Abstract
The ability of plants to withstand drought, a potentially major constraint to yield and production, is influenced by abscisic acid (ABA). ABA is synthesized in the cytosol from plastid carotenoid pathway derived precursors, and later inactivated by the action of ABA hydroxylases. Endogenous accumulation of ABA is controlled by both its synthesis and catabolism. Enzymatic activity of ABA 8′-hydroxylase (ABA8Ox), also referred to as CYP707A, is considered one of the key steps in modulating ABA levels that control numerous physiological processes. To investigate the role of this enzyme, maize ABA8Ox gene family members were identified. ABA8Ox gene expression was then analyzed in different tissues and roots during the drought-stress response in maize. These genes were found to be expressed in all tissues, with a high degree of specificity to each tissue and some degree of overlap. Maize ABA8Ox1a and ABA8Ox1b were shown to be the major transcript components for regulating ABA catabolism in drought-stressed roots. Phylogenetic and gene-structure analyses were performed to extend the implications and infer the cause of ABA catabolism in other cereal crops.
Keywords: ABA, abiotic stress, carotenoids, maize, sorghum, rice
1. Introduction
Human survival depends on food production, and drought is a potentially major constraint to yield and production of food crops. In maize, for example, drought accounts for at least 15% of the crop loss in sub-Saharan Africa [1]. Abscisic acid (ABA) is a plant hormone that influences the plant's ability to withstand abiotic stresses such as drought, cold, salt and wounding [2]. ABA has additional roles in regulating embryo and seed development, maintenance of seed dormancy and germination, seed desiccation tolerance, vegetative development, general seedling growth, and in mediating pathogen defense responses (reviewed in [3]). Transgenic plants overexpressing ABA biosynthetic genes showed improved drought tolerance [4], while overexpression of ABA 8′ hydroxylases reduced ABA levels and produced ABA-deficient phenotypes [5]. Thus, the amount of ABA is determined by the balance between biosynthesis and catabolism [6], and one strategy for achieving maximum drought tolerance in crop plants, and thereby avoiding losses, is to increase the synthesis of endogenous ABA while controlling catabolism.
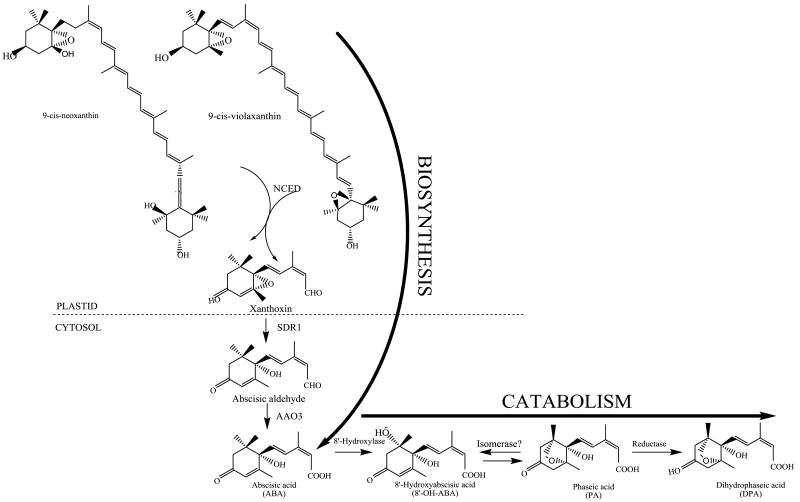
ABA-biosynthesis-related genes [7, 8] and their regulatory mechanisms [3, 9, 10] have been identified in a wide range of plants. The first committed step in ABA biosynthesis is the oxidative cleavage of a 9-cis-epoxycarotenoid (C40) to form xanthoxin (C15). Xanthoxin is oxidized to form abscisic aldehyde, and then further oxidized to ABA [7, 11]. The major route to inactivation of ABA is mediated by the cytochrome P450 monooxygenase ABA 8′ hydroxylase (ABA8Ox, also referred to as CYP707A) [6]. ABAOx hydroxylates ABA to yield 8′-hydroxy ABA thus depleting the active ABA pool (Fig. 1).
Fig. 1.
