Abstract
Carotenoids in skin have been known to play a role in photoprotection against UV radiation. We performed dermal biopsies of healthy humans (N=27) and collected blood samples for pair-wise correlation analyses of total and individual carotenoid content by high performance liquid chromatography (HPLC). The hydrocarbon carotenoids (lycopene and beta-carotene) made up the majority of carotenoids in both skin and plasma, and skin was somewhat enriched in these carotenoids relative to plasma. Beta-cryptoxanthin, a monohydroxycarotenoid, was found in similar proportions in skin as in plasma. In contrast, the dihydroxycarotenoids, lutein and zeaxanthin, were relatively lacking in human skin in absolute and relative levels as compared to plasma. Total carotenoids were significantly correlated in skin and plasma (r = 0.53, p<0.01). Our findings suggest that human skin is relatively enriched in lycopene and beta-carotene, compared to lutein and zeaxanthin, possibly reflecting a specific function of hydrocarbon carotenoids in human skin photoprotection.
Keywords: carotenoids, lycopene, beta-carotene, lutein, zeaxanthin, beta-cryptoxanthin, skin
1.0 INTRODUCTION
There is considerable interest in possible health effects of carotenoids in skin as recently reviewed by Goralczyk and Wertz [1]. Carotenoids are known to accumulate in human skin, with the levels of carotenoids reflecting dietary intake and bioavailability from the food source [2]. The most common carotenoids in the Western diet are alpha-carotene, beta-carotene, beta-cryptoxanthin, lycopene, lutein, and zeaxanthin [3]. After absorption in the intestine, carotenoids are transported through the bloodstream by lipoproteins to various target tissues [4–5]. Recent evidence suggests that cholesterol transporters, such as scavenger receptor class B1 type 1 protein (SR-B1) and Cluster of Differentiation 36 membrane protein (CD 36), facilitate absorption of carotenoids in the intestine [6]. There is suggestive evidence that these transporters may also facilitate carotenoid absorption in the epidermal layers of the skin [7]. Carotenoids are lipophilic molecules found in anatomical sites where the stratum corneum, the upper most skin layer, is thick [8]. Body sites highest in total carotenoid levels include the sole of the foot, forehead, and palm of the hand, which has a high lipid to protein ratio [9]. Adipose tissue is another main accumulation site for carotenoids due to the large volume in the human body [2]. The levels of carotenoids found in human adipose tissue are considered to be markers of usual intake because adipose tissue is a more stable repository for carotenoids compared with plasma, in which carotenoids have a shorter-half life [10].
Perhaps the best-studied potential health effect of carotenoids beyond their provitamin A activity is a promising role in photoprotection, that is, the protection against erythema and sunlight damage [11–12]. Beta-carotene has established efficacy in the treatment of erythropoietic protoporphyria, a photosensitivity disease [13–14]. In humans without this disease, there is also evidence from controlled studies that carotenoids such as beta-carotene have efficacy in the protection from sunburn [15], although the sun protection factor is modest (SPF approximately equal to 2). This meta-analysis observed a significant protective effect for beta-carotene supplementation vs. placebo on the development of a sunburn reaction. Carotenoids are known to quench singlet oxygen and other free radical species generated in the skin by exposure to UVA [13]. Finally, several recent studies have examined the potential protective effects of carotenoids against premature photoaging of the skin, marked by signs such as wrinkling, pigmentation, dryness, and inelasticity. There is suggestive evidence for a protective effect of beta-carotene on photoaging [16]. Evidence also suggests that higher levels of lycopene in the skin results in lower levels of skin roughness [17].
The variability in the photoprotective effect of carotenoids observed across human studies can be attributed to several factors. The bioavailability of the food source or supplementation affects the amount of carotenoids absorbed by the body and taken up by target tissues, including skin [2]. Additionally, greater UV exposure and skin sensitivity to UV radiation can decrease the photoprotective effect of these micronutrients [18–19]. Finally, lifestyle factors, notably smoking status [20], have also been associated with significantly lower dermal carotenoid levels in human skin, while genetics factors have been associated with lower plasma beta-carotene levels [21–22].
Arguably the most appropriate technique to examine the distribution and levels of individual carotenoids in human skin is HPLC analysis of dermal biopsies. Absorption spectroscopy has been used in some studies to estimate carotenoid content, but cannot differentiate between the various carotenes, xanthophylls, and their isomers. As noted by Goralczyk and Wertz, “Unfortunately, reports on carotenoid concentrations in skin of humans or laboratory animals are rare, many of them old and most referring to beta-carotene only” [1]. Goralczyk and Wertz note four studies that measured beta-carotene or total carotenoid levels in human skin using HPLC analysis to assess the photoprotective effects of moderate to high-dose carotenoid supplements [23–26]. Another study [27] from Peng et al. [23], examined the correlation between individual carotenoids measured by HPLC analysis of plasma samples and skin biopsies in a sample of adults (N=96) in the context of a skin cancer chemoprevention trial. In this study, there was significant correlation between levels of the individual carotenoids measured in plasma and skin. Additionally, the levels of carotenoids in skin were lower in smokers and higher for supplement users, even after adjustment for potential covariates [27].
