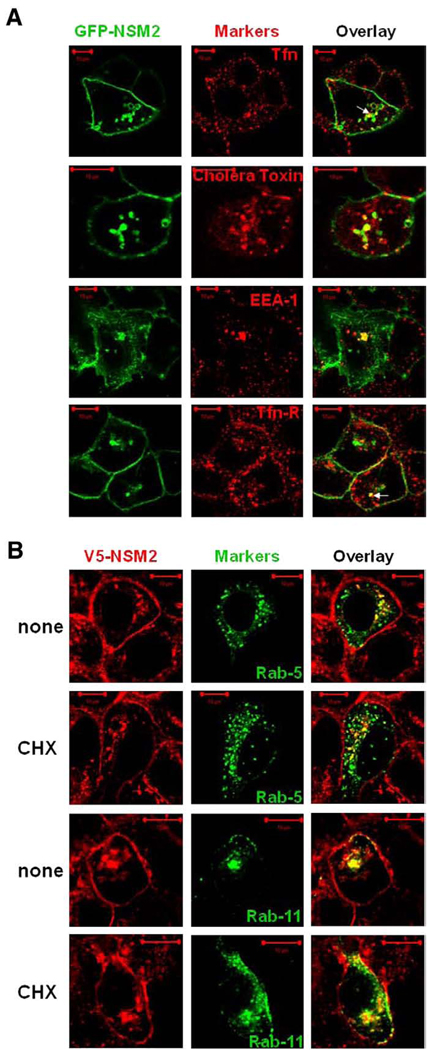
If nSMase2 recycles from the PM to the Golgi, the recycling endosomes may represent an intermediate compartment during trafficking. To investigate this possibility, co-localization studies with different endosomal/recycling markers were performed. In the presence of CHX, GFP-nSMase2 co-localized partly with the early endosomal marker EEA-1 and three different markers of the recycling endosomes: human transferrin, human transferrin-receptor, and β-cholera toxin (Fig. 6A). This indicates localization of nSMase2 to the early/recycling endosomes.
Fig. 6.
GFP-nSMase2 and V5-nSMase2 co-localize with markers of early and recycling endosomes. (A) MCF-7 cells were plated at low density (0.3 × 106/dish) and 24 h later were transfected with 3′-GFP-tagged nSMase2 (0.5 µg/dish). After 18 to 20 h, cells were treated for 4 h with 50 µg/ml CHX. One hour before the end of treatment, cells were incubated with 10 µg/ml human transferrin-Alexa Fluor 555 (Tfn) or with 1 µg/ml cholera toxin subunit β-Alexa Fluor 594 for the remaining time. Cells were then washed and fixed. Alternatively, cells were washed, fixed, permeabilized, and stained with anti-EEA-1 or anti-transferrin receptor (Tfn-R) antibodies as described in Materials and methods. (B) MCF-7 cells stably overexpressing V5-nSMase2 were transfected with GFP-Rab5 or GFP-Rab11 (0.5 µg/dish). After 24 h transfection, cells were treated with (CHX) or without (none) 50ug/ml CHX for 2 h. Cells were fixed, permeabilized and stained with anti-V5 antibody (green or red) as described in Materials and methods. Co-localization of GFP-nSMase2 with the different markers and of V5-nSMase2 with GFP-Rab5 or Rab11 was analyzed by confocal microscopy. Pictures are representative of at least 4 fields taken from three independent experiments.
The GTPase proteins Rab-5 and Rab-11 are involved in retrograde transport from endosomes to the TGN, specifically regulating fusion processes in the early/sorting endosomes and the recycling endosomes respectively. Therefore, to further investigate the potential recycling of V5-nSMase2 from the PM to the Golgi, overexpression of GFP-Rab-5 and GFP-Rab-11 was performed in MCF-7 cells stably overexpressing V5-nSMase2. Following transfection, cells were treated with or without CHX to increase the putative “recycling” pool. A clear co-localization of the intracellular pool of V5-nSMase2 with GFP-Rab-5 and GFP-Rab-11 was observed both in the presence and absence of CHX (Fig. 6B). Taken together, this suggests recycling of V5-nSMase2 through the early and the recycling endosomes.
