Abstract
With growing use of anticancer complementary and alternative medicines (CAMs) worldwide, there is a need to assess and screen commercially available natural products for relative tumoricidal properties under standard experimental conditions. In the current study, we screened and ranked 264 traditional Chinese and Egyptian herbal medicines for tumoricidal potency against malignant neuroblastoma in vitro. The data obtained show that tumoricidal potencies of plants were randomly dispersed throughout similar orders, families and genera under the Division: Magnoliophyta, class: Magnoliopsida, subclasses: Asteridae, Caryophyllidae, Dilleniidae, Hamamelididae, Magnoliidae and Rosidae. The most potent plant extracts (LC50 < 0.08 mg/ml) were prepared from gromwell root also known as ‘Hong Tiao Zi Cao’ (Lithospermum Erythrorhizon) Family (Boraginaceae) > beth root (Trillium Pendulum), Family (Liliaceae) and galbanum (Ferula Galbaniflua), Family (Apiaceae). Gromwell root is traditionally used in the preparation of Chinese medicinal tea. In addition, galbanum was highly regarded for its sacred and medicinal value according to ancient texts and the bible. Future research will be required to isolate and identify chemical constituents within these plants which are responsible for tumoricidal effects.
Keywords: herbs, screening, cancer, bible, galbanum, beth root, Lithospermum erythrorhizon root
INTRODUCTION
There is an increase of individuals seeking self-administration of complementary and alternative medicine (CAM)s worldwide to aid in the fight against cancer. The term CAM generally refers to the use of potential holistic therapeutic practices that contribute to the integrated health of mind, body and spirit. While a number of studies provide helpful statistics on the types of individuals who use CAM modalities (Ferrucci et al., 2009; Owens et al., 2009), there continues to be a lack of established research evaluating the relative efficacy of various CAMs to treat or assist in the treatment of cancer.
With regard to chemoprevention, popular consumer choices are known to include the oral administration of antioxidant supplements, glutamine, arginine, zinc, omega-3, fatty acids, probiotics, prebiotics, garlic and phytochemical rich spices such as turmeric, red chilli, cloves, ginger, nutmeg, fennel, fenugreek and black cumin (Blot, 1997; Rosenberg et al., 2002; Conney, 2003; Kraft, 2009). Once cancer is established and diagnosed, self-administration of CAMs can occur without apprising the primary care physician (Clerici et al., 2009; Richardson et al., 2000; Ohno et al., 2009) often including oral administration of selenium, beta-carotene (van Tonder et al., 2009), herbal teas, green tea (Boon et al., 2000; Yates et al., 2005; Scott et al., 2005; Molassiotis et al., 2006), mistletoe, ginseng, cayenne, chamomile, don quai, feverfew, kava kava, milk thistle, licorice, meadowsweet, motherwort, senna leaf, shepherds purse and stinging nettle (Advance Data, CDC, 2004; Dy et al., 2004; Hu et al., 2005; Kumar et al., 2005; Gerson-Cwilich et al., 2006; Melnick, 2006; Tarhan et al., 2009). Although a number of reports suggest that the prevalence of self-administered CAMs is greatest when the disease prognosis is poor (Kristoffersen et al., 2009) or in instances of pediatric cancers (Genc et al., 2009; Clerici et al., 2009), there remains meager research on the relative potencies or efficacy of CAMs utilized in late stage cancers.
In our first report entitled ‘In Vitro Screening of Tumoricidal Properties of International Medicinal Herbs’ (Mazzio and Soliman, 2009), the tumoricidal potencies of the most popular plant based CAMs were evaluated and ranked. Hundreds of international medicines, herbs and plants which are distributed and available to the public worldwide were also tested. While the data showed significant tumoricidal effects for green tea, feverfew, senna leaf, nutmeg, ginger and clove, promising herbs with the lowest LC50s included: wild yam root, balm of gilead bud, chapparal, frankincense and bakuchi seed. In contrast, many popular CAMs used against cancer such as chamomile, milk thistle, motherwort, shepherd’s purse and fennel seed etc., showed weak, or a lack of, tumoricidal properties where the LC50 exceeded 5 mg/mL in vitro. In the current study, we continue to rank the relative efficacy of a diverse range of Chinese and Egyptian herbal medicines for tumoricidal cytotoxic properties in malignant neuroblastoma under uniform extraction and experimental conditions in vitro.
MATERIALS AND METHODS
Neuro-2A cells (N-2A) cells were purchased from American Type Culture Collection (Manassas, VA). Dulbecco’s modified Eagle medium (DMEM), l-glutamine, fetal bovine serum – heat inactivated (FBS), phosphate buffered saline (PBS), Hank’s balanced salt solution (HBSS) and penicillin/streptomycin were purchased from Fischer Scientific, Mediatech, (Pittsburgh, PA, USA). Chinese herbal medicines were purchased from Mayway Herbs (Oakland, CA) with all other herbs being obtained from Kalyx Natural Marketplace (Camden, NY, USA), Frontier Natural Brands, (Norway, Iowa, USA), Mountain Rose Herbs (Eugene, OR, USA), Scents of the Earth (Cape May, NJ) and Monterey Bay Spice Company (Santa Cruz, CA). Chemicals and research supplies were purchased from Sigma Chemical (St Louis, MO, USA).
