Abstract
It has been proposed in human colorectal cancers (CRC), a minority subset of cancer cells within tumors able to initiate tumor growth, defined as cancer stem cells (CSC). Solid human primary colonic and its ovarian metastatic cancer tissues were collected from fresh surgical samples and subsequent xenografts were established in non-obese diabetic/severe combined immunodeficient (NOD/SCID) mice. The resulting tumors were disaggregated into single-cell suspensions and a CD133 negative cell line (NANK) was newly established and analyzed by flow cytometry. Surface markers of progenitor cells were immunophenotypically analyzed, and expression of stem cell and cancer-related genes were characterized. Secreted angiogenesis-associated molecules were investigated by proteomic array technology. Finally, different number of NANK was implanted and their tumor-initiating properties were investigated in NOD/SCID mice. Intraperitoneal injection of NANK in NOD/SCID mice induced tumors with developing progressive peritoneal dissemination and ascites. NANK cells maintained a differentiated phenotype and reproduced the full morphologic and phenotypic heterogeneity of their parental lesions. Noticeably, NANK lacked the expression of conventional CSC markers CD133 and CD44, self-renewal genes Oct-4 and Nanog, but showed the expression of an important gastrointestinal development marker CDX-2 and BMI-1 that is essential in regulating the proliferative activity of normal and leukemic stem cells. In addition, NANK secreted high amounts of important angiogeneic cytokines.
These results provide a novel and extensive model in human CSC for studying the generation and maintenance of phenotypic heterogeneity in CRC.
Keywords: Cell line, cancer, metastasis, intraperitoneal dissemination, gene expression, cytokines, angiogenesis
INTRODUCTION
Colon carcinoma is considered to be the third most common form of cancer and the second leading cause of cancer related death in the Western world, causing 655, 000 deaths worldwide each year (18). Although advances have been made in colon cancer therapies, most cancer deaths still result from metastatic disease, being the largest hurdle for successful tumor treatment. Unfortunately, there is a poor understanding of the molecular mechanisms responsible for the local or disseminated metastasis, thus impeding progress on effective therapies.
Colon carcinoma often metastasizes to different locations, among them the ovary where 5% to 10 % of malignant tumors involving this organ are metastatic and are frequently mistaken for the primary ovarian carcinoma (47). Colonic adenocarcinoma accounts for 37% to 45% of all metastatic ovarian tumors (25) and the distinction of metastatic ovarian carcinoma from the primary malignant ovarian neoplasm is crucial to its subsequent management since diagnostic misinterpretation may have important adverse consequences for the patient.
Recently, it has been proposed that stem cells play a role in the development and progression of tumors. The concept of cancer stem cells (CSC) has been suggested as cells with the ability to self-renew and to produce the heterogeneous lineages of cancer cells that comprise tumors (24,35). More importantly, CSC may not only be associated with tumor initiation and growth but may also play a crucial role in tumor metastasis (14). The prognosis of patients with metastatic colorectal carcinoma is poor despite recent advances in diagnostic and therapeutic techniques (9,21,42). An understanding of the biological nature of this neoplasm is needed to improve the prognosis of patients. In vitro stable growing human CRC cell lines can be the most suitable tool for this purpose, because such cells are applicable to a variety of experiments to understand tumor biology and the role of CSC.
We have established a tumor-initiating cell line NANK that does not share characteristics with the previously reported CSC. In the present study, we described the cell phenotype of NANK, including the in vitro and in vivo growth characteristics and the gene expression profile.
Utilization of NANK could contribute to the development of CSC research by providing a novel biological feature of CSC, which may lead to find the future therapeutic targets on colon cancer-initiating cells.
MATERIALS AND METHODS
Tumor tissues and xenograft preparation
Tumor tissues were obtained from colorectal primary and metastasis to the ovary from a 41-year-old Japanese female, with a T4 N4 M1 Stage IV advanced colon cancer. The Ethics Committee of Okayama University Hospital approved this study and the subject gave her informed consent for participation in the present study. Briefly, the colonic and ovarian tissue specimens were minced with scissors into small (2-mm3) fragments and implanted intraperitoneally through a small incision on the animal’s abdominal cavity into 6–8 week-old NOD/SCID mice (Charles River Laboratories). The tissue fragments were implanted separately in different mice.
Isolation and in vitro expansion of tumor cells from a xenograft derived from colon metastasis to the ovary
A solid tissue collected from mouse xenograft was mechanically and enzymatically disaggregated into single-cell suspension and incubated with 200units/mL of collagenase Type IV (Sigma) and DNase I (Roche) for 1h at 37 °C to obtain enzymatic dissociation. Cells were then resuspended by pippeting and serially filtered by using sterile gauze and, 100-μm and 40-μm nylon meshes. After filtration, cells were plated at 10,000 cells/ml in a T25 flask on DMEM (GibcoBRL/Invitrogen, Co.) supplemented with 10% FBS (Cansera International, Inc, Ontario, Canada) and 120 μg/ml penicillin, 100 μg/ml streptomycin, at 37 °C in a humidified atmosphere with 5% carbon dioxide. The medium was routinely changed every 2–3 days. Cells derived from this ovarian tissue were designated as NANK.
Immunofluorescence study
NANK, human colon adenocarcinoma cell line Colo320 (ATCC), human ovarian mucinous adenocarcinoma cell line JHOS-2 (purchased from Summit Pharmaceuticals Intl. Corp., Japan) were grown in 8-chamber culture slides, then fixed with 4% paraformaldehyde. The slides were subjected to double immunofluorescence using anti-CDX 2 mAb and anti-actin mAb (Millipore Japan, Tokyo, Japan). Cy3-labeled goat anti-rabbit IgG and FITC-labeled goat anti-mouse IgG (Jackson ImunoResearch Laboratories, Inc., West Grove, PA) were used as secondary antibodies. Hoechst33258 (Sigma Aldrich Japan, Japan) was used for nuclear counterstaining. In another set of experiments, NANK, Colo320 and another human colon cancer cell line WiDr, were stained to evaluate the expression of cytokeratin 7 (CK 7) and cytokeratin 20 (CK 20) using anti-human CK 7 mAb (Abcam, clone Ks20.8, Tokyo, Japan) anti-human CK 20 mAb (Abcam, clone OV-TL 12/30, Tokyo, Japan). Cy3-labeled goat anti-mouse IgG (Jackson ImunoResearch Laboratories, Inc., West Grove, PA) was used as secondary antibody.
