Abstract
Cell-based technologies to support/restore liver function represent one of the most promising opportunities in the treatment of acute liver failure. However, the understanding of the constituent cell types that interact to achieve liver-specific structure and function as not been achieved in the development of liver assist devices (LADs). Here we show that hepatocytes migrate toward and adhere and formed sinusoids-like structures in conjunction with liver non-parenchymal cells, and that this liver organoid formed sophisticated tissue after 7 days in an implanted LADs in rodents. Hepatocytes only or in combination with human non-parenchymal liver cell lines (endothelial, cholangiocytes and stellate cells) were cultured in Matrigel, Ultra-structural showed that the hepatocyte-decorated endothelial vascular structures resemble in vivo sinusoids containing plate-like structures, bile canaliculi, and lumen. The sinusoid-like structures retained albumin secretion and drug-metabolism capabilities. In addition, LADs containing cocultures of human liver non-parenchymal cells were transplanted in animals for a week, the liver tissue formed sophisticated structures resembling the liver. These results demonstrate the importance of non-parenchymal cells in the cellular composition of LADs. The novelty of the culture’s sinusoid-like organization and function strongly support the integration of liver non-parenchymal units into hepatocyte-coculture based LADs as a potential destination therapy for liver failure.
Keywords: Organoid, Liver Support, Liver Cell Therapy, Liver Failure
INTRODUCTION
Fulminant hepatic failure is a life threatening condition that is treated by transplantation of the liver when a donor organ can be found. Because the natural history of acute failure varies so widely, even transient hepatic failure, when severe, must be treated by transplantation and life-long immune suppression (2,7,22) but this therapy is limited due to the shortage of donor livers and high costs. Therefore, treatment of hepatic failure could be dramatically improved by development of methods for temporary hepatic support (2,15). Thus, the use of a liver assist devices (LADs) has greater theoretic potential as a minimally intrusive therapy, as incorporated hepatocytes might provide additional metabolic capacity and physiologically active molecules important for recovery of native hepatic function (14).
However, concerns exist related to the use of liver assist devices; the feasibility and risk when the direct connection to the circulatory system, a reliable cell source; the structure, function, and viability of the parenchymal cells seeded in the device. The expected cell type used in LADs is clearly a human hepatocyte to support the systemic dysfunction that occurs after liver failure, other cell sources include porcine hepatocytes and lately stem cell-derived hepatocytes (1,2,12,16,19). However, a supportive cell type cocultured with hepatocytes in the device can aid in meeting these design criteria in a secondary manner. Ideally, a supportive cell could reduce the hepatic cell mass needed in a device by enhancing hepatocyte function ex vivo and enhance the preservability of parenchymal cells during storage. It is well known that heterotypic cell interactions are required for the phenotypic stability of the parenchymal cells as well as for proper liver function (13). Hepatocytes clusters naturally are invaded by matrix depositing stellate cells, followed by growing endothelial cells that form capillaries (20) restoring normal liver architecture. The interactions between hepatocytes and other liver non-parenchymal cells leading to liver morphogenesis and its use in LADs have yet to be recaptured. Several studies have highlighted the importance of hepatic function of hepatocytes when supported with non-parenchymal liver cells (10,11).
In this study, we sought to determine if supportive cell types can influence the outcome of liver assist devices. We have currently developed a new type of bioartificial device that allows a three-dimensional cell culture based on a poly-amino-urethan (PAU)-coated, non-woven polytetrafluoroethylene (PTFE) fabric previously developed by our laboratory (4,17,18). Here, we demonstrate the ability of liver non-parenchymal cells including endothelial, stellate and cholangyocytes to support liver sinusoid formation in an in vitro Matrigel assay then we inoculated these liver organoid in a liver assist device that was intra-peritoneally implanted for a week. The formed organoid resemble sinusoid-like structures and retained albumin secretion, and drug metabolism capacities significantly greater than that of cultures consisting in only hepatocytes. Our ability to establish liver-like organization and function offers the possibility to study the heterotypic cell–cell interactions shown to be important for proper liver function, under carefully controlled conditions, and suggests a new method to design large-scale liver assist devices in the future.
MATERIALS AND METHODS
Hepatocyte Isolation
Mouse primary hepatocytes were isolated from Balb/c mice (Nippon CLEA, Tokyo, Japan) weighing 25 g by a 2-step collagenase digestion method, as previously reported (18). Cell viability was assessed by a trypan blue dye exclusion method in all cases. In the hepatocyte isolation and culture experiments, the isolated hepatocytes of which viabilities were more than 90% were used in all cases. In all groups, hepatocytes were seeded onto Matrigel ®(500 μL) (BD, Becton Dickinson) that was thawed overnight at 4°C, layered on ice-cold 12-well culture plates, and incubated at 37°C for 30 min to allow the gel to form. Hepatocytes were cultured with Williams’ E solution (Sigma, St. Louis, MO) supplemented with 10% fetal bovine serum (Sigma), 1×107 mol/L insulin (GibcoBRL), 25 ng/L EGF (Sigma), 1×106 mol/L dexamethasone (Sigma), 1×105 U/L penicillin and 100 mg/L streptomycin (Sigma) and deleted variant of HGF (100 ng/ml) (kindly provided by Daiichi Pharmaceutical Co., Ltd., Tokyo, Japan). In the following experiments, cell cultures functions were measured per unit of time per mg of cellular protein at each of time points. The data were analyzed from 4 independent experiments in mouse hepatocyte cultures each of the experiments had 3 samples.