Extraction and sample preparation
All crude plants were weighed (0.25 g), pulverized, macerated/homogenized and extracted in 1000 µL of absolute ethanol for 7 days at 4°C (Chakraborty et al., 2004) in the absence of light. A stock solution for each extract was subsequently prepared by dilution to 10 mL with HBSS + 5 mm (N-[2-hydroxyethylpiperazine-N′-[2-ethanesulfonic acid]) (HEPES), pre-adjusted to a pH of 7.4. Dilutions of each experimental extract were prepared from the stock solution in order to span a 1000-fold concentration range with the highest final plating concentration set at 5 mg/mL (w/v).
Cell culture
Neuro-2A cells (N-2A) were used to screen for tumoricidal effects, as they were originally derived from a malignant spontaneous tumor and deemed appropriate for evaluation of chemotherapy drugs (Klebe and Ruddle, 1969; Finklestein et al., 1975; Mazzio et al., 2003). Briefly, N-2A cells were cultured in DMEM containing phenol red supplemented with 10% FBS, 4 mm l-glutamine, 20 µm sodium pyruvate and penicillin/streptomycin (100 Units/0.1 mg, mL). The cultures were maintained at 37°C in 5% CO2/atmosphere and sub-cultured every 2–3 days. Experimental plating media consisted of DMEM (- phenol red) supplemented with 1.8% FBS, penicillin/streptomycin (100 Units/0.1 mg/mL), 20 µm sodium pyruvate and 4 mm l-glutamine. The cells were plated in 96-well plates at a density of ~0.5 × 106 cells/mL. A three tier process was established where all extracts were evaluated at 0.5–5 mg/mL (tier 1). Those inducing cell death at any level were then re-examined at (0.1–0.5 mg/mL) (tier 2), and those inducing cell death at any level of tier 2, where further evaluated at tier 3 (.05–0.1 mg/mL). Any extract that was lethal within this range was re-tested, and further assessed at lower concentrations.
Evaluation of cellular toxicity
Cell viability was assessed by resazurin-almar blue indicator dye as described previously (Mazzio et al., 2003). Experimental blanks and extract controls were run simultaneously with samples, in order to detect any interferences or reactivity with the dye or cell viability. Briefly, almar blue was dissolved in sterile PBS (0.5 mg/mL) and the cell viability was assessed by quantifying the reduction of the dye to its corresponding fluorescent intermediate – resorufin. The use of fluorescence for cell viability eliminates significant interferences introduced by experimental compounds themselves, otherwise presented during UV detection using spectrophotometric dyes. The fluorescence intensity was analysed using a microplate fluorometer – Model 7620 version 5.02 (Cambridge Technologies Inc, Watertown, Mass) with settings held at [550/580], [excitation/emission].
Evaluation of cell death
Fluorescein diacetate (FD) was used to corroborate the loss of cell viability (Mazzio et al., 2003). FD is cleaved by viable esterases in living cells where a loss of fluorescence is indicative of cell death. Samples were analysed photographically using an Olympus IX-70 inverted microscope and images were captured using a MD35 Electronic Eyepiece (Zhejiang Jincheng Science and Technology Co., Ltd, China) with acquisition using C-imaging systems confocal PCI-Simple software (Compix Inc. Cranberry Township, PA, USA).
Data analysis
Statistical analysis was performed using both Origin Lab Scientific Evaluation Software (version 7.5 SR6) (Original Lab Corp., Northampton, MA, USA) and Graphpad Prism (version 3.0), (Graphpad Software Inc. San Diego, CA, USA). The lethal concentrations (LC50) were established from dose-dependent data with Origin Lab 7.5 SR6 and significance of difference between the groups was assessed using a one-way analysis of variance (ANOVA), followed by a Tukey post-hoc means comparison test using Graphpad Prism Ver 3.0 software.
RESULTS
The data in Table 1 list each natural product that was examined by the common name and respective LC50 which was calculated from dose dependent toxicity in malignant neuroblastoma across three tiers and nine concentrations ranging from 0.005–5 mg/mL (n = 4). A taxonomical cross-reference with specific Latin names, families and plant parts are presented in Table 2. The data are listed with the most potent tumoricidal properties first and separated into five classifications based on LC50 where Category 1 (Table 1A) list the strongest agents LC50 =[0.015–0.553 mg/mL]; Category 2 (Table 1B) moderate to strong LC50 =[0.554–1.504 mg/mL]; Category 3 (Table 1C), moderate LC50 =[1.509–3.026 mg/mL]; Category 4 (Table 1D), weak to moderate LC50 = [3.03–4.47 mg/mL] and Category 5 (Table 1E), weak – listing those with no tumoricidal effects and an LC50 > 5.0 mg/mL.
Table 1.