Reverse transcriptase-polymerase chain reaction (RT-PCR) analysis
Total RNA was extracted from NANK cells using TRIzol (Invitrogen) reagent according to the manufacturer’s instructions. Reverse transcription (RT) was performed at 22°C for 10 minutes, followed by 42°C for 20 minutes, using specific primers with 2.0 μg of RNA per reaction. The following primers used were: Oct-4, sense: 5′ -GAGCAAAACCCGGAGGAGT-3′, antisense: 5′ -TTCTCTTTCGGGCCTGCAC-3′; nanog, sense: 5′ -GCTTGCCTTGCTTTGAAGCA-3′, antisense: 5′ -TTCTTGACTGGGACCTTGTC-3′; BMI-1, sense: 5′ -GGAGACCAGCAAGTATTGTCCTTTTG-3′ antisense: 5′ -CATTGCGCTGGGCATCGTAAG-3′; P53, sense: 5′-CTGAGGTTGGCTCTGACTGTACCACCATCC-3′ antisense: 5′-CTCATTCAGCTCTCGGAACATCTCGAAGCG-3′; APC, sense: 5′TCATGATAAGGATGATATGTCGC3′ antisense: 5′AATTCTGCAATGGCCTGTAGTC3′; Bcl_xL, sense: 5′-GGAAAGCGTAGA CAAGGAGATGC-3′ antisense: 5- GGTGGGAGGGTAGAGTGGATGGT-3′; caspase 3, sense 5′-TGATGATGTGGAAGAACTTAGG-3′, antisense 5′-ACGGCTCCGCACCTGCTGAGGC-3′; CD133, sense: 5′ -ACATGAAAAGACCTGGGGG-3′ antisense: 5′ -GATCTGGTGTCCCAGCATG-3′; CD44, sense: 5′ -CCA AGA TGA TCA GCC ATT CTG G-3′, antisense: 5′ -AAG ACA TCT ACC CCA GCA AC-3′; and GAPDH, sense: 5′-TCCCCATCACCATCTTCCAG-3′ antisense: GAGTCCTTCCACGATACCAA-3′. PCR products were resolved on 1% agarose gels and visualized by ethidium bromide staining. Human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as an internal control for cDNA synthesis. mRNA expression levels were normalized relative to GAPDH and expressed as folds of relative intensity.
Irradiation experiments, MTS and FACS analyses
NANK and SW620 were cultured at a density of 3×104 cells per well in flat-bottomed 96-well plates. After 24 hrs cells were irradiated with 4Gy. Forty-eight hrs after irradiation, the cell viability was determined MTS assay, according to the manufactures instructions, and the absorbance was measured at 492nm. To determine the percentage of CD133 and CD44 cells after irradiation in NANK, 1×106 cells were plated in T75 flasks in DMEM supplemented with 10% FBS. Twenty-four hrs later, cells were subjected to irradiation at 4Gy. The cells were collected 48 hrs after irradiation, stained with anti-CD133 and anti-CD44 antibodies, and analyzed by FACS. As an isotype control, CD133-APC and IgG2b-APC (Miltenyi Biotec) and IOTest CD44-FITC and IgG1-FITC (Miltenyi Biotec) were used for CD133 and CD44 staining, respectively.
Protein and cytokine assays
NANK was maintained in DMEM, supplemented with 10% FBS and 100 units/ml penicillin, 0.1 mg/ml streptomycin. When confluent (>90% of the surface is covered) in T25-flasks, the cells were shifted to serum free DMEM medium and cultured 24 hrs, at 37°C and 5% CO2. Conditioned medium (CM) was then collected and passed through a 0.45 μm filter membrane (Sartorius, Hannover, Germany). The composition of NANK-derived conditioned medium was analyzed using a high-density protein array according to the manufacturer’s instructions. Serum free DMEM was used as a control.
Flow cytometry and cell sorting experiments
NANKwas magnetically labeled and separa ted by double passage using a human CD133 cell isolation kit (Miltenyi Biotec). Before separation, samples were assessed using a FACSCalibur flow cytometer, mouse IgG2b conjugated to APC was used as isotype control (Miltenyi Biotec). CD133 expression was assessed using human anti-CD133/1(AC133)-Biotin (Miltenyi Biotec). At least 10,000 events were acquired for each sample and all cells positive for propidium iodide were gated out. After magnetic bead separation, samples were assessed by flow cytometry to define purity.
Transplantation of NANK cells in NOD/SCID mice
Six to eight week-old NOD/SCID mice (Charles River Laboratories) were used as recipients. All animal procedures were approved by the Okayama University Institutional Animal Care and Use Committee, and thus within the guidelines for human care of laboratory animals.
CD133-negative NANK at a density of 1×104, 5×104 and 1×105 were diluted in 500μl of Matrigel and transplanted into the peritoneal cavity of the mice (n=3) for each dilution. All mice were sacrificed when tumors were palpable measured 10 mm, or at the first sign of suffering, between 8 and 12 weeks post-transplantation.
Histological and immunohistochemical analyses
At the time of sacrifice, tumors were removed and fixed in 20% formalin, and embedded in paraffin. Serial tissue sections were cut and stained with hematoxylin and eosin. Fixed, paraffin-embedded tissue sections were then examined immunohistochemically using the mouse monoclonal antibody to human CK 7, CK 20 (DakoCytomation, Carpinteria, CA), p53, p21 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), PCNA (NOVOCASTRA Laboratories, UK) rabbit antibody to human CDX-2 (BioGenex, San Ramon, CA). Tissue sections were stained with an automated immunostainer (Ventana XT system BENCHMARK, Ventana, Japan) using heat-induced epitope retrieval and standard DAB detection kit (Ventana). For detection of each signal, endogenous peroxidase activity was quenched by immersion in 3% hydrogen peroxide for 10 minutes, before the process by automated imunohistochemistry. Tissue sections were also stained with CD133/1 (AC133 Miltenyi Biotec) or with anti-human CD44 (G44-26, BD Pharmingen™) by employing the automated immunostainer. Samples were examined under a confocal laser scanning microscope (LSM510, Carl Zeiss, Germany). Primary colon, metastatic ovarian tissue samples obtained from the patient, as well as colon and ovarian xenograft tissues were also prepared for staining.
Statistical analyses
Mean values are presented with standard deviations (SD). Significance was determined by t-test using p<0.05 as significant.
RESULTS
Patient characteristics
We obtained colon and ovary tumor specimens from a 41-year-old female with advanced colon cancer Stage IV. Before surgery her CEA serum levels were 741 μg/L (normal range is 0 to 2.5 μg/L (less than 3 ng/mL), CA19-9 levels were 1607 U/mL (normal levels less than 40 U/mL) and the serum marker CA125 for ovarian carcinoma was 33.1 U/mL (normal values range from 0 to 35 U/mL).
