Abstract
Lutein and zeaxanthin are two dietary carotenoids that compose the macular pigment of the primate retina. Another carotenoid, meso-zeaxanthin, is formed from lutein in the retina. A membrane location is one possible site where these dipolar, terminally dihydroxylated carotenoids, named macular xanthophylls, are accumulated in the nerve fibers and photoreceptor outer segments. Macular xanthophylls are oriented perpendicular to the membrane surface, which ensures their high solubility, stability, and significant effects on membrane properties. It was recently shown that they are selectively accumulated in membrane domains that contain unsaturated phospholipids, and thus are located in the most vulnerable regions of the membrane. This location is ideal if they are to act as lipid antioxidants, which is the most accepted mechanism through which lutein and zeaxanthin protect the retina from age-related macular degeneration. In this mini-review, we examine published data on carotenoid-membrane interactions and present our hypothesis that the specific orientation and location of macular xanthophylls maximize their protective action in membranes of the eye retina.
Keywords: macular xanthophylls, lutein, zeaxanthin, membrane, AMD, POS
Introduction
Lutein and zeaxanthin—dipolar, terminally dihydroxylated carotenoids—selectively accumulate at an extremely high concentration in membranes of the primate eye retina [1–3] from blood plasma (where more than 20 other carotenoids are available [4]). These two carotenoids can impede the onset of age-related macular degeneration (AMD) [5–8] and have been recently added to the list of potentially beneficial nutrients provided by leafy greens [9]. What role can lutein and zeaxanthin play in protecting the retina? Blue-light filtration [10] and antioxidant functions [11] are often effects that are assumed for these carotenoids. Although, blue-light absorption can be considered an indirect antioxidant action because it prevents blue light from generating reactive oxygen species that can damage photoreceptor cells [12]. Reacting as antioxidants with free radicals and reactive oxygen species, macular xanthophylls protect the retina against peroxidation and photo-damage [13,14]. The ability of macular xanthophylls to quench singlet oxygen and triplet states of photoactive molecules is especially significant [15].
Why have lutein and zeaxanthin been selected from the more than 20 carotenoids present in human plasma? The ability of these xanthophylls to filter out blue light is not better than that of other carotenoids. Likewise, their abilities to quench singlet oxygen [15] and to scavenge free radicals [16] in organic solvents are not better. Therefore, it must be that some specific property or properties of these xanthophylls can help explain their presence in the primate retina. One such property is their behavior in biological membranes [17,18]. In this mini-review, we will present data that show the unique lutein-and zeaxanthin-membrane interaction that distinguishes these carotenoids from others available from blood plasma. We will also present data that support our hypothesis that the specific orientation and location of macular xanthophylls maximize their protective action in the membranes of the eye retina.
The precise location of macular xanthophylls in the Henle’s fiber layer of photoreceptor axons and in photoreceptor outer segments (POS) is not known. There are two major hypotheses about this localization. According to the first, macular xanthophylls transversely incorporate in the lipid-bilayer portion of membranes of the human retina [19,20]. High-energy, short-wave visible light promotes the formation of reactive oxygen species that can initiate lipid peroxidation in membranes of the human retina—a tissue that is abundantly illuminated, has large respiratory demands for oxygen, and is rich in long-chain polyunsaturated fatty acids (such as docosahexaenoic acid [DHA] [21]), which are quite vulnerable to lipid peroxidation. Macular xanthophylls are thought to combat light-induced damage mediated by reactive oxygen species by absorbing the most damaging incoming wavelength of light prior to the formation of reactive oxygen species (a function expected of carotenoids in Henle’s fibers) and by chemically and physically quenching reactive oxygen species once they are formed (a function expected of carotenoids in POS). The membrane location of macular xanthophylls is ideal for these actions. According to the second hypothesis, macular xanthophylls are protein-bound by membrane-associated, xanthophyll-binding proteins [22,23]. Bernstein’s group has identified and characterized a zeaxanthin-binding protein in human macula that enhances the antioxidant activity of zeaxanthin [24,25]. This membrane-associated, xanthophyll-binding protein binds zeaxanthin (but not lutein) with high specificity and affinity. Recently, the Bernstein group also reported the presence of a lutein-binding protein in human macula [26]. Lastly, there is the question of whether the amount of these proteins is sufficient to bind and to store all xanthophyll molecules, which accumulate in the retina in extremely high concentrations. Both interactions of lutein and zeaxanthin with lipid-bilayer membranes and specific proteins are significant. However, in this mini-review, we will focus on carotenoid-membrane interactions.
Transport of macular xanthophylls and their solubility and orientation in membranes
There are data that show that polar carotenoids are more efficiently transported from the lumen of the gastrointestinal tract into intestinal mucosal cells than nonpolar carotenes, which results in their better bioavailability [27]. Carotenoids appear to be absorbed by mucosal cells by a mechanism involving passive diffusion [27–29], although During et al. [30] suggest that intestinal transport of carotenoids might be facilitated by the participation of a specific epithelial transporter. Owing to their more polar nature, xanthophylls like lutein and zeaxanthin can more easily be incorporated into the outer portions of lipid micelles within the gastrointestinal tract and therefore can be more easily taken up by enterocyte membranes and, eventually, chylomicrones [27].
Carotenoids are transported in human blood plasma exclusively by lipoproteins. Nonpolar carotenoids are transported primarily in light density lipoproteins (LDL), whereas more polar carotenoids are more evenly distributed between LDL and high density lipoproteins (HDL) [23,28]. It is thought that most tissues obtain carotenoids via the LDL receptor route [28]. However, in the case of lutein and zeaxanthin transport, we believe that receptors for HDL should be involved instead. It has been suggested that this role can be played by receptors that are similar to those found in the central nervous system for HDL particles containing ApoE [23,31]. However, there is a lack of convincing evidence that lipoproteins containing ApoE are responsible for delivery and accumulation of macular xanthophylls in the retina. Nevertheless, data presented above suggest that the segregation of polar and nonpolar carotenoids already occurs on the level of carotenoid transport.
Macular xanthophylls (dipolar, terminally dihydroxylated carotenoids) are well soluble in lipid bilayers. The reported xanthophyll solubility thresholds (concentration of xanthophylls at which aggregation initiates) in fluid-phase model membranes lie in the area of 10 mol% [32]. Also, our results show that xanthophylls affect membrane properties at the concentration up to 10 mol% without indicating saturation of the observed effect [33–35]. However, lower values of xanthophyll solubility, such as 5 and 2 mol%, were reported for small unilamellar vesicles and lipid multibilayers [36–38]. Nonpolar β-carotene starts to aggregate at a concentration as low as 0.5 mol% [39], although values of the solubility threshold as high as 1 mol% have also been reported [40]. Monopolar β-cryptoxanthin is also less soluble in the lipid bilayer than macular xanthophylls [41]. Interestingly, the tendency of cis-isomers of xanthophylls to aggregate is usually much less than their all-trans counterparts [42,43], and they also affect membrane properties more strongly (the effect of zeaxanthin on membrane properties increases in the direction: all-trans < 9-cis ≤ 13-cis [44]). Cis-isomers are also more readily solubilized, absorbed, and transported [42]. It can be hypothesized that the high solubility of lutein and zeaxanthin in the membrane determines the transport and selective accumulation of macular xanthophylls in the retina of the eye. We hypothesize that the high membrane solubility of macular xanthophylls is one of the major characteristics that distinguishes them from other dietary carotenoids.