ABA biosynthesis and catabolism in higher plants. Carotenoid precursors of ABA are synthesized in the plastid. The cis-xanthophylls are cleaved by a family of 9-cis-epoxycarotenoid dioxygenases (NCED) to form the first 15-carbon precursor, xanthoxin. Xanthoxin moves to the cytosol and is converted to abscisic aldehyde by a short-chain dehydrogenase reductase (SDR1), which is then oxidized to ABA by an abscisic aldehyde oxidase (AAO3). ABA is hydroxylated to 8′-hydroxy ABA in the presence of 8′ hydroxylase (ABA8Ox), and subsequently converted to phaseic acid and dihydrophaseic acid. The genes involved in these downstream steps are still unknown.
Extensive studies have been conducted on the small gene families that encode the ABA8Ox catabolic enzymes from Arabidopsis (CYP707A1-CYP707A4) [6, 12], barley (HvABA8Ox1 and HvABA8Ox2) [13], and beans (PvCYP707A1-PvCYP707A3) [14]. These studies showed that ABA catabolism under stress and recovery is mainly regulated at the transcriptional level.
Although there is a wealth of knowledge on ABA catabolism in model species, ABA catabolism in staple food crops is not as well understood. Cereal crops of global importance, such as maize, sorghum, wheat and rice, are evolutionarily related as members of the Poaceae (grasses) family. The aim of this study was to characterize the ABA8Ox gene family in maize and to elucidate the expression of ABA-catabolism-related genes in unstressed maize tissues and in maize roots affected by drought stress. Using comparative genomics, orthologs were also identified in other grass genomes, creating the possibility of breeding these plants for drought tolerance.
2. Materials and Methods
Plant materials and stress treatments
Maize (Zea mays L.) inbred line B73 plants were field grown in the Bronx, New York, sibling-pollinated, and endosperm and embryo tissues were dissected 20 days after pollination (DAP). Non-stressed leaf and root tissues and drought stressed root samples were collected at the six-leaf stage. B73 was grown in a greenhouse as described previously [8]. Dissected tissues were stored at -80°C until analysis.
Sequence analyses
The genomic DNA sequences of ABA hydroxylases of Arabidopsis thaliana, Oryza sativa, Sorghum bicolor and Zea mays were obtained from NCBI GenBank, Gramene, Phytozome and PlantGDB, respectively (Table 1). The sequences were analyzed using Vector NTI Suite, Version 9.0 (InforMax, North Bethesda, MD), and processed for gene-structure analysis, while deducing cDNA and protein sequences. Sequence comparisons for maize ABAOx proteins are shown in Table 2. Amino acid sequences were aligned using ClustalW and a neighbor-joining tree was constructed with 500 bootstrap replication support using MEGA3 software [14]. Chromosomal mapping was performed using the WebAGCoL package [15] or MaizeGDB.
Table 1.
Summary of ABA8Ox enzymes and genes in grasses (maize, rice, and sorghum) and Arabidopsis. CYP, cytochrome P450.
| Enzyme | Arabidopsis thaliana | Oryza sativa | Zea mays | Sorghum bicolor | |
|---|---|---|---|---|---|
| Gramene | BAC clones/ESTs | MAP | Phytozome | ||
| ABA8Ox1 | At4g19230 (CYP707A1) At5g45340 (CYP707A3) |
LOC_Os02g47470 |
AC194862 (ABA8Ox1a) DR806072; EC902187; DY688015; CO520018; EB706067; DR803102; EE157729 |
5.06 | Sb04g030660 |
|
AC182107 (ABA8Ox1b) CD433445; EE190691; EE169703; EE036846; EE023371; EC902781; EE036847; EC894886; EE169704; EC884504; EC898571; EC884505 |
4.06 | ||||
| ABA8Ox2 | At2g29090 (CYP707A2) At3g19270 (CYP707A4) |
LOC_Os08g36860 |
AC212409 (ABA8Ox2) CO460095; CO456959; CO459318; DV494257; EB701816; AI670285; EE040561; CO456726; DV529214; CA398898; DY623142; DY239249; EB701815 |
4.04 | Sb07g022990 |
| ABA8Ox3 | LOC_Os09g28390 |
AC190490 (ABA8Ox3a) CD941324; CD941122; CD955590 |
2.06 | Sb02g026600 | |
|
AC195926 (ABA8Ox3b) EC904849; DV527897; EE043845; DR785156; EE175998; EE036989 DV514713; DR962771; DV519081 |
7.02 | ||||
Table 2.