We conducted a human study aimed at validating resonance Raman spectroscopy (RRS) for use in the non-invasive assessment of dermal carotenoids for epidemiologic research. As part of the validation of the RRS method, we needed to perform dermal biopsies of healthy humans, and analyze the total carotenoid content of these biopsies by HPLC. In that same study, we also collected blood samples, to examine how dermal carotenoid levels correlated with plasma carotenoids, with both assessed by HPLC, because plasma carotenoids have been the most commonly used measure of carotenoid status for epidemiologic research. This provided us with an opportunity to examine correlations for each of the major carotenoids found in vivo between paired blood and skin samples. The limited data estimating the accumulation of individual carotenoids, other than beta-carotene, in skin by HPLC underscores the need for the current research. If particular carotenoids preferentially accumulate in dermal tissue, it may suggest a specific function of these carotenoids in skin photoprotection. Below we describe the results of our analyses of carotenoid levels in paired skin and plasma samples from healthy humans.
2.0 MATERIALS AND METHODS
2.1 Subjects
Our goal was to recruit a total of 30 normal healthy adults between the ages of 21 and 65. Participants were part of a larger parent study examining resonance Raman spectroscopy (RRS) as an objective measure of carotenoid status and were not routine supplement users (Mayne et al., manuscript submitted for publication). We attempted to recruit men and women, as well as smokers and nonsmokers (plasma carotenoid levels of smokers are known to be lower than those of nonsmokers [28]). For this portion of the research, subjects had to be willing to undergo phlebotomy and dermal biopsy. After obtaining signed informed consent, participants were interviewed to obtain demographic data, and then completed dermal biopsy and phlebotomy at the same clinic visit.
2.2 Dermal Biopsies and Phlebotomy
A dermatologic surgeon performed the dermal biopsy in the posterior hip area of each subject. Participants were given injected anesthetic (Lidocaine) at the biopsy site. Once the skin was numb, the dermatologic surgeon removed a 3 mm punch biopsy of skin. Residual adipose tissue was removed from the sample, prior to placing it into a cryovial. Biopsy samples were snap frozen immediately using liquid nitrogen. Biopsy sites were sutured in order to facilitate rapid healing.
Blood samples (10 ml) were obtained by venipuncture by a trained phlebotomist and collected into heparinized tubes. Tubes were protected from light, chilled but not frozen, and then centrifuged to obtain plasma. Plasma aliquots were obtained and stored at −70°C prior to HPLC analysis.
2.3 Extraction and HPLC Analyses
Extractions were carried out as described elsewhere [29]. 280 μl of Phosphate buffered saline was added to the skin punch tissue. 35 μl collagenase solution (50 mg/ml; Sigma Cat # E -1644) was added, vortex-mixed and incubated at 37° C for 1 hr. Tissues were homogenized on ice, and 35 μl of protease solution (20 mg/ml; Sigma catalog # 11360) was added, vortex-mixed, and incubated at 37° C for 0.5 hr. Four mL of sodium dodecyl sulfate (SDS)-ethanol-butylated hydroxytoluene (BHT) solution was added and vortexed for 60 sec. Samples were then extracted twice with hexane (2 X 500 μl), and dried down prior to HPLC injection.
Plasma samples (100 μl) were treated with ethanol and hexane containing 0.1% (w/v) BHT, and centrifuged to remove the proteins. The proteins were re-extracted with hexane (3 × 300 μl), and the combined extract was evaporated to dryness under reduced pressure at below 40°C. After evaporation of the solvent, the residue was reconstituted in the appropriate HPLC solvents and centrifuged at 2000 × g prior to analysis.
The chromatographic conditions for carotenoid separation and quantitation were similar to those reported earlier [30]. The mobile phase was an isocratic mixture of acetonitrile: isopropanol: ethyl acetate (50:40:10 v/v), at a flow rate of 0.7 ml per minute. The analysis was performed on a reversed-phase Luna C18(2) analytical column, [250 mm length × 4.6 mm id, (Phenomenex, Torrance, CA, USA); particle size 5 μm; pore size 100 Angstrom]. The dried pigments (after extraction) were re-dissolved in 200 μl of HPLC mobile phase. The column was maintained at room temperature, and the HPLC detector was operated at 450 nm. Peak identities were confirmed by photodiode-array (PDA) spectra, mass spectra and by coelution with authentic standards as necessary. To avoid overloading of the mass spectrometer with eluted molecules, 50% of the eluant was directed to waste with the help of diverter valve. Lutein and zeaxanthin coeluted but were quantitated based on single ion monitoring (SIM) method [31].
2.4 Mass Spectrometry Equipment and Analysis
MS analysis was performed using Thermo Electron MSQ single quadrupole mass spectrometer (San Jose, CA), equipped with an atmospheric pressure chemical ionization (APCI) source. The protonated precursor molecular ions were initially acquired in two full scan modes (A. 200–500 & B. 500–800 Da) with 0.2 step size and 2 ms dwell time. Selected ion monitoring (SIM) was performed using dwell time of 200 ms for each channel. In SIM mode the channel m/z used was 551±2 for lutein (dehydrated form), 569±2 for zeaxanthin, 552±2 for beta-cryptoxanthin and 537±2 for beta-carotene and lycopene. Typical conditions were corona discharge current 5 μA, RF lens bias voltage 0.1V, cone voltage 80V and heater temperature 500 °C. The ion source and tuning lens parameters were optimized automatically by infusing carotenoid standards via the built-in injector operated manually. For routine quantitative analysis, the ThemoElectron MSQ QUAN BROWSER (Xcaliber) was used. The software provides a peak integration of the ion intensity for each of the programmed mass ranges. Automated analysis was followed by manual confirmation of in source fragmentation patterns. For calibration, standard solutions were injected in different volumes so as to achieve final injected levels ranging from 1–1000 pg, and 1–8 ng. Accuracy and precision of the method were determined by generating intra- and inter-day variability data from a series of samples in the range of 1–1000 pg injected five times on a single day.