In the human retina, the concentration of carotenoids reaches a level between 0.1 and 1 mM in the central fovea [3], which is about 1000 times higher than in other tissues. The extremely high level of macular xanthophylls in the retina does not reflect their content in POS membranes. Macular xanthophylls in POS constitute about 10% of the amount in the entire retina [45], although values as high as 25% have also been reported in the outer segment [46]. Despite the lower percentage, the local concentration of macular xanthophylls in membranes of the rod outer segment is ~70% higher than in residual retina membranes [46]. As indicated in the introduction, we will focus on xanthophyll-membrane interactions. Based on measurements with model lipid-bilayer membranes, Bone and Landrum [19] concluded that lutein is located in the membrane and oriented perpendicular to the bilayer surface. This orientation in lipid-bilayer membranes was confirmed for zeaxanthin [36]. However, linear dichroism analysis of the mean orientation of the dipole transition moment of zeaxanthin and lutein incorporated to the oriented EYPC multibilayers revealed essentially different orientation of zeaxanthin and lutein in the membranes [47]. Zeaxanthin was found to adopt roughly vertical orientation with respect to the plane of the membrane, showing the 33° orientation angle between the transition dipole and the axis normal to the plane of the membrane. The relatively large orientation angle of 67° found in the case of lutein was interpreted as a representation of the existence of two orthogonally oriented pools of lutein, one following the orientation of zeaxanthin and the second parallel with respect to the plane of the membrane. We direct readers to a review by Gruszecki [48] where possible orientations of carotenoids in the lipid bilayer membranes are discussed in details and to a review by Krinski [18] and our paper [41] where the unusual results obtained for lutein are critically evaluated. Nevertheless, in this review, we will assume only the transmembrane localization of xanthophylls because the polar hydroxyl groups at each end of the xanthophyll molecule encourage a membrane-spanning configuration in the lipid bilayer. The presence of xanthophylls in biological membranes is ideal if they are to act as a lipid antioxidant, although lipid association alone cannot explain the extraordinary specific uptake of xanthophylls into the macula.
The transmembrane localization of a significant portion of macular xanthophylls in the retinal cells seems to be obvious. Such localization of macular xanthophylls can explain their very slow removal from the retina, observed after discontinuation of a lutein supplement given to healthy volunteers in a study by Landrum et al. [49]. After discontinuing a 140-day lutein supplement, Landrum et al. [49] observed a relatively fast decrease of lutein concentration in the serum, whereas the level of lutein in the retina remained unchanged and at a high concentration for up to six months. Similar effects were observed by Hammond et al. [50]. These observations suggest that anchoring xanthophyll molecules at opposite membrane surfaces is significant not only in enhancing their effects on membrane properties [41] (see also reviews by Gruszecki [51] and Gruszecki and Strzalka [52]), but also in stabilization of these molecules in membranes of the human retina. Thus, transmembrane orientation can also be included as a characteristic that distinguishes macular xanthophylls from other dietary carotenoids.
Antioxidant potency of macular xanthophylls in membranes
For organisms with a high carotenoid content in their membranes (for example, bacteria, and in some situations, plants, in which the local carotenoid concentration in the lipid bilayer can reach a value up to a few mol%), it is most important to understand how carotenoids affect the membrane’s physical properties, structure, and dynamics. It has been shown that membranes of halophyles and thermophylic bacteria contain a fairly large amount of polar carotenoids [53,54]. These bacteria, which live in extreme conditions, should possess stable membranes that provide a high barrier for nonspecific permeation of small molecules. Incorporation of dipolar carotenoids into the membrane serves this purpose well. Dipolar carotenoids stabilize both halves of the lipid bilayer like transmembrane “rivets”, increasing membrane rigidity by ordering the alkyl chains of lipids [33,34], and raising the membrane hydrophobic barrier for polar molecules and ions [35]. Our results support Rohmer’s hypothesis [55] that polar carotenoids regulate membrane properties in prokaryotes in a manner similar to cholesterol in eukaryotes (see also discussions in [35] and [56]). Polar carotenoids are also present transiently in the lipid-bilayer portion of thylakoid membranes at a high enough concentration to regulate membrane fluidity during the xanthophyll cycle [57].
In animals, the highest carotenoid concentration is found in the eye retina of primates, but even here the carotenoid concentration in the lipid-bilayer portion of the membrane is much lower than 1 mol% [19]. Therefore, it seems advisable that to act as antioxidants in vivo, carotenoids should be incorporated in tissues in the correct location and/or selectively accumulated to present a local concentration high enough to protect vulnerable molecules. Thus, for systems with a low carotenoid concentration, it is especially important to understand how the membrane itself—its composition, structure, and lateral organization—affects the organization of carotenoids in the lipid bilayer, including orientation (transmembrane vs. parallel) and localization (distribution between membrane domains).
Orientation of polar and nonpolar carotenoids (including their cis-forms) in the lipid-bilayer membrane has been investigated by many laboratories [19,44,47,58,59]. It is suggested that the presence of polar hydroxyl groups at the ends of carotenoid molecules and their transmembrane orientation (as in the case of zeaxanthin and lutein) enhance their antioxidant properties, as compared with the antioxidant properties of monopolar (β-cryptoxanthin) and nonpolar (β-carotene) carotenoids [40,60]. Although dipolar zeaxanthin and nonpolar β-carotene show similar antioxidant properties in organic solutions, they differ when incorporated into membranes. Zeaxanthin was shown to react with free radicals slightly more effectively than β-cryptoxanthin and much more effectively than β-carotene [40,61]. β-carotene and lycopene are able to react efficiently only with radicals generated in the inner part of the membrane, whereas zeaxanthin and lutein, with their end groups exposed to an aqueous environment, can also scavenge free radicals generated in the aqueous phase [42].
We hypothesize that the transmembrane orientation of xanthophylls may also enhance the antioxidant properties of these carotenoids indirectly—by changing membrane properties so that the membrane becomes less sensitive to oxidative damage. It has been shown that perpendicularly oriented dipolar carotenoids (but not nonpolar carotenoids [62]) significantly affect membrane properties, including membrane fluidity [33,34], vertical fluctuations of alkyl chains [63], the hydrophobicity of the membrane interior [35], and the oxygen transport rate within the membrane [64]. McNulty et al. [65] tried to relate the physical interactions of carotenoids with the membrane to carotenoids’ antioxidant effects. According to this group, an ordering effect of carotenoids is accompanied by a strong antioxidant action, as seen for the dipolar xanthophyll, astaxanthin. On the contrary, nonpolar carotenoids like β-carotene and lycopene disordered the membranes and acted as prooxidants. β-carotene—because of its low membrane solubility as a monomer and low incorporation efficiency, and, therefore, weak effects on membranes—does not protect membranes against lipid peroxidation. Moreover, at a high oxygen concentration, it may act as a prooxidant [66]. It has to be emphasized, however, that possible indirect antioxidant action of carotenoids via their alteration of membrane properties would require a high concentration of carotenoids in the membrane.
Distribution of macular xanthophylls between membrane domains
The lipid-bilayer portion of biological membranes is currently depicted not as a passive matrix in which membrane proteins are immersed, but as an active membrane component that controls a variety of biological functions through selective accumulation [67–70] or exclusion [71,72] of certain proteins and lipids [73] from specific membrane domains. Raft domains have been postulated to enhance signal transduction [71,72,74], and are also involved in lipid sorting [75] and protein trafficking/recycling [69,76]. It has been shown that in membranes of retinal pigment epithelium and photoreceptors, raft domains are present [77–80]. Rafts in membranes of photoreceptor cells are involved in regulation of the G-protein-mediated pathway of photo-transduction [79]. Aggregation of small, unstable rafts in bigger platforms (observed, for example, in retinal pigment epithelium cells) is supposed to enhance signal transduction to the cell interior and cause a specific reaction in the cell, such as apoptosis [80].