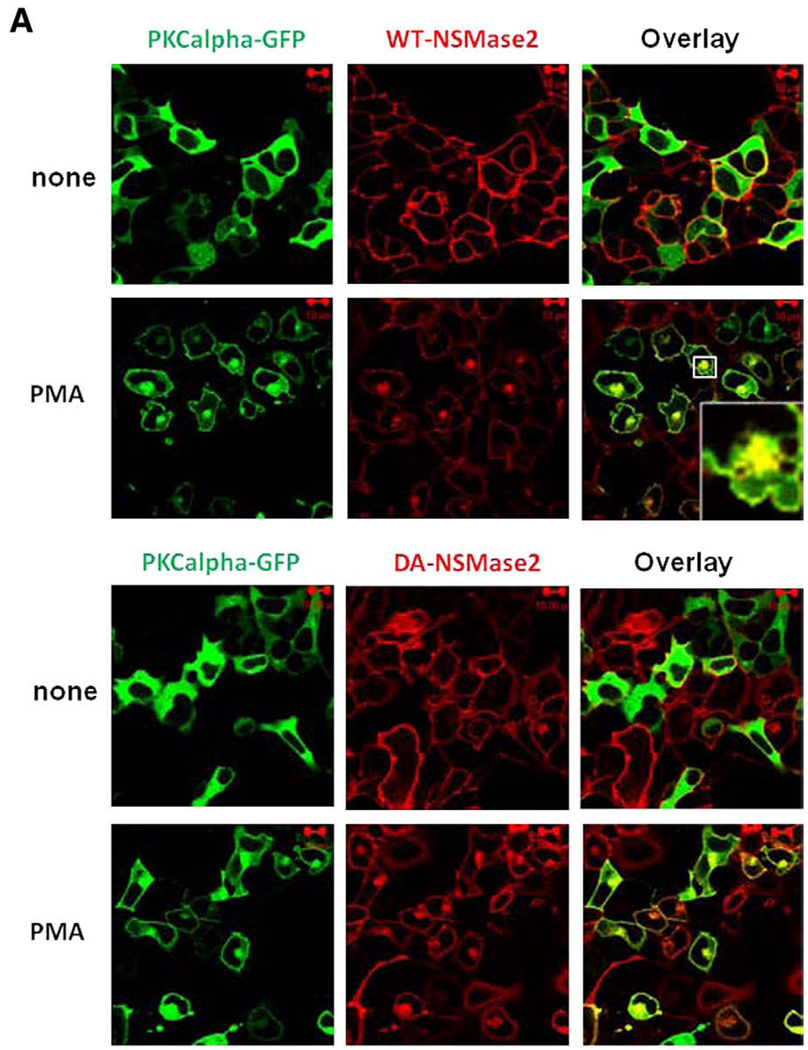
Previous studies from our laboratory have defined and characterized a novel, protein kinase C (PKC)- and phospholipase D (PLD)-dependent subset of the perinuclear (Rab11-positive) recycling endosomes, termed the pericentrion [26,27]. Sustained activation of classical PKC (cPKC) α and βII isoforms was shown to induce sequestration of PKC and other recycling plasma membrane components into this compartment [28]. As we observed some co-localization of GFP-Rab-11 with V5-nSMase2, we wanted to further corroborate the presence of nSMase2 within the recycling compartment and if this regulated by determining if nSMase2 localizes to the pericentrion upon stimulation of PKC. Accordingly, V5-nSMase2 (stable) and PKCα-GFP or GFP-PKCβII (transient) were co-expressed in HEK293 cells, and phorbol myristate acetate (PMA) was used to activate PKC. Consistent with the above data in A549 and MCF-7 cells, stably overexpressed nSMase2 localized predominantly to the PM in HEK293 cells.
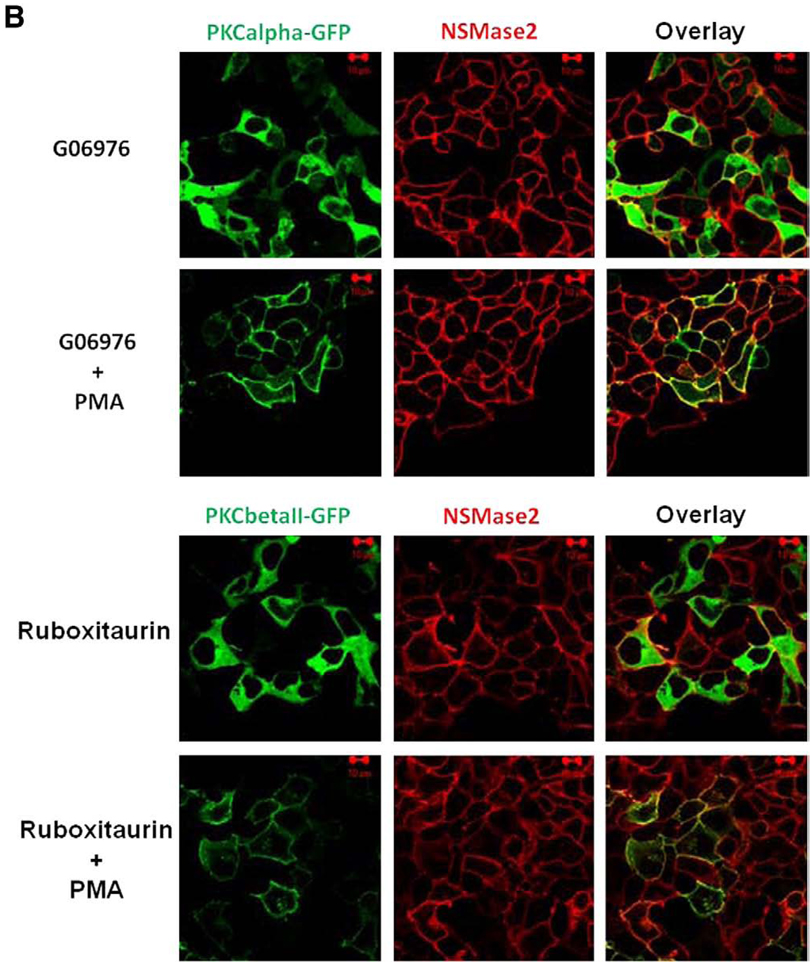
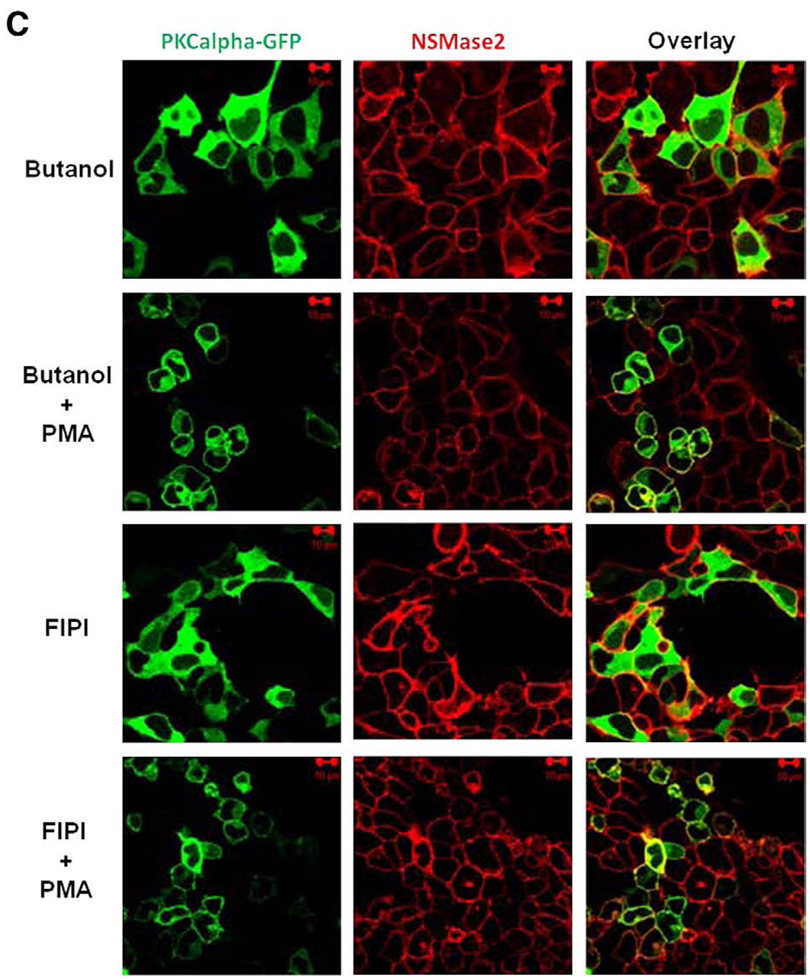
Stimulation of cells with PMA induced translocation of both PKC and nSMase2 to the pericentrion (Fig. 7A, upper panels). Notably, this was not dependent on nSMase2 activity as the catalytically inactive DA-nSMase2 also translocated to the pericentrion (Fig. 7A, lower panels). Consistent with previous studies, this phenomenon was