The xenograft tissues recapitulated the histological characteristics of the parental tumors
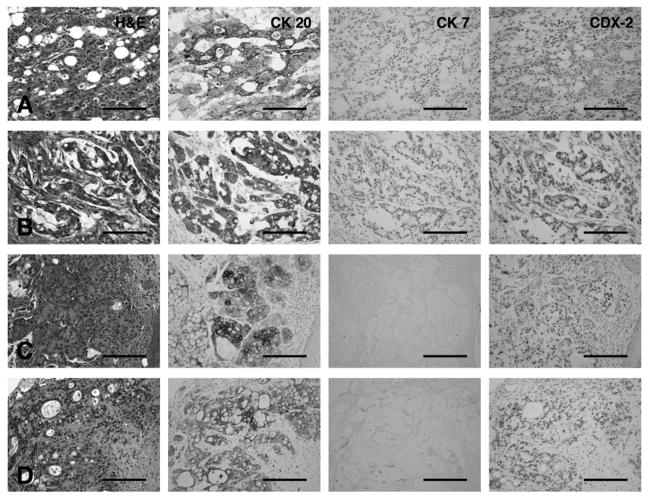
The colon primary tumor and its metastatic foci to the ovary as well as the xenografts in NOD/SCID mice shared similar characteristics (Fig. 1). On microscopic examination, both the colon primary tumor and its metastatic foci to the ovary showed predominantly moderately differentiated adenocarcinoma with fused glands, and mucin production, which was mixed with poorly differentiated adenocarcinoma at deeper site of invasion (Fig. 1A, B). Noticeably, both the colon and the ovary xenografted tumors were as well, moderately differentiated adenocarcinoma in combination with multiple poorly differentiated adenocarcinoma foci, some parts of which showed mucin production and they histologically were partially similar to those in primary lesion from the patient (Fig. 1C, D).
Figure 1. Immunohistological characterization of the primary and xenograft tumor tissues.
H&E for morphological appearance of the tumor and immunohistochemistry for CK 20, CK 7 and CDX-2 of human and xenograft cancer tissues. The expression of CK 20, CK 7 and CDX-2 is indicated by brown signal.
(A) Primary tissue of colon cancer
(B) Colon metastatic tumor tissue to the ovary
(C) Primary colon cancer xenograft
(D) metastatic ovarian xenograft formed intraperitoneally in NOD/SCID mice. Scale bar: 200 μm
The primary colon and metastatic ovarian tumors were positive for CK 20 and CDX-2 (Fig. 1A, B), an important nuclear transcription factor involved in intestinal-type differentiation, and they showed absence of CK 7 (Fig. 1A, B). This combination of markers has been useful to distinguish between primary and metastatic tumors from colon origin (41). Xenograft tissues showed a very similar pattern, with positivity for CK 20 and CDX-2 and absence of CK 7 (Fig. 1C, D).
Establishment and long-term culture of NANK
An intraperitoneal xenograft model was used in an attempt to establish a cell line derived from a metastatic ovarian tumor of a patient with primary colon cancer. After 6–8 weeks, the xenograft tissue was explanted and isolation of the cells was performed. We successfully established a new cell line from the ovarian tumor specimen and it was designated NANK. The NANK cells grew as an adherent monolayer, showing spindle-like morphology when sub-confluent, whereas when confluent the cells showed cobblestone-like shape (Fig. 2A). NANK maintained the consistent morphology from the primary culture to the following passages. After cryopreservation, thawed NANK was able to propagate in culture without noticeable change in growth and morphology (Fig. 2B). NANK has grown continuously in vitro for more than 18 months.
Figure 2. In vitro morphological and Immunohistochemical characteristics of NANK cells.
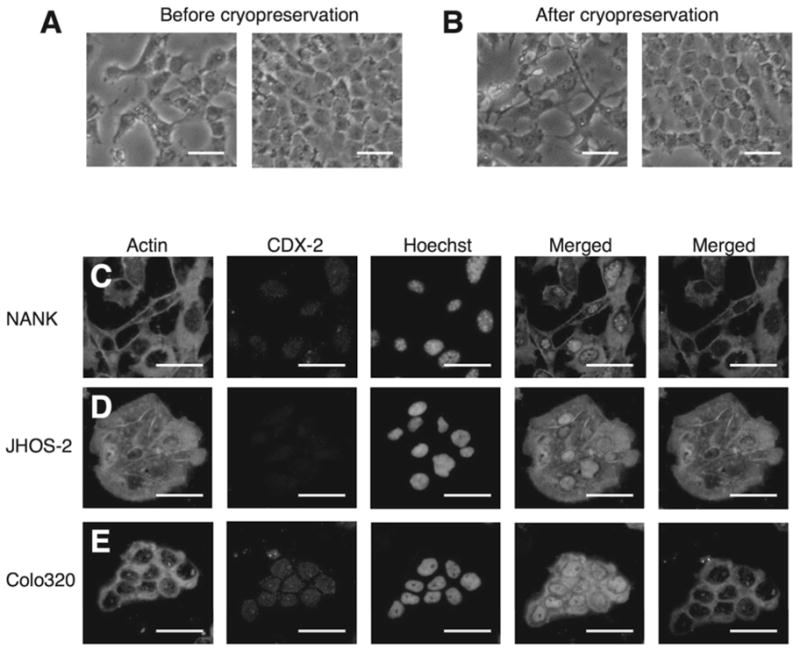
(A) Morphological appearance of NANK in vitro. No change in morphology (cobblestone like shape) when the cells reach confluency.