Membranes of the human retina are abundant in long-chain, polyunsaturated fatty acids, such as docosahexaenoic acid (DHA), with a six double-bond chain [21,45,82]. Additionally, POS discs contain nearly equimolar concentrations of unsaturated fatty acids (DHA), saturated fatty acids (myristoyl + palmitoyl + stearoyl), and cholesterol [83], which makes them very similar to raft-forming mixtures. Indeed, rafts were isolated as a detergent-resistant membrane (DRM) fraction from POS disc membranes [77–80]. Interestingly, the remaining detergent-soluble membrane (DSM) fraction, which is formed by the bulk lipid domain surrounding the raft domain in POS disc membranes, is rich in long-chain, polyunsaturated fatty acids. Additionally, rhodopsin, the main protein of POS membranes (comprising more than 90% of all proteins in these membranes) that is responsible for the first stages of visual signal transduction, is also located in the bulk domain of the POS membrane [84] and can be isolated mostly with the DSM fraction [77,78,80]. Also, Wang et al [85] using fluorescence recovery after photobleaching technique showed that in the dark and immediately after photoexcitation, rhodopsin diffuses freely in the bulk lipid phase. It is worth mentioning that rhodopsin requires the presence of polyunsaturated lipids (DHA) for its activity [86–88], and thus their co-localization is functionally justified. Surprisingly, in the model of POS membranes, macular xanthophylls were also about 14 times more concentrated in the unsaturated bulk domain (enriched in polyunsaturated DHA and isolated as DSM) and excluded from the raft domain (enriched in saturated lipids and cholesterol and isolated as DRM) [89] (Fig. 1B). Similar distribution was also found in membranes made of raft-forming mixture where macular xanthophylls lutein and zeaxanthin were about eight times more concentrated in the bulk, unsaturated domain than in the raft domain [90] (Fig. 1A). A similar distribution has been observed for monopolar β-cryptoxanthin, but not for nonpolar β-carotene, which is more uniformly distributed between DRM and DSM domains (Fig. 1A).
Fig. 1.
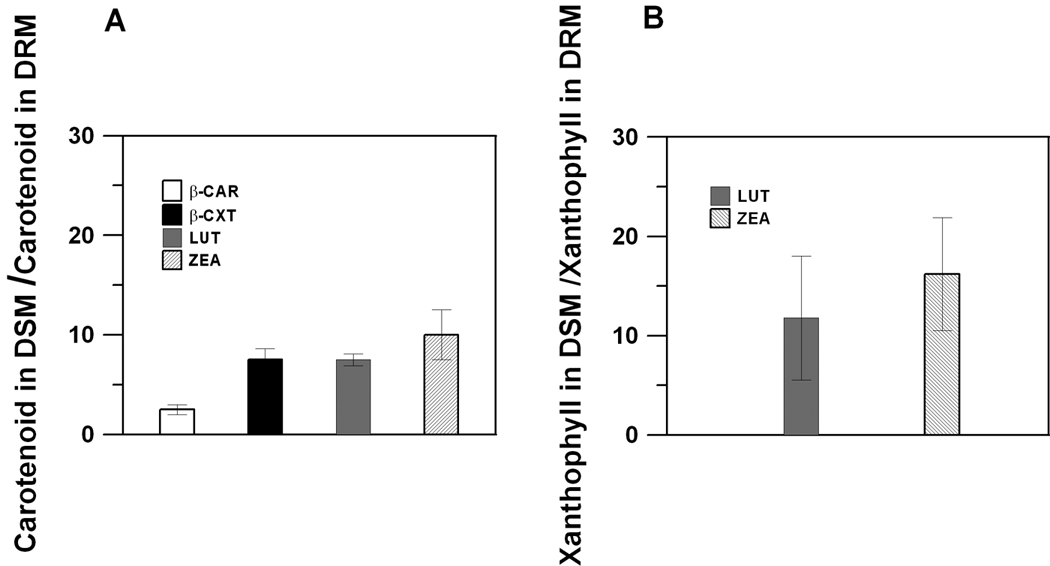
(Mole ratio of carotenoids and total lipids in DSM)/(mole ratio of carotenoids and total lipids in DRM) indicated on the y-axis as (Carotenoid in DSM)/(Carotenoid in DRM) in domains isolated from membranes made of raft-forming mixture (equimolar ternary mixture of dioleoylphosphatidylcholine/sphingomyelin/cholesterol) with 1 mol% carotenoid added (see Ref. [90] for more detail) (A). (Mole ratio of xanthophylls and total lipids in DSM)/(mole ratio of xanthophylls and total lipids in DRM) indicated on the y-axis as (Xanthophyll in DSM)/(Xanthophyll in DRM) in domains isolated from the model of POS membranes (equimolar ternary mixture of 1-palmitoyl-2-docosahexaenoylphosphatidylcholine/distearoylphosphatidylcholine/cholesterol) with 1 mol% lutein or zeaxanthin added (see Ref. [89] for more detail) (B).
Localization of macular xanthophylls in domains rich in unsaturated lipids is ideal if they are to act as a lipid antioxidant, which is the most accepted mechanism through which lutein and zeaxanthin protect the retina from age-related macular diseases [17,18,91]. Polyunsaturated DHA, localized in this domain and extremely susceptible to lipid peroxidation, needs to be protected in its direct proximity. Also, the oxygen transport parameter (oxygen diffusion-concentration product) is two to four times greater in the bulk domain than in the raft domain [89], which makes polyunsaturated lipids located in the former domain even more susceptible to oxidative damage. Finally, photoactivation of rhodopsin (also located in the unsaturated bulk domain) leads to isomerization of its chromophore, 11-cis-retinal to all-trans-retinal (ATR), which under certain conditions can act as a photosensitizer. During intense light illumination, upon regeneration of rhodopsin, ATR can be released from the opsin to the inner leaflet of the disc membrane before being reduced to all-trans-retinol [92]. Free ATR may absorb light and transfer energy from its excited triplet state to molecular oxygen, generating singlet oxygen [93]. Owing to close proximity of xanthophylls, which are able to effectively quench excited triplet states of molecules, the energy transfer from excited ATR to xanthophyll is possible, which prevents singlet oxygen generation by this photosensitizer [92]. The unsaturated bulk domain is fluid (more fluid than the raft domain [95,96]), so xanthophyll molecules can diffuse there faster and encounter a greater number of reactive species. The schematic drawing illustrating the distribution of macular xanthophylls between domains in the POS membrane is presented in Fig. 2. As indicated in the figure, the bulk domain contains rhodopsin surrounded by unsaturated (and polyunsaturated) lipids, which, during illumination, makes this domain even more vulnerable to oxidative damage. Thus, accumulation of xanthophylls directly in this domain is more than perfect.
Fig. 2.