The effect of natural products on cell viability in murine neuroblastoma cells originally derived from a spontaneous malignant tumor. The data represent the Common English name or Chinese name and the LC50 (mg/mL) calculated from 3–9 concentrations spanning a thousand-fold dilution range (n = 4)
| A. Anti-Cancer Screen – Category 1: Strongest | |||||
|---|---|---|---|---|---|
LC50 = [0.015–0.553 mg/ml] 
| |||||
| Gromwell root | [0.015] | Burningbush | [0.375] | Cao Dou Kou | [0.531] |
| Beth root | [0.032] | Silk Tree [1] | [0.378] | Lilytree | [0.532] |
| Galbanum | [0.078] | Akebia | [0.401] | Baikal Scullcap | [0.533] |
| Asafetida | [0.253] | Damask Rose | [0.451] | Ji Xue Teng | [0.534] |
| Yuan Zhi | [0.255] | Southern Chinese Pine | [0.464] | Szechuan Pepper | [0.538] |
| Zhi Mu | [0.261] | California Yerba Santa | [0.485] | Official Burnet | [0.545] |
| Tumeric | [0.269] | Common Tansy | [0.502] | Daisy | [0.548] |
| White Edge Morning Glory | [0.28] | Woodland Figwort | [0.505] | Ramanas Rose | [0.549] |
| Locust | [0.287] | Common Hop | [0.508] | Moutain Peony | [0.549] |
| Chinese Rhubarb | [0.353] | Common Selfheal | [0.527] | Cinnamon Twig | [0.55] |
| Common Pricklyash | [0.373] | Oriental Arbovitae | [0.528] | Fang Feng | [0.553] |
| B. Anti-Cancer Screen – Category 2: Moderate to Strong | |||||
|---|---|---|---|---|---|
| LC50 = [0.554–1.504 mg/ml] → | |||||
| Dang Gui (Wei) | [0.554] | Florida fishpoison tree | [0.746] | Jing Jie | [1.084] |
| Korean Epimedium | [0.554] | Sassafras | [0.784] | Foetid Bugbane | [1.108] |
| Hou Po | [0.563] | Bi Xie | [0.89] | Oregano | [1.123] |
| Cow Cockle | [0.577] | Gravelroot | [0.892] | Partidgeberry | [1.126] |
| Clove Bud | [0.577] | Thoroughwort | [0.92] | Hu Po (Succinum Resin) | [1.164] |
| Himalayan Teasel Root | [0.6] | Chinese Rhubarb | [0.921] | Yin Chai Hu | [1.197] |
| Lesser Galangal | [0.603] | Evodia Fruit | [0.941] | Soybean | [1.214] |
| Wu Jia Pi | [0.61] | Qian Hu | [0.951] | Japanese Gentian | [1.237] |
| Hai Feng Teng | [0.61] | Sacred Lotus | [0.968] | European Dogbane | [1.263] |
| Fang Ji | [0.622] | Cinnamon Bark | [0.969] | Sha Ren Guang | [1.323] |
| Cultivated Radish | [0.627] | Qiang Hua | [0.972] | Cornmint | [1.329] |
| Arbovitae | [0.629] | Indian Madder | [0.982] | Chuan Hua Jiao | [1.357] |
| Common Mullein | [0.665] | Sappanwood | [0.983] | Japenese Persimmon | [1.377] |
| Huang Lian | [0.665] | Licorice Root [1] | [1.003] | Madder | [1.438] |
| Common Juniper | [0.676] | Yi Zhi Ren | [1.008] | Balloon Flower | [1.448] |
| Queens Delight | [0.682] | Red Sage | [1.027] | Licorice Root [2] | [1.478] |
| Cang Zhu | [0.704] | Sandalwood | [1.082] | Sang Ji Sheng | [1.49] |
| Cultivated Radish | [0.741] | Black Cardamom | [1.082] | Flowered Wintergreen | [1.504] |
| C. Anti-Cancer Screen – Category 3: Moderate | |||||
|---|---|---|---|---|---|
LC50 = [1.509–3.026 mg/ml] 
| |||||
| Largebracted Plantain | [1.509] | False Starwort | [2.249] | Hu Huang Lian | [2.571] |
| Hairy Agrimony | [1.519] | Mosla | [2.253] | Egyptian Senna | [2.578] |
| Loosestrife | [1.562] | British Yellowhead | [2.256] | Flowering Quince | [2.656] |
| Ze Xie | [1.601] | Balsampear | [2.262] | Tangerine (peel immature) | [2.695] |
| Tian San Qi | [1.8] | Sour Orange | [2.317] | Blackberry Lily | [2.723] |
| Chinese Corktree | [1.801] | Fu Pen Zi | [2.334] | Dang Gui (Tou) | [2.824] |
| Bai Zhu | [1.919] | Giant Puffball | [2.369] | Wine Grape | [2.841] |
| Evergreen Spice Bush | [1.943] | Japanese Pagoda Tree | [2.384] | Xiang Jia Pi | [2.901] |