(B) Morphological appearance of NANK in vitro after cryopreservation. NANK maintained the same features even after crypreservation. Scale bar: 50 μm
(C) Representative immunofluorescent images of Actin and CDX-2 in NANK
(D) JHOS-2, used as a negative control (E) Colo320, used as a positive control. Green signal in the cytoplasm was for actin, and red signal in the nuclei was for CDX-2. Hoechst was used for nuclear counterstaining. Scale bar: 50 μm
Characterization of NANK
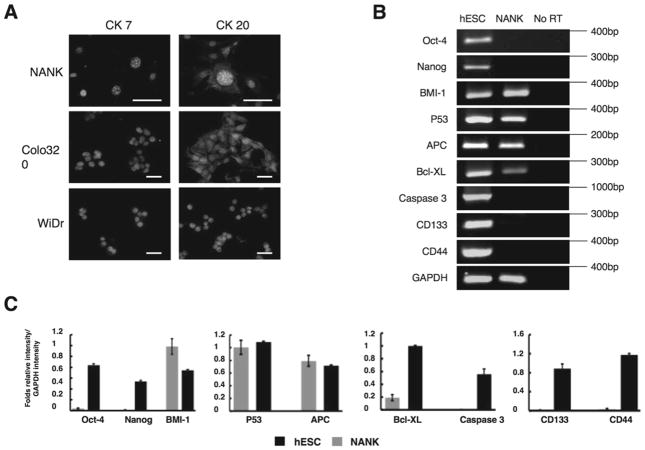
The expression of CDX-2, an intestine-specific transcription factor that regulates both proliferation and differentiation in intestinal epithelial cells, was evaluated in NANK. NANK revealed nuclear positive staining of CDX-2 (Fig. 2C), but the human ovarian adenocarcinoma cell line JHOS-2, used as a negative control, was almost negative for CDX-2 expression (Fig. 2D). On the other hand, CDX-2 was clearly expressed in Colo320 (Fig. 2E). To further confirm whether NANK is of colonic origin, we examined the expression of CK7 and CK20 in NANK on the basis of the previously accumulated surveys (9,18). Typically, most of the colonic adenocarcinomas are CK7−/CK20+, whereas ovarian adenocarcinomas are usually CK7+/CK20−. In accordance with the staining pattern of CK 7 and CK 20 observed in the primary colon and metastatic ovarian tumors, the expressional pattern of these two markers in NANK was completely consistent with those of the parental colon tumor (CDX-2+/CK20+/CK7−) (Fig. 3A). Examining the two representative human colon cancer cell lines Colo320 and WiDr as comparative examples for NANK, CK7 expression was almost negative in Colo320, whereas in WiDr faintly expressed it (Fig. 3A). To further characterize NANK, a genetic expression analysis was performed and shown in Fig. 3B. NANK lacked the expression of the putative stem cell markers Oct-4 and Nanog. However, it had a very intense expression of the proto-oncogene BMI-1 (Fig. 3B, C). Both of the tumor suppressor genes p53 and APC were present in NANK cells. Two markers well known for being mutated in most cancer cell lines (28,40). The anti-apoptotic gene Bcl-XL was expressed in NANK, whereas the pro-apoptotic gene caspase-3 was absent. As for the surface markers, CD133 and CD44, both were absent in NANK cells (Fig. 3B, C). Taken these data together, NANK cells display specific tumor properties. Reactivation of cytoprotective pathways may provide NANK with the ability to escape conventional anticancer treatments.
Figure 3. Protein and gene expression profiles of NANK.
(A) Representative immunofluorescent images of CK 7 and CK 20 in NANK, Colo320 and WiDr. Red signal in the cytoplasm was for CK7 and CK20, and Dapi was used for nuclear counterstaining. Scale bar: 25 μm.
(B) Gene expression profile of Oct-4, Nanog, BMI-1, p53, APC, Bcl_XL, caspase 3, CD133 and CD44 were analyzed in NANK by RT-PCR. hESC served as a positive control and no RT products served as negative controls. GAPDH was used as an internal control.
(C) mRNA expression levels were normalized by relative to the internal control GAPDH. The data are representative of at least three independent experiments with similar results.
CSC markers (CD 133/CD44) did not emerge in NANK after irradiation
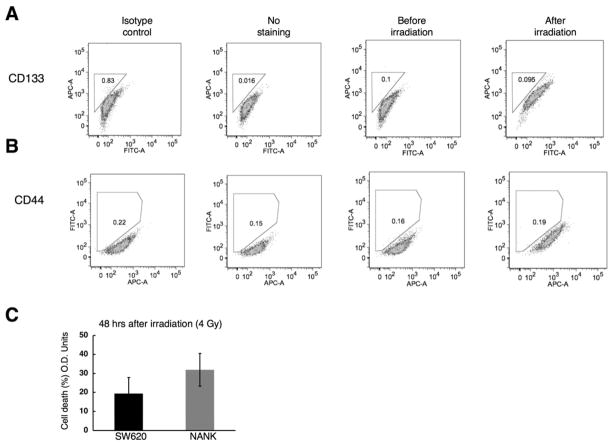
It has been reported that cancer stem cells, specifically CD133 and CD44 populations can be enriched after irradiation (3,33). To evaluate whether irradiation could enhance CD133 or CD44 subsets in our cell line, NANK cells were exposed to 4 Gy of irradiation. Forty-eight hours after irradiation, CD133 and CD44 populations were evaluated in NANK and we found no significant increase in these two populations (for CD133 expression of NANK; before=0.1%, after=0.095%, isotype control=0.83%; for CD44 expression of NANK; before=0.16%, after=0.19%, isotype control=0.22%) (Fig. 4A, B). At least three independent experiments were performed in different passages of NANK. Next we investigated a biological difference in irradiation-sensitivity between NANK and SW620, which contains high percentage of conventional CSC markers CD133 and CD44 (unpublished data), by exposing the cells to 4 Gy of irradiation. NANK was more sensitive to irradiation than SW620, showing a non-significant decrease in the cell survival (Fig. 4C). The finding is in accordance with the previous reports in which CD133+ cells have shown to be more resistance to irradiation than CD133- cells (5,49).
Figure 4. Expression of CD133 and CD44 in NANK after irradiation.
(A) The expression of CD133 was assessed in NANK before and 48 hrs after irraditation (4Gy) by FACS analysis. No positivity for CD133 was found in NANK.
(B) The expression of CD44 was assessed in NANK before and 48 hrs after irradiation (4Gy) by FACS analysis. No positivity for CD44 was detected in NANK. Mouse IgG2b conjugated to APC (CD133) and Mouse IgG1 conjugated to FITC (CD44) were used as an isotype control and cells without staining served as a negative control.
(C) Percentage of cell death quantification by MTS assay in NANK and SW620 48 hrs after irradiation.
Production of cytokines and growth factors in NANK
We analyzed the conditioned medium of NANK to know weather NANK produced any cytokine responsible for the tumorigenic potential. NANK secreted many cytokines involved in angiogenesis, one of the conditions necessary for cancer metastasis and to sustain tumor growth, such as angiogenin, epidermal growth factor (EGF), granulocyte colony-stimulation factor (GCSF), tumor necrosis factor alpha (TNF-a), vascular endothelial growth factor (VEGF), angiopoietin, and ENA-78. NANK cells also produced considerable amounts of IL-1a and IL-1b, which have also been shown to promote tumor invasiveness and angiogenesis (44).