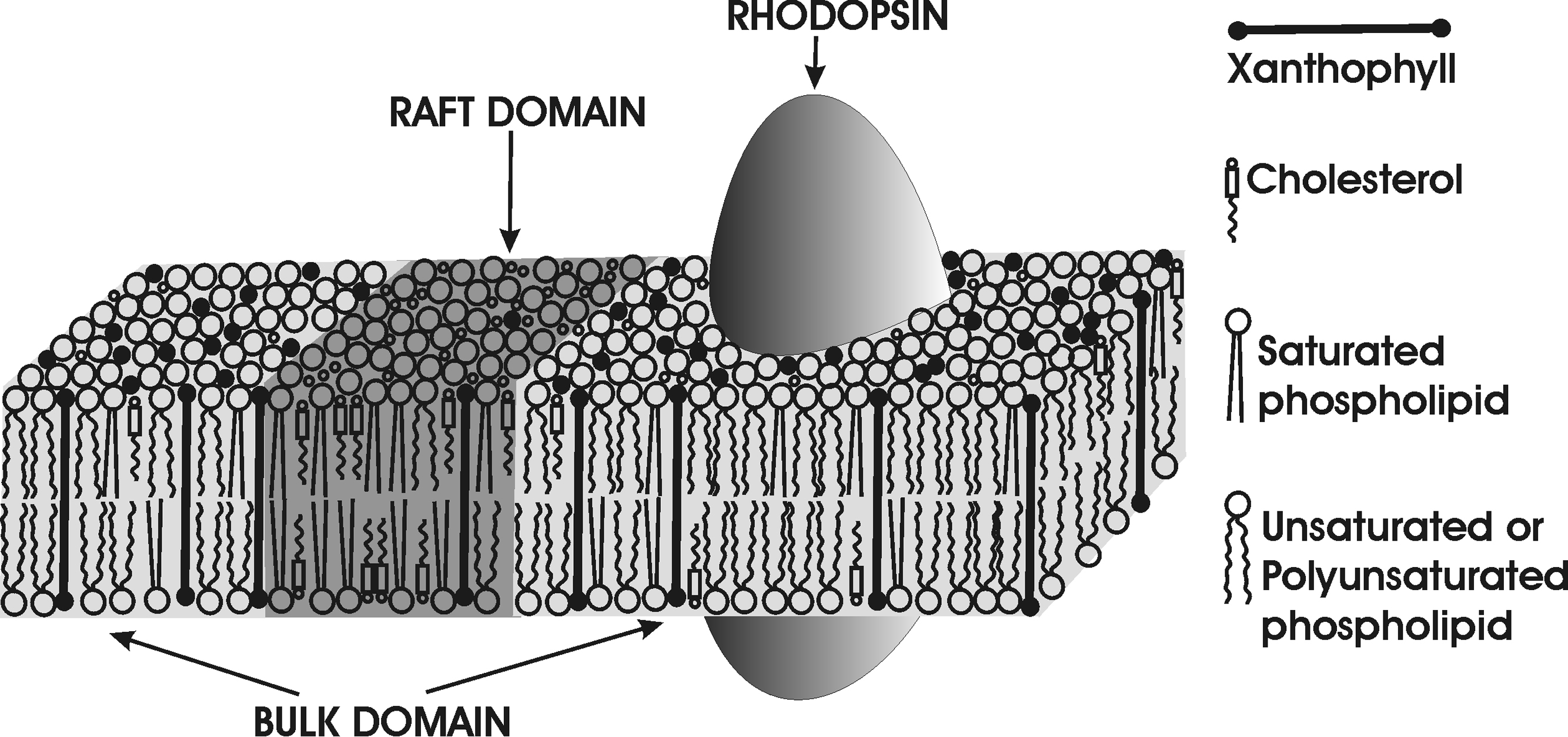
Schematic drawing of the distribution of xanthophyll molecules between the saturated raft domain and the unsaturated bulk domain in the membrane of the photoreceptor outer segment. In this illustration, the integral membrane protein, rhodopsin, which is located in the unsaturated membrane domain, is also included to show its co-localization with unsaturated lipids and xanthophylls. As was demonstrated by X-ray diffraction and linear dichroism (in thin membranes), xanthophyll molecules are inclined with respect to the bilayer normal [59]. This inclination decreases with membrane thickness [33] (see Ref. [99] for membrane thicknesses). The thickness of the POS membranes, which contain not only long-chain fatty acids such as DHA [82] but also very-long-chain fatty acids [100,101], should be significantly greater than those investigated in Refs. [33] and [59]. Therefore, we depict xanthophylls as perpendicular to the membrane surface.
The presented data clearly demonstrate that macular xanthophylls are excluded from cholesterol-rich membrane domains. They are also poorly soluble in membranes with a high cholesterol content [97]. This has been confirmed by Wisniewska et al. [98] who showed that spin-labeled lutein was completely insoluble in saturated PC membranes containing 30 mol% cholesterol. All these suggest that the xanthophyll-cholesterol interaction is weaker than the xanthophyll-phospholipid interaction. In the lipid bilayer, the rigid bar-like xanthophyll molecule does not conform to the cholesterol molecule, which has a rigid, plate-like tetracyclic ring structure and flexible isooctyl chain. Two polar groups of the xanthophyll molecule interact with opposite surfaces of the membrane, and its rigid bar-like portion crosses the entire membrane. In contrast, the molecule of cholesterol is located in one leaflet of the bilayer, and its rigid plate-like portion extends to the depth of the seventh to ninth carbon in the lipid bilayer. The cross-section of the isooctyl chain of the cholesterol molecule is much smaller than the cross-section of the rigid steroid ring. When rigid xanthophyll and cholesterol molecules are located next to each other in the cholesterol-rich, lipid-bilayer domain, a free space is created in the membrane center (indicated by the ring in Fig. 3). Cholesterol molecules are forced to sink deeper into the bilayer, which is energetically unfavorable because it allows water to access the hydrophobic surface of alkyl chains. Thus, macular xanthophylls are excluded from cholesterol-rich domains, as illustrated in Figs. 2 and 3.
Fig. 3.
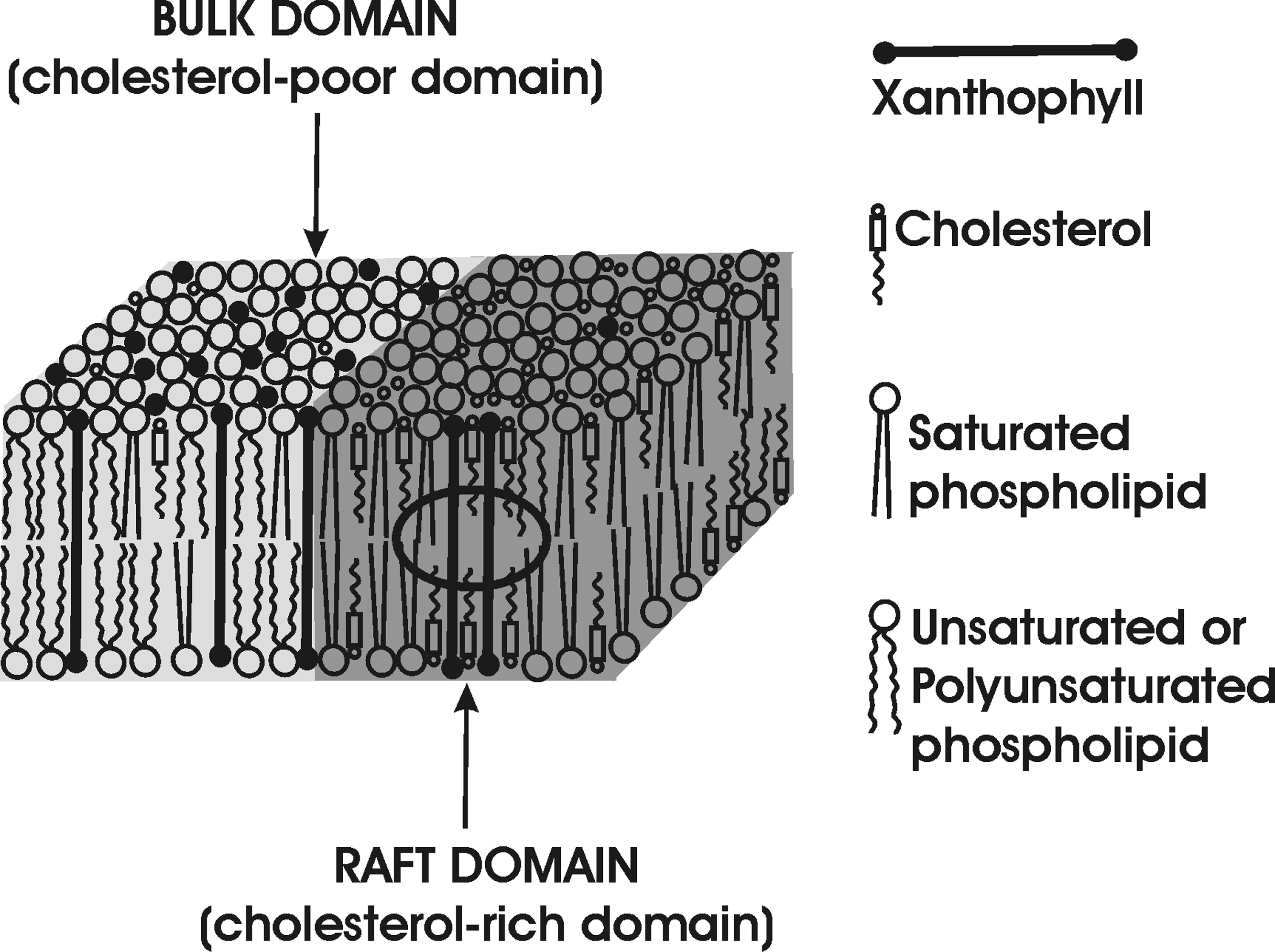
Schematic drawing showing the localization of xanthophyll molecules in the cholesterol-rich raft domain and the cholesterol-poor bulk domain. Unfavorable interaction of rigid xanthophylls with cholesterol, which creates a free space in the membrane center, is indicated by the circle. When cholesterol is surrounded by phospholipids, such as in the cholesterol-pure domain, the created free space is filled by the flexible hydrocarbon chains of phospholipids.