Human Liver Non-Parenchymal Cell Lines Establishment
The human endothelial (TMNK-1) (9) cell line was generated from commercially available normal human liver endothelial cells (Dinippon Sumitomo Parma, cat. no. CS-ABI-566) by transduction with recombinant retrovirus vectors SSR#69, which transfers expression of the simian virus 40 large T antigen (SV40T), and SSR#197, which transfers human telomerase reverse transcriptase (hTERT) expression. The human stellate (TWNT-1) (21) cell line was generated from human LI 90 cells, which were derived from a mesenchymal liver tumor removed from a 55-year-old Japanese woman. The cells were transduced with the SSR#197 recombinant retroviral vector, which transfers human telomerase reverse transcriptase (hTERT) expression. The human cholangiocyte (MMNK-1) (8) cell line was generated from OUMS-21 cells, which were derived from human fetal liver by transduction with the SSR#197 recombinant retrovirus vector (hTERT). Human liver non-parenchymal cell-lines, were maintained in T75 tissue culture flasks (Falcon) in 10 ml of DMEM (GibcoBRL/Invitrogen, Co.) and 10% FBS (Cansera International, Inc, Ontario, Canada) and 1% penicillin/streptomycin (100 units/ml penicillin, 0.1 mg/ml streptomycin).
Liver Organoid Engineering on Matrigel
7.5×105 primary Fresh isolated mice hepatocytes were seeded in each well of a 6 well plate (Corning Costar, Action, MA). A combination of same cell number of each human liver non-parenchymal cell lines (endothelial, cholangiocytes and stellate cells) were layered on top of the gel at various ratios (combination of human liver non-parenchymal cells: hepatocytes at 1:1, 1:3 and 1:5) in 2 mL of hepatocyte medium previously described in material and methods section. Co-culture medium was replaced every 24 hrs and the spent media was stored at −80°C for subsequent analysis.
Morphological Assessment
During the time of culture, morphological appearance of liver organoids was observed using a phase contrast microscope (Olympus CK40-SL Japan). On days 1 and 5, the cells inoculated onto matrigel and on day 7 after been implanted into the LADs were subjected to micro-structural analysis using a scanning electron microscope (SEM) (Hitachi S-2300, Hitachi Co. Ltd., Tokyo, Japan) and transitional electron microscope (TEM) (Hitachi H-7100, Hitachi Co. Ltd.). For SEM, the samples were washed with PBS followed by fixation with 2% glutaraldehyde for 2 h at 37°C, and gently washed with PBS. The samples were then post-fixed with osmium tetraoxide for 2 h and dehydration was accomplished using a graded series of ethanol (50%, 60%, 70%, 80%, 90% and 99%). The samples were then dried at critical point for 2 h in absolute alcohol and mounted on an aluminum stub and sputter-coated with gold before viewing under SEM. For TEM, the cells were fixed, first in 2.5% glutaraldehyde in 0.1 M PB, and then in 1.0 % OsO4 in 0.1 M PB (pH 7.2). The samples were dehydrated through graded concentrations of ethanol and embedded in Epon. Ultra thin sections of the samples were double-stained with uranyl, and observed under TEM. Ten different areas were randomly chosen and examined.
Cell Death Assay
Cell death assay was assessed by measuring lactate dehydrogenase (LDH) release into the culture medium with the CytoTox 96 non-radioactive cytotoxicity assay kit (Promega). Maximal LDH release was measured on days 3 and 5. LDH values for different co-culture ratios (1:1, 1:3, 1:5) were expressed as optical density units (OD) during the time of evaluation.
Evaluation of Hepatic Metabolic and Synthetic Capacities of the Liver Organonide
At different time points of culture, hepa tocytes cultured on collagen or unwoven PTFE were subjected to metabolic and synthetic tests. Ammonium sulfate (0.56 mM) and lidocaine (1 mg/ml) were added to individual wells of 12-well-plates and the amount of each substrate remaining in the media after culture for 4 h was measured. The ammonia concentration was determined using a Fuji Dri-Chem slide (Fuji Co., Tokyo, Japan) and concentration of lidocaine was measured by SRL (Tokyo, Japan). Four hours after ammonia loading, 10 micrometer of culture medium was collected for urea synthesis, as previously reported. Albumin secretion into the culture medium for 24 h was measured by an albumin enzyme-linked immunosorbent assay (ELISA) kit for mouse albumin secretion into the culture medium for 24 h ELISA kit (Shibayagi; Gunma, Japan) was used.
