Abstract
The Diabetes Prevention Trial – type 1 (DPT-1) tested whether a combination of SQ and IV insulin therapy would delay the onset of disease in individuals at high risk of progression. We investigated whether this regimen altered T cell responses to human islet proteins using cellular immunoblotting. Among the 10 treated and 7 control subjects studied, we found that there was a significant effect of treatment on cellular immunoblotting responses. We conclude that parenteral insulin may suppress proliferation to islet antigens in individuals at risk for diabetes, but this effect may be transient. Further study is needed to determine whether a therapy that results in sustained suppression of T cell proliferation could yield a measurable clinical benefit.
INTRODUCTION
Advances in our understanding of the course of the type 1 diabetes disease process, coupled with the availability of novel immunomodulating agents have resulted in a myriad of clinical trials to interrupt the immune mediated beta cell destructive process before or after the onset of clinical disease. Prior to diagnosis, the trial endpoint is generally clinical onset of disease, and post diagnosis, the primary outcome measure for these studies has been stimulated C-peptide reflecting endogenous insulin secretion [5]. Due to the variance in this measure between individuals with type 1 diabetes, the prolonged preservation of insulin secretion post diagnosis in some subjects, and the time frame before diagnosis for those at less than very high risk, these trials must involve a relatively large number of subjects studied for several years to achieve clinical significance.
As a result, parallel to efforts to refine prediction of disease and tests of new therapies, considerable effort has gone into development of “surrogate markers” to determine whether there is a measurable impact of therapy on autoimmunity. Such information may eventually allow for smaller and shorter clinical trials, and moreover should enhance the information we obtain from trials by allowing us to better understand disease or therapeutic mechanisms and/or by suggesting that further clinical development along a particular path is warranted even in the absence of a positive outcome.
One such possible surrogate marker is determination of the proliferative responses to multiple islet antigens. In our studies to date, most patients within the first year of diagnosis of type 1 diabetes have proliferative responses to multiple islet antigens [1;3]. Further, though the T-cell stimulating antigens have not been individually identified, in two multicenter studies using masked samples, this assay demonstrated reproducibility and reliably distinguished subjects with type 1 diabetes from controls with excellent sensitivity and specificity [7;15].
The “high risk” arm of the Diabetes Prevention Trial (DPT-1) tested whether parenteral insulin could delay or prevent the onset of disease in individuals at >50% five year risk of type 1 diabetes. Subjects in the DPT-1 treatment arm received both daily sub-cutaneous insulin and a yearly four day course of continuous IV insulin. Results from this DPT-1 study demonstrated that this combination of IV and sub-cutaneous antigen (insulin) administration did not prevent clinical disease [4]. As an ancillary study to that DPT-1 clinical trial, we investigated T cell proliferative responses to islet antigens using cellular immunoblotting.
RESEARCH DESIGN and METHODS
DPT-1 ancillary study
Following an IRB approved protocol, all subjects and/or their parents signed an informed consent. Ten subjects randomized to the treatment arm of the DPT-1 high risk study, and eight in the control arm participated in this ancillary study. One of the 8 control subjects had only one sample obtained and the results from that assay were not interpretable. The ancillary study did not begin until after the clinical trial had started, so baseline samples prior to randomization were not available for 3/7 control subjects and 9/10 treated subjects. Samples were obtained at variable intervals at least 3 months apart according to the DPT-1 clinical trial schedule and subject compliance. Samples were also obtained on two individuals at study end when they were off DPT-1 treatment and on five individuals after diagnosis of diabetes when they were receiving subcutaneous insulin therapeutically. As previously described, insulin was administered over an annual four day period as a variable IV infusion aimed to keep glucose within defined parameters. As outpatient, subjects received a total daily dose of 0.25 u/kg of ultralente insulin administered in two doses[4].
Masking
Samples were coded and provided to the laboratory in pairs consisting of one sample from a DPT-1 subject and one sample from an irrelevant subject (either type 1 diabetes subject not part of the intervention studies or healthy control). The laboratory was also masked to consecutive samples from the same subject. Results were reported by coded sample number and the code was not broken until study conclusion.