prevented by inhibitors of classical PKC (Gö6976; Fig. 7B, upper panel). In addition, the specific PKCβ inhibitor (ruboxistaurin) was also able to inhibit nSMase2 and PKCβII localization within the pericentrion (Fig. 7B, lower panels). Moreover, inhibition of PLD with 1-butanol or the recently described inhibitor 5-Fluoro-2-indolyl des-chlorohalopemide (FIPI) [29] also prevented nSMase2 sequestration into the pericentrion (Fig. 7C). Taken together, these results confirm the presence of nSMase2 in the recycling endosomes and indicate that recycling of nSMase2 is independent of its catalytic activity.
Fig. 7.
Localization of nSMase2 to the pericentrion. HEK293 cells stably overexpressing wild type (WT-nSMase2) or catalytically inactive V5-nSMase2 (DA-nSMase2) were transfected with PKCα-GFP or GFP-PKCβII (1 µg/dish) for 24 h as indicated and then treated with (PMA) or without (none) 100nM PMA for 1 h (A). Alternatively, before the PMA treatment, cells were pretreated with classical PKC inhibitor (3µMGö6976, 1 h), PKCβ inhibitor (300 nM ruboxistaurin, 2 h) (B) or with PLD inhibitors (0.4% 1-butanol, 10 min or 750 nM FIFI, 4 h) (C). Cells were fixed, permeabilized and stained with anti-V5 antibody (red) as described in Materials and methods. Co-localization of V5-nSMase2 with PKCα-GFP or GFP-PKCβII was analyzed by confocal microscopy. Pictures are representative of at least 4 fields taken from three independent experiments.