| Florist’s Daisy | [2.007] | Silk Tree [2] | [2.453] | Zhang Nao | [2.958] |
| Greater Burdock | [2.147] | Textile Bamboo | [2.458] | Gumweed | [2.99] |
| Monnier’s Snowparsley | [2.159] | Du Huo | [2.471] | Canadian Wildginger | [3.026] |
| D. Anti-Cancer Screen – Category 4: Weak to Moderate | |||||
|---|---|---|---|---|---|
LC50 = [3.03–4.47 mg/ml] 
| |||||
| Richweed | [3.03] | Dang Gui | [3.598] | Indian Trumpet Flower | [4.087] |
| Marijuana | [3.071] | Chinese Violet | [3.629] | Bai Zhi | [4.122] |
| Poontahai | [3.071] | Licorice Root [3] | [3.761] | Oriental Arbovitae | [4.154] |
| Yin Chen Hao | [3.15] | Bai Jiang Cao | [3.767] | Confederate Jasmine | [4.299] |
| Narrowleaf Cattail | [3.166] | Mao Dong Qing | [3.773] | Bitter Lettuce | [4.315] |
| Cherokee Rose | [3.196] | Tangerine (seed) | [3.783] | Cape Jasmine | [4.389] |
| Dandelion | [3.292] | Horseradish | [3.785] | Simple Leaf Chastetree | [4.393] |
| Sweet Wormwood | [3.344] | Formosan Gum | [3.794] | European Centaury | [4.47] |
| Beefsteakplant | [3.347] | Scouringrush Horsetail | [3.929] | ||
| Bitter Ash | [3.431] | Cocklebur | [3.935] | ||
| Peach | [3.514] | Spike Moss | [3.987] | ||
| Loquat | [3.558] | Sorrell | [3.997] | ||
| E. Anti-Cancer Screen – Category 5: Weak | |||
|---|---|---|---|
| LC50 > [5.0 mg/ml] | |||
| American Plum | Da Quing Ye | Little Hogweed | Shi Chang Pu |
| Asiatic Dogwood | Da Zao | Locust | Siberian Ginseng |
| Bai Bu | Dai Zhe Shi (Haematitum) | Long Chi (Dens Draconis) | Silver Cock’s Comb |
| Bai Dou Kou | Devils Horsewhip | Long Gu (Os Draconis) | Snake Needle Grass |
| Bai Fu Zi | Dill | Longan | Snow Fungus |
| Beefsteakplant | Duckmeat | Lou Lu | Solomon’s Seal |
| Big Leaf Gentian | Dwarf Lilyturf | Mang Xiao (Natrii Sulfas) | Sour Orange |
| Bile Arisaema | E Zhu | Matrimony Vine | Spiketail |
| Cao Wu (Zhi) | English Walnut | Mayapple | Suan Zao Ren |
| Carmichael’s Monkshood | European Lily of the Valley | Mexican Tea | Swallow-wort |
| Chaun Bei Mu | False Daisy | Ming Fan (Alumen) | Tangerine (peel) |
| Chi Shi Zhi (Halloysitum Rubrum | Fringed Pink | Mu Tong | Thistle |
| Chinese Asparagus | Fuller’s Earth | No-Binu | Tian Ha |
| Chinese Cinquefoil | Ge Gen | Nutgrass | Tiger’s Claw |
| Chinese Cobra Lily | Gou Teng | Paper Mulberry | Ting Li Zi |
| Chinese Cucumber (fruit) | Gu Jing Cao | Peach | Tuckahoe [1] |
| Chinese Cucumber (peel) | Herb of the Cross | Puntinpole Bamboo | Tuckahoe [2] |
| Chinese Cucumber (seed) | Hong Kong Lily | Qing Dai | Waxgourd |
| Chinese Cucumber (root) | Hua Shi (Talcum) | Qing Feng Teng | While Mulberry (fruit) |
| Chinese Haw | Huai Niu Xi | Qing Meng Shi (Lapis Chloriti) | While Mulberry (leaf) |
| Chinese Lobelia | Huang Jing | Rangoon Creeper | While Mulberry (root bark) |
| Chinese Motherwort | Huang Qin | Red Tangerine Peel | While Mulberry (twig) |
| Chinese Peony | Hyacinthbean | Ricebean (seed) [1] | Ya Dan Zi |
| Chinese Yam | Indian Mulberry | Ricebean (seed) [2] | Yan Hu Suo |
| Chuan Niu Xi | Japanese Apricot | Ricebean (root) | Yang Qi Shi (Actinolitum) |
| Ci Shi (Magnetitum) | Japanese Bush Cherry | Ricebean (fruit) | Yu Jin |
| Citron | Japanese Pagoda Tree | Sacred Lotus | Zhe Bei Mu |
| Cluster Mallow | Japenese Climbing Fern | San Leng | Zhu Ling |
| Common Barley | Ji Gu Cao | Sesame Roots | Zi Ran Tong (Pyritum) |
| Common Rush | Job’s Tears | Sesame Seed | Zi Shi Ying (Fluoritum) |
| Common Wheat | Largebracted Plantain | Sha Yuan Zi | |
| Crowdipper | Ling Zhi (Hong) | Shan Dou Gen | |
Table 2.