Comparison of sequence similarity (top) and identity (bottom) between Zea mays ABA8Ox proteins.
| ABA8Ox1a | ABA8Ox1b | ABA8Ox2 | ABA8Ox3a | |
|---|---|---|---|---|
| ABA8Ox1b | 90.4% 88.1% |
|||
| ABA8Ox2 | 63.1% 49.9% |
60.4% 48.3% |
||
| ABA8Ox3a | 66.5% 54.4% |
64.7% 53.0% |
70.2% 61.7% |
|
| ABA8Ox3b | 66.2% 54.0% |
64.7% 52.7% |
72.8% 63.0% |
82.6% 78.3% |
Quantitative real-time PCR
RNA extraction and quantitative real-time PCR were performed using gene-specific primers (Table 3) and normalized to actin, as previously described [16]. Total RNA was isolated using the RNeasy Plant Mini Kit (Qiagen Sciences, Maryland), and DNase I-treated (Invitrogen, Carlsbad, CA) prior to first strand cDNA synthesis using oligo (dT) as a primer and Superscript™ III RT (Invitrogen, Carlsbad, CA). One ul of 50μM oligo (dT)20 and 1ul of 10mM dNTP mix were mixed with 8μl of DNase I treated total RNA (∼1 μg) and incubated at 65°C for 5 min, and left on ice for at least 1 min. Ten μl of cDNA synthesis mix (2μl of 10X RT buffer, 4μl of 25mM MgCl2, 2μl of 0.1M DTT, 1μl of RNaseOUT™ (40 U/μl), 1ul of Superscript™ III RT (200 U/μl) were added and incubated for 50min at 50°C and reactions terminated at 85°C for 5min. Samples were collected by brief centrifugation and 1μl of RNase H added and incubated for 20 min at 37°C. cDNA samples were amplified on the MyIQ Single-Color Real-Time PCR detection system (Bio-Rad, Hercules, CA), using iQTM SYBR Green Supermix (Bio-Rad, Hercules, CA). Two μl (5ng/μl) of cDNA; 15μl of 2X iQTM SYBR Green Supermix; 11μl of nuclease-free water; 1 μl (20ρm/μl) of each gene-specific primer were used in a 30μl reaction volume. Thermal cycling conditions included an initial incubation at 94°C for 10s, followed by 35 cycles of 95°C for 10s, 58°C for 35s, and 72°C 10s. Melt curve analysis was performed to verify primer specificity, and PCR products were confirmed by sequencing. The relative quantity of the transcripts was calculated by using the comparative threshold cycle (CT) method. Actin mRNA was amplified simultaneously for normalization between samples. Values were expressed as the mean of three RT-PCR replicates +/- standard deviation.
Table 3.
Primers used in this study of maize ABA8Ox genes.