3.5. The recycling of nSMase2 from the PM to the Golgi regulates its enzymatic activity
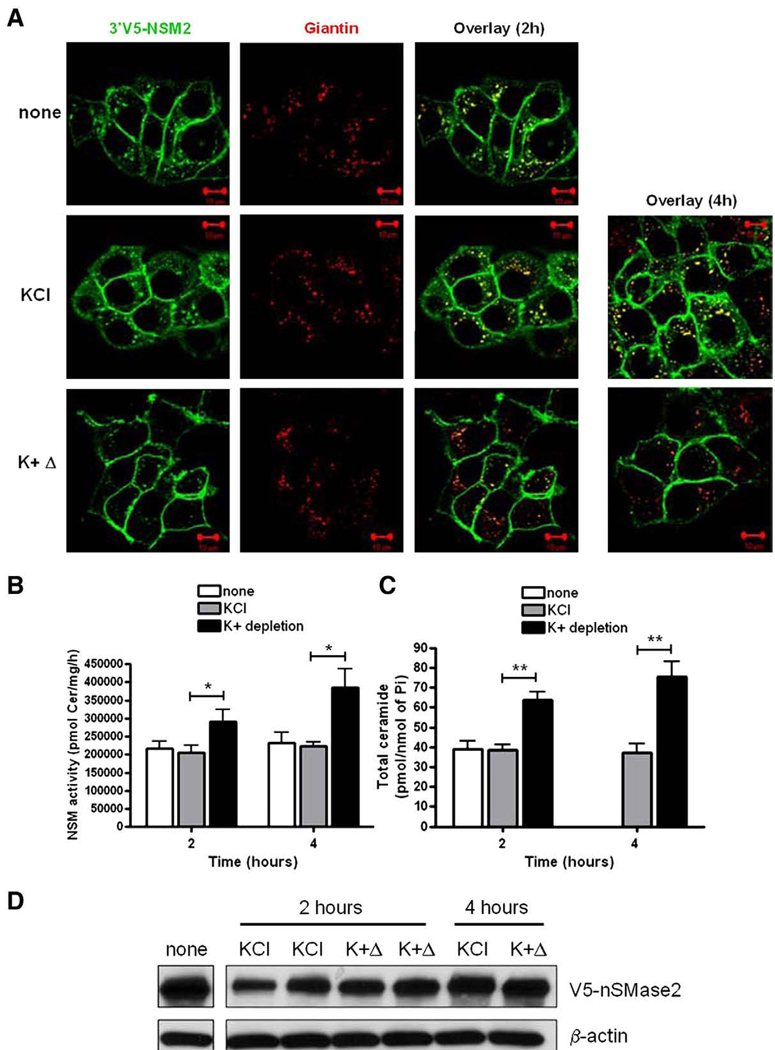
In order to recycle from the PM, proteins must first be endocytosed into PM-derived vesicles. To confirm that this is the case for nSMase2, the effect of inhibition of endocytosis on the localization of stably overexpressed V5-nSMase2 in MCF-7 cells was evaluated (Fig. 8A). For this, cells were briefly exposed to hypotonic shock and incubated for 2 or 4 h in potassium-depleted buffer (K+Δ), previously found to inhibit clathrin-dependent endocytosis by preventing the formation of new coated-pits [30]. The efficiency of endocytosis inhibition by K+depletion was verified using transferrin-555 as a positive control (data not shown). As above, in both control cells (none) and those treated with control buffer (KCl), V5-nSMase2 localized both at the PM and Golgi (Fig. 8A). Importantly, the inhibition of endocytosis for 2 and 4 h strongly depleted the intracellular pool of V5-nSMase2 including that in the Golgi compartment (Fig. 8A). This suggests that nSMase2 is endocytosed from the PM and retrieved to the Golgi through the recycling compartments.
Fig. 8.
K + depletion affects the Golgi localization of V5-nSMase2 and increases nSMase activity. MCF-7 cells stably overexpressing V5-nSMase2 were briefly exposed to hypotonic shock and then incubated in the presence of potassium-depleted buffer (K+Δ), with control buffer containing 10 mM KCl (KCl), or remained untreated (none) as indicated. (A) Cells were fixed, permeabilized and stained with anti-V5 (green) and anti-giantin (red) antibodies. Co-localization of V5-nSMase2 with giantin was analyzed by confocal microscopy. Pictures are representative of at least 4 fields taken from three independent experiments. (B) In vitro nSMase activity in total cell lysate was measured as described in Materials and methods. (C) Total ceramide levels were quantified by MS analysis. (D) Total protein was extracted and immunoblotted with anti-V5 and anti-actin antibodies as described in Materials and methods. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Although nSMase2 activity was not necessary for recycling of the enzyme, we wanted to determine if recycling was required for regulation of nSMase activity. To do this, nSMase2 recycling was blocked by inhibition of endocytosis and the effect on nSMase2 activity was evaluated. To ensure that nSMase2 activity was being measured, experiments were performed in MCF-7 cells stably over-expressing nSMase2. As can be seen, inhibition of endocytosis significantly increased nSMase2 activity after both 2 and 4 h (Fig. 8B) when compared to control conditions (none and control buffer KCl). To assess the functional consequences of this, total ceramide levels were evaluated (Fig. 8C). Consistent with the increased activity, endocytosis inhibition led to an increased total ceramide levels in a time-dependent manner. Importantly, treatment of the cells with phenyl arsine oxide (PAO) (10 µM, 2 h), a distinct inhibitor of clathrin-dependent endocytosis [31], had the same effects on nSMase2 localization and nSMase2 activity (data not shown). To address the specificity of the effect of endocytosis inhibition on nSMase2 activity, the quantification of nSMase2 protein level was evaluated. The treatment slightly decreased the levels of nSMase2 suggesting that the increased activity seen is not due to increased nSMase2 protein (Fig. 8D). Consequently, this likely reflects an increase in specific activity of nSMase2. Taken together, these results suggest that the recycling of nSMase2 from the PM to the Golgi is necessary for negative regulation of its enzymatic activity.
4. Discussion
Previous studies have reported localization of nSMase2 both in the Golgi and at the PM, and have suggested regulated trafficking of nSMase2. Despite these observations, the underlying mechanism of nSMase2 transport has remained unclear. In the current study, we have clarified the trafficking of nSMase2 between the Golgi compartment and the PM and observed both an anterograde and a retrograde transport of nSMase2. Interestingly, recycling of nSMase2 from the PM appears to be important in controlling its enzymatic activity and the production of ceramide. Taken together, these data shed light on the constitutive regulation of nSMase2 and further implicate the PM as a primary site of nSMase2 action.