2.5 Statistical Analyses
Descriptive statistics (mean and median levels) were obtained for individual and total carotenoids measured in plasma and skin, as well as for the demographic characteristics of the study sample. Pair-wise Pearson correlation coefficients were calculated from the biopsy and plasma data for individual and total carotenoids. Additionally, scatter plots were constructed to graphically display the correlations. Potential outliers were identified using several diagnostic techniques, including box-plots, stem-and-leaf plots, dot plots of residuals, and residual plots against fitted values. All statistical computations were conducted using SAS v. 9.1 (SAS Institute, Inc. Cary, NC) with a p-value of 0.05 considered statistically significant.
3.0 RESULTS
We recruited a total of 27 subjects, all of whom had paired data values available for HPLC analyses from dermal biopsies and plasma samples. Our study subjects were mostly Caucasian (88.9%), normal weight (70.4%), non-smoking (88.9%), females (63.0%). The average age of participants was 33.3 (±10.6) years.
Box-plots, stem-and-leaf plots, and dot plots of residuals revealed no major outliers in the data. Additionally, plots of studentized residuals against fitted values showed no major outliers among individual carotenoids or total carotenoids (i.e. > 3 or <−3) which indicated that the assumption of constant error variance had not been violated. As a result, all observations were retained in this analysis (N=27).
Table 1 shows the median carotenoid levels in plasma and in skin, as well as the results of the Pearson correlation analysis for individual and total carotenoids measured in the paired skin biopsies and plasma samples. The most common individual carotenoid measured in plasma, lycopene (median level: 640 nmol/L), was also most common in skin (median level: 6.1 pmol/punch). Beta-carotene was the second most common individual carotenoid measured in plasma (median level: 530 nmol/L) and skin (median level 3.9 pmol/punch). The hydrocarbon carotenoids, lycopene and beta-carotene, accounted for 64% of measured total carotenoids in plasma vs. 76% in skin, with somewhat greater enrichment of lycopene relative to beta-carotene. The monohydroxycarotenoid, beta-cryptoxanthin, accounted for 12% of total carotenoids in plasma and 11% of total carotenoids in skin. In contrast, the dihydroxycarotenoids, lutein and zeaxanthin (which are more polar), were decidedly lacking in skin, accounting for 21% of total carotenoids in plasma but only 3% of total carotenoids in skin.
Table 1.
Median carotenoid levels in plasma and dermal biopsies, paired samples (N=27)
| Median Plasma Levels (nmol/L) | Median Skin Levels (pmol/punch) | Pearson r | p-value | |
|---|---|---|---|---|
| Lycopene | 640 | 6.1 | 0.37 | 0.05 |
| Beta-carotene | 530 | 3.9 | 0.34 | 0.09 |
| Beta-cryptoxanthin | 225 | 1.4 | 0.07 | 0.72 |
| Lutein | 220 | 0.2 | 0.08 | 0.70 |
| Zeaxanthin | 155 | 0.2 | 0.25 | 0.21 |
| Total Carotenoids | 1828 | 13.1 | 0.53 | <0.01 |
Lycopene in plasma was significantly correlated with lycopene in skin (r = 0.37; p=0.05), and we observed a strong positive trend between beta-carotene in plasma and beta-carotene in skin (r = 0.34; p=0.09). Hydrocarbon carotenoids account for the majority of carotenoids in plasma, so it is not surprising that total carotenoids measured in the plasma was significantly correlated with total carotenoids measured in the skin (r = 0.53; p<0.01). In contrast, plasma-skin correlations were lower and not statistically significant for any of the xanthophyllic carotenoids (zeaxanthin r = 0.25; p=0.21; lutein r = 0.08; p=0.70; and beta-cryptoxanthin r = 0.07; p=0.72).
Scatter plots for individual (Figs. 1–5) and total carotenoid levels (Fig. 6) measured by HPLC analysis in plasma samples and dermal biopsies graphically demonstrate the correlations for the pair-wise comparisons. The scatter plots constructed for the hydrocarbon carotenoids, lycopene (Fig. 1) and beta-carotene (Fig. 2) suggest a dose-response relationship between plasma and skin. In contrast, the scatter plot for the monohydroxycarotenoid, beta-cryptoxanthin (Fig. 3), and the dihydroxycarotenoids, lutein (Fig. 4) and zeaxanthin (Fig. 5) demonstrate no evidence of a dose-response relationship between plasma and skin.
Figure 1.
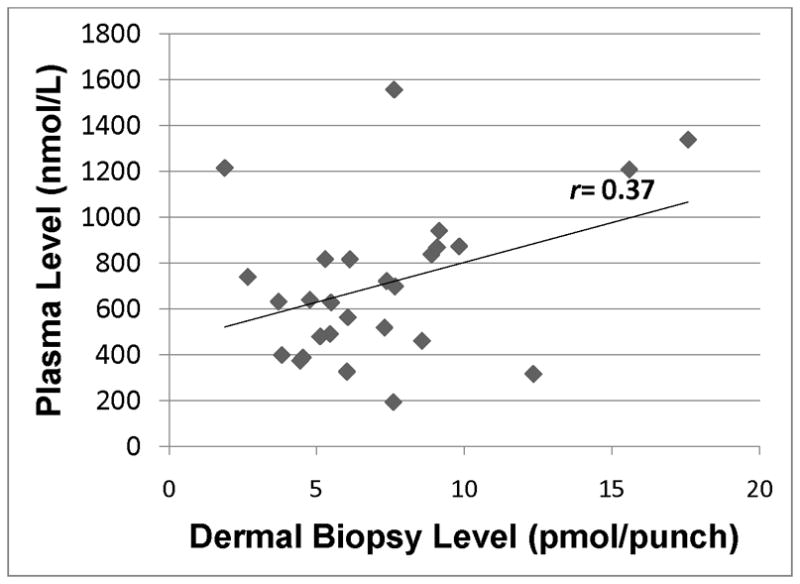
Lycopene level in plasma as assessed by HPLC analysis of blood samples vs. dermal lycopene in skin as assessed by HPLC analysis of dermal biopsy. r=0.37 (p=0.05).