Cellular Immunoblotting assay
Detailed methods have been previously described [3]. In brief, human islet cells were subjected to SDS-PAGE and electroblotted onto nitrocellulose membranes. Human islets were obtained from the NIH Islet Consortium. These membranes were then cut into 18 strips based on molecular weight, solubilized, reprecipitated, and used to stimulate freshly isolated PMBCs in vitro. Cells were cultured for 5 days with triplicate wells containing nitrocellulose particles with and without antigen or cells alone. Positive controls were tetanus toxoid and Con A. Counts per minute after tritiated thymidine incorporation were used to determine a stimulation index (SI). The SI was calculated as mean CPM in experimental wells/mean CPM in control wells (cells without antigen). Positive proliferation is a stimulation index ≥2.0. The number of blot sections with positive proliferation was then totaled per subject. A subject was considered to have a positive response in the assay when more than 3 blot sections were positive.
Reproducibility of this assay has been studied in both healthy control subjects and those with type 1 diabetes [3;7;8]. In the latter study, the reproducibility of the assay with masked samples shipped from multiple locations was 82%, just below that found for autoantibodies (86%).
Analysis
A linear mixed-effect (LME) model was used with the treatment group as predictor of interest and each subject treated as a cluster of data. A test for trend over time or slope was not significant. In addition, unpaired T-Tests were performed to determine differences in T cell responses to islet proteins at first and last sample. Values are mean number ± SD.
RESULTS
Demographic information, antibody status and HLA DR type for subjects in the DPT-1 study are shown in Table 1.
TABLE 1.
DPT-1 subjects
| Age at baseline visit |
ICA* | IAA | GAD | ICA512 | mIAA | HLA DR | |
|---|---|---|---|---|---|---|---|
| C 1 | 9.6 | Positive | Positive | Positive | Positive | Positive | 0101/0301 |
| C 2 | 21.2 | Positive | Negative | Positive | Positive | Negative | 0301/1301 |
| C 3 | 12.7 | Positive | Positive | Positive | Positive | Negative | |
| C 4 | 24.0 | Positive | Positive | Positive | Negative | - | 0401/0403 |
| C 5 | 10.8 | Positive | Positive | Positive | Negative | Negative | 0408 |
| C 6 | 40.0 | Positive | Negative | Negative | Negative | - | 0301/0408 |
| C 7 | 18.8 | Positive | Positive | Positive | Negative | - | 0301/0401 |
| Rx 1 | 39.5 | Positive | Negative | Positive | Negative | Negative | 0401/1301 |
| Rx 2 | 9.5 | Positive | Positive | Positive | Negative | Negative | 0101/0301 |
| Rx 3 | 33.1 | Positive | Negative | Positive | Negative | Negative | 0401/0301 |
| Rx 4 | 46.0 | Positive | Negative | Positive | Negative | Negative | 0404/0301 |
| Rx 5 | 12.5 | Positive | Positive | Positive | Positive | Negative | |
| Rx 6 | 19.1 | Positive | Negative | Positive | Positive | Negative | 0401/1302 |
| Rx 7 | 13.5 | Positive | Positive | Negative | Positive | - | 0301/0401 |
| Rx 8 | 11.5 | Positive | Positive | Positive | Positive | Positive | 0402/1601 |
| Rx 9 | 6.7 | Positive | Positive | Positive | Negative | Negative | 0301/0301 |
| Rx 10 | 16.5 | Positive | Negative | Negative | Positive | Negative | 0301/0201 |