4.1. Transport of nSMase2 from Golgi to PM
Using MCF-7 cells as a model system, we investigated the mechanism of nSMase2 transport with a variety of tagged nSMase2 constructs and by a combination of stable and transient transfection. In accordance with previous studies from our laboratory, results confirmed the effect of confluence on nSMase2 localization within the Golgi and PM observing that, as cellular density increased, the population of cells with solely PM localization of nSMase2 also increased. In addition, since the co-localization of nSMase2 with markers of the TGN was noted here (Fig. 1) and previously [11], it is speculated that this transport is likely to be through TGN-derived vesicles. Importantly, nSMase2 activity is not necessary for its transport to the PM (Fig. 3). Thus, although nSMase2 was implicated in the transport of Golgi secretory vesicles [24], this does not appear to directly influence its own transport from Golgi to PM.
Others studies have also reported the Golgi localization of overexpressed membrane proteins [32,33]. As done here, CHX is commonly used to discriminate between a pool of newly synthesized proteins and a permanent localization [33,34]. From these results, it was evident that Golgi-localized nSMase2 does, in part, represent newly synthesized nSMase2 en route to the PM via the secretory pathway. Notably, the confluence-induced nSMase2 ‘translocation’ from the Golgi to the PM could be explained only in part as a consequence of a global reduction of protein synthesis during growth arrest [35].
4.2. Transport of nSMase2 from PM to Golgi
In addition to exploring Golgi to PM transport of nSMase2, we also report here on the retrograde transport of nSMase2 from the PM back to the Golgi via recycling endosomes. This was indicated by several lines of evidence. CHX did not affect the Golgi localization of stably overexpressed nSMase2 (Fig. 2B) and partially affected the Golgi localization of GFP-nSMase2 indicating that new protein synthesis alone cannot account for Golgi-localized nSMase2. Consistent with the existence of a second Golgi population of nSMase2, recovery of intracellular fluorescence after photobleaching was still observed in the presence of CHX. Furthermore, CHX treatment of transiently overexpressing cells increased the population of cells with intracellular nSMase2 that was not associated with the Golgi suggesting possible localization in the endosomes. By using various markers for the endosomal compartments, the presence of both GFP-nSMase2 and V5-nSMase2 in the early and recycling endosomes was confirmed (Fig. 5). This was further supported by the translocation of nSMase2 to the pericentrion, a novel PKC-dependent subset of Rab-11 positive recycling endosomes, in HEK293 cells overexpressing PKCα or PKCβII. PKCα or PKCβII may thus regulate nSMase2 recycling, but it is important to note that this endosomal pool of nSMase2 could come from either the Golgi, as described for newly synthesized vesicular stomatitis virus glycoprotein-G en route to the cell surface [36] or the PM, as most endocytosed membrane proteins recycle back to the PM via the endocytic recycling pathway (for review [37]) or, indeed, from both compartments. However, the predominantly PM localization of nSMase2 and the fluorescence recovery of intracellular GFP-nSMase2 in the presence of CHX (Fig. 4) favor a recycling of nSMase2 from the PM. Consistent with this theory, inhibitors of clathrin-dependent endocytosis had a strong effect on the intracellular localization of nSMase2. Although the inhibitors utilized here (K + depletion and PAO) suggest endocytosis of nSMase2 through clathrin-coated pits, we cannot exclude a clathrin-independent endocytosis of nSMase2. Notably, some studies have reported localization of nSMase activity in the raft fractions of the PM and there is indirect evidence that this may be attributable to nSMase2 (reviewed in [16]). Additionally, intracellular nSMase2 co-localized with cholera toxin, an enterotoxin endocytosed from the raft domain of the PM to the Golgi by multiple mechanisms such as caveole/raft- and clathrin-dependent pathways but also by dynamin-independent non-clathrin pathways [38,39]. With this in mind, the possibility of multiple endocytic mechanisms regulating nSMase2 warrants further investigation. This would be of particular interest if distinct mechanisms of endocytosis of nSMase2 are important for deciding the cellular fate of the enzyme as, for example, was recently shown for the TGF-β receptor [40].
Retrograde transport from the endosomes to the TGN has been described for different types of proteins such as the acid-hydrolase receptor, mannose 6-phosphate receptors (M6PR), transmembrane enzymes (furin and SNAREs) and some bacterial and plant toxins (Shiga and Cholera toxin, ricin and abrin) (reviewed in [41]). Notably, the cation-dependent M6PRs are palmitoylated on cysteine residues in their cytoplasmic domains, and this modification is essential for their retrieval to the TGN [42]. Interestingly, previous results from our group have also reported palmitoylation of nSMase2 and demonstrated its importance in regulating the PM localization of the enzyme [10]. Thus, nSMase2 palmitoylation may play a role in its recycling from the PM to the Golgi and this is currently under investigation in our laboratory. Additionally, membrane proteins that undergo clathrin-mediated endocytosis often contain internalization signals in their cytoplasmic domains. These signals are recognized by adaptor protein (AP) and GGAs (Golgi-localized, γ-ear containing, ARF-binding proteins), both important for clathrin-coated vesicle transport from the PM or TGN to the endosomes (reviewed in [43]). Currently, two types of signals are known: tyrosine-based, YXXO (where X is any amino acid and O is an amino acid with a bulky hydrophobic side chain) or dileucine-based (DXXXLL), although in the former case, the signal remains functional when a phenyalanine replaces the tyrosine, particularly when hydrophobic pockets flank the amino acid [44]. Notably, the nSMase2 sequence contains two putative internalization motifs within the C-terminus: an acidic dileucine motif (Leu549 and Leu550) and an aromatic residue based motifs (Phe627) (N. Marchesini, T-G. Truong and Y.A. Hannun, unpublished observations) further supporting the recycling of nSMase2. Consequently, further studies investigating the functional role of these motifs in endocytosis and recycling of nSMase2 are currently underway in our laboratory.