Figure 5.
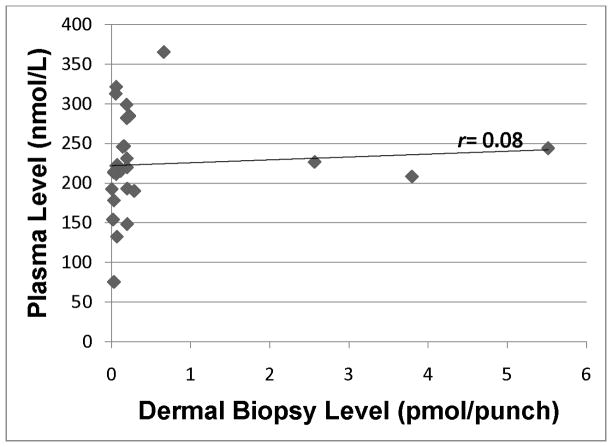
Zeaxanthin level in plasma as assessed by HPLC analysis of blood samples vs. dermal zeaxanthin in skin as assessed by HPLC analysis of dermal biopsy. r=0.25 (p=0.21).
Figure 6.
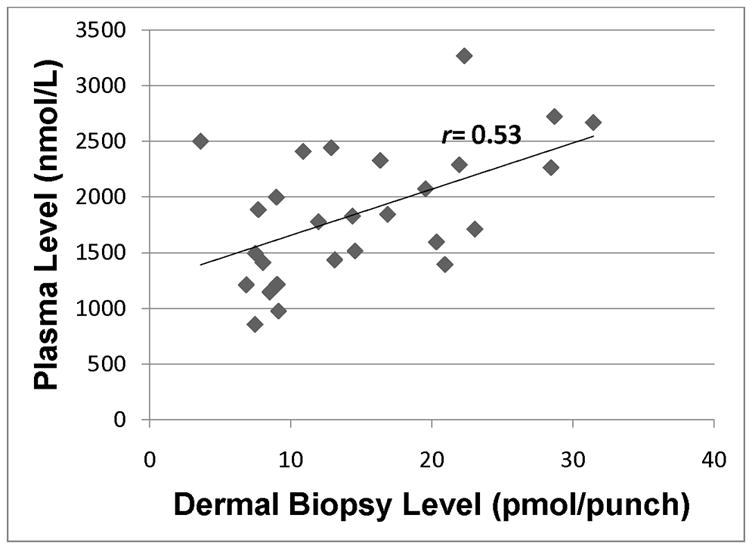
Total carotenoid level in plasma as assessed by HPLC analysis of blood samples vs. total dermal carotenoid in skin as assessed by HPLC analysis of dermal biopsy. r=0.53 (p<0.01).
Figure 2.
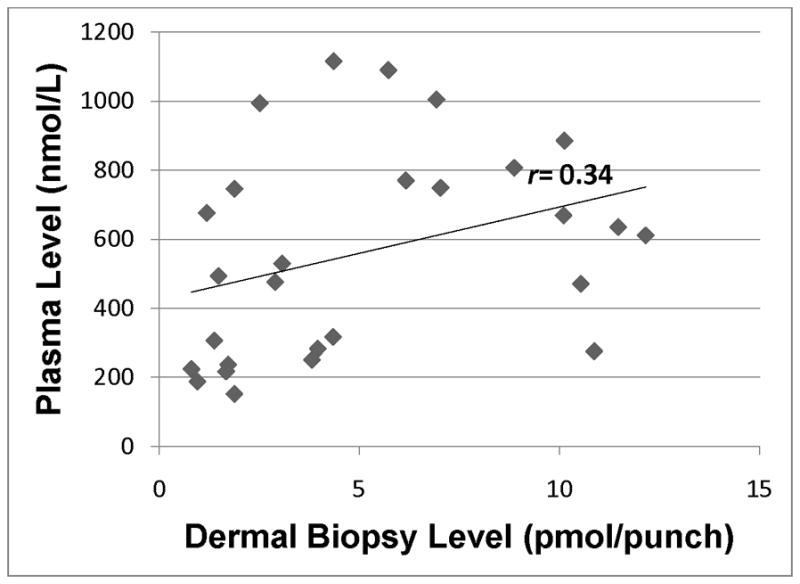
Beta-carotene level in plasma as assessed by HPLC analysis of blood samples vs. dermal beta-carotene in skin as assessed by HPLC analysis of dermal biopsy. r=0.34 (p=0.09).
Figure 3.