Construction of the implantable LADs
The LAD was constructed using a polyethylene-vinyl alcohol membrane (30 nm pore size), which inhibits passage of immune competent cells and C3 and thus provides a degree of immune isolation, and a PAU-coated PTFE non-woven fabric, which provides a substrate for cell adhesion. The 15 × 15mm PTFE fabric was covered with an EVAL membrane and layered with a piece of polyester supporting fabric of equal size to generate an inside volume of 1.125 ml. A cell injection port was then attached. To facilitate angiogenesis around the implanted module, both surface layers of the LAD module were coated with gelatinized FGF-2 prior to intraperitoneal implantation.
Transplantation Experiments
To examine the feasibility to transplant in the intra-peritoneal cavity this implantable LAD containing primary hepatocytes only and the hepatic organoid on mice with hepatectomized livers, eight week-old female Balb/c mice (n=6) (Nippon CLEA) underwent 50% hepatectomy to induce hepatic regeneration. All the following procedures performed on the mice were approved by the Okayama University Institutional Animal Care and Use Committee. Both sides of a LAD module were coated by 2 mg of gelatinized FGF-2 microspheres as previously described (17). Directly after hepatectomy, a LAD module containing only hepatocytes (5×106 cells) or Hepatic organoid (5×106 hepatoctes, 1×106 of each cell human liver non-parenchymal cell line with 200 μl Matrigel (BD Biosciences) and 500 μl culture medium into the BAL module through a subcutaneously fixed cell injection port) was implanted in the intra-peritoneal cavity and the abdominal wall was closed. After 7 days animals were sacrificed and LADs were extracted and ultra-structural analysis was performed.
Statistical Analyses
Mean values are presented with standard deviations (SDs). A two-tailed Student’s t test was used to calculate the significance of difference in mean values.
RESULTS
Co-Culture of Hepatocytes and Non-Parenchymal cells Formed Sinusoid-like structures and Maintained Viability
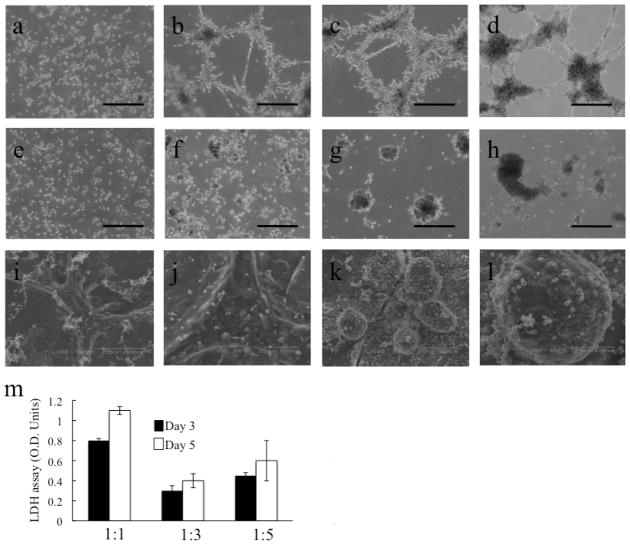
Matrigel is a commonly used cell culture subtract assay and has been shown to preserve hepatocyte function in vitro (10). In order to study the interaction between non-parenchymal cells and hepatocytes, we seeded either mice hepatocytes alone, or mice hepatocytes and the human liver non-parenchymal cell lines on Matrigel. The endothelial, stellate cells and cholangiocytes rapidly formed tube-like structures (Fig. 1a–d). Freshly isolated mice (n=7) hepatocytes were regrouped and migrated around the pre-established vascular tubes and remained adherent to them (Fig. 1a–d). We never observed hepatocyte migration away from the nearest tube-like structure. When hepatocytes alone were plated on Matrigel in the same base medium but in the absence of human liver non-parenchymal cells, they failed to aggregate in caused formation of ducts along the plates, and they formed just independent spheroids without any connection on to the other (n=10, Fig. 1e-h). These results were corroborated by ultra-structural studies using scanning electron microscope, when using co-cultures of hepatocytes with liver non-parenchymal cells interconnected tube-like structures were observed (Fig. 1i and j) in contrast when only hepatocytes were used duct-like structures failed to formed (Fig. 1k,l).
Figure 1. Morphological transformations in co-cultures of hepatocytes and human liver non-parenchymal cells.