| Gene | No. | Primer Sequence | Orientation | Accession No. |
|---|---|---|---|---|
| ABA8Ox1a | 1774 | ATGCTCGTGCTCTTCCACCACCT | Forward | AC194862 |
| 1769 | TATACCGCCATACCATATCCATCCGCC | Reverse | ||
| ABA8Ox1b | 1774 | ATGCTCGTGCTCTTCCACCACCT | Forward | AC182107 |
| 1772 | GGAAGCGGTTTTTCGCGTTCCTGG | Reverse | ||
| ABA8Ox2 | 1785 | AGCCTACGAGGAGAACGATG | Forward | AC212409 |
| 1786 | TCAGGAACCCTTGGTAGTGC | Reverse | ||
| ABA8Ox3a | 1783 | ACAGAAAGGGGCGTGAGACCGA | Forward | AC190490 |
| 1778 | AGGCGAGCAAAGAAGAATTTCAA | Reverse | ||
| ABA8Ox3b | 1784 | ACAGAAATGGCCGCCATGAGACCGA | Forward | AC195926 |
| 1781 | ATTTCTTCTCCCCCTCAAGGTAAT | Reverse | ||
| Actin | 1134 | CGATTGAGCATGGCATTGTCA | Forward | J01238 |
| 1135 | CCCACTAGCGTACAACGAA | Reverse |
3. Results and Discussion
Identification and characterization of ABA 8′-hydroxylases in maize
Five putative ABA8Ox genes were found in maize (ABA8Ox1a, ABA8Ox1b, ABA8Ox2, ABA8Ox3a, ABA8Ox3b). These genes are homologs of the four known genes in Arabidopsis [6, 12] and three genes in rice [17]. The ABA8Ox proteins are predicted to localize to the cytosol, the predominant site for ABA catabolism to phaseic acid and dihydrophaseic acid (Fig. 1). Table 1 summarizes the putative genes encoding ABA8Ox enzymes and their chromosomal positions in maize along with homologs in Arabidopsis, rice, and sorghum. The sequence similarity and identity between the Zea mays ABA8Ox proteins are presented in Table 2 and alignments of the grass ABA8Ox proteins are shown in Supplementary Fig. S1.
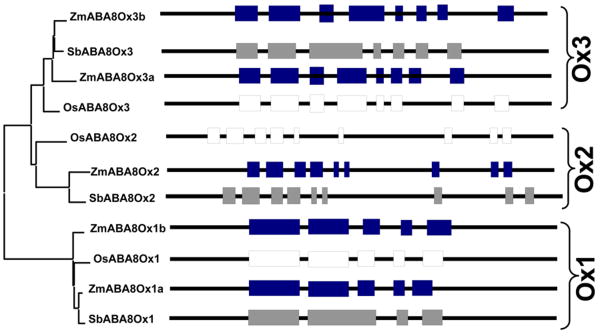
The exon-intron boundaries were deduced and confirmed through selected expressed sequence tags (ESTs) where available (Table 1). All members of the ABA8Ox gene family from all three grass species contain multiple introns (Fig. 2). To better understand the evolutionary origin of the various gene copies, phylogenetic analysis was conducted using the deduced protein sequences. The structures of the genes are shown with their orthologs grouped into one of three clades, ABA8Ox1 (Ox1), ABA8Ox2 (Ox2), and ABA8Ox3 (Ox3). Orthologs in the three species shared exon-intron organization that distinguished each paralog, except Sorghum which has fewer exons compared to its orthologous genes in the Ox3 clade. The Ox1 clade had the fewest number of exons (five) whereas the Ox2 clade had the most (nine) (Fig. 2). Classification of clades based on gene structure was similar to the protein phylogenetic organization.
Fig. 2.
ABA8Ox gene family. Gene structures (with boxes indicating exons and thin lines representing introns) for paralogs and orthologs of the ABA8Ox gene family in Zea mays (Zm), Oryza sativa (Os) and Sorghum bicolor (Sb) (accession numbers are presented in Table 1). Gene structures are compared to protein phylogenetic tree on left which was produced based on sequences shown in Supplementary Fig. S1.