Concluding remarks
As presented above, dipolar macular xanthophylls are largely excluded from DRMs extracted from xanthophyll-containing membranes made of raft-forming mixtures [90] or from models of POS membranes [89] and remain concentrated in DSMs. DRMs and DSMs isolated from model and cell membranes, thought to be related to membrane domains, have similar lipid compositions as rafts and bulk domains, respectively [68,73]. All these suggest that in POS membranes macular xanthophylls should also be concentrated in the bulk domain enriched in polyunsaturated lipids, where rhodopsin is also located.
Co-localization of rhodopsin with polyunsaturated phospholipids has its functional purpose [86–88]. However, it creates a dangerous situation for both, especially during illumination when reactive oxygen species can be produced by photosensitizers. Thus, nature has used xanthophylls as an effective protector that can neutralize photosensitizers and reactive oxygen species. Co-localization of these molecules, together with polyunsaturated phospholipids and rhodopsin, should significantly enhance their effectiveness as protector, especially when the local concentration of xanthophylls in the membrane is not so high. This is possible because of the domain structure of the POS membrane and the ability of these domains to select and exclude specific classes of lipids and proteins. Thus, the membrane domain structure can also play a significant role in the protection of vulnerable molecules and processes by co-localizing them with protective molecules and processes. We consider this a function of membrane domains that has not yet been explored and that can be included with already accepted domain functions such as signal transduction, lipid sorting, and protein trafficking/recycling. Nevertheless, the domain structure allows location of macular xanthophylls in the most vulnerable regions of POS membranes, which should significantly enhance their ability to prevent AMD.
Acknowledgements
This work was supported by grants EY015526, EB002052, and EB001980 of the National Institutes of Health and by the POL-POSTDOC III PBZ/MNiSW/07/2006/01 grant of the Polish Ministry of Higher Education and Science.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bone RA, Landrum JT, Tarsis SL. Preliminary identification of the human macular pigment. Vision Res. 1985;25:1531–1535. doi: 10.1016/0042-6989(85)90123-3. [DOI] [PubMed] [Google Scholar]
- 2.Bone RA, Landrum JT, Fernandez L, Tarsis SL. Analysis of the macular pigment by HPLC: Retinal distribution and age study. Invest. Ophthalmol. Vis. Sci. 1988;29:843–849. [PubMed] [Google Scholar]
- 3.Landrum JT, Bone RA, Moore LL, Gomea CM. Analysis of zeaxanthin distribution within individual human retinas. Methods Enzymol. 1999;229:457–467. doi: 10.1016/s0076-6879(99)99043-2. [DOI] [PubMed] [Google Scholar]
- 4.Khachik F, Spangler CJ, Smith JC, Jr, Canfield LM, Steck A, Pfander H. Identification, quantification, and relative concentrations of carotenoids and their metabolites in human milk and serum. Anal. Chem. 1997;69:1873–1881. doi: 10.1021/ac961085i. [DOI] [PubMed] [Google Scholar]
- 5.Gale CR, Hall NF, Phillips DIW, Martyn CN. Lutein and zeaxanthin status and risk of age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 2003;44:2461–2465. doi: 10.1167/iovs.02-0929. [DOI] [PubMed] [Google Scholar]
- 6.Mares-Perlman JA, Millen AE, Ficek TL, Hankinson S. The body of evidence to support a protective role for lutein and zeaxanthin in delaying chronic disease, Overview. J. Nutr. 2002;132:518–524. doi: 10.1093/jn/132.3.518S. [DOI] [PubMed] [Google Scholar]
- 7.Mares JA. Carotenoids and eye disease: Epidemiological evidences. In: Krinsky NI, Mayne ST, Sies H, editors. Carotenoids in Health and Disease. New York: Marcel Dekker; 2004. pp. 427–444. [Google Scholar]
- 8.Cho E, Seddon JM, Rosner B, Willett WC, Hankinson SE. Prospective study of intake of fruits, vegetables, vitamins, and carotenoids and risk of age-related maculopathy. Arch. Ophthalmol. 2004;122:883–892. doi: 10.1001/archopht.122.6.883. [DOI] [PubMed] [Google Scholar]
- 9.Mares-Perlman JA, Erdman JW., Jr Can lutein protect against chronic disease? A multidisciplinary approach involving basic science and epidemiology to weigh evidences and design analytic strategies. Introduction. J. Nutr. 2002;132:517S. [Google Scholar]
- 10.Kirschfeld K. Carotenoid pigments: Their possible role in protecting against photooxidation in eyes and photoreceptor cells. Proc. R. Soc. Lond. 1982;B216:71–85. doi: 10.1098/rspb.1982.0061. [DOI] [PubMed] [Google Scholar]
- 11.Martin H-D, Ruck C, Schmidt M, Sell S, Beutner S, Mayer B, Walsh R. Chemistry of carotenoid oxidation and free radical reactions. Pure Appl. Chem. 1999;71:2253–2262. [Google Scholar]
- 12.Wooten BR, Hammond BR. Macular pigment: Influence on visual acuity and visibility. Prog. Retin. Eye Res. 2002;21:225–240. doi: 10.1016/s1350-9462(02)00003-4. [DOI] [PubMed] [Google Scholar]
- 13.Krinsky NI. Antioxidant functions of carotenoids. Free Radic. Biol. Med. 1989;7:617–635. doi: 10.1016/0891-5849(89)90143-3. [DOI] [PubMed] [Google Scholar]
- 14.Edge R, McGarvey DJ, Truscott TG. The carotenoids as anti-oxidants—A review. J. Photochem. Photobiol. B. 1997;41:189–200. doi: 10.1016/s1011-1344(97)00092-4. [DOI] [PubMed] [Google Scholar]
- 15.Cantrell A, MacGarvey DJ, Truscott TG, Rancan F, Böhm F. Singlet oxygen quenching in model membrane environment. Arch. Biochem. Biophys. 2003;412:47–54. doi: 10.1016/s0003-9861(03)00014-6. [DOI] [PubMed] [Google Scholar]
- 16.Stahl W, Junghans A, de Boer B, Driomina ES, Briviba K, Sies H. Carotenoid mixtures protect multilamellar liposomes against oxidative damage: Synergistic effects of lycopene and lutein. FEBS Lett. 1998;427:305–308. doi: 10.1016/s0014-5793(98)00434-7. [DOI] [PubMed] [Google Scholar]
- 17.Krinsky NI, Landrum JT, Bone RA. Biological mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu. Rev. Nutr. 2003;23:171–201. doi: 10.1146/annurev.nutr.23.011702.073307. [DOI] [PubMed] [Google Scholar]
- 18.Krinsky NI. Possible biological mechanisms for protective role of xanthophylls. J. Nutr. 2002;132:540S–542S. doi: 10.1093/jn/132.3.540S. [DOI] [PubMed] [Google Scholar]
- 19.Bone RA, Landrum JT. Macular pigment in Henle fiber membranes: A model for Haidinger’s brushes. Vis. Res. 1984;24:103–108. doi: 10.1016/0042-6989(84)90094-4. [DOI] [PubMed] [Google Scholar]
- 20.Bone RA, Landrum JT, Cains A. Optical density spectra of the macular pigment in vivo and in vitro. Vision Res. 1992;32:105–110. doi: 10.1016/0042-6989(92)90118-3. [DOI] [PubMed] [Google Scholar]