Taxonomy of natural products listed in Table 1A–E. in alphabetical order by Common Name, [Family]; Genus Species and Parts
| Akebia [Lardizabalaceae] Akebia trifoliata fruit |
| American Plum [Rosaceae] Prunus armeniaca seed |
| Arbovitae [Cupressaceaea] Thuja occidentalis Twigs |
| Asafetida [Apiaceae] Ferula Assa-Foetida |
| Asiatic Dogwood [Cornaceae] Cornus officinalis fruit |
| Bai Bu [Stemonaceae] Stemona sessilifolia root |
| Bai Dou Kou [Zingiberaceae] Amomum kravanh fruit |
| Bai Fu Zi [Araceae] Typhonium giganteum rhizome |
| Bai Jiang Cao [Dipsacaceae] Patrinia villosa herb |
| Bai Zhi [Apiaceae] Angelica dahurica root |
| Bai Zhu [Asteraceae] Atractylodes macrocephala rhizome |
| Baikal Scullcap [Lamiaceae] Scutellaria baicalensis root |
| Balloon Flower [Campanulaceae] Platycodon grandiflorum root |
| Balsampear [Cucurbitaceae] Momordica cochinchinensis seed |
| Beefsteakplant [Lamiaceae] Perilla frutescens fruit |
| Beefsteakplant [Lamiaceae] Perilla frutescens stem |
| Beth root [Liliaceae] Trillium pendulum root |
| Bi Xie [Dioscoreaceae] Dioscorea Collettii var hypoglauca rhizome |
| Big Leaf Gentian [Gentianaceae] Gentiana macrophylla root |
| Bile Arisaema [Araceae] Arisaema cum bile |
| Bitter Ash [Simaroubaceae] Picraena excelsa |
| Bitter Lettuce [Asteraceae] Lactuca virosa latex, leaves |
| Black Cardamom [Zingiberaceae] Amomum tsao-ko fruit |
| Blackberry Lily [Iridaceae] Belamcanda chinensis rhizome |
| British Yellowhead [Asteraceae] Inula britannica flower |
| Burningbush [Chenopodiaceae] Kochia scoparia fruit |
| California Yerba Santa [Hydrophyllaceae] Eriodictyon californicum |
| Canadian Wildginger [Aristolochiaceae] Asarum canadense root rhizome |
| Cang Zhu [Asteraceae] Atractylodes lancea rhizome |
| Cao Dou Kou [Zingiberaceae] Alpinia katsumadae seed |
| Cao Wu (Zhi) [Ranunculaceae] Aconitum kusnezoffi i root prepared |
| Cape Jasmine [Rubiaceae] Gardenia jasminoides fruit |
| Carmichael’s Monkshood [Ranunculaceae] Aconitum carmichaeli root prep |
| Chaun Bei Mu [Ranunculaceae] Fritillaria cirrhosa bulb |
| Cherokee Rose [Rosaceae] Rosa Laevigata fruit |
| Chinese Asparagus [Liliaceae] Asparagus cochinchinensis tuber |
| Chinese Cinquefoil [Rosaceae] Potentilla chinensis herb |
| Chinese Cobra Lily [Araceae] Arisaema erubescens rhizome |
| Chinese Corktree [Rutaceae] Phellodendron chinense bark |
| Chinese Cucumber (fruit) [Cucurbitaceae] Trichosanthes kirilowii fruit |
| Chinese Cucumber (peel) [Cucurbitaceae] Trichosanthes kirilowii peel |
| Chinese Cucumber (root) [Cucurbitaceae] Trichosanthes kirilowii root |
| Chinese Cucumber (seed) [Cucurbitaceae] Trichosanthes kirilowii seed |
| Chinese Haw [Rosaceae] Crataegus pinnatifida fruit |
| Chinese Lobelia [Campanulaceae] Lobelia chinensis herb |
| Chinese Motherwort [Lamiaceae] Leonurus japonicus herb |
| Chinese Peony [Paeoniaceae] Paeonia lactiflora root |
| Chinese Rhubarb [Polygonaceae] Rheum palmatum root |
| Chinese Rhubarb [Polygonaceae] Rheum palmatum root & rhizome |
| Chinese Violet [Violaceae] Viola yezoensis herb |
| Chinese Yam [Dioscoreaceae] Dioscorea oppositifolia rhizome |
| Chuan Hua Jiao [Rutaceae] Zanthoxylum bungeanum peel |
| Chuan Niu Xi [Amaranthaceae] Cyathula officinalis root |
| Cinnamon Bark [Lauraceae] Cinnamomum cassia bark |
| Cinnamon Twig [Lauraceae] Cinnamomum cassia twig |
| Citron [Rutaceae] Citrus medica finger – fruit |
| Clove Bud [Myrtaceae] Eugenia caryophyllata flower bud |
| Cluster Mallow [Malvaceae] Malva verticillata seed |
| Cocklebur [Asteraceae] Xanthium sibiricum fruit |
| Common Barley [Poaceae] Hordeum vulgare fruit |
| Common Hop [Cannabaceae] Humulus lupulus |
| Common Juniper [Cupressaceaea] Juniperus communis |
| Common Mullein [Scrophulariaceae] Verbascum thapsus flower, leaf |