antibody data from pre-randomization testing
Comparisons between groups
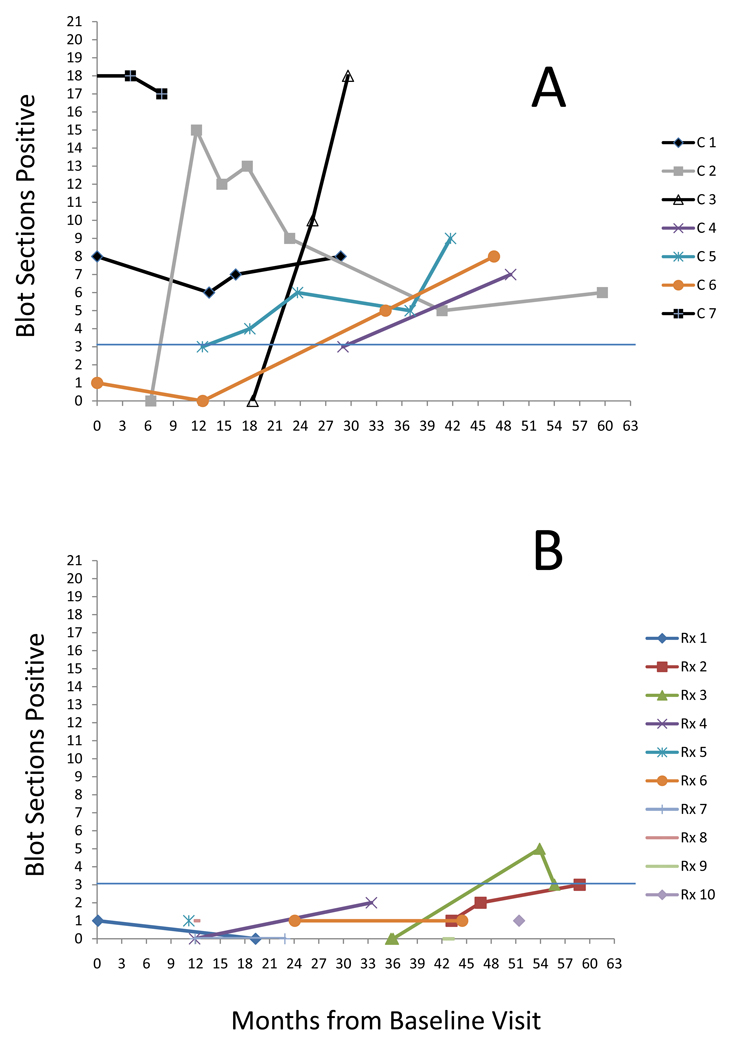
Figure 1 depicts the cellular responses over time in individuals in the control (1a) and treatment (1b) arms.
Figure 1.
Cellular Immunoblotting Responses in subjects enrolled in DPT-1 parenteral insulin trial. (a) Control arm subjects, n=7 (b) Treatment arm subjects, n=10.
Subjects with more than 3 immunoblot sections are considered to have a positive cellular assay response. Circles indicate date of diagnosis of type 1 diabetes in 5 subjects.
The mean number of blot sections positive at first sample obtained was 4.7 +/− 6.5 in control subjects. The mean number of blot sections positive at first sample obtained was 0.6 +/− 0.5 in treated subjects. All control subjects had positive responses and all treated subjects had negative responses at the last sample obtained at end of DPT-1. At that sample, the number of blot sections positive in the control subjects as a group (mean, SD: 10.4 ± 4.9) was markedly different than those in the treatment group (mean, SD:1.5 ± 1.4; p <0.002).
Using the linear mixed-effect model, the mean number of blot sections for control subjects overall was 8.04 with a treatment effect of 6.99 indicating that the overall mean number of blot sections in treatment group using this model is 1.05. This was highly significant for a treatment effect, p<0.001).
Progression to type 1 diabetes
Five subjects developed type 1 diabetes during the study time period; three in the control arm and two in the treatment arm. All three subjects in the control arm had positive blot sections at the time of diagnosis, while the two subjects in the treatment arm who developed type 1 diabetes had negative responses at the time of diagnosis. Samples obtained 6.8 and 10.4 months after diagnosis of T1DM from these two individuals demonstrated positive proliferative responses at 6 and 4 blot sections respectively.
Therapy withdrawal
When DPT-1 ended, subjects were instructed to stop SQ insulin therapy and no further IV insulin infusions were performed. Samples were obtained 13 and 19 months off therapy in two individuals who did not develop diabetes. In each of these cases (rx4, rx7) the number of blot sections increased from before to after end of treatment going from 2 to 16 blot sections positive and from 0 to 1.
Discussion
Results from the DPT-1 study in subjects at >50% risk for development of diabetes demonstrated that IV and SQ antigen (insulin) administration did not prevent clinical disease [4]. As an ancillary study to that clinical trial, we investigated T cell proliferative responses to islet antigens using cellular immunoblotting and found that control subjects had sustained proliferative responses over time whereas the treated subjects had suppressed responses. This data suggests that while clinical efficacy was not demonstrated, DPT-1 insulin therapy may have had an effect on the immune response. Since samples obtained in four treated subjects three to twelve months after the end of DPT-1 therapy demonstrated a loss of this suppression, the data also suggest that IV insulin did not have a prolonged effect on the immune response. If true, this may account for the lack of clinical efficacy seen in the DPT-1 trial.