- 21.Beatty S, Kok H, Phil M, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv. Ophthalmol. 2000;45:115–134. doi: 10.1016/s0039-6257(00)00140-5. [DOI] [PubMed] [Google Scholar]
- 22.Bhosale P, Bernstein PS. Vertebrate and invertebrate carotenoid-binding proteins. Arch. Biochem. Biophys. 2007;458:121–127. doi: 10.1016/j.abb.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loane E, Nolan JM, O’Donovan O, Bhosale P, Bernstein PS, Beatty S. Transport and retinal capture of lutein and zeaxanthin with reference to age-related macular degeneration. Surv. Ophthalmol. 2008;53:68–81. doi: 10.1016/j.survophthal.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 24.Bhosale P, Larson AJ, Frederick JM, Southwick K, Thulin CD, Bernstein PS. Identification and characterization of a Pi isoform of glutathione S-transferase (GSTP1) as a zeaxanthin-binding protein in the macula of the human eye. J. Biol. Chem. 2004;279:49447–49454. doi: 10.1074/jbc.M405334200. [DOI] [PubMed] [Google Scholar]
- 25.Bhosale P, Bernstein PS. Synergistic effects of zeaxanthin and its binding protein in the prevention of lipid membrane oxidation. Biochim. Biophys. Acta. 2005;1740:116–121. doi: 10.1016/j.bbadis.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 26.Bhosale P, Li B, Sharifzadeh M, Gellermann W, Frederick JM, Tsuchida K, Bernstein PS. Purification and partial characterization of a lutein-binding protein from human retina. Biochemistry. 2009;48:4798–4807. doi: 10.1021/bi9004478. [DOI] [PubMed] [Google Scholar]
- 27.Yeum K-J, Russell RM. Carotenoid bioavailability and bioconversion. Annu. Rev. Nutr. 2002;22:483–504. doi: 10.1146/annurev.nutr.22.010402.102834. [DOI] [PubMed] [Google Scholar]
- 28.Parker RS. Absorption, metabolism and transport of carotenoids. FASEB J. 1996;10:542–551. [PubMed] [Google Scholar]
- 29.Beatty S, Nolan J, Kavanagh H, O’Donovan O. Macular pigment optical density and its relationship with serum and dietary levels of lutein and zeaxanthin. Arch. Biochem. Biophys. 2004;430:70–76. doi: 10.1016/j.abb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 30.During A, Hussain MM, Morel DW, Harrison EH. Carotenoid uptake and secretion by CaCo-2 cells: β-carotene isomer selectivity and carotenoid interactions. J. Lipid Res. 2002;43:1086–1095. doi: 10.1194/jlr.m200068-jlr200. [DOI] [PubMed] [Google Scholar]
- 31.Thomson LR, Toyoda Y, Langner A, Delori FC, Garnett KM, Craft N, Nichols CR, Cheng KM, Dorey CK. Elevated retinal zeaxanthin and prevention of light-induced photoreceptor cell death in quail. Invest. Ophthalmol. Vis. Sci. 2002;43:3538–3549. [PubMed] [Google Scholar]
- 32.Milon A, Lazrak T, Albrecht A-M, Wolff G, Weill G, Ourisson G, Nakatani Y. Osmotic swelling of unilamellar vesicles by the stopped-flow light scattering method. Influence of vesicle size, solute, temperature, cholesterol and three a,ω-dihydroxycarotenoids. Biochim. Biophys. Acta. 1986;859:1–9. [Google Scholar]
- 33.Subczynski WK, Markowska E, Sielewiesiuk J. Spin-label studies on phosphatidylcholine polar carotenoid membranes: Effects of alkyl chain length and unsaturation. Biochim. Biophys. Acta. 1993;1150:173–181. doi: 10.1016/0005-2736(93)90087-g. [DOI] [PubMed] [Google Scholar]
- 34.Subczynski WK, Markowska E, Gruszecki WI, Sielewiesiuk J. Effects of polar carotenoids on dimyristoylphosphatidylcholine membranes: Spin-label studies. Biochim. Biophys. Acta. 1992;1105:97–108. doi: 10.1016/0005-2736(92)90167-k. [DOI] [PubMed] [Google Scholar]
- 35.Wisniewska A, Subczynski WK. Effects of polar carotenoids on the shape of the hydrophobic barrier of phospholipids bilayer. Biochim. Biophys. Acta. 1998;1368:235–246. doi: 10.1016/s0005-2736(97)00182-x. [DOI] [PubMed] [Google Scholar]
- 36.Gabrielska J, Gruszecki WI. Zeaxanthin (dihydroxy-β-carotene) but not β-carotene rigidifies lipid membranes: A 1H-NMR study of carotenoid-egg phosphatidylcholine liposomes. Biochim. Biophys. Acta. 1996;1285:167–174. doi: 10.1016/s0005-2736(96)00152-6. [DOI] [PubMed] [Google Scholar]
- 37.Sujak A, Okulski W, Gruszecki WI. Organization of xanthophyll pigments lutein and zeaxanthin in lipid membranes formed with dipalmitoylphosphatidylcholine. Biochim. Biophys. Acta. 2000;1509:255–263. doi: 10.1016/s0005-2736(00)00299-6. [DOI] [PubMed] [Google Scholar]
- 38.Sujak A, Mazurek P, Gruszecki WI. Xanthophyll pigments lutein and zeaxanthin in lipid multibilayers formed with dimyristoylphosphatidylcholine. J. Photochem. Photobiol. 2002;B 68:39–44. doi: 10.1016/s1011-1344(02)00330-5. [DOI] [PubMed] [Google Scholar]
- 39.Kennedy TA, Liebler DC. Peroxyl radical scavenging by beta-carotene in lipid bilayers. Effect of oxygen partial pressure. J. Biol. Chem. 1992;267:4658–4663. [PubMed] [Google Scholar]
- 40.Woodall AA, Britton G, Jackson MJ. Antioxidant activity of carotenoids in phosphatidylcholine vesicles: Chemical and structural considerations. Biochem. Soc. Trans. 1995;23:133S. doi: 10.1042/bst023133s. [DOI] [PubMed] [Google Scholar]
- 41.Wisniewska A, Widomska J, Subczynski WK. Carotenoid-membrane interactions in liposomes: Effect of dipolar, monopolar, and nonpolar carotenoids. Acta Biochim. Pol. 2006;53:475–484. [PubMed] [Google Scholar]
- 42.Britton G. Structure and properties of carotenoids in relation to function. FASEB J. 1995:1551–1558. [PubMed] [Google Scholar]
- 43.Milanowska J, Polit A, Wasylewski Z, Gruszecki WI. Interaction of isomeric forms of xanthophyll pigment zeaxanthin with dipalmitoylphosphatidylcholine studied in monomolecular layers. J. Photochem. Photobiol. B: Biol. 2003;72:1–9. doi: 10.1016/j.jphotobiol.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 44.Widomska J, Subczynski WK. Transmembrane localization of cis-isomers of zeaxanthin in the host dimyristoylphosphatidylcholine bilayer membrane. Biochim. Biophys. Acta. 2008;1778:10–19. doi: 10.1016/j.bbamem.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rapp LM, Maple SS, Choi JH. Lutein and zeaxanthin concentrations in rod outer segment membranes from perifoveal and peripheral human retina. Invest. Ophthalmol. Vis. Sci. 2000;41:1200–1209. [PubMed] [Google Scholar]
- 46.Sommerburg OG, Siems WG, Hurst JS, Lewis JW, Kliger DS, van Kuijk FJ. Lutein and zeaxanthin are associated with photoreceptors in the human retina. Curr. Eye Res. 1999;19:491–495. doi: 10.1076/ceyr.19.6.491.5276. [DOI] [PubMed] [Google Scholar]
- 47.Sujak A, Gabrielska J, Grudzinski W, Borc R, Mazurek P, Gruszecki WI. Lutein and zeaxanthin as protectors of lipid membranes against oxidative damage: The structural aspects. Arch. Biochem. Biophys. 1999;371:301–307. doi: 10.1006/abbi.1999.1437. [DOI] [PubMed] [Google Scholar]