4.3. Biological role of nSMase2 recycling
Although nSMase2 activity was not required for its anterograde or retrograde transport, the blocking of nSMase2 recycling with endocytosis inhibitors resulted in a significant increase in both nSMase2 activity and total ceramide levels (Fig. 8), suggesting a functional role of nSMase2 recycling as negatively regulating enzymatic activity. As nSMase2 has been implicated in inflammatory and cell death signaling pathways, this suggests that nSMase2 endocytosis may function to downregulate the activation of such signaling at the PM and, consequently, regulate the associated biological functions. Consistent with this theory, a previous study utilizing an endocytosis-deficient TNF-α receptor reported enhanced TNF-α-dependent nSMase2 activity and subsequent caspase activation in fibroblasts [45]. Notably, in Alzheimer's disease, the production of amyloid β peptide was correlated with a low level of retromer, a complex involved in retrograde transport between the endosomes and the TGN (reviewed in [41]). Moreover, nSMase2 dowregulation prevented the cytotoxic effects of amyloid β peptide and nSMase2 inhibition decreases amyloid β peptide levels (reviewed in [4]). This suggests the possibility that the disruption of retrograde transport of nSMase2 may increase nSMase2 activity at the PM which could be involved in the neuronal toxicity described in this pathology. Aside from these speculations, the biological function of nSMase2 recycling needs to be further investigated. In addition, a limitation of our study is the use of tagged proteins to monitor nSMase2 trafficking. Unfortunately, due to a lack of nSMase2 antibodies of sufficient quality, we were unable to probe the endogenous localization of the enzyme. However, when these tools become available, an important first step for future studies would be to repeat key observations described here.
In conclusion, in this study we have explored the trafficking of nSMase2 in MCF-7 cells. We find that newly synthesized nSMase2 traffics from the Golgi to the PM, likely through TGN derived vesicles. Moreover, for the first time, we show the retrograde transport of nSMase2 from the PM to the Golgi through the recycling endosomes and demonstrate its importance in regulating nSMase2 activity and cellular ceramide levels. Manipulation of the endocytosis or recycling of nSMase2 may be an attractive means to modulate the cellular levels of ceramide and the signaling pathways downstream of nSMase2 activation.
Acknowledgments
This work was supported by GM43825 grant (to YAH). We thank the Lipidomics Core at Medical University of South Carolina for LC-MS analysis. Work conducted in the Lipidomics Core was supported by NIH grants C06 RR01882 from the extramural research program of the National Center for Research Resources. We also thank the Hollings Cancer Center Molecular Imaging Facility at the Medical University of South Carolina for the use of confocal microscope (grant P30 CA 138313).
Abbreviations
- nSMase2
neutral sphingomyelinase-2
- CHX
cycloheximide
- PM
plasma membrane
- PKCα
protein kinase C-alpha
- PLD
phospholipase D
- PMA
phorbol myristate acetate
- ER
endoplasmic reticulum
- PA
phosphatidic acid
- TGN
trans-golgi network
- FRAP
fluorescence recovery after photobleaching
- PAO
phenyl arsine oxide
- NMDA
N-methyl-d-aspartate
- HEK
human embryonic kidney cells
- TGFβ
tumor growth factor-beta
- TNF-α
tumor necrosis factor alpha
- PBS
phosphate-buffered saline
- GFP
green fluorescent protein
- Il-1β
interleukin-1-beta
- EEA-1
early endosomal antigen 1
- MS
mass spectrometer
References
- 1.Bartke N, Hannun YA. Bioactive sphingolipids: metabolism and function. J. Lipid Res. 2009;50 Suppl:S91–S96. doi: 10.1194/jlr.R800080-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirschberg K, Rodger J, Futerman AH. The long-chain sphingoid base of sphingolipids is acylated at the cytosolic surface of the endoplasmic reticulum in rat liver. Biochem. J. 1993;290(Pt 3):751–757. doi: 10.1042/bj2900751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 4.Clarke CJ, Snook CF, Tani M, Matmati N, Marchesini N, Hannun YA. The extended family of neutral sphingomyelinases. Biochemistry. 2006;45:11247–11256. doi: 10.1021/bi061307z. [DOI] [PubMed] [Google Scholar]
- 5.Mizutani Y, Tamiya-Koizumi K, Nakamura N, Kobayashi M, Hirabayashi Y, Yoshida S. Nuclear localization of neutral sphingomyelinase 1: biochemical and immunocytochemical analyses. J. Cell Sci. 2001;114:3727–3736. doi: 10.1242/jcs.114.20.3727. [DOI] [PubMed] [Google Scholar]
- 6.Sawai H, Domae N, Nagan N, Hannun YA. Function of the cloned putative neutral sphingomyelinase as lyso-platelet activating factor-phospholipase C. J. Biol. Chem. 1999;274:38131–38139. doi: 10.1074/jbc.274.53.38131. [DOI] [PubMed] [Google Scholar]