In vivo tumor-initiating ability of NANK
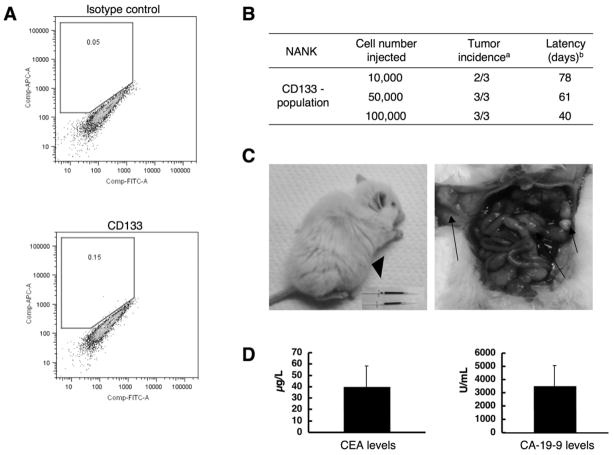
In order to identify the presence of cancer stem cells in NANK, by means of the surface marker CD133, which has been demonstrated to be present in several normal and malignant stem cells of different tissues (14,31,34), and have been considered to be tumor-initiating cells, NANK cells were labeled with CD133 antibodies and analyzed by FACS. FACS analysis revealed the absence of CD133+ cells in NANK (Fig. 5A). In order to test whether this CD133- population was capable of initiating tumor formation, as was previously reported (36), NANK cells at a density of 1×104, 5×104 and 1×105 were intraperitoneally transplanted in NOD/SCID mice. When 1×104 CD133- cells were transplanted, 2 out of 3 mice developed tumors, whereas in mice transplanted with 5×104 and 1×105 cells, tumors were developed in all of them and in a shorter period of time (Fig. 5B). The animals showed peritoneal dissemination with ascities (Fig. 5C). Evaluation of the ascitic fluid revealed high levels of CEA and CA-19-9 (Fig. 5D).
Figure 5. Intraperitoneal transplantation of NANK in NOD/SCID mice.
(A) NANK showed no significant CD133 expression by FACS.
(B) Different cell numbers of NANK were injected intraperitoneally in NOD/SCID mice. a Number of tumors formed from the total mice injected. b Average in days of the tumor formation.
(C) Representative images of the tumors formed by NANK in NOD/SCID mice. An Arrowhead indicates the presence of ascities and arrows depict the peritoneal dissemination.
(D) CEA and CA-19-9 levels in the ascites in mice (n=3).
Analysis of tumors developed after NANK cells transplant in mice
H&E staining of tumors grown in mice after injection of CD133- NANK revealed the formation of tumors producing mucin, with glandular formation, thus sharing some of the characteristics from the xenograft tumors. These tumors were predominantly poorly differentiated adenocarcinomas. When stained for CDX-2, only focal expression was found in very few areas, mainly with no reactivity for this marker (Fig. 6A). In contrast, a diffuse immunoreactivity for both CK 7 and CK 20 was positively observed in NANK-initiating tumors (Fig. 6A). The tumors derived from NANK cells injection demonstrated a positive and diffuse immunoreactivity for p53, indicating the presence of a mutated form of p53 protein, whereas p21, which is a downstream of p53, was almost absent in the tumor tissues (Fig. 6A). We found a very clear and diffuse immunoreactivity for PCNA, a marker of cell cycle, in the tumors. NANK-derived tumors were immunohistologically compared with those derived from the xenograft tissues developed after the implantation of the primary colon and ovary metastatic tumors. While the colon and ovarian xenograft tissues maintained a very similar immunohistological characterization with exception of p21 which was not analyzed in these tumors (Fig. 6B, C), tumors derived from NANK showed two main immunohistological alterations, the loss of CDX-2 and the acquisition of CK 7 (Fig. 6A).
Figure 6. Immunohistological characterization of the tumors formed by NANK in comparison with the xenograft tumor tissues.
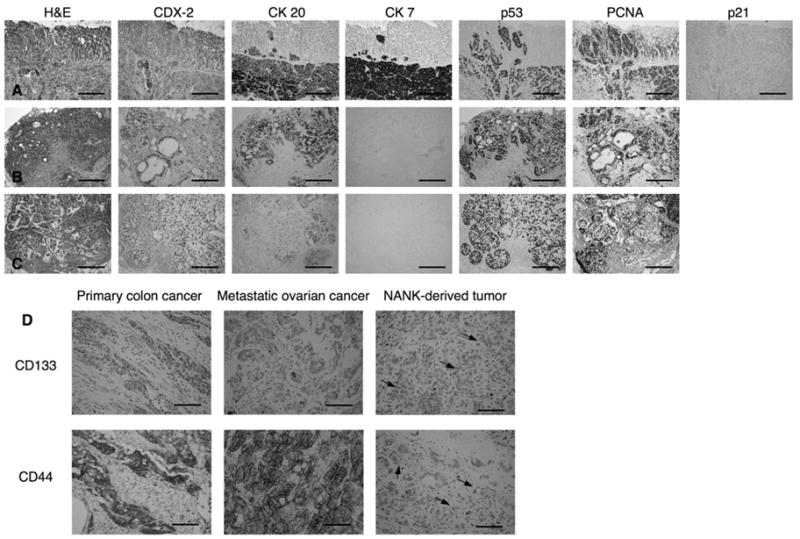
(A, B, C) Images of H&E staining and immunohistochemistry for CDX-2, CK 20, CK 7, p53, PCNA and p21 of NANK-forming tumors in NOD/SCID mice (A), primary colon xenograft (B) and metastatic ovarian xenograft (C) formed intraperitoneally in NOD/SCID mice. Scale bar: 200 μm.
(D) Images for CD133 and CD44. Scale bar: 200 μm.
Tumors derived from NANK cells transplant recapitulated the phenotype of the primary colonic and metastatic ovarian cancers in terms of CSC markers
Next, the expression of CD133 and CD44, both of which were previously reported as consensus CSC markers, were examined in the primary colon cancer, its metastatic cancer to the ovary, and tumors derived from NANK cell injection by immunohistochemistry. Both CD133 and CD44 were positive in the primary colon tumor and ovarian metastatic tumor (Fig. 6D). Interestingly, the tumor derived from NANK comprised the mixture of CD133 negative (major population) and weakly CD133 positive cells (very minor population), which was similarly observed as a mixture of CD44 negative and weak CD44 positive cells. These results indicated that NANK, not totally but partially recapitulated the same immunophenotypes as their primary and metastatic counterparts (Fig. 6D).
DISCUSSION
In the process of tumorigenesis many factors are believed to play an important role. Among them, CSCs are believed to have the tumor-initiating capacity in many cancers. The isolation of these cells have been possible in a variety of tumors (14,31,34,36) through different surface markers, such as CD133 for colon and brain tumors where it is believed that only the CD133 positive population can initiate tumor development (31,34,38). Although it has been recently proposed that not only the CD133+ cells but also the CD133- cells are endowed with tumorigenic capabilities when derived from metastatic tumors (36).
Up to date, there is a wide variety of markers used to identify CSCs from other cancers: CD44+/CD24− for breast tumors (1), Sca-1+/CD45−/PECAM−/CD34+ for adenocarcinomas (22) and CD44+/alpha2beta1+/CD133 for prostate cancers (8), which suggests that there are not perfect markers to represent CSCs. In addition, the ability to form tumors depends not only on properties inherent to cancer cells, but also on the host microenvironment, which plays a major role in tumor initiation and progression (13,29).
We here demonstrated that a cell line derived from colon metastasis to the ovary, NANK, lacking the surface marker CD133, was able to initiate tumors when transplanted intraperitoneally into NOD/SCID mice.