This data is in contrast with data from newly diagnosed patients with type 1 diabetes who though routinely treated with subcutaneous insulin have proliferative responses [1–3]. One difference in the two groups is that the DPT-1 subjects received both IV and subcutaneous insulin, while patients with type 1 diabetes receive only subcutaneous therapy. This suggests the possibility that it was the IV component of the DPT-1 protocol that resulted in the suppression of the T cell responses, although this hypothesis requires further study.
Unfortunately, our data do have some limitations. Since the ancillary study started after the DPT-1 began, baseline samples were not obtained on most subjects and on several subjects only a single time point was available. Only one DPT-1 site was involved in this ancillary study, so the numbers of subjects tested was small. Even with the above noted limitations the cellular immunoblotting assay used has been repeatedly demonstrated as one of the most specific and sensitive tests of cellular responses in individuals with type 1 diabetes [3;8;15]. In addition, considerable thought was put into the operations to ensure masking of samples. Research samples were coded and samples from subjects in this trial were always accompanied by samples obtained from other subjects with diabetes. In this way, laboratory personnel did not know which samples were pertinent to this ancillary study, nor were they able to link any sample to the same subject at a different time point. Unmasking did not occur until results were confirmed as final and sent to the clinical team.
Our data is consistent with the hypothesis that IV and not subcutaneous insulin alters immune responses. While complete beta cell rest was not achieved by either regimen, the degree of suppression of endogenous beta cell secretion was considerably greater during IV as compared with subcutaneous administration. This could have been sufficient to decrease antigen presentation as had been demonstrated in early in vitro studies [6;12]. The IV insulin was certainly sufficient to control glucose excursions and this correction of hyperglycemia may have also been important in suppression of responses. This interpretation is consistent with previous work demonstrating enhanced insulin-specific T regulatory cells after exogenous insulin therapy soon after diagnosis[16]. Alternatively, basic immunologic principals suggest that the immune responses to IV and subcutaneous antigens may differ [9–11;13;14]. Thus, the effects seen here may reflect the route rather than the dose of insulin delivered.
This report is the first to demonstrate a possible effect of the DPT-1 high risk therapy on a potential surrogate marker – specifically on cellular immune responses to islet proteins. Our data also implies that the effect may have depended upon IV administration of insulin and that the effect may not last beyond a few months. It may be worthwhile to test whether a therapeutic regimen resulting in more sustained suppression of T cell proliferation as measured by cellular immunoblotting would yield a measurable clinical benefit.
Acknowledgements
The DPT-1 study was supported by cooperative agreements with the Division of Diabetes, Endocrinology and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Diseases; the National Institute of Allergy and Infectious Diseases; the National Institute of Child Health and Human Development; the National Center for Research Resources; the American Diabetes Association; and the Juvenile Diabetes Research Foundation. Supplies were provided by Eli Lilly, Bayer, Becton Dickinson, International Technidyne, LifeScan, the Mead Johnson Nutritionals Division of Bristol-Myers Squibb, the Medisense Division of Abbott Laboratories, MiniMed, and Roche Diagnostics. Harvey Chiu was an Endocrinology fellow supported (in part) by the Medical Research Services of the Department of Veterans Affairs. This research was also supported in part by NIH/NIDDK grants P30 DK17047, U01 DK46601 and a Clinical Research grant from the American Diabetes Association.
DPT-1 subjects in this ancillary study and those in the pilot study for individuals with type 1 diabetes were seen at the GCRC of the University of Washington. This is currently funded under NCRR Grant M01-RR-00037. Additional support was provided in part by the Buse Diabetes Research Fund at the Benaroya Research Institute.