- 48.Gruszecki WI. Carotenoids in Lipid Membranes. In: Landrum JT, editor. Carotenoids. Physical, Chemical, and Biological Function and Properties. Boca Raton, London, New York: Taylor & Francis Group; 2009. pp. 19–30. [Google Scholar]
- 49.Landrum JT, Bone RA, Joa H, Kilburn MD, Moore LL, Sprague KE. A one year study of the macular pigment: the effect of 140 days of a lutein supplement. Exp. Eye Res. 1997;65:57–62. doi: 10.1006/exer.1997.0309. [DOI] [PubMed] [Google Scholar]
- 50.Hammond BR, Jr, Johnson EJ, Russell RM, Krinsky NI, Yeum KJ, Edwards RB, Snodderly DM. Dietary modification of human macular pigment density. Invest. Ophthalmol. Vis. Sci. 1997;38:1795–1801. [PubMed] [Google Scholar]
- 51.Gruszecki WI. Carotenoids in membranes. In: Frank HA, Young AJ, Britton G, editors. The Photochemistry of Carotenoids. Dordrecht: Kluwer Academic; 1999. pp. 363–379. [Google Scholar]
- 52.Gruszecki WI, Strzalka K. Carotenoids as modulators of lipid membrane physical properties. Biochim. Biophys. Acta. 2005;1740:108–115. doi: 10.1016/j.bbadis.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 53.Anwar M, Khan TB, Prebble J, Zagalsky PF. Membrane-bound carotenoid in Micrococcus luteus protects naphthoquinone from photodynamic action. Nature. 1977;270:538–540. doi: 10.1038/270538a0. [DOI] [PubMed] [Google Scholar]
- 54.Yokoyama A, Sandmann G, Hashino T, Adachi K, Sakai M, Shizari Y. Thermozeaxanthins, new carotenoid-glycoside-esters from thermophilic eubacterium Thermus thermophilus. Tetrahedron Lett. 1995;36:4901–4904. [Google Scholar]
- 55.Rohmer M, Bouvier P, Ourisson G. Molecular evolution of biomembranes: Structural equivalents and phylogenetic precursors of sterols. Proc. Natl. Acad. Sci. USA. 1979;76:847–851. doi: 10.1073/pnas.76.2.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Subczynski WK, Wisniewska A, Yin J-J, Hyde JS, Kusumi A. Hydrophobic barriers of lipid-bilayer membranes formed by reduction of water penetration by alkyl chain unsaturation and cholesterol. Biochemistry. 1994;33:7670–7681. doi: 10.1021/bi00190a022. [DOI] [PubMed] [Google Scholar]
- 57.Gruszecki WI, Strzalka K. Does the xanthophyll cycle take part in the regulation of fluidity of the thylakoid membrane? Biochim. Biophys. Acta. 1991;1060:310–314. [Google Scholar]
- 58.van der Ven M, Kattenberg M, van Ginkel G, Levine YK. Study of the orientational ordering of carotenoids in lipid bilayers by resonance-Raman spectroscopy. Biophys. J. 1984;45:1203–1210. doi: 10.1016/S0006-3495(84)84269-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gruszecki WI, Sielewiesiuk J. Orientation of xanthophylls in phosphatidylcholine multibilayer. Biochim. Biophys. Acta. 1990;1023:405–412. doi: 10.1016/0005-2736(90)90133-9. [DOI] [PubMed] [Google Scholar]
- 60.Woodall AA, Britton G, Jackson MJ. Carotenoids and protection of phospholipids in solution or in liposomes against oxidation by peroxyl radicals: Relationship between carotenoid structure and protective ability. Biochim. Biophys. Acta. 1997;1336:575–586. doi: 10.1016/s0304-4165(97)00007-x. [DOI] [PubMed] [Google Scholar]
- 61.Woodall AA, Lee SW, Weesie RJ, Jackson MJ, Britton G. Oxidation of carotenoids by free radicals: Relationship between structure and reactivity. Biochim. Biophys. Acta. 1997;1336:33–42. doi: 10.1016/s0304-4165(97)00006-8. [DOI] [PubMed] [Google Scholar]
- 62.Subczynski WK, Wisniewska A. Effects of β-carotene on physical properties of lipid membranes—Comparison with effects of polar carotenoids. Curr. Top. Biophys. 1998;22:44–51. [Google Scholar]
- 63.Yin J-J, Subczynski WK. Effects of lutein and cholesterol on alkyl chain bending in lipid bilayers: A pulse electron spin resonance spin labeling study. Biophys. J. 1996;71:832–839. doi: 10.1016/S0006-3495(96)79284-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Subczynski WK, Markowska E, Sielewiesiuk J. Effect of polar carotenoids on the oxygen diffusion-concentration product in lipid bilayers. An EPR spin label study. Biochim. Biophys. Acta. 1991;1068:68–72. doi: 10.1016/0005-2736(91)90061-c. [DOI] [PubMed] [Google Scholar]
- 65.McNulty HP, Byun J, Lockwood SF, Jacob RF, Mason RP. Differential effects of carotenoids on lipid peroxidation due to membrane interactions: X-ray diffraction analysis. Biochim. Biophys. Acta. 2007;1768:167–174. doi: 10.1016/j.bbamem.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 66.Palozza P, Serini S, Trombino S, Lauriola L, Ranelletti FO, Calviello G. Dual role of beta-carotene in combination with cigarette smoke aqueous extract on the formation of mutagenic lipid peroxidation products in lung membranes: Dependence on pO2. Carcinogenesis. 2006;27:2383–2391. doi: 10.1093/carcin/bgl074. [DOI] [PubMed] [Google Scholar]
- 67.Benting J, Rietveld A, Ansorge I, Simons K. Acyl and alkyl chain length of GPI-anchors is critical for raft association in vitro. FEBS Lett. 1999;462:47–50. doi: 10.1016/s0014-5793(99)01501-x. [DOI] [PubMed] [Google Scholar]
- 68.Moffett S, Brown DA, Linder ME. Lipid-dependent targeting of G proteins into rafts. J. Biol. Chem. 2000;275:2191–2198. doi: 10.1074/jbc.275.3.2191. [DOI] [PubMed] [Google Scholar]
- 69.Ridyard MS, Robbins SM. Fibroblast growth factor-2-induced signaling through lipid raft-associated fibroblast growth factor receptor substrate 2 (FRS2) J. Biol. Chem. 2003;278:13803–13809. doi: 10.1074/jbc.M210245200. [DOI] [PubMed] [Google Scholar]
- 70.Sharma P, Varma R, Sarasij RC, Ira, Gousset K, Krishnamoorthy G, Rao M, Mayor S. Nanoscale organization of multiple GPI-anchored proteins in living cell membranes. Cell. 2004;116:577–589. doi: 10.1016/s0092-8674(04)00167-9. [DOI] [PubMed] [Google Scholar]
- 71.Janes PW, Ley SC, Magee AI. Aggregation of lipid rafts accompanies signaling via the T cell antigen receptor. J. Cell Biol. 1999;147:447–461. doi: 10.1083/jcb.147.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Young RM, Zheng X, Holowka D, Baird B. Reconstitution of regulated phosphorylation of FcεRI by a lipid raft-excluded protein-tyrosine phosphatase. J. Biol. Chem. 2005;280:1230–1235. doi: 10.1074/jbc.M408339200. [DOI] [PubMed] [Google Scholar]
- 73.London E, Brown DA. Insolubility of lipids in Triton X-100: Physical origin and relationship to sphingolipid/cholesterol membrane domains (rafts) Biochim. Biophys. Acta. 2000;1508:182–195. doi: 10.1016/s0304-4157(00)00007-1. [DOI] [PubMed] [Google Scholar]