Phase contrast microscopy. Time-lapse of hepatocytes and human liver non-parenchymal cells (endothelial, stellate cells and cholangiocytes) migration toward endothelial tube-like structures (a-d) or only hepatocytes (e-h) cultured on matrigel at 0h (a,e), 24h (b,f), 72h (c,g) and 120h (d, h). Hepatocytes selectively migrate toward and adhere to endothelial tube-like structures, creating sinusoid-like structures. Scanning electron microscope (SEM) images after 120h of co-culture of hepatocytes and human liver non-parenchymal cells (i,j) or only hepatocytes (k,l). Note the difference of formations, where co-cultures induced organotypic structures similar to liver sinosoide-like structures with tight and interconnected cells. LDH release study (m) revealed a significant maintenance of cell viability when evaluated by at different ratios of cells indicating better viability during the time (Optica density units, O.D. units). (a-h) bar= 200 micrometer.
The morphogenetic patterns formed in these co-cultures when placed in Matrigel suggest that hepatocytes alone have innate tendencies to form sinusoids-like structures. The photographs presented are representative of all the experiments performed. Moreover, to determine the best co-culture conditions of human liver non-parenchymal cells and fresh isolated hepatocytes, cells were simultaneously seeded at different ratios (1:1, 1:3 and 1:5 ratio) of liver non-parenchymal cells to hepatocytes. In a cell death assay lactate dehydrogenase (LDH) for viability of the hepatic organoid during the time, absorbance of an LDH regent was compared among the different ratios to evaluate cell death among the cells in co-culture according to the optical density units (O.D. units). Co-cultures with ratio 1:3 maintained significantly better cell viability compared to other co-culture ratios at days 3 and 5 after seeding (Fig. 1m).
Ultra-Structural Analysis of Liver Organoids reveals Sinusoid-like Structures
Images obtained by transmission electron microscopy showed that the sinusoid structures were composed of cells with differentiated hepatocyte morphology including abundant glycogen granules, extensive rough and numerous endoplasmic reticulum, mitochondria, and bile canaliculi between the hepatocytes with mature junctional complexes, with a thick, and electron dense layer of extracellular matrix with several fibroblastic type cells residing in the matrix and around the hepatocytes (Fig. 2a–d). Microvilli extended along the seams between hepatocytes, as well as along the bile canalicular spaces (Fig. 2c). Transmission electron microscopy images of the encapsulated hepatocyte–endothelial structures revealed several long endothelial cells, and neighboring hepatocyte plate-like structures (Fig. 2c,d). Ultrastructurally, the hepatocytes at the capsule periphery appeared well preserved after 7 days of in vitro culture, whereas those in the structure interior had enlarged peroxisomes.
Figure 2. Ultra-structural Analysis and Functional Capacities of the Engineered Liver Organoid.
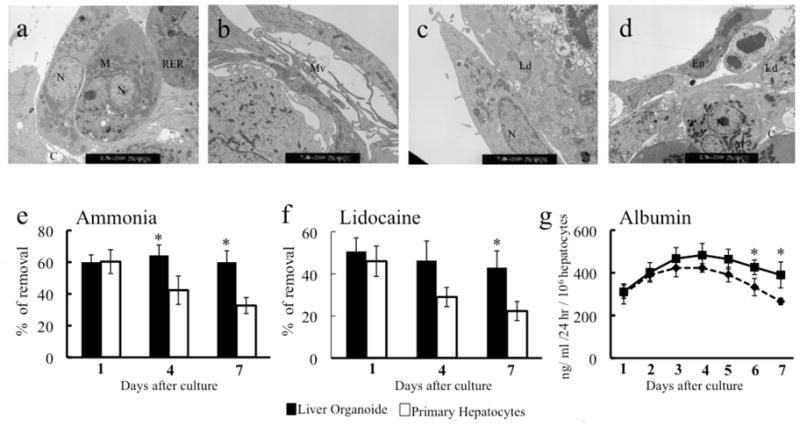
Transmission electro microscope study (TEM) revealed as shown at low power electron micrographs (a-d) that acinar structure were formed after 120h in co-cultures of freshly isolated hepatocytes and human liver non-parenchymal cells. Evident are the duct-like canalicular structures in the center of the acinar structure. Cells contain extensive endoplasmic reticulum and numerous mitochondria, endhotelial and stellate cells covered the hepatocytes forming sinusoid-like structures. N; nucleus, M; mitochondria, RER; golgi elements, C; canaliculi, Mv; microvilli, Ld; lipid droplets, Ed, endothelial cell. Magnification (a, d) 2,500X; (b, c) 7,000X. Engineering of a liver organoid using freshly isolated hepatocyte and human liver non-parenchymal cells significantly enhanced ammonia-metabolizing activity four hours after loading ammonium sulfate in the culture medium at the final concentration of 0.56 mM, ammonia when compared to the same hepatocyte cell mass alone cultured on matrigel (e). After adding lidocaine (1 mg/ml) in the culture medium, respectively, metabolic rates were compared after 4 h. The engineered liver organoid significantly metabolized lidocaine compared to hepatocytes only cultured on matrigel. Significantly better production of albumin was observed in the engineered liver organoid (per 106 hepatocytes) cultured onto matrigel. * indicates p < 0.05 for Engineered Liver Organoid vs only hepatocytes respectively.