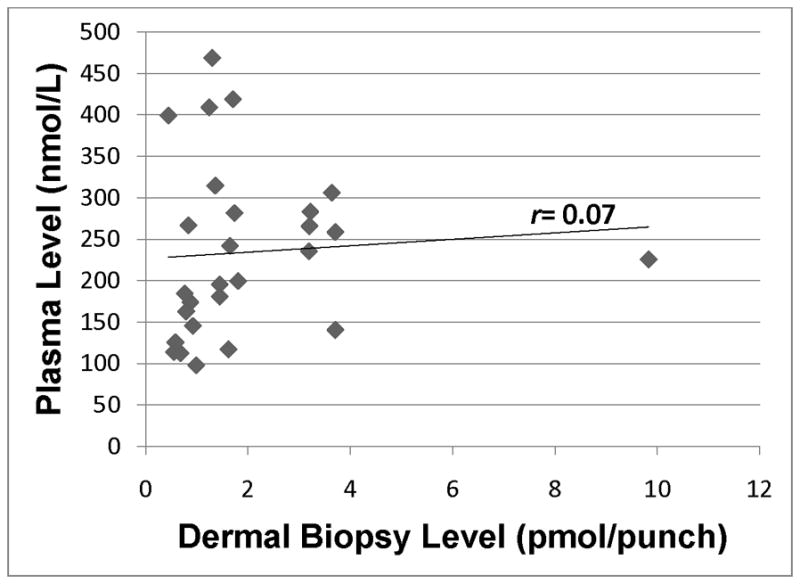
Beta-cryptoxanthin level in plasma as assessed by HPLC analysis of blood samples vs. dermal beta-cryptoxanthin in skin as assessed by HPLC analysis of dermal biopsy. r=0.07 (p=0.72).
Figure 4.
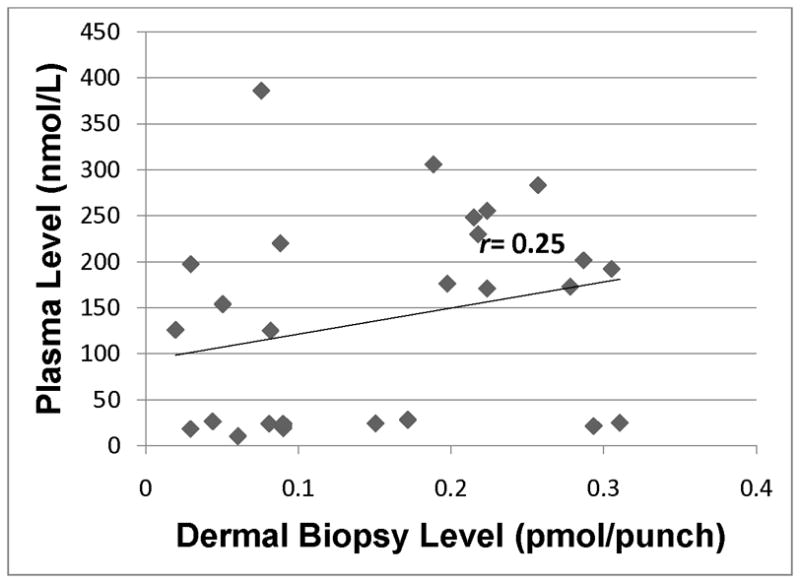
Lutein level in plasma as assessed by HPLC analysis of blood samples vs. dermal lutein in skin as assessed by HPLC analysis of dermal biopsy. r=0.08 (p=0.70).
Of note, Fig. 4 indicates that the level of lutein in skin was low for the majority of our subjects, with the exception of three participants who had > 2 pmol lutein/3 mm punch, with no evidence of a linear relationship between plasma and skin. Finally, Fig. 6 provides graphical evidence of a linear relationship between total carotenoid levels in paired plasma and skin samples from our healthy population.
4.0 DISCUSSION
Consistent with other reports [27], our findings indicate that beta-carotene and lycopene were present in relatively high levels in human skin samples, with somewhat less of the xanthophyll carotenoids, in comparison to blood. The highest correlations for individual carotenoids were observed for lycopene and beta-carotene, which were also the highest levels in blood and skin. Peng et al. also found the highest plasma:skin correlations for beta-carotene and lycopene, although they observed somewhat higher correlations for cryptoxanthin than what we observed [27]. As beta-carotene and lycopene are the predominant carotenoids in human plasma in the U.S. population, total carotenoids measured in skin and plasma samples were significantly correlated.
In human tissues, carotenoids are known to accumulate in varying levels. Specifically, evidence suggests that the liver, adrenals, adipose, and reproductive tissues have particularly high levels of carotenoids, compared to other body sites [2]. Evidence suggests that uptake of carotenoids by tissues is dependent on the amount of low-density lipoprotein (LDL) receptors, along with scavenger receptor class B type I protein (SR-BI) [32], and the degradation of these lipoproteins by enzymes. Preferential uptake by various body tissues seemingly occurs for different carotenoids, as well as geometrical isomers of individual carotenoids [2].