Moreover, we also analyzed the possibility of NANK to contain a sub-population positive for the other well-known stem cell marker CD44 and we found no expression of CD44 by RT-PCR and FACS analyses (Figs. 3,4). In spite of these findings, NANK cells were capable of developing aggressive peritoneal tumor dissemination in the mice with ascities formation and showing high levels of tumor markers CEA and CA19-9 in the ascitic fluid. The tumors recapitulated the features of the parental colon and its metastatic ovarian tumors.
First, we sought to determine the origin of the metastatic ovarian tumor. Clinically, the expression of CK20, as well as CK7 and CDX-2 have been used to discern the primary lesions from metastatic adenocarcinomas in the ovary (41,46). The expression of CK20 is restricted to a limited group of adenocarcinomas. It is detectable in almost all colon cancers, about half of gastric and pancreato-biliary carcinomas, and about 30% of transitional cell carcinomas (6). Although several patterns have been reported (6,15), our findings are in accordance with some reports in where metastatic ovarian carcinomas from lower gastrointestinal tract origin are CDX-2+/CK20+/CK7−. The xenograft tissues from which NANK cells were derived preserved the same pattern of CDX-2+/CK20+/CK7−.
CDX-2 is a member of the caudal-related homeobox family and is an intestine-specific transcription factor that regulates both proliferation and differentiation in intestinal epithelial cells. It has been shown to be expressed in a high proportion of colon carcinoma (61–100%) (20,41,46), or be down-regulated in the later stages of human colon carcinogenesis, namely, high grade dysplasia and invasive carcinoma (10). We found CDX-2 to be expressed in NANK, which might account for the spontaneous differentiation of the cells once they reach confluency (Fig. 2A). In addition, we found the expression of CK 20 and absence of CK 7 in NANK in vitro, similarly to the pattern found in the primary colon and its metastatic ovarian cancers as well as in the xenograft tissues (Figs. 2, 3).
Self-renewing activity and extensive proliferation are some of the hallmarks of stem/progenitor cells in culture, and at some extent, these capabilities are believed to be conferred by the expression of Nanog and Oct-4 genes (11,30). NANK cells did not express these self-renewal markers, but instead they had another mechanism to proliferate by expressing the polycomb group gene BMI-1, which is recognized to play an essential role in regulating the proliferative activity of both normal and leukemia stem cells (17,26,32). Over-expression of BMI-1 was in fact described in several types of cancers (23,43), and it is believed to confer a high degree of malignancy when BMI-1 is co-expressed with other proteins from the PcG, such as EZH2 (4). Moreover, NANK expressed p53 and APC, which are well-known markers to be mutated in several cancer cell lines. These markers in combination with the apoptosis inhibitor Bcl_xL conferred NANK cells a mechanism to survive and proliferate malignantly.
Angiogenesis performs a critical role in the development of cancers and influences the dissemination of cancer cells throughout the entire body, eventually leading to metastasis formation. NANK cells produced several pro-angiogenic factors such as VEGF-D, IL-1α, and IL-1β, which are required for in vivo angiogenesis and have been associated with the promotion of metastatic spread of tumor cells (39,44). VEGF expressed in tumors is identified as responsible for the formation of tumor vessels that not only allow for progression of the primary tumors, but also facilitate cancer cells to access the tumor vessels (12,44). In accordance with these findings, we can speculate that the high expression of growth factors related with angiogenesis by NANK could be one of the many factors bestowing them with a metastatic potential.
As previously mentioned, NANK cells did not express CD133 or CD44, expressed in normal and cancer stem cells, nor did they express the self-renewal genes Oct4 and Nanog. However, NANK cells had the potential to initiate tumor formation when a small number of cells was intraperitoneally transplanted, in accordance with the previous reports in which transplantation of a CD133-negative population (36) was capable of developing tumors. It is also possible that the presence of a small subset of cancer stem cells is contained in NANK. Identification of such a subset that may be responsible for its malignant potential is now under investigation.
The property of resistance to chemotherapy and irradiation is the major clinical criterion to characterize “cancer stem-like cells” (19). The existence of cancer stem-like cells may explain why conventional anti-cancer therapies are able only to suppress or shrink a tumor but often cannot completely eradicate such cells, resulting in eventual recurrence of tumors (7,19). NANK cells in vitro were comparably sensitive to irradiation (to slightly higher extent) when compared with SW620 that possess a high percentage of CD133 and CD44 positive cells (unpublished data). The finding may indicate that NANK is not endowed with neither of the well-known CSC markers CD133 and CD44, thus leading to be irradiation-sensitive in vitro.
Interestingly, when NANK was transplanted in NOD/SCID mice, CD133 and CD44 expressing cells were emerged in the resultant tumors in vivo. Detailed mechanism of this phenomenon still remain unclear at this moment, however, similar findings have been reported by previous studies using in vivo brain tumor models (45).
The tumors derived from NANK cells were able to recapitulate the morphological characteristics from the primary colon, metastatic ovarian, and xenograft tumors. While the expression of CK 20 was preserved, the tumors acquired CK 7 expression, which was absent in the parental tumors (Figs. 1A–D, 6A–C). Although it is well recognized that the majority of tumors derived from the gastrointestinal tract lack the expression of CK 7, there have been other reports showing its presence but in a quite low frequency (48). In addition, CK 7 expression is associated in combination with CK20 expression in advance colorectal cancers. Therefore, the acquisition of CK7 expression may correlate with the progression of the clinical staging (15). On the other hand, tumors derived from NANK dramatically lost CDX-2 expression (Fig. 6A), which remains a possible consequence of the dysregulation that accompanies tumor progression. The decreased expression in CDX-1 and/or CDX-2 is associated with progression to the more advanced stages of colorectal tumorigenesis (16,27). The decreased CDX-2 expression in NANK-derived tumors is correlated with the histological status of poorly differentiated adenocarcinomas. When CDX-2 was diffusely expressed in the tumors, they were diagnosed as moderately differentiated adenocarcinomas. The histological findings of NANK-derived tumor are in accordance with previous studies suggesting the critical role of CDX-2 in the maintenance of the intestinal phenotype (37). CDX-2 is also suggested as a proliferation inhibitor through the up-regulation of the p21 gene, considered to be a transcriptional target of CDX-2 (2).
The absence or almost no expression of CDX-2 in NANK-derived tumors resulted in the down-regulation of p21. In addition, the status of p53 was mutated in NANK tumors, leading to the loss of the effect in p21. Therefore, cell cycle inhibition was not accomplished in the tumor cells, which was supported by the wide expression of PCNA (Fig. 6A).