Liver Organoids Maintain Liver Function
Hepatocytes in monoculture are known to exhibit poor metabolic function when seeded on collagen coated surfaces (3). However, aggregation of hepatocytes around vascular tubes preserved hepatocyte function during short-term culture. Significantly higher metabolic capacities of ammonia were observed in co-cultures of human liver non-parenchymal cells and hepatocytes (1:3) than those seeded only with hepatocytes (n=3, Fig. 2e). Percent of metabolized ammonia at the end of the culture period at a 1:3 ratio of human liver non-parenchymal cells:hepatocytes was day 1 liver organoid 60±4.5, only hepatocytes 60±7.5, day 4 liver organoid 64±6.5, only hepatocytes 42±9, day 7 liver organoid 60±7.2, only hepatocytes 32.6±5 (Fig 2e). Lidocaine metabolism was used as another indicator of hepatocyte metabolism and showed similar trends between both groups, liver organoids led to a stable drug metabolism over the 7 days evaluated, day 1 liver organoid 50.6±6.4, only hepatocytes 46±7, day 4 liver organoid 46±9, only hepatocytes 29±4.5, day 7 liver organoid 43±8, only hepatocytes 22±4.5 (Fig 2f). Without coculture, hepatocytes failed to maintain drug metabolism activities over time and had no significant metabolic function. Overall, these in vitro studies suggest that human liver non-parenchymal cell can support and stabilize hepatocyte function. However, long-term studies should be done in the future.
The daily albumin production of the liver organoids was compared for 7 days along the two different culture conditions. Albumin production was significantly maintained in the sinusoid-like structures compared to the cultured hepatocytes only (n=3, Fig. 2g). In contrast, aggregates consisting in only hepatocytes decreased its albumin secretion significantly by day 5.Data are means+ SD. After a week of culture, hepatocyte spheroids began to collapse and brake, leading to loss of specific hepatic function, however, sinusoid like structures co-cultures maintained hepatic functions at high levels for longer periods of time (data not shown).
Liver Organoid Ultra-Structural Characterization After Implantation in LAD into Hepatectomized Mice
In order to evaluate the survival, growth and morphological analysis of the engineered liver organoid inside of a liver assist device, we implanted a LAD in the intraperitoneal cavity in 50% hepatectomized mice. We hypothesize that the regenerative effect of the hepatectomy could induce benefic effects for the liver organoid contained in the implanted LAD. Notably, after 72 h of co-culture we transplanted the engineered liver organoid and after 7 days showed organized tissue in the study using SEM, (Fig. 3a–i), which were consistent with the findings observed in phase contrast microscopy in vitro. Surprisingly, SEM revealed a sophisticated organotypic structures occurring in three dimensions during liver development, such as ducts, plates, and spaces between plates reminiscent of sinusoidal areas, the large monolager patches of liver tissue ranging from 1–5 mm in diameter (Fig. 3a–f). These structures appeared at the rate of 7–10 per LAD. These patches had a cytoarchitecture of striking similar to the sections of liver acinus. Complete canalicular networks developed throught the entire length of each of the plates. Occassional ducts were also present in random locations along the plate structures. Transmission electron microscope (TEM) revealed that the engineered liver organoids showed Gap-junction between the cells (Fig. 3g) and well-developed canaliculi, indicated by (C) (Fig. 3h), the images are typical for hepatocytes with most features present in liver, including abundant rough endoplasmic reticulum, microbodies and mature junction complexes, with glycogen granules (Fig. 3i). The photographs presented are representative of all the experiments performed. In contrast transplanted hepatic spheroids did not survive enough to be analyzed.
Figure 3. Ultrastructural Analysis by Electron Microscopy of the Engineered Liver Organoid after LAD Implantation in Mice.