The best example of the preferential uptake of carotenoids occurs in the human eye. Of more than 15 carotenoids and isomers that can be measured in human serum, only two are present in the macula of the eye: (3R,3′R,6′R)-lutein and (3R,3′S,meso)-zeaxanthin [33]. These carotenoids accumulate in the macula lutea of the central retina, which implies a specific biological function. Evidence suggests that the absorption spectrum of lutein and zeaxanthin includes near-to-UV blue light (λ=488 nm), which has the highest energy, and is therefore the most damaging wavelength of light that reaches the retina [33]. Lutein and zeaxanthin may also serve as an antioxidant defense against damaging free radicals in the macula [34]. Several trials suggest supplementation with lutein and zeaxanthin can increase macular pigment optical density and improve human visual performance [35–36]. Recently, xanthophyll binding proteins have been identified in the macula of the human eye, including the Pi isoform of glutathione S-transferase (GSTP1) [37]. It was observed that GSTP1 had a greater affinity for (3R,3′S-meso)-zeaxanthin and dietary (3R,3′R)-zeaxanthin, compared to (3R,3′R,6′R)-lutein [38]. The identification of these xanthophyll binding proteins provides further evidence of the preferential uptake of carotenoids by human tissues for potential health benefits.
In contrast to what is observed in the macula, our findings suggest that hydrocarbon carotenoids, specifically lycopene and beta-carotene, preferentially accumulate in the skin. There are several hypotheses to explain the tissue enrichment we observed. First, beta-carotene and lycopene are the most hydrophobic of the carotenoids, and their preferential accumulation may derive from lipid solubility of the carotenoids [2]. Alternatively, binding proteins could preferentially take up hydrocarbon carotenoids into skin, but the identity of these proteins, if any, is not known.
While the mechanism for enhanced uptake of hydrocarbon carotenoids into skin is not known, there is a clear biological rationale for this phenomenon. That is, evidence suggests that lycopene in particular, but also beta-carotene, are the most efficient quenchers of singlet oxygen, while xanthophylls, especially lutein, are the least efficient [39]. The function of carotenoids in skin may well be linked to their singlet oxygen quenching properties, which is supported by evidence both in vivo and in vitro [1]. An intriguing hypothesis is that the body reserves lutein and zeaxanthin for tissue accumulation in the eye, where they are thought to play a unique function, and beta-carotene and lycopene for tissue accumulation in the skin, with a different function. The eye and the skin are the two human organs most in need of photoprotection, a known function of carotenoids in plants and in photosynthetic bacteria [1, 13, 26].
Strengths of this study include the use of HPLC to measure total carotenoids, as well as individual carotenoids, in tissue and plasma samples from a healthy population. A limitation was the relatively homogeneous population, which was primarily Caucasian. However, Caucasians are the population most in need of photoprotection, so this is obviously a highly relevant population for this work. Another limitation is that we obtained blood at only one point in time; we would expect to see higher correlation coefficients had we obtained multiple blood measures for carotenoid status. Additionally, some of the correlations for individual carotenoids in plasma and skin were marginally significant (e.g. lycopene and beta-carotene). Due to a small sample size, we may have had insufficient power to detect statistically significant correlations. Therefore, a larger sample size may be needed to replicate the results of our Pearson correlation analyses. Despite these limitations, we observed significant correlation between total carotenoids measured in blood and skin, and observed comparatively higher levels of hydrocarbon carotenoids in our skin samples. The predominant carotenoids in blood in our population and in the U.S. population in general are lycopene and beta-carotene; it is not known if this finding would persist in populations that have different carotenoid profiles resulting from differing carotenoid intake patterns. If so, this would lend further support to the hypothesis that skin prefers hydrocarbon carotenoids, while the macula prefers xanthophyllic carotenoids.
5.0 CONCLUSIONS
Our findings indicate that skin is relatively enriched in lycopene and in beta-carotene especially in comparison to lutein and zeaxanthin. Future interventions examining the role of carotenoids for various measures of skin health may want to target these carotenoids specifically for potential health benefits.
Acknowledgments
We wish to thank the participants in this study. This research was supported by an R01 CA96838 grant from the National Cancer Institute; National Institutes of Health. Additional support provided by an R01 EY-11600 grant from the National Eye Institute; National Institutes of Health and a departmental grant from Research to Prevent Blindness (New York, New York).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7.0 REFERENCES