| Common Pricklyash [Rutaceae] Zanthoxylum americanum bark, berries |
| Common Rush [Juncaceae] Juncus effusus |
| Common Selfheal [Lamiaceae] Prunella vulgaris |
| Common Tansy [Asteraceae] Tanacetum vulgare flowering tops |
| Common Wheat [Poaceae] Triticum aestivum fruit |
| Confederate Jasmine [Apocynaceae] Trachelospermum jasminoides stem |
| Cornmint [Lamiaceae] Mentha haplocalyx herb |
| Cow Cockle [Caryophyllaceae] Vaccaria segetalis seed |
| Crowdipper [Araceae] Pinellia ternata rhizome |
| Cultivated Radish [Brassicaceae] Raphanus sativus bark |
| Cultivated Radish [Brassicaceae] Raphanus sativus seed |
| Da Quing Ye [Polygonaceae] Polygonum tinctorium leaf |
| Da Zao [Rhamnaceae] Ziziphus jujuba fruit-black |
| Daisy [Asteraceae] Chrysanthemum indicum flower |
| Damask Rose [Rosaceae] Rosa damascena |
| Dandelion [Asteraceae] Taraxacum mongolicum herb |
| Dang Gui [Apiaceae] Angelica sinensis root-palm sliced |
| Dang Gui (Tou) [Apiaceae] Angelica sinensis root-head |
| Dang Gui (Wei) [Apiaceae] Angelica sinensis root-tail |
| Devils Horsewhip [Amaranthaceae] Achyranthes aspera root |
| Dill [Apiaceae] Anethum graveolens seeds, leaf |
| Du Huo [Apiaceae] Angelica pubescens root |
| Duckmeat [Lemnaceae] Spirodela polyrhiza |
| Dwarf Lilyturf [Liliaceae] Ophiopogon japonicus tuber |
| E Zhu [Zingiberaceae] Curcuma wenyujin rhizome |
| Egyptian Senna [Fabaceae] Cassia angustifolia leaf |
| English Walnut [Juglandaceae] Juglans regia seed |
| European Centaury [Gentianaceae] Centaurium erythraea root, leaf, flower |
| European Dogbane [Apocynaceae] Apocynum venetum herb |
| European Lily of the Valley [Liliaceae] Convallaria majalis flower, leaf |
| Evergreen Spice Bush [Lauraceae] Lindera aggregata root |
| Evodia Fruit [Rutaceae] Evodia rutaecarpa fruit |
| False Daisy [Asteraceae] Eclipta prostrata herb |
| False Starwort [Caryophyllaceae] Pseudostellaria heterophylla root |
| Fang Feng [Apiaceae] Saposhnikovia divaricata root |
| Fang Ji [Menispermaceae] Stephania tetranda root |
| Florida fishpoison tree [Fabaceae] Piscidia erythrina root bark |
| Florist’s Daisy [Asteraceae] Chrysanthemum morifolium flower |
| Flowered Wintergreen [Pyrolaceae] Pyrola calliantha herb |
| Flowering Quince [Rosaceae] Chaenomeles speciosa fruit |
| Foetid Bugbane [Ranunculaceae] Cimicifuga feotida rhizome |
| Formosan Gum [Hamamelidaceae] Liquidambar formosana fruit |
| Fringed Pink [Caryophyllaceae] Dianthus superbus herb |
| Fu Pen Zi [Rosaceae] Rubus chingii fruit |
| Galbanum [Apiaceae] Ferula Galbaniflua |
| Ge Gen [Fabaceae] Pueraria thomsonii root |
| Giant Puffball [Lycoperdaceae] Calvatia gigantea |
| Gou Teng [Rubiaceae] Uncaria rhynchophylla twig and thorn |
| Gravelroot [Asteraceae] Eupatorium purpureum root |
| Greater Burdock [Asteraceae] Arctium lappa fruit |
| Gromwell root [Boraginaceae] Lithospermum erythrorhizon root |
| Gu Jing Cao [Eriocaulaceae] Eriocaulon buergerianum flos |
| Gumweed [Asteraceae] Grindelia camporum leaf, flowering top |
| Hai Feng Teng [Piperaceae] Piper kadsura stem |
| Hairy Agrimony [Rosaceae] Agrimonia pilosa herb |
| Herb of the Cross [Verbenaceae] Verbena officinalis herb |
| Himalayan Teasel Root [Dipsacaceae] Dipsacus asperoides root |
| Hong Kong Lily [Liliaceae] Lilium brownii bulb |
| Horseradish [Brassicaceae] Armoracia rusticana root, leaf |
| Hou Po [Magnoliaceae] Magnolia officinalis bark |
| Hu Huang Lian [Plantaginaceae] Picrorhiza scropulariaeflora rhizome |
| Huai Niu Xi [Amaranthaceae] Achyranthes bidentata root |
| Huang Jing [Liliaceae] Polygonatum sibiricum rhizome |
| Huang Lian [Ranunculaceae] Coptis chinensis rhizome |
| Huang Qin [Fabaceae] Astragalus membranaceus root |
| Hyacinthbean [Fabaceae] Lablab purpureus seed |