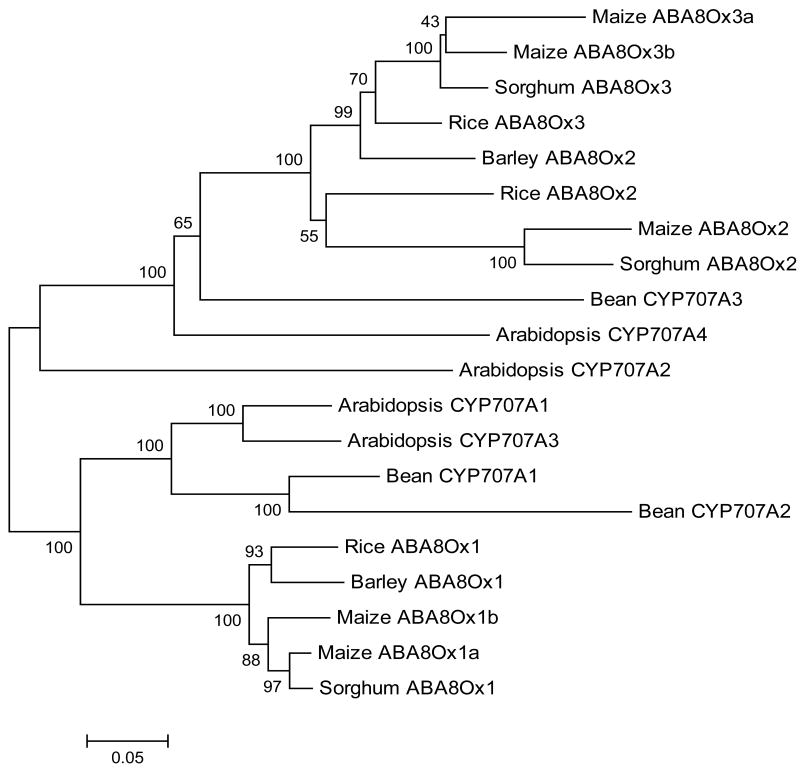
Phylogenetic analysis of ABA8Ox protein sequences from the grasses and selected dicots are shown in Fig. 3. For the grass clades, ABA8Ox2 and ABA8Ox3 appear to form sister clades that are evolutionarily diverged from a common ancestor of the ABA8Ox1 clade. During the speciation events among monocots, it appears that some grasses lost some of their ABA8Ox genes. This assumption is partially supported by the absence of ABA8Ox2 in barley (data not shown). The ABA8Ox2 (labeled as such) from barley is actually a true ABA8Ox3 based on its sequence similarity with other grass genes (Supplementary Fig. S1). The absence of an ABA8Ox2 gene in barley could be due to either incomplete genome sequencing or, at least in part, evolutionary mechanisms.
Fig. 3.
Phylogenetic analysis of proteins encoded by members of the CYP707A gene subfamily for representative monocots and dicots. A neighbor-joining tree was constructed using the following protein sequences (given as accession numbers): Zea mays (ABA8Ox1a, DR806072; ABA8Ox1b, CD433445; ABA8Ox2, CO460095; ABA8Ox3a, EC904849; ABA8Ox3b, CD941324), Oryza sativa (ABA8Ox1, LOC_Os02g47470; ABA8Ox2, LOC_Os08g36860; ABA8Ox3, LOC_Os09g28390), Sorghum bicolor (ABA8Ox1, Sb04g030660; ABA8Ox2, Sb07g022990; ABA8Ox3, Sb02g026600), Hordeum vulgare (ABA8Ox1, DQ145930; ABA8Ox2, DQ145931), Arabidopsis thaliana (CYP707A1, At4g19230; CYP707A2, At2g29090; CYP707A3, At5g45340; CYP707A4, At3g19270), Phaseolus vulgaris (CYP707A1, DQ352541; CYP707A2, DQ352542; CYP707A3, DQ352543). Amino acid distances were subjected to Poisson correction. Numbers indicate bootstrap support for individual nodes. Bootstrap support higher than 40% is indicated at respective nodes (n = 500).