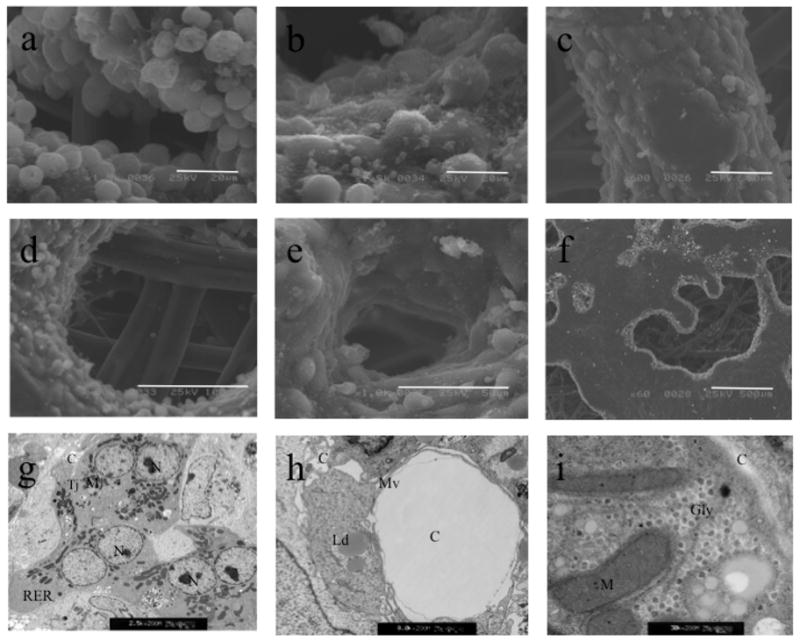
Scanning electron micrograph of the engineered liver tissue formed in the implantable LAD after 7 days of intra-peritoneal implantation in 50% hepatectomized mice (n=5) (a-f). a, b; bar=20 micrometer, c, e; bar=50 micrometer, d; bar=100 micrometer, f; bar=500 micrometer. Evident are the duct-like canalicular structures in the center of the acinar structures. Sophisticated liver organotypic structures similar to natural sinusoid-like structures are shown. By transmission electron microscope (TEM) Cells demonstrated extensive numerous and healthy mitochondria, abundant microvilli (Mv), extensive rough endoplasmic reticulum (RER), mitochondria (M), and glycogen granules can be seen (Gly). Tight junctions (TJ) delimit a small bile canalicular space (C) between the cells and the presence of cells with lipid droplets (Ld). Magnification (g) 2,500X, (h) 8,000X, (i) 30,000X.
DISCUSSION
There is evidence suggesting that interaction between endothelium and surrounding tissue plays an intimate role in tissue differentiation, maintenance, and organization (10,13,14,20), therefore, bio-artificial devices that incorporate primary cells for therapeutic use may require non-parenchymal cells to support tissue-specific functions ex vivo. In this study, we have combined the non-parenchymal cell types that can be critical to the formation of liver structures that may have a potential long-term efficacy of a liver assist device.
The stabilization of hepatocyte function by fibroblasts has been previously demonstrated (6), also, there are few reports describing the fate of hepatocytes cocultured with bone marrow-derived stem cells (5) but the actual organization of endothelial vascular structures coated by hepatocytes, stellate cells and cholangiocytes is unique to our system. These results may solely rely on the supportive secretome of the human liver non-parenchymal cell lines that have been previously studied. Our sinusoid-like structures retained drug metabolism activity and albumin secretion in vitro longer than when cultured alone on matrigel. Hepatocyte only spheroids cultured under the same conditions fail after 72 hrs.
We previously reported that an implantable liver assist device seeded with ES cell-derived hepatocytes could provide a short-term survival benefit to mice undergoing acute liver failure when compared to an acellular device (17). In addition, we have shown that molecules secreted by human liver non-parenchymal cells lines are bioactive and can modulate the hepatic differentiation of ES cell-derived hepatocytes (17,19). However, these results were predominantly due to trophic support provided on differentiation process and were assessed with a short-term study endpoint.
We observed that early hepatocellular metabolism in basal conditions became stabilized with human liver non-parenchymal cell lines. These in vitro results suggested that non-parenchymal cells might utilize mechanisms to stabilize hepatocellular function, although this warrants further investigation for long-term culture. Recent studies by our group have demonstrated that human liver non-parenchymal-derived molecules include many growth factors, cytokines and chemokines that collectively can enhance hepatocyte replication and protect against hepatocyte death (17). A comprehensive proteomic analysis of the conditioned medium is underway to identify the active ingredient(s) within human liver non-parenchymal conditioned medium.
We next determined if the resulting liver organoid could be a substitute in the development of a coculture LAD. Post-LAD implantation, only composite devices showed healthy tissue-like structures growth. Future experiments are focus on the reduction of total cell numbers seeded in the bioreactor to minimize the overall cell number of the devices, which can allow for smaller devices and decreased dead volume that is outside of the patient and in the perfusion circuit. More importantly, given the observations in this study, we speculate that the development of human-sized bioartificial liver devices can potentially reduce the number of primary hepatocytes or allow for “less functional” hepatocytes (e.g. porcine or human hepatoma cells) to be used for future LADs.
In conclusion, these findings demonstrate the importance of non-parenchymal cells in the cellular composition of LADs. We therefore suggest that this system and the resulting sinusoid-like structures may serve as a unique in vitro model of the liver for tissue- engineering applications. This experimental evidence supports the notion of LADs containing co-cultures of human liver non-parenchymal cell and hepatocytes as a potential destination therapy for acute liver failure. We visualize cultures of perfused liver sinusoids suitable for the study of pathologic hepatic microcirculation and the drug discovery studies in the pharmaceutical industry.