| Indian Madder [Rubiaceae] Rubia cordifolia root & rhizome |
| Indian Mulberry [Rubiaceae] Morinda officinalis root |
| Indian Trumpet Flower [Bignoniaceae] Oroxylum indicum seed |
| Japanese Apricot [Rosaceae] Prunus mume fruit |
| Japanese Bush Cherry [Rosaceae] Prunus japonica seed |
| Japanese Gentian [Gentianaceae] Gentiana scabra root |
| Japanese Pagoda Tree [Fabaceae] Sophora japonica flower |
| Japanese Pagoda Tree [Fabaceae] Sophora japonica fruit |
| Japenese Climbing Fern [Lygodiaceae] Lygodium japonicum spore |
| Japenese Persimmon [Ebenaceae] Diospyros Kaki calyx & receptacle |
| Ji Gu Cao [Fabaceae] Abrus cantoniensis herb |
| Ji Xue Teng [Fabaceae] Spatholobus suberectus vine |
| Jing Jie [Lamiaceae] Schizonepeta tenuifolia herb |
| Job’s Tears [Poaceae] Coix lachryma jobi seed |
| Korean Epimedium [Berberidaceae] Epimedium koreanum |
| Largebracted Plantain [Plantaginaceae] Plantago asiatica |
| Largebracted Plantain [Plantaginaceae] Plantago asiatica seed |
| Lesser Galangal [Zingiberaceae] Alpinia offi cinarum rhizome |
| Licorice Root [1] [Fabaceae] Glycyrrhiza uralensis root prepared |
| Licorice Root [2] [Apiaceae] Ligusticum chuanxiong zhizome |
| Licorice Root [3] [Apiaceae] Ligusticum sinese root |
| Lilytree [Magnoliaceae] Magnolia denudata flower |
| Ling Zhi (Hong) [Gandodermataceae] Ganoderma lucidum fungus – red |
| Little Hogweed [Portulacaceae] Portulaca oleracea herb |
| Locust [Fabaceae] Gleditsia sinesis fruit |
| Locust [Fabaceae] Gleditsia sinesis spine |
| Longan [sapindaceae] Dimocarpus longan fruit |
| Loosestrife [Primulaceae] Lysimachia christiniae |
| Loquat [Rosaceae] Eriobotrya japonica leaf |
| Lou Lu [Asteraceae] Rhaponticum uniflorum root |
| Madder [Rubiaceae] Rubia tinctorum root |
| Mao Dong Qing [Aquifoliaceae] Ilex pubescens root |
| Marijuana [Cannabaceae] Cannabis sativa fruit |
| Matrimony Vine [Solanaceae] Lycium barbarum fruit |
| Mayapple [Berberidaceae] Podophyllum peltatum Root |
| Mexican Tea [Chenopodiac eae] Chenopodium ambrosiodes |
| Monnier’s Snowparsley [Apiaceae] Cnidium monnieri fruit |
| Mosla [Lamiaceae] Mosla chinesis herb |
| Moutain Peony [Paeoniaceae] Paeonia suffructicose root, bark |
| Mu Tong [Ranunculaceae] Clematis armandii stem |
| Narrowleaf Cattail [Typhaceae] Typha angustifolia pollen |
| No-Binu [Liliaceae] Allium macrostemon bulb |
| Nutgrass [Cyperaceae] Cyperus rotundus rhizome |
| Official Burnet [Rosaceae] Sanguisorba officinalis root |
| Oregano [Lamiaceae] Origanum vulgare |
| Oriental Arbovitae [Cupressaceaea] Platycladus orientalis seed |
| Oriental Arbovitae [Cupressaceaea] Platycladus orientalis twig/leaf |
| Paper Mulberry [Moraceae] Broussonetia papyrifera fruit |
| Partidgeberry [Rubiaceae] Mitchella repens berries, aerial parts |
| Peach [Rosaceae] Prunus persica |
| Peach [Rosaceae] Prunus persica seed |
| Poontahai [Sterculiaceae] Sterculia Lychnophorum seed |
| Puntinpole Bamboo [Poaceae] Bambusa tuldoides shavings |
| Qian Hu [Apiaceae] Peucedanum praeruptorum root |
| Qiang Hua [Apiaceae] Notopterygium incisium root |
| Qing Dai [Polygonaceae] Polygonum tinctorium levis |
| Qing Feng Teng [Menispermaceae] Sinomenium acutum stem |
| Queens Delight [Euphorbiaceae] Stillingia sylvatica root |
| Ramanas Rose [Rosaceae] Rosa rugosa flower |
| Rangoon Creeper [Combretaceae] Quisqualis indica fruit |
| Red Sage [Lamiaceae] Salvia miltiorrhiza root |
| Red Tangerine Peel [Rutaceae] Citrus rubrum peel |
| Ricebean (fruit) [Poaceae] Orzya sativa fruit |
| Ricebean (root) [Poaceae] Oryza sativa root |
| Ricebean (seed) [1] [Phaseolus] Phaseolus calcaratus seed |
| Ricebean (seed) [2] [Phaseolus] Phaseolus radiatus seed |
| Richweed [Lamiaceae] Collinsonia canadensis root, leaf |
| Sacred Lotus [Nelumbonaceae] Nelumbo nucifera plumule |