In conclusion, we have shown that our newly established human colon cancer cell line NANK lacks the important conventional CSC markers such as CD133 and CD44, Nanog, and Oct/4, while being capable of initiating tumors and developing aggressive progression with CEA and CA-19-9 synthesis and metastatic potential in NOD/SCID mice. These findings may contribute to provide another novel aspect of CSC biology.
Abbreviations
- CRC
colorectal cancer
- CSC
cancer stem cell
- NOD/SCID mice
non-obese severe combined immunodeficiency mice
- PD
Peritoneal dissemination
Footnotes
Competing interests statement
The authors declare no competing financial interests.
References
- 1.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100(7):3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bai YQ, Miyake S, Iwai T, Yuasa Y. CDX2, a homeobox transcription factor, upregulates transcription of the p21/WAF1/CIP1 gene. Oncogene. 2003;22(39):7942–7949. doi: 10.1038/sj.onc.1206634. [DOI] [PubMed] [Google Scholar]
- 3.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 4.Cao R, Tsukada Y, Zhang Y. Role of Bmi-1 and Ring1A in H2A ubiquitylation and Hox gene silencing. Mol Cell. 2005;20(6):845–854. doi: 10.1016/j.molcel.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Chen YC, Hsu HS, Chen YW, Tsai TH, How CK, Wang CY, Hung SC, Chang YL, Tsai ML, Lee YY, Ku HH, Chiou SH. Oct-4 expression maintained cancer stem-like properties in lung cancer-derived CD133-positive cells. PLoS ONE. 2008;3(7):e2637. doi: 10.1371/journal.pone.0002637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chu P, Wu E, Weiss LM. Cytokeratin 7 and cytokeratin 20 expression in epithelial neoplasms: a survey of 435 cases. Mod Pathol. 2000;13(9):962–972. doi: 10.1038/modpathol.3880175. [DOI] [PubMed] [Google Scholar]
- 7.Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, Visvader J, Weissman IL, Wahl GM. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66(19):9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 8.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65(23):10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 9.Ducreux M, Raoul JL, Marti P, Merrouche Y, Tigaud JM, Rebischung C, Boige V. High-dose irinotecan plus LV5FU2 or simplified LV5FU (HD-FOLFIRI) for patients with untreated metastatic colorectal cancer: a new way to allow resection of liver metastases. Oncology. 2008;74(1–2):17–24. doi: 10.1159/000138352. [DOI] [PubMed] [Google Scholar]
- 10.Ee HC, Erler T, Bhathal PS, Young GP, James RJ. Cdx-2 homeodomain protein expression in human and rat colorectal adenoma and carcinoma. Am J Pathol. 1995;147(3):586–592. [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandes AM, Meletti T, Guimaraes R, Stelling MP, Marinho PA, Valladao AS, Rehen SK. Worldwide survey of published procedures to culture human embryonic stem cells. Cell Transplant. 2010 Jan 6; doi: 10.3727/096368909X485067. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Fidler IJ, Ellis LM. The implications of angiogenesis for the biology and therapy of cancer metastasis. Cell. 1994;79(2):185–188. doi: 10.1016/0092-8674(94)90187-2. [DOI] [PubMed] [Google Scholar]
- 13.Heppner GH, Miller FR. The cellular basis of tumor progression. Int Rev Cytol. 1998;177:1–56. doi: 10.1016/s0074-7696(08)62230-5. [DOI] [PubMed] [Google Scholar]
- 14.Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1(3):313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Hernandez BY, Frierson HF, Moskaluk CA, Li YJ, Clegg L, Cote TR, McCusker ME, Hankey BF, Edwards BK, Goodman MT. CK20 and CK7 protein expression in colorectal cancer: demonstration of the utility of a population-based tissue microarray. Hum Pathol. 2005;36(3):275–281. doi: 10.1016/j.humpath.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 16.Hinoi T, Tani M, Lucas PC, Caca K, Dunn RL, Macri E, Loda M, Appelman HD, Cho KR, Fearon ER. Loss of CDX2 expression and microsatellite instability are prominent features of large cell minimally differentiated carcinomas of the colon. Am J Pathol. 2001;159(6):2239–2248. doi: 10.1016/S0002-9440(10)63074-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobs JJ, Scheijen B, Voncken JW, Kieboom K, Berns A, van Lohuizen M. Bmi-1 collaborates with c-Myc in tumorigenesis by inhibiting c-Myc-induced apoptosis via INK4a/ARF. Genes Dev. 1999;13(20):2678–2690. doi: 10.1101/gad.13.20.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56(2):106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 19.Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. 2006;355(12):1253–1261. doi: 10.1056/NEJMra061808. [DOI] [PubMed] [Google Scholar]
- 20.Kaimaktchiev V, Terracciano L, Tornillo L, Spichtin H, Stoios D, Bundi M, Korcheva V, Mirlacher M, Loda M, Sauter G, Corless CL. The homeobox intestinal differentiation factor CDX2 is selectively expressed in gastrointestinal adenocarcinomas. Mod Pathol. 2004;17(11):1392–1399. doi: 10.1038/modpathol.3800205. [DOI] [PubMed] [Google Scholar]
- 21.Khan OA, Ranson M, Michael M, Olver I, Levitt NC, Mortimer P, Watson AJ, Margison GP, Midgley R, Middleton MR. A phase II trial of lomeguatrib and temozolomide in metastatic colorectal cancer. Br J Cancer. 2008;98(10):1614–1618. doi: 10.1038/sj.bjc.6604366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121(6):823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 23.Kim JH, Yoon SY, Kim CN, Joo JH, Moon SK, Choe IS, Choe YK, Kim JW. The Bmi-1 oncoprotein is overexpressed in human colorectal cancer and correlates with the reduced p16INK4a/p14ARF proteins. Cancer Lett. 2004;203(2):217–224. doi: 10.1016/j.canlet.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi N, Navarro-Alvarez N, Soto-Gutierrez A, Kawamoto H, Kondo Y, Yamatsuji T, Shirakawa Y, Naomoto Y, Tanaka N. Cancer stem cell research: current situation and problems. Cell Transplant. 2008;17(1–2):19–25. doi: 10.3727/000000008783906982. [DOI] [PubMed] [Google Scholar]
- 25.Lash RH, Hart WR. Intestinal adenocarcinomas metastatic to the ovaries. A clinicopathologic evaluation of 22 cases. Am J Surg Pathol. 1987;11(2):114–121. doi: 10.1097/00000478-198702000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Lessard J, Sauvageau G. Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature. 2003;423(6937):255–260. doi: 10.1038/nature01572. [DOI] [PubMed] [Google Scholar]