- 7.Krut O, Wiegmann K, Kashkar H, Yazdanpanah B, Kronke M. Novel tumor necrosis factor-responsive mammalian neutral sphingomyelinase-3 is a C-tail-anchored protein. J. Biol. Chem. 2006;281:13784–13793. doi: 10.1074/jbc.M511306200. [DOI] [PubMed] [Google Scholar]
- 8.Corcoran CA, He Q, Ponnusamy S, Ogretmen B, Huang Y, Sheikh MS. Neutral sphingomyelinase-3 is a DNA damage and nongenotoxic stress-regulated gene that is deregulated in human malignancies. Mol. Cancer Res. 2008;6:795–807. doi: 10.1158/1541-7786.MCR-07-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marchesini N, Luberto C, Hannun YA. Biochemical properties of mammalian neutral sphingomyelinase 2 and its role in sphingolipid metabolism. J. Biol. Chem. 2003;278:13775–13783. doi: 10.1074/jbc.M212262200. [DOI] [PubMed] [Google Scholar]
- 10.Tani M, Hannun YA. Neutral sphingomyelinase 2 is palmitoylated on multiple cysteine residues. Role of palmitoylation in subcellular localization. J. Biol. Chem. 2007;282:10047–10056. doi: 10.1074/jbc.M611249200. [DOI] [PubMed] [Google Scholar]
- 11.Clarke CJ, Truong TG, Hannun YA. Role for neutral sphingomyelinase-2 in tumor necrosis factor alpha-stimulated expression of vascular cell adhesion molecule-1 (VCAM) and intercellular adhesion molecule-1 (ICAM) in lung epithelial cells: p38 MAPK is an upstream regulator of nSMase2. J. Biol. Chem. 2007;282:1384–1396. doi: 10.1074/jbc.M609216200. [DOI] [PubMed] [Google Scholar]
- 12.Marchesini N, Osta W, Bielawski J, Luberto C, Obeid LM, Hannun YA. Role for mammalian neutral sphingomyelinase 2 in confluence-induced growth arrest of MCF7 cells. J. Biol. Chem. 2004;279:25101–25111. doi: 10.1074/jbc.M313662200. [DOI] [PubMed] [Google Scholar]
- 13.Levy M, Castillo SS, Goldkorn T. nSMase2 activation and trafficking are modulated by oxidative stress to induce apoptosis. Biochem. Biophys. Res. Commun. 2006;344:900–905. doi: 10.1016/j.bbrc.2006.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clarke CJ, Guthrie JM, Hannun YA. Regulation of neutral sphingomyelinase-2 (nSMase2) by tumor necrosis factor-alpha involves protein kinase C-delta in lung epithelial cells. Mol. Pharmacol. 2008;74:1022–1032. doi: 10.1124/mol.108.046250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nikolova-Karakashian M, Karakashian A, Rutkute K. Role of neutral sphingomyelinases in aging and inflammation. Subcell. Biochem. 2008;49:469–486. doi: 10.1007/978-1-4020-8831-5_18. [DOI] [PubMed] [Google Scholar]
- 16.Milhas D, Clarke CJ, Hannun YA. Sphingomyelin metabolism at the plasma membrane: implications for bioactive sphingolipids. FEBS Lett. 2009 doi: 10.1016/j.febslet.2009.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wheeler D, Knapp E, Bandaru VV, Wang Y, Knorr D, Poirier C, Mattson MP, Geiger JD, Haughey NJ. Tumor necrosis factor-alpha-induced neutral sphingomyelinase-2 modulates synaptic plasticity by controlling the membrane insertion of NMDA receptors. J. Neurochem. 2009;109:1237–1249. doi: 10.1111/j.1471-4159.2009.06038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brugger B, Simons M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 19.Gruenberg J, Stenmark H. The biogenesis of multivesicular endosomes. Nat. Rev. Mol. Cell Biol. 2004;5:317–323. doi: 10.1038/nrm1360. [DOI] [PubMed] [Google Scholar]
- 20.Feng X, Hannun YA. An essential role for autophosphorylation in the dissociation of activated protein kinase C from the plasma membrane. J. Biol. Chem. 1998;273:26870–26874. doi: 10.1074/jbc.273.41.26870. [DOI] [PubMed] [Google Scholar]
- 21.Bielawski J, Szulc ZM, Hannun YA, Bielawska A. Simultaneous quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods. 2006;39:82–91. doi: 10.1016/j.ymeth.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Van Veldhoven PP, Bell RM. Effect of harvesting methods, growth conditions and growth phase on diacylglycerol levels in cultured human adherent cells. Biochim. Biophys. Acta. 1988;959:185–196. doi: 10.1016/0005-2760(88)90030-6. [DOI] [PubMed] [Google Scholar]
- 23.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 24.Stoffel W, Jenke B, Block B, Zumbansen M, Koebke J. Neutral sphingomyelinase 2 (smpd3) in the control of postnatal growth and development. Proc. Natl Acad. Sci. USA. 2005;102:4554–4559. doi: 10.1073/pnas.0406380102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kenworthy AK. Fluorescence-based methods to image palmitoylated proteins. Methods. 2006;40:198–205. doi: 10.1016/j.ymeth.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 26.Becker KP, Hannun YA. Isoenzyme-specific translocation of protein kinase C (PKC)betaII and not PKCbetaI to a juxtanuclear subset of recycling endosomes: involvement of phospholipase D. J. Biol. Chem. 2004;279:28251–28256. doi: 10.1074/jbc.M400770200. [DOI] [PubMed] [Google Scholar]