- 1.Goralczyk R, Wertz K. Skin Photoprotection by Carotenoids. In: Britton G, Liaaen-Jensen S, Pfander H, editors. Carotenoids Volume 5: Nutrition and Health. Birkhauser Verlag; Basel, Switzerland: 2009. pp. 335–362. [Google Scholar]
- 2.Canene-Adams K, Erdman J, JW . Absorption, Transport, Distribution in Tissues and Bioavailability. In: Britton G, Liaaen-Jensen S, Pfander H, editors. Carotenoids Volume 5: Nutrition and Health. Birkhauser Verlag; Basel, Switzerland: 2009. [Google Scholar]
- 3.Institute of Medicine, National Academy of Sciences, Food and Nutrition Board. Panel on Dietary Antioxidants and Related Compounds, Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. National Academy Press; Washington: 2000. [Google Scholar]
- 4.Lin S, Quaroni L, White WS, Cotton T, Chumanov G. Localization of carotenoids in plasma low-density lipoproteins studied by surface-enhanced resonance Raman spectroscopy. Biopolymers. 2000;57:249–256. doi: 10.1002/1097-0282(2000)57:4<249::AID-BIP6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 5.Lowe GM, Bilton RF, Davies IG, Ford TC, Billington D, Young AJ. Carotenoid composition and antioxidant potential in subfractions of human low-density lipoprotein. Ann Clin Biochem. 1999;36( Pt 3):323–332. doi: 10.1177/000456329903600304. [DOI] [PubMed] [Google Scholar]
- 6.van Bennekum A, Werder M, Thuahnai ST, Han CH, Duong P, Williams DL, Wettstein P, Schulthess G, Phillips MC, Hauser H. Class B scavenger receptor-mediated intestinal absorption of dietary beta-carotene and cholesterol. Biochemistry. 2005;44:4517–4525. doi: 10.1021/bi0484320. [DOI] [PubMed] [Google Scholar]
- 7.Tsuruoka H, Khovidhunkit W, Brown BE, Fluhr JW, Elias PM, Feingold KR. Scavenger receptor class B type I is expressed in cultured keratinocytes and epidermis. Regulation in response to changes in cholesterol homeostasis and barrier requirements. J Biol Chem. 2002;277:2916–2922. doi: 10.1074/jbc.M106445200. [DOI] [PubMed] [Google Scholar]
- 8.Darvin ME, Fluhr JW, Caspers P, van der Pool A, Richter H, Patzelt A, Sterry W, Lademann J. In vivo distribution of carotenoids in different anatomical locations of human skin: comparative assessment with two different Raman spectroscopy methods. Exp Dermatol. 2009 doi: 10.1111/j.1600-0625.2009.00946.x. [DOI] [PubMed] [Google Scholar]
- 9.Ermakov IV, Ermakova MR, McClane RW, Gellermann W. Resonance Raman detection of carotenoid antioxidants in living human tissues. Opt Lett. 2001;26:1179–1181. doi: 10.1364/ol.26.001179. [DOI] [PubMed] [Google Scholar]
- 10.Rock CL, Swendseid ME, Jacob RA, McKee RW. Plasma carotenoid levels in human subjects fed a low carotenoid diet. J Nutr. 1992;122:96–100. doi: 10.1093/jn/122.1.96. [DOI] [PubMed] [Google Scholar]
- 11.Stahl W, Heinrich U, Wiseman S, Eichler O, Sies H, Tronnier H. Dietary tomato paste protects against ultraviolet light-induced erythema in humans. J Nutr. 2001;131:1449–1451. doi: 10.1093/jn/131.5.1449. [DOI] [PubMed] [Google Scholar]
- 12.Stahl W, Heinrich U, Jungmann H, Sies H, Tronnier H. Carotenoids and carotenoids plus vitamin E protect against ultraviolet light-induced erythema in humans. Am J Clin Nutr. 2000;71:795–798. doi: 10.1093/ajcn/71.3.795. [DOI] [PubMed] [Google Scholar]
- 13.Mathews-Roth MM. Photoprotection by carotenoids. Fed Proc. 1987;46:1890–1893. [PubMed] [Google Scholar]
- 14.Mathews-Roth MM. Treatment of erythropoietic protoporphyria with beta-carotene. Photodermatol. 1984;1:318–321. [PubMed] [Google Scholar]
- 15.Kopcke W, Krutmann J, Kopcke W, Krutmann J. Protection from sunburn with beta-Carotene--a meta-analysis. Photochem Photobiol. 2008;84:284–288. doi: 10.1111/j.1751-1097.2007.00253.x. [DOI] [PubMed] [Google Scholar]
- 16.Eicker J, Kurten V, Wild S, Riss G, Goralczyk R, Krutmann J, Berneburg M. Betacarotene supplementation protects from photoaging-associated mitochondrial DNA mutation. Photochem Photobiol Sci. 2003;2:655–659. doi: 10.1039/b300808h. [DOI] [PubMed] [Google Scholar]
- 17.Darvin M, Patzelt A, Gehse S, Schanzer S, Benderoth C, Sterry W, Lademann J. Cutaneous concentration of lycopene correlates significantly with the roughness of the skin. Eur J Pharm Biopharm. 2008;69:943–947. doi: 10.1016/j.ejpb.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 18.Alaluf S, Heinrich U, Stahl W, Tronnier H, Wiseman S, Alaluf S, Heinrich U, Stahl W, Tronnier H, Wiseman S. Dietary carotenoids contribute to normal human skin color and UV photosensitivity. J Nutr. 2002;132:399–403. doi: 10.1093/jn/132.3.399. [DOI] [PubMed] [Google Scholar]
- 19.Biesalski HK, Obermueller-Jevic UC. UV light, beta-carotene and human skin--beneficial and potentially harmful effects. Arch Biochem Biophys. 2001;389:1–6. doi: 10.1006/abbi.2001.2313. [DOI] [PubMed] [Google Scholar]
- 20.Darvin ME, Patzelt A, Knorr F, Blume-Peytavi U, Sterry W, Lademann J. One-year study on the variation of carotenoid antioxidant substances in living human skin: influence of dietary supplementation and stress factors. J Biomed Opt. 2008;13:044028. doi: 10.1117/1.2952076. [DOI] [PubMed] [Google Scholar]