- 74.Simons K, Toomre D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 75.Simons K, Ikonen E. How cells handle cholesterol. Science. 2000;290:1721–1726. doi: 10.1126/science.290.5497.1721. [DOI] [PubMed] [Google Scholar]
- 76.Ikonen E. Roles of lipid rafts in membrane transport. Curr. Opin. Cell Biol. 2001;13:470–471. doi: 10.1016/s0955-0674(00)00238-6. [DOI] [PubMed] [Google Scholar]
- 77.Seno K, Kishimoto M, Abe M, Higuchi Y, Mieda M, Owada Y, Yoshiyama W, Liu H, Hayashi F. Light- and guanosine 5'-3-O-(thio)triphosphate-sensitive localization of a G protein and its effect on detergent-resistant membrane rafts in rod photoreceptor outer segments. J. Biol. Chem. 2001;276:20813–20816. doi: 10.1074/jbc.C100032200. [DOI] [PubMed] [Google Scholar]
- 78.Boesze-Battaglia K, Dispoto J, Kahoe MA. Association of a photoreceptor-specific tetraspain protein, ROM-1, with triton X-100-resistant membrane rafts from rod outer segment disk membranes. J. Biol. Chem. 2002;277:41843–41849. doi: 10.1074/jbc.M207111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nair KS, Balasubramanian N, Slepak VZ. Signal-dependent translocation of transducin, RGS9-1-Gbeta5L complex, and arrestin to detergent-resistant membrane rafts in photoreceptors. Curr. Biol. 2002;12:421–425. doi: 10.1016/s0960-9822(02)00691-7. [DOI] [PubMed] [Google Scholar]
- 80.Martin RE, Elliott MH, Brush RS, Anderson RE. Detailed characterization of the lipid composition of detergent-resistant membranes from photoreceptor rod outer segment membranes, Invest. Ophthalmol. Vis. Sci. 2005;46:1147–1154. doi: 10.1167/iovs.04-1207. [DOI] [PubMed] [Google Scholar]
- 81.Lincoln JE, Boling M, Parikh AN, Yeb Y, Gilchrist DG, Morse LS. Fas signaling induces raft coalescence that is blocked by cholesterol depletion in human RPE cells undergoing apoptosis. Invest. Ophthalmol. Vis. Sci. 2006;47:2172–2178. doi: 10.1167/iovs.05-1167. [DOI] [PubMed] [Google Scholar]
- 82.Stimson AM, Wigand RD, Anderson RE. Fatty acid and molecular species composition of phospholipids and diacylglycerols from rat retina membranes. Exp. Eye Res. 1991;52:213–218. doi: 10.1016/0014-4835(91)90261-c. [DOI] [PubMed] [Google Scholar]
- 83.Boesze-Battaglia K, Schimmel RJ. Cell membrane lipid composition and distribution: Implications for cell function and lessons learned from photoreceptors and platelets. J. Exp. Biol. 1997;200:2927–2936. doi: 10.1242/jeb.200.23.2927. [DOI] [PubMed] [Google Scholar]
- 84.Polozova A, Litman BJ. Cholesterol dependent recruitment of di22:6-PC by a G protein-coupled receptor into lateral domains. Biophys. J. 2000;79:2632–2643. doi: 10.1016/S0006-3495(00)76502-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang Q, Zhang X, Zhang L, He F, Zhang G, Jamrich M, Wensel TG. Activation-dependent hindrance of photoreceptor G protein diffusion by lipid microdomains. J. Biol. Chem. 2008;283:30015–30024. doi: 10.1074/jbc.M803953200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Anderson RE, Benolken RM, Dudley PA, Landis DJ, Wheeler TG. Polyunsaturated fatty acids of photoreceptor membranes. Exp. Eye Res. 1974;18:205–213. doi: 10.1016/0014-4835(74)90149-3. [DOI] [PubMed] [Google Scholar]
- 87.Mitchell DC, Straume M, Litman BJ. Role of sn-1-saturated, sn-2-polyunsaturated phospholipids in control of membrane receptor conformational equilibrium: Effects of cholesterol and acyl chain composition on the metarhodopsin I-metarhodopsin II equilibrium. Biochemistry. 1992;31:662–670. doi: 10.1021/bi00118a005. [DOI] [PubMed] [Google Scholar]
- 88.Litman BJ, Mitchell DC. A role of phospholipid polyunsaturation in modulating membrane protein function. Lipids. 1996;31:S193–S197. doi: 10.1007/BF02637075. [DOI] [PubMed] [Google Scholar]
- 89.Wisniewska A, Subczynski WK. Distribution of macular xanthophylls between domains in model of photoreceptor outer segment membranes. Free Radic. Biol. Med. 2006;41:1257–1265. doi: 10.1016/j.freeradbiomed.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 90.Wisniewska A, Subczynski WK. Accumulation of macular xanthophylls in unsaturated membrane domains. Free Radic. Biol. Med. 2006;40:1820–1826. doi: 10.1016/j.freeradbiomed.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 91.Landrum JT, Bone RA. Mechanistic evidence for eye diseases and carotenoids. In: Krinsky NI, Mayne ST, Sies H, editors. Carotenoids in Health and Disease. New York: Marcel Dekker; 2004. pp. 445–472. [Google Scholar]
- 92.Różanowska M, Sarna T. Light-induced damage to the retina: Role of rhodopsin chromophore revisited. Photochem. Photobiol. 2005;81:1305–1330. doi: 10.1562/2004-11-13-IR-371. [DOI] [PubMed] [Google Scholar]
- 93.Delmelle M. An investigation of retinal as a source of singlet oxygen. Photochem. Photobiol. 1978;27:731–734. [Google Scholar]
- 94.Kim SR, Nakanishi K, Itagaki Y, Sparrow JR. Photooxidation of A2-PE, a photoreceptor outer segment fluorophore, and protection by lutein and zeaxanthin. Exp. Eye Res. 2006;82:828–839. doi: 10.1016/j.exer.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 95.Brown DA, London E. Structure and origin of ordered lipid domains in biological membranes. J. Membr. Biol. 1998;164:103–114. doi: 10.1007/s002329900397. [DOI] [PubMed] [Google Scholar]
- 96.de Almeida RFM, Fedorov A, Prieto M. Sphingomyelin/phosphatidylcholine/cholesterol phase diagram: Boundaries and composition of lipid rafts. Biophys. J. 2003;85:2406–2416. doi: 10.1016/s0006-3495(03)74664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Socaciu C, Jessel R, Diehl HA. Competitive carotenoid and cholesterol incorporation into liposomes: Effects on membrane phase transition fluidity, polarity and anisotropy. Chem. Phys. Lipids. 2000;106:79–88. doi: 10.1016/s0009-3084(00)00135-3. [DOI] [PubMed] [Google Scholar]
- 98.Wisniewska A, Draus J, Subczynski WK. Is fluid mosaic model of biological membranes fully relevant? Studies on lipid organization in model and biological membranes. Cell. Mol. Biol. Lett. 2003;8:147–154. [PubMed] [Google Scholar]
- 99.Cornell BA, Separovic F. Membrane thickness and acyl chain length. Biochim. Biophys. Acta. 1983;733:189–193. doi: 10.1016/0005-2736(83)90106-2. [DOI] [PubMed] [Google Scholar]
- 100.Aveldano MI. Phospholipid species containing long and very long polyenoic fatty acids remain with rhodopsin after hexane extraction of photoreceptor membranes. Biochemistry. 1988;27:1229–1239. doi: 10.1021/bi00404a024. [DOI] [PubMed] [Google Scholar]
- 101.Suh M, Clandinin MT. 20:5n–3 but not 22:6n–3 is a preferred substrate for synthesis of n–3 very long-chain fatty acids (C24–C36) in retina. Curr. Eye Res. 2005;30:959–968. doi: 10.1080/02713680500246957. [DOI] [PubMed] [Google Scholar]