Reference List
- 1.Brooks-Worrell B, Gersuk VH, Greenbaum C, Palmer JP. Intermolecular antigen spreading occurs during the preclinical period of human type 1 diabetes. J.Immunol. 2001;166:5265–5270. doi: 10.4049/jimmunol.166.8.5265. [DOI] [PubMed] [Google Scholar]
- 2.Brooks-Worrell BM, Pihoker C, Greenbaum CJ, Palmer JP. Presence of islet reactive T cells and autoantibodies in children diagnosed clinically with either type 1, type 2, or atypical diabetes. 4th Immunology of Diabetes Society. 1999 [Google Scholar]
- 3.Brooks-Worrell BM, Starkebaum GA, Greenbaum C, Palmer JP. Peripheral blood mononuclear cells of insulin-dependent diabetic patients respond to multiple islet cell proteins. J.Immunol. 1996;157:5668–5674. [PubMed] [Google Scholar]
- 4.Diabetes Prevention Trial Study Group. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N.Engl.J.Med. 2002;346:1685–1691. doi: 10.1056/NEJMoa012350. [DOI] [PubMed] [Google Scholar]
- 5.Greenbaum CJ, Harrison LC. Guidelines for intervention trials in subjects with newly diagnosed type 1 diabetes. Diabetes. 2003;52:1059–1065. doi: 10.2337/diabetes.52.5.1059. [DOI] [PubMed] [Google Scholar]
- 6.Hao W, Lindsong L, Mehta V, Lernmark A, Palmer J. The Functional State of the Beta Cell Affects Expression of Both Forms of Glutamic Acid Decarboxylase. Autoimmunity. 1993;15:73. doi: 10.1097/00006676-199409000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Herold KC Type 1 Diabetes TrialNet study group. Report of the TrialNet T cell validation study in Type 1 diabetes. Diabetes. 2007:1229. Ref Type: Abstract. [Google Scholar]
- 8.Herold KC, Brooks-Worrell B, Palmer J, Dosch HM, Peakman M, Gottlieb P, Reijonen H, Arif S, Spain LM, Thompson C, Lachin JM. Validity and Reproducibility of Measurement of Islet Autoreactivity by T-cell Assays in Subjects with Early Type 1 diabetes. Diabetes. 2009 doi: 10.2337/db09-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobs MJ, van den Hoek AE, van de Putte LB, van den Berg WB. Anergy of antigen-specific T lymphocytes is a potent mechanism of intravenously induced tolerance. Immunology. 1994;82:294–300. [PMC free article] [PubMed] [Google Scholar]
- 10.Lagrange PH, Mackaness GB, Miller TE. Influence of dose and route of antigen injection on the immunological induction of T cells. J.Exp.Med. 1974;139:528–542. doi: 10.1084/jem.139.3.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lelchuk R, Carrier M, Kahl L, Liew FY. Distinct IL-3 activation profile induced by intravenous versus subcutaneous routes of immunization. Cell Immunol. 1989;122:338–349. doi: 10.1016/0008-8749(89)90082-8. [DOI] [PubMed] [Google Scholar]
- 12.McCulloch DK, Barmeier H, Neifing JL, Palmer JP. Metabolic state of the pancreas affects end-point titre in the islet cell antibody assay. Diabetologia Sep. 1991;34:622–625. doi: 10.1007/BF00400990. [DOI] [PubMed] [Google Scholar]
- 13.Mukasa A, Itoh M, Tokunaga Y, Hiramine C, Hojo K. Inhibition of a novel model of murine experimental autoimmune orchitis by intravenous administration with a soluble testicular antigen: participation of CD8+ regulatory T cells. Clin.Immunol.Immunopathol. 1992;62:210–219. doi: 10.1016/0090-1229(92)90074-x. [DOI] [PubMed] [Google Scholar]
- 14.Myers LK, Stuart JM, Seyer JM, Kang AH. Identification of an immunosuppressive epitope of type II collagen that confers protection against collagen-induced arthritis. J.Exp.Med. 1989;170:1999–2010. doi: 10.1084/jem.170.6.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seyfert-Margolis V, Gisler TD, Asare AL, Wang RS, Dosch HM, Brooks-Worrell B, Eisenbarth GS, Palmer JP, Greenbaum CJ, Gitelman SE, Nepom GT, Bluestone JA, Herold KC. Analysis of T-cell assays to measure autoimmune responses in subjects with type 1 diabetes: results of a blinded controlled study. Diabetes. 2006;55:2588–2594. doi: 10.2337/db05-1378. [DOI] [PubMed] [Google Scholar]
- 16.Tiittanen M, Huupponen JT, Knip M, Vaarala O. Insulin treatment in patients with type 1 diabetes induces upregulation of regulatory T-cell markers in peripheral blood mononuclear cells stimulated with insulin in vitro. Diabetes. 2006;55:3446–3454. doi: 10.2337/db06-0132. [DOI] [PubMed] [Google Scholar]