- 27.Mallo GV, Rechreche H, Frigerio JM, Rocha D, Zweibaum A, Lacasa M, Jordan BR, Dusetti NJ, Dagorn JC, Iovanna JL. Molecular cloning, sequencing and expression of the mRNA encoding human Cdx1 and Cdx2 homeobox. Down-regulation of Cdx1 and Cdx2 mRNA expression during colorectal carcinogenesis. Int J Cancer. 1997;74(1):35–44. doi: 10.1002/(sici)1097-0215(19970220)74:1<35::aid-ijc7>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 28.Menendez M, Gonzalez S, Obrador-Hevia A, Dominguez A, Pujol MJ, Valls J, Canela N, Blanco I, Torres A, Pineda-Lucena A, Moreno V, Bachs O, Capella G. Functional characterization of the novel APC N1026S variant associated with attenuated familial adenomatous polyposis. Gastroenterology. 2008;134(1):56–64. doi: 10.1053/j.gastro.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 29.Mueller MM, Fusenig NE. Friends or foes - bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4(11):839–849. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 30.Navarro-Alvarez N, Soto-Gutierrez A, Yuasa T, Yamatsuji T, Shirakawa Y, Nagasaka T, Sun SD, Javed MS, Tanaka N, Kobayashi N. Long-term culture of Japanese human embryonic stem cells in feeder-free conditions. Cell Transplant. 2008;17(1–2):27–33. [PubMed] [Google Scholar]
- 31.O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445(7123):106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 32.Park IK, Qian D, Kiel M, Becker MW, Pihalja M, Weissman IL, Morrison SJ, Clarke MF. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature. 2003;423(6937):302–305. doi: 10.1038/nature01587. [DOI] [PubMed] [Google Scholar]
- 33.Phillips TM, McBride WH, Pajonk F. The response of CD24(−/low)/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst. 2006;98(24):1777–1785. doi: 10.1093/jnci/djj495. [DOI] [PubMed] [Google Scholar]
- 34.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445(7123):111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 35.Ricci-Vitiani L, Pagliuca A, Palio E, Zeuner A, De Maria R. Colon cancer stem cells. Gut. 2008;57(4):538–548. doi: 10.1136/gut.2007.127837. [DOI] [PubMed] [Google Scholar]
- 36.Shmelkov SV, Butler JM, Hooper AT, Hormigo A, Kushner J, Milde T, StClair R, Baljevic M, White I, Jin DK, Chadburn A, Murphy AJ, Valenzuela DM, Gale NW, Thurston G, Yancopoulos GD, D’Angelica M, Kemeny N, Lyden D, Rafii S. CD133 expression is not restricted to stem cells, and both CD133+ and CD133− metastatic colon cancer cells initiate tumors. J Clin Invest. 2008;118(6):2111–2120. doi: 10.1172/JCI34401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silberg DG, Swain GP, Suh ER, Traber PG. Cdx1 and cdx2 expression during intestinal development. Gastroenterology. 2000;119(4):961–971. doi: 10.1053/gast.2000.18142. [DOI] [PubMed] [Google Scholar]
- 38.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 39.Stacker SA, Caesar C, Baldwin ME, Thornton GE, Williams RA, Prevo R, Jackson DG, Nishikawa S, Kubo H, Achen MG. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med. 2001;7(2):186–191. doi: 10.1038/84635. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka M, Jin G, Yamazaki Y, Takahara T, Takuwa M, Nakamura T. Identification of candidate cooperative genes of the Apc mutation in transformation of the colon epithelial cell by retroviral insertional mutagenesis. Cancer Sci. 2008;99(5):979–985. doi: 10.1111/j.1349-7006.2008.00757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vang R, Gown AM, Wu LS, Barry TS, Wheeler DT, Yemelyanova A, Seidman JD, Ronnett BM. Immunohistochemical expression of CDX2 in primary ovarian mucinous tumors and metastatic mucinous carcinomas involving the ovary: comparison with CK20 and correlation with coordinate expression of CK7. Mod Pathol. 2006;19(11):1421–1428. doi: 10.1038/modpathol.3800698. [DOI] [PubMed] [Google Scholar]
- 42.Vincenzi B, Santini D, Galluzzo S, Russo A, Fulfaro F, Silletta M, Battistoni F, Rocci L, Zobel BB, Adamo V, Dicuonzo G, Tonini G. Early magnesium reduction in advanced colorectal cancer patients treated with cetuximab plus irinotecan as predictive factor of efficacy and outcome. Clin Cancer Res. 2008;14(13):4219–4224. doi: 10.1158/1078-0432.CCR-08-0077. [DOI] [PubMed] [Google Scholar]
- 43.Vonlanthen S, Heighway J, Altermatt HJ, Gugger M, Kappeler A, Borner MM, van Lohuizen M, Betticher DC. The bmi-1 oncoprotein is differentially expressed in non-small cell lung cancer and correlates with INK4A-ARF locus expression. Br J Cancer. 2001;84(10):1372–1376. doi: 10.1054/bjoc.2001.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Voronov E, Shouval DS, Krelin Y, Cagnano E, Benharroch D, Iwakura Y, Dinarello CA, Apte RN. IL-1 is required for tumor invasiveness and angiogenesis. Proc Natl Acad Sci USA. 2003;100(5):2645–2650. doi: 10.1073/pnas.0437939100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang J, Sakariassen PO, Tsinkalovsky O, Immervoll H, Boe SO, Svendsen A, Prestegarden L, Rosland G, Thorsen F, Stuhr L, Molven A, Bjerkvig R, Enger PO. CD133 negative glioma cells form tumors in nude rats and give rise to CD133 positive cells. Int J Cancer. 2008;122(4):761–768. doi: 10.1002/ijc.23130. [DOI] [PubMed] [Google Scholar]
- 46.Werling RW, Yaziji H, Bacchi CE, Gown AM. CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin: an immunohistochemical survey of 476 primary and metastatic carcinomas. Am J Surg Pathol. 2003;27(3):303–310. doi: 10.1097/00000478-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 47.Young RH, Scully RE. Metastatic tumors in the ovary: a problem-oriented approach and review of the recent literature. Semin Diagn Pathol. 1991;8(4):250–276. [PubMed] [Google Scholar]
- 48.Zhang PJ, Shah M, Spiegel GW, Brooks JJ. Cytokeratin 7 immunoreactivity in rectal adenocarcinomas. Appl Immunohistochem Mol Morphol. 2003;11(4):306–310. doi: 10.1097/00129039-200312000-00005. [DOI] [PubMed] [Google Scholar]
- 49.Zito G, Richiusa P, Bommarito A, Carissimi E, Russo L, Coppola A, Zerilli M, Rodolico V, Criscimanna A, Amato M, Pizzolanti G, Galluzzo A, Giordano C. In vitro identification and characterization of CD133(pos) cancer stem-like cells in anaplastic thyroid carcinoma cell lines. PLoS ONE. 2008;3(10):e3544. doi: 10.1371/journal.pone.0003544. [DOI] [PMC free article] [PubMed] [Google Scholar]