Acknowledgments
We thank the support of the American Liver Foundation to A.S.G. Funding from the US National Institutes of Health (NIH) K99DK083556-01 to A.S.G supported this work.
Footnotes
Conflict of interest statement: The authors declare no conflict of interest related to the work presented in this publication.
References
- 1.Basma H, Soto-Gutierrez A, Yannam GR, Liu L, Ito R, Yamamoto T, Ellis E, Carson SD, Sato S, Chen Y, Muirhead D, Navarro-Alvarez N, Wong RJ, Roy-Chowdhury J, Platt JL, Mercer DF, Miller JD, Strom SC, Kobayashi N, Fox IJ. Differentiation and Transplantation of Human Embryonic Stem Cell-Derived Hepatocytes. Gastroenterology. 2009;136(3):990–999. doi: 10.1053/j.gastro.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen SC, Mullon C, Kahaku E, Watanabe F, Hewitt W, Eguchi S, Middleton Y, Arkadopoulos N, Rozga J, Solomon B, Demetriou AA. Treatment of severe liver failure with a bioartificial liver. Ann N Y Acad Sci. 1997;831:350–360. doi: 10.1111/j.1749-6632.1997.tb52210.x. [DOI] [PubMed] [Google Scholar]
- 3.Dunn JC, Yarmush ML, Koebe HG, Tompkins RG. Hepatocyte function and extracellular matrix geometry: long-term culture in a sandwich configuration. FASEB J. 1989;3(2):174–177. doi: 10.1096/fasebj.3.2.2914628. [DOI] [PubMed] [Google Scholar]
- 4.Ikeda H, Kobayashi N, Tanaka Y, Nakaji S, Yong C, Okitsu T, Oshita M, Matsumoto S, Noguchi H, Narushima M, Tanaka K, Miki A, Rivas-Carrillo JD, Soto-Gutierrez A, Navarro-Alvarez N, Tanaka K, Jun HS, Tanaka N, Yoon JW. A newly developed bioartificial pancreas successfully controls blood glucose in totally pancreatectomized diabetic pigs. Tissue Eng. 2006;12(7):1799–1809. doi: 10.1089/ten.2006.12.1799. [DOI] [PubMed] [Google Scholar]
- 5.Isoda K, Kojima M, Takeda M, Higashiyama S, Kawase M, Yagi K. Maintenance of hepatocyte functions by coculture with bone marrow stromal cells. J Biosci Bioeng. 2004;97(5):343–346. doi: 10.1016/S1389-1723(04)70217-0. [DOI] [PubMed] [Google Scholar]
- 6.Khetani SR, Bhatia SN. Microscale culture of human liver cells for drug development. Nat Biotechnol. 2008;26(1):120–126. doi: 10.1038/nbt1361. [DOI] [PubMed] [Google Scholar]
- 7.Lee WM. Acute liver failure. N Engl J Med. 1993;329(25):1862–1872. doi: 10.1056/NEJM199312163292508. [DOI] [PubMed] [Google Scholar]
- 8.Maruyama M, Kobayashi N, Westerman KA, Sakaguchi M, Allain JE, Totsugawa T, Okitsu T, Fukazawa T, Weber A, Stolz DB, Leboulch P, Tanaka N. Establishment of a highly differentiated immortalized human cholangiocyte cell line with SV40T and hTERT. Transplantation. 2004;77(3):446–451. doi: 10.1097/01.TP.0000110292.73873.25. [DOI] [PubMed] [Google Scholar]
- 9.Matsumura T, Takesue M, Westerman KA, Okitsu T, Sakaguchi M, Fukazawa T, Totsugawa T, Noguchi H, Yamamoto S, Stolz DB, Tanaka N, Leboulch P, Kobayashi N. Establishment of an immortalized human-liver endothelial cell line with SV40T and hTERT. Transplantation. 2004;77(9):1357–1365. doi: 10.1097/01.tp.0000124286.82961.7e. [DOI] [PubMed] [Google Scholar]
- 10.Michalopoulos GK, Bowen WC, Zajac VF, Beer-Stolz D, Watkins S, Kostrubsky V, Strom SC. Morphogenetic events in mixed cultures of rat hepatocytes and nonparenchymal cells maintained in biological matrices in the presence of hepatocyte growth factor and epidermal growth factor. Hepatology. 1999;29(1):90–100. doi: 10.1002/hep.510290149. [DOI] [PubMed] [Google Scholar]
- 11.Mitaka T, Sato F, Mizuguchi T, Yokono T, Mochizuki Y. Reconstruction of hepatic organoid by rat small hepatocytes and hepatic nonparenchymal cells. Hepatology. 1999;29(1):111–125. doi: 10.1002/hep.510290103. [DOI] [PubMed] [Google Scholar]