Spatial analysis of ABA 8′-hydroxylase transcripts
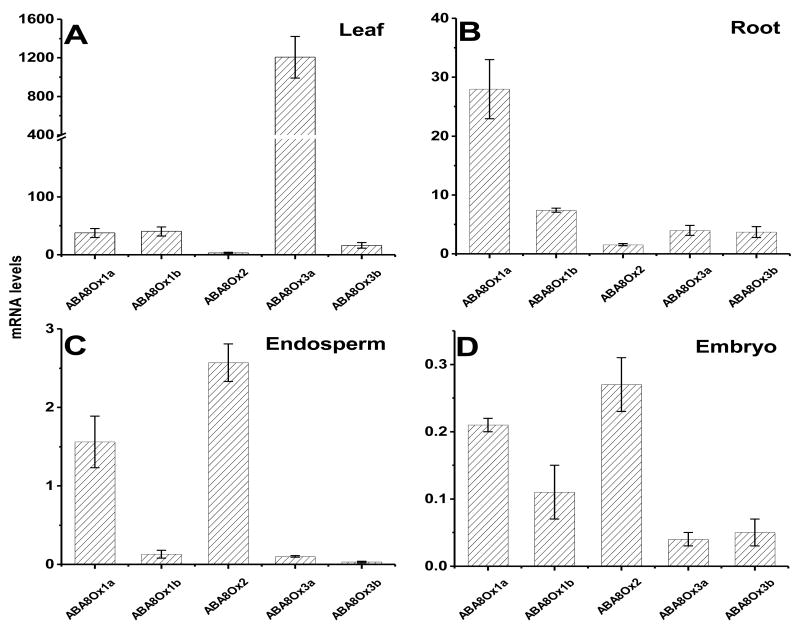
Among the sampled tissues, maize paralogs of ABA8Ox varied in relative expression, suggesting that in each tissue, different combinations of ABA8Ox are responsible for ABA catabolism. ABA8Ox3a was found to be predominantly expressed in leaves, ABA8Ox1a in roots, ABAOx2 and ABAOx1a in the endosperm, and ABA8Ox2, ABA8Ox1a and ABA8Ox1b in the embryo (Fig. 4). The abundance of maize ABA8Ox1a in the roots suggests a prominent role in root ABA catabolism. In this study, ABA8Ox3a exhibited over 1000-fold higher expression in leaves than the other paralogs, a much higher difference than that exhibited by paralogs expressed in other sampled tissues. Therefore, maize ABA8Ox3a could be an important target in the control of maize leaf ABA catabolism, given that ABA can be mobilized from any tissue. Tissue specific patterns of mRNA accumulation have also been observed for ABA8Ox gene family members in other species. In Arabidopsis, CYP707A3 (an Ox1 homolog, based on Fig. 3) plays a prominent role in ABA catabolism in the vegetative tissues while CYP707A2 was found to play a significant role in seed dormancy [6, 18, 19]. In Phaseolus vulgaris, high ABA8Ox activity in leaves during dehydration was found to be due solely to an increase in CYP707A3 transcripts while no change was observed in transcript levels of CYP707A1 and CYP707A2 [20].
Fig. 4.
Transcript profiles of maize inbred line B73 genes encoding ABA 8′-hydroxylases. Means of three replicates ± SD are presented. Embryo and endosperm were collected at 20 DAP from field-grown plants; leaf and root samples were collected from seedlings at the six-leaf stage.
Modulation and contribution of ABA 8′-hydroxylases during water deficiency in roots
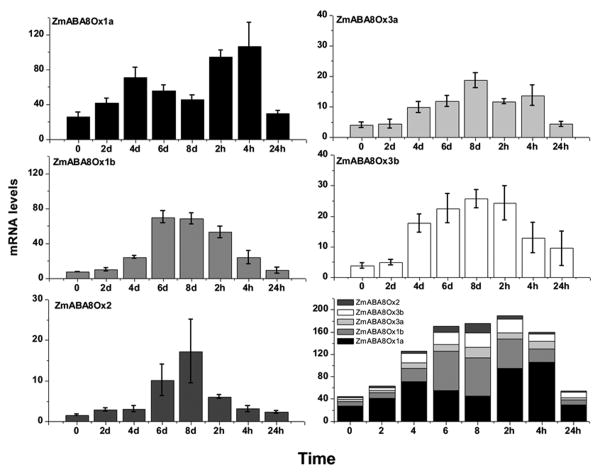
Because drought stress is first detected by the roots, it is likely that this organ can sense and respond to abiotic stress, leading to the mechanics of ABA accumulation. This suggests that ABA8Ox modulation in roots can determine drought tolerance in the entire plant. Data presented in Fig. 5 suggest that all five maize ABA8Ox genes are modulated by drought stress: their relative transcript levels paralleled dehydration and rehydration (Fig. 5) and modulation of ABA levels [measured in the same samples, as reported earlier in 8]. In roots, expression of ABA8Ox1a, followed temporally by that of ABA8Ox1b, suggests an important role for these two genes in modulating root ABA levels. ABA8Ox1a levels peaked at 4 days of drought stress, whereas ABA8Ox1b transcript levels peaked at 6 days. ABA8Ox1a, but not ABA8Ox1b, also showed further elevation in response to rehydration within 2 h of watering on day 8 (Fig. 5). These data also suggest that since all ABA8Ox genes are expressed in roots, it is reasonable to assume that each of them contributes to the modulation of ABA concentration in that organ. Pooled transcript levels suggested temporal, compensatory variations in expression of each of the paralogous genes, particularly between ABA8Ox1a and ABA8Ox1b. ABA8Ox1a was found to contribute the most to root ABA8Ox transcript levels in early responses to drought stress, followed by ABA8Ox1b, ABA8Ox3b, ABA8Ox3a and ABA8Ox2 (Fig. 5).