- 27.Idkowiak-Baldys J, Baldys A, Raymond JR, Hannun YA. Sustained receptor stimulation leads to sequestration of recycling endosomes in a classical protein kinase C- and phospholipase D-dependent manner. J. Biol. Chem. 2009;284:22322–22331. doi: 10.1074/jbc.M109.026765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Idkowiak-Baldys J, Becker KP, Kitatani K, Hannun YA. Dynamic sequestration of the recycling compartment by classical protein kinase C. J. Biol. Chem. 2006;281:22321–22331. doi: 10.1074/jbc.M512540200. [DOI] [PubMed] [Google Scholar]
- 29.Su W, Yeku O, Olepu S, Genna A, Park JS, Ren H, Du G, Gelb MH, Morris AJ, Frohman MA. 5-Fluoro-2-indolyl des-chlorohalopemide (FIPI), a phospholipase D pharmacological inhibitor that alters cell spreading and inhibits chemotaxis. Mol. Pharmacol. 2009;75:437–446. doi: 10.1124/mol.108.053298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hansen SH, Sandvig K, van Deurs B. Molecules internalized by clathrin-independent endocytosis are delivered to endosomes containing transferrin receptors. J. Cell Biol. 1993;123:89–97. doi: 10.1083/jcb.123.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xia W, Wong EW, Mruk DD, Cheng CY. TGF-beta3 and TNFalpha perturb blood-testis barrier (BTB) dynamics by accelerating the clathrin-mediated endocytosis of integral membrane proteins: a new concept of BTB regulation during spermatogenesis. Dev. Biol. 2009;327:48–61. doi: 10.1016/j.ydbio.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng H, McKay J, Buss JE. H-Ras does not need COP I- or COP II-dependent vesicular transport to reach the plasma membrane. J. Biol. Chem. 2007;282:25760–25768. doi: 10.1074/jbc.M700437200. [DOI] [PubMed] [Google Scholar]
- 33.Choy E, Chiu VK, Silletti J, Feoktistov M, Morimoto T, Michaelson D, Ivanov IE, Philips MR. Endomembrane trafficking of ras: the CAAX motif targets proteins to the ER and Golgi. Cell. 1999;98:69–80. doi: 10.1016/S0092-8674(00)80607-8. [DOI] [PubMed] [Google Scholar]
- 34.Sato I, Obata Y, Kasahara K, Nakayama Y, Fukumoto Y, Yamasaki T, Yokoyama KK, Saito T, Yamaguchi N. Differential trafficking of Src, Lyn, Yes and Fyn is specified by the state of palmitoylation in the SH4 domain. J. Cell Sci. 2009;122:965–975. doi: 10.1242/jcs.034843. [DOI] [PubMed] [Google Scholar]
- 35.Meyuhas O. Synthesis of the translational apparatus is regulated at the translational level. Eur. J. Biochem. 2000;267:6321–6330. doi: 10.1046/j.1432-1327.2000.01719.x. [DOI] [PubMed] [Google Scholar]
- 36.Ang AL, Taguchi T, Francis S, Folsch H, Murrells LJ, Pypaert M, Warren G, Mellman I. Recycling endosomes can serve as intermediates during transport from the Golgi to the plasma membrane of MDCK cells. J. Cell Biol. 2004;167:531–543. doi: 10.1083/jcb.200408165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maxfield FR, McGraw TE. Endocytic recycling. Nat. Rev. Mol. Cell Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 38.Pang H, Le PU, Nabi IR. Ganglioside GM1 levels are a determinant of the extent of caveolae/raft-dependent endocytosis of cholera toxin to the Golgi apparatus. J. Cell Sci. 2004;117:1421–1430. doi: 10.1242/jcs.01009. [DOI] [PubMed] [Google Scholar]
- 39.Torgersen ML, Skretting G, van Deurs B, Sandvig K. Internalization of cholera toxin by different endocytic mechanisms. J. Cell Sci. 2001;114:3737–3747. doi: 10.1242/jcs.114.20.3737. [DOI] [PubMed] [Google Scholar]
- 40.Chen YG. Endocytic regulation of TGF-beta signaling. Cell Res. 2009;19:58–70. doi: 10.1038/cr.2008.315. [DOI] [PubMed] [Google Scholar]
- 41.Bonifacino JS, Rojas R. Retrograde transport from endosomes to the trans-Golgi network. Nat. Rev. Mol. Cell Biol. 2006;7:568–579. doi: 10.1038/nrm1985. [DOI] [PubMed] [Google Scholar]
- 42.Schweizer A, Kornfeld S, Rohrer J. Cysteine34 of the cytoplasmic tail of the cation-dependent mannose 6-phosphate receptor is reversibly palmitoylated and required for normal trafficking and lysosomal enzyme sorting. J. Cell Biol. 1996;132:577–584. doi: 10.1083/jcb.132.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Derby MC, Gleeson PA. New insights into membrane trafficking and protein sorting. Int. Rev. Cytol. 2007;261:47–116. doi: 10.1016/S0074-7696(07)61002-X. [DOI] [PubMed] [Google Scholar]
- 44.Teuchert M, Maisner A, Herrler G. Importance of the carboxyl-terminal FTSL motif of membrane cofactor protein for basolateral sorting and endocytosis. Positive and negative modulation by signals inside and outside the cytoplasmic tail. J. Biol. Chem. 1999;274:19979–19984. doi: 10.1074/jbc.274.28.19979. [DOI] [PubMed] [Google Scholar]
- 45.Neumeyer J, Hallas C, Merkel O, Winoto-Morbach S, Jakob M, Thon L, Adam D, Schneider-Brachert W, Schutze S. TNF-receptor I defective in internalization allows for cell death through activation of neutral sphingomyelinase. Exp. Cell Res. 2006;312:2142–2153. doi: 10.1016/j.yexcr.2006.03.014. [DOI] [PubMed] [Google Scholar]