- 12.Nagata H, Nishitai R, Shirota C, Zhang JL, Koch CA, Cai J, Awwad M, Schuurman HJ, Christians U, Abe M, Baranowska-Kortylewicz J, Platt JL, Fox IJ. Prolonged survival of porcine hepatocytes in cynomolgus monkeys. Gastroenterology. 2007;132(1):321–329. doi: 10.1053/j.gastro.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 13.Nahmias Y, Schwartz RE, Hu WS, Verfaillie CM, Odde DJ. Endothelium-mediated hepatocyte recruitment in the establishment of liver-like tissue in vitro. Tissue Eng. 2006;12(6):1627–1638. doi: 10.1089/ten.2006.12.1627. [DOI] [PubMed] [Google Scholar]
- 14.Navarro-Alvarez N, Rivas-Carrillo JD, Soto-Gutierrez A, Yuasa T, Okitsu T, Noguchi H, Matsumoto S, Takei J, Tanaka N, Kobayashi N. Reestablishment of microenvironment is necessary to maintain in vitro and in vivo human islet function. Cell Transplant. 2008;17(1–2):111–119. doi: 10.3727/000000008783907125. [DOI] [PubMed] [Google Scholar]
- 15.Roger V, Balladur P, Honiger J, Baudrimont M, Delelo R, Robert A, Calmus Y, Capeau J, Nordlinger B. Internal bioartificial liver with xenogeneic hepatocytes prevents death from acute liver failure: an experimental study. Ann Surg. 1998;228(1):1–7. doi: 10.1097/00000658-199807000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soto-Gutierrez A, Basma H, Navarro-Alvarez N, Uygun B, Yarmush M, Kobayashi N, Fox IJ. ifferentiating stem cells into liver. Biotechnol Genet Eng Rev. 2008;25:149–164. doi: 10.5661/bger-25-149. [DOI] [PubMed] [Google Scholar]
- 17.Soto-Gutierrez A, Kobayashi N, Rivas-Carrillo JD, Navarro-Alvarez N, Zhao D, Okitsu T, Noguchi H, Basma H, Tabata Y, Chen Y, Tanaka K, Narushima M, Miki A, Ueda T, Jun HS, Yoon JW, Lebkowski J, Tanaka N, Fox IJ. Reversal of mouse hepatic failure using an implanted liver-assist device containing ES cell-derived hepatocytes. Nat Biotechnol. 2006;24(11):1412–1419. doi: 10.1038/nbt1257. [DOI] [PubMed] [Google Scholar]
- 18.Soto-Gutierrez A, Navarro-Alvarez N, Rivas-Carrillo JD, Tanaka K, Chen Y, Misawa H, Okitsu T, Noguchi H, Tanaka N, Kobayashi N. Construction and transplantation of an engineered hepatic tissue using a polyaminourethane-coated nonwoven polytetrafluoroethylene fabric. Transplantation. 2007;83(2):129–137. doi: 10.1097/01.tp.0000250561.14108.03. [DOI] [PubMed] [Google Scholar]
- 19.Soto-Gutierrez A, Navarro-Alvarez N, Zhao D, Rivas-Carrillo JD, Lebkowski J, Tanaka N, Fox IJ, Kobayashi N. Differentiation of mouse embryonic stem cells to hepatocyte-like cells by co-culture with human liver nonparenchymal cell lines. Nat Protoc. 2007;2(2):347–356. doi: 10.1038/nprot.2007.18. [DOI] [PubMed] [Google Scholar]
- 20.Stolz DB, Ross MA, Salem HM, Mars WM, Michalopoulos GK, Enomoto K. Cationic colloidal silica membrane perturbation as a means of examining changes at the sinusoidal surface during liver regeneration. Am J Pathol. 1999;155(5):1487–1498. doi: 10.1016/S0002-9440(10)65464-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watanabe T, Shibata N, Westerman KA, Okitsu T, Allain JE, Sakaguchi M, Totsugawa T, Maruyama M, Matsumura T, Noguchi H, Yamamoto S, Hikida M, Ohmori A, Reth M, Weber A, Tanaka N, Leboulch P, Kobayashi N. Establishment of immortalized human hepatic stellate scavenger cells to develop bioartificial livers. Transplantation. 2003;75(11):1873–1880. doi: 10.1097/01.TP.0000064621.50907.A6. [DOI] [PubMed] [Google Scholar]
- 22.Williams R, Wendon J. Indications for orthotopic liver transplantation in fulminant liver failure. Hepatology. 1994;20(1 Pt 2):S5–10S. doi: 10.1016/0270-9139(94)90265-8. [DOI] [PubMed] [Google Scholar]