Fig. 5.
Drought-stress-modulated levels of transcripts encoded by Zea mays (Zm) ABA8Ox genes controlling inactivation of ABA in maize roots. Means of three replicates ± SD are presented. See Table 1 for GenBank accession numbers. Bottom right panel shows the sum (stacked) of all transcripts controlling ABA catabolism. 0-8d are days under drought and 2h-24h are hours under rehydration with water.
Conclusions
Many factors contribute to whole-plant adaptation and tolerance to drought stress. This hypothesis is consistent with the existence of various quantitative trait loci reported in previous studies on drought stress [21, 22]. Here, evidence is provided for control of ABA levels in roots mediated by ABA8Ox1a as the main early-response catabolic enzyme followed temporally by ABA8Ox1b. Drawing from the phylogenetic analysis, ABA8Ox1 orthologs can be considered potential targets for the engineering of drought-tolerance across the grass family.
Supplementary Material
Acknowledgments
This research was supported by grants (to ETW) from the National Institutes of Health (GM081160).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Badu-Apraku B, Fakorede MAB, Lum AF, Akinwale R. Improvement of yield and other traits of extra-early maize under stress and nonstress environments. Agronomy Journal. 2009;101:381–389. [Google Scholar]
- 2.Finkelstein RA, Gampala SSL, Rock CD. Abscisic acid signalling in seeds and seedlings. The Plant Cell Supplement. 2002:S15–S45. doi: 10.1105/tpc.010441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR. Abscisic acid: emergence of a core signaling network. Annual Review of Plant Biology. 2010;61:651–679. doi: 10.1146/annurev-arplant-042809-112122. [DOI] [PubMed] [Google Scholar]
- 4.Iuchi S, Kobayashi M, Taji T, Naramoto M, Seki M, Kato T, Tabata S, Kakubari Y, Yamaguchi-Shinozaki K, Shinozaki K. Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J. 2001;27:325–333. doi: 10.1046/j.1365-313x.2001.01096.x. [DOI] [PubMed] [Google Scholar]
- 5.Nitsch LM, Oplaat C, Feron R, Ma Q, Wolters-Arts M, Hedden P, Mariani C, Vriezen WH. Abscisic acid levels in tomato ovaries are regulated by LeNCED1 and SICYP707A1. Planta. 2009;229:1335–1346. doi: 10.1007/s00425-009-0913-7. [DOI] [PubMed] [Google Scholar]
- 6.Kushiro T, Okamoto M, Nakabayashi K, Yamagishi K, Kitamura S, Asami T, Hirai N, Koshiba T, Kamiya Y, Nambara E. The Arabidopsis cytochrome P450 CYP707A encodes ABA 8′-hydroxylases: key enzymes in ABA catabolism. The EMBO Journal. 2004;23:1647–1656. doi: 10.1038/sj.emboj.7600121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwartz SH, Qin X, Zeevaart JAD. Elucidation of the indirect pathway of Abscisic Acid Biosynthesis by Mutants. Genes and Enzymes Plant Physiology. 2003;131:1591–1601. doi: 10.1104/pp.102.017921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li F, Vallabhaneni R, Wurtzel ET. PSY3, a new member of the phytoene synthase gene family conserved in the Poaceae and regulator of abiotic-stress-induced root carotenogenesis. Plant Physiol. 2008b;146:1333–1345. doi: 10.1104/pp.107.111120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiong L, Zhu JK. Regulation of abscisic acid biosynthesis. Plant Physiology. 2003;133:29–36. doi: 10.1104/pp.103.025395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seo M, Koshiba T. Complex regulation of ABA biosynthesis in plants. Trends in Plant Science. 2002;7:41–48. doi: 10.1016/s1360-1385(01)02187-2. [DOI] [PubMed] [Google Scholar]