- 21.Leung WC, Hessel S, Meplan C, Flint J, Oberhauser V, Tourniaire F, Hesketh JE, von Lintig J, Lietz G. Two common single nucleotide polymorphisms in the gene encoding beta-carotene 15,15′-monoxygenase alter beta-carotene metabolism in female volunteers. FASEB J. 2009;23:1041–1053. doi: 10.1096/fj.08-121962. [DOI] [PubMed] [Google Scholar]
- 22.Ferrucci L, Perry JR, Matteini A, Perola M, Tanaka T, Silander K, Rice N, Melzer D, Murray A, Cluett C, Fried LP, Albanes D, et al. Common variation in the beta-carotene 15,15′-monooxygenase 1 gene affects circulating levels of carotenoids: a genome-wide association study. Am J Hum Genet. 2009;84:123–133. doi: 10.1016/j.ajhg.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng YM, Peng YS, Lin Y, Moon T, Baier M. Micronutrient concentrations in paired skin and plasma of patients with actinic keratoses: effect of prolonged retinol supplementation. Cancer Epidemiol Biomarkers Prev. 1993;2:145–150. [PubMed] [Google Scholar]
- 24.Ribaya-Mercado JD, Garmyn M, Gilchrest BA, Russell RM. Skin lycopene is destroyed preferentially over beta-carotene during ultraviolet irradiation in humans. J Nutr. 1995;125:1854–1859. doi: 10.1093/jn/125.7.1854. [DOI] [PubMed] [Google Scholar]
- 25.Biesalski HK, Hemmes C, Hopfenmuller W, Schmid C, Gollnick HP. Effects of controlled exposure of sunlight on plasma and skin levels of beta-carotene. Free Radic Res. 1996;24:215–224. doi: 10.3109/10715769609088019. [DOI] [PubMed] [Google Scholar]
- 26.Greul AK, Grundmann JU, Heinrich F, Pfitzner I, Bernhardt J, Ambach A, Biesalski HK, Gollnick H. Photoprotection of UV-irradiated human skin: an antioxidative combination of vitamins E and C, carotenoids, selenium and proanthocyanidins. Skin Pharmacol Appl Skin Physiol. 2002;15:307–315. doi: 10.1159/000064534. [DOI] [PubMed] [Google Scholar]
- 27.Peng YM, Peng YS, Lin Y, Moon T, Roe DJ, Ritenbaugh C. Concentrations and plasma-tissue-diet relationships of carotenoids, retinoids, and tocopherols in humans. Nutr Cancer. 1995;23:233–246. doi: 10.1080/01635589509514378. [DOI] [PubMed] [Google Scholar]
- 28.Lagiou P, Benetou V, Tebelis N, Papas A, Naska A, Trichopoulou A. Plasma carotenoid levels in relation to tobacco smoking and demographic factors. Int J Vitam Nutr Res. 2003;73:226–231. doi: 10.1024/0300-9831.73.3.226. [DOI] [PubMed] [Google Scholar]
- 29.Peng YM, Peng YS, Lin Y. A nonsaponification method for the determination of carotenoids, retinoids, and tocopherols in solid human tissues. Cancer Epidemiol Biomarkers Prev. 1993;2:139–144. [PubMed] [Google Scholar]
- 30.Weissenberg M, Schaeffler I, Menagem E, Barzilai M, Levy A. Isocratic non-aqueous reversed-phase high-performance liquid chromatographic separation of capsanthin and capsorubin in red peppers (Capsicum annuum L.), paprika and oleoresin. J Chromatogr A. 1997;757:89–95. doi: 10.1016/s0021-9673(96)00665-6. [DOI] [PubMed] [Google Scholar]
- 31.Bhosale P, Serban B, Zhao da Y, Bernstein PS. Identification and metabolic transformations of carotenoids in ocular tissues of the Japanese quail Coturnix japonica. Biochemistry. 2007;46:9050–9057. doi: 10.1021/bi700558f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kiefer C, Sumser E, Wernet MF, Von Lintig J. A class B scavenger receptor mediates the cellular uptake of carotenoids in Drosophila. Proc Natl Acad Sci USA. 2002;99:10581–10586. doi: 10.1073/pnas.162182899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Landrum JT, Bone RA. Lutein, zeaxanthin, and the macular pigment. Arch Biochem Biophys. 2001;385:28–40. doi: 10.1006/abbi.2000.2171. [DOI] [PubMed] [Google Scholar]
- 34.Davies NP, Morland AB. Macular pigments: their characteristics and putative role. Prog Retin Eye Res. 2004;23:533–559. doi: 10.1016/j.preteyeres.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 35.Kvansakul J, Rodriguez-Carmona M, Edgar DF, Barker FM, Kopcke W, Schalch W, Barbur JL. Supplementation with the carotenoids lutein or zeaxanthin improves human visual performance. Ophthalmic Physiol Opt. 2006;26:362–371. doi: 10.1111/j.1475-1313.2006.00387.x. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez-Carmona M, Kvansakul J, Harlow JA, Kopcke W, Schalch W, Barbur JL. The effects of supplementation with lutein and/or zeaxanthin on human macular pigment density and colour vision. Ophthalmic Physiol Opt. 2006;26:137–147. doi: 10.1111/j.1475-1313.2006.00386.x. [DOI] [PubMed] [Google Scholar]
- 37.Bhosale P, Li B, Sharifzadeh M, Gellermann W, Frederick JM, Tsuchida K, Bernstein PS. Purification and partial characterization of a lutein-binding protein from human retina. Biochemistry. 2009;48:4798–4807. doi: 10.1021/bi9004478. [DOI] [PubMed] [Google Scholar]
- 38.Bhosale P, Larson AJ, Frederick JM, Southwick K, Thulin CD, Bernstein PS. Identification and characterization of a Pi isoform of glutathione S-transferase (GSTP1) as a zeaxanthin-binding protein in the macula of the human eye. J Biol Chem. 2004;279:49447–49454. doi: 10.1074/jbc.M405334200. [DOI] [PubMed] [Google Scholar]
- 39.Cantrell A, McGarvey DJ, Truscott TG, Rancan F, Bohm F. Singlet oxygen quenching by dietary carotenoids in a model membrane environment. Arch Biochem Biophys. 2003;412:47–54. doi: 10.1016/s0003-9861(03)00014-6. [DOI] [PubMed] [Google Scholar]