- 11.Nambara E, Marion-Poll A. Abscisic acid biosynthesis and catabolism. Annu Rev Plant Biol. 2005;56:165–185. doi: 10.1146/annurev.arplant.56.032604.144046. [DOI] [PubMed] [Google Scholar]
- 12.Saito S, Hirai N, Matsumoto C, Ohigashi H, Ohta D, Sakata K, Mizutani M. Arabidopsis CYP707As encode (+)-abscisic acid 8′-hydroxylase, a key enzyme in the oxidative catabolism of abscisic acid. Plant Physiol. 2004;134:1439–1449. doi: 10.1104/pp.103.037614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Millar AA, Jacobsen JV, Ross JJ, Helliwell CA, Poole AT, Scofield G, Reid JB, Gubler F. Seed dormancy and ABA metabolism in Arabidopsis and barley: the role of ABA 8′-hydroxylase. Plant J. 2006;45:942–954. doi: 10.1111/j.1365-313X.2006.02659.x. [DOI] [PubMed] [Google Scholar]
- 14.Kumar S, Tamura K, Jakobsen IB, Nei M. MEGA2: molecular evolutionary genetics analysis software. Bioinformatics. 2001:1244–1245. doi: 10.1093/bioinformatics/17.12.1244. [DOI] [PubMed] [Google Scholar]
- 15.Pampanwar V, Engler F, Hatfield J, Blundy S, Gupta G, Soderlund C. FPC web tools for rice, maize, and distribution. Plant Physiol. 2005;138:116–126. doi: 10.1104/pp.104.056291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vallabhaneni R, Wurtzel ET. Timing and biosynthetic potential for carotenoid accumulation in genetically diverse germplasm of maize. Plant Physiol. 2009;150:562–572. doi: 10.1104/pp.109.137042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saika H, Okamoto M, Miyoshi K, Kushiro T, Shinoda S, Jikumaru Y, Fujimoto M, Arikawa T, Takahashi H, Ando M, Arimura Si, Miyao A, Hirochika H, Kamiya Y, Tsutsumi N, Nambara E, Nakazono M. Ethylene promotes submergence-induced expression of OsABA8ox1, a gene that encodes ABA 8′-hydroxylase in rice. Plant Cell Physiol. 2007;48:287–298. doi: 10.1093/pcp/pcm003. [DOI] [PubMed] [Google Scholar]
- 18.Okamoto M, Kuwahara A, Seo M, Kushiro T, Asami T, Hirai N, Kamiya Y, Koshiba T, Nambara E. CYP707A1 and CYP707A2, which encode abscisic acid 8′-hydroxylases, are indispensable for proper control of seed dormancy and germination in Arabidopsis. Plant Physiology. 2006;141:97–107. doi: 10.1104/pp.106.079475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Umezawa T, Okamoto M, Kushiro T, Nambara E, Oono Y, Seki M, Kobayashi M, Koshiba T, Kamiya Y, Shinozaki K. CYP707A3, a manjor ABA 8′ hydroxylase involved in dehydration and rehydration response in Arabidopsis thaliana. The Plant Journal. 2006;46:171–182. doi: 10.1111/j.1365-313X.2006.02683.x. [DOI] [PubMed] [Google Scholar]
- 20.Yang SH, Zeevaart JAD. Expression of ABA 8′-hydroxylases in relation to leaf water relations and seed development in bean. The Plant Journal. 2006;47:675–686. doi: 10.1111/j.1365-313X.2006.02815.x. [DOI] [PubMed] [Google Scholar]
- 21.Tuberosa R, Salvi S, Corinna Sanguineti M, Landi P, Maccaferri M, Conti S. Mapping QTLs regulating morpho-physiological traits and yield: Case studies, shortcomings and persepectives in drought-stressed maize. Annals of Botany. 2002;89:941–963. doi: 10.1093/aob/mcf134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sari-Gorla M, Krajewski P, Di Fonzo N, Villa M, Frova C. Genetic analysis of drought tolerance in maize by molecular markers. II. Plant height and flowering. Theor Appl Genet. 1999;99:289–295. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.