| Sacred Lotus [Nelumbonaceae] Nelumbo nucifera seed – white |
| San Leng [Sparganiaceae] Sparganium stoloniferum rhizome |
| Sandalwood [Santalaceae] Santalum album wood |
| Sang Ji Sheng [Loranthaceae.] Taxillus chinensis stem & leaf |
| Sappanwood [Fabaceae] Caesalpinia sappan wood |
| Sassafras [Lauraceae] Sassafras officinale |
| Scouringrush Horsetail [Equisetaceae] Equisetum hyemale herb |
| Sesame Roots [Pedaliaceae] Sesamum indicum seeds, roots |
| Sesame Seed [Pedaliaceae] Sesamum indicum seed |
| Sha Ren Guang [Zingiberaceae] Amomum villosum fruit |
| Sha Yuan Zi [Fabaceae] Astragalus complanatus seed |
| Shan Dou Gen [Fabaceae] Sophora tonkinensis root |
| Shi Chang Pu [Acoraceae] Acorus tatarinowii rhizome |
| Siberian Ginseng [Araliaceae] Acanthopanax senticosus root |
| Silk Tree [1] [Fabaceae] Albizia julibrissin flower |
| Silk Tree [2] [Fabaceae] Albizia julibrissin bark |
| Silver Cock’s Comb [Amaranthaceae] Celosia argentea seed |
| Simple Leaf Chastetree [Verbenaceae] Vitex trifolia fruit |
| Snake Needle Grass [Rubiaceae] Oldenlandia diffusa herb |
| Snow Fungus [Tremellaceae] Tremella fuciformis |
| Solomon’s Seal [Liliaceae] Polygonatum odoratum rhizome |
| Sorrell [Polygonaceae] Rumex acetosella aerial parts |
| Sour Orange [Rutaceae] Citrus aurantium fruit – immature |
| Sour Orange [Rutaceae] Citrus aurauntium fruit – ripe |
| Southern Chinese Pine [Pinaceae] Pinus tabulaeformis modular branch |
| Soybean [Fabaceae] Glycine max seed-prepared |
| Spike Moss [Selaginellaceae] Selaginella doederleinii herb |
| Spiketail [Stachyuraceae] Stachyurus himalaicus |
| Suan Zao Ren [Rhamnaceae] Ziziphus jujuba seed – prepared |
| Swallow-wort [Asclepiadaceae] Cynanchum stauntonii rhizome |
| Sweet Wormwood [Asteraceae] Artemesia annua herb |
| Szechuan Pepper [Rutaceae] Zanthoxylum simulans |
| Tangerine (peel immature) [Rutaceae] Citrus reticulata peel-immature |
| Tangerine (peel) [Rutaceae] Citrus reticulata peel |
| Tangerine (seed) [Rutaceae] Citrus reticulata seed |
| Textile Bamboo [Poaceae] Bambusa textilis tabasheer |
| Thistle [Asteraceae] Cirsium setosum herb |
| Thoroughwort [Asteraceae] Eupatorium fortunei herb |
| Tian Ha [Orchidaceae] Gastrodia elata rhizome |
| Tian San Qi [Araliaceae] Panax notoginseng root |
| Tiger’s Claw [Fabaceae] Erythrina variegata bark |
| Ting Li Zi [Brassicaceae] Lepidium apetalum seed |
| Tuckahoe [1] [Polyporaceae] Poria cocos – spirit |
| Tuckahoe [2] [Polyporaceae] Poria cocus |
| Tumeric [Zingiberaceae] Curcuma longa |
| Waxgourd [Cucurbitaceae] Benincasa hispida seed |
| White Mulberry (fruit) [Moraceae] Morus alba fruit |
| White Mulberry (leaf) [Moraceae] Morus alba leaf |
| White Mulberry (root bark) [Moraceae] Morus alba root-bark |
| White Mulberry (twig) [Moraceae] Morus alba twig |
| White Edge Morning Glory [Convolvulaceae] Pharbitis nil seed |
| Wine Grape [Vitaceae] Vitis vinifera seed, stem, leaf, fruit |
| Woodland Figwort [Scrophulariaceae] Scrophularia nodosa aerial tops |
| Wu Jia Pi [Araliaceae] Acanthopanax gracilistylus root-bark |
| Xiang Jia Pi [Asclepiadaceae] Periploca sepium root-bark |
| Ya Dan Zi [Simaroubaceae] Brucea javanica fruit |
| Yan Hu Suo [Fumariaceae] Corydalis yanhusuo rhizome |
| Yi Zhi Ren [Zingiberaceae] Alpinia oxyphylla fruit |
| Yin Chai Hu [Caryophyllaceae] Stellaria dichotoma root |
| Yin Chen Hao [Asteraceae] Artemisia capillaris herb |
| Yu Jin [Zingiberaceae] Curcuma phaeocaulis tuber |
| Yuan Zhi [Polygonaceae] Polygala tenufolia root |
| Ze Xie [Alismataceae] Alisma orientale rhizome |
| Zhang Nao – Camphor |
| Zhe Bei Mu [Liliaceae] Fritillaria thunbergii bulb |
| Zhi Mu [Liliaceae] Anemarrhena asphodeloides rhizome |
| Zhu Ling [Polyporaceae] Polyporus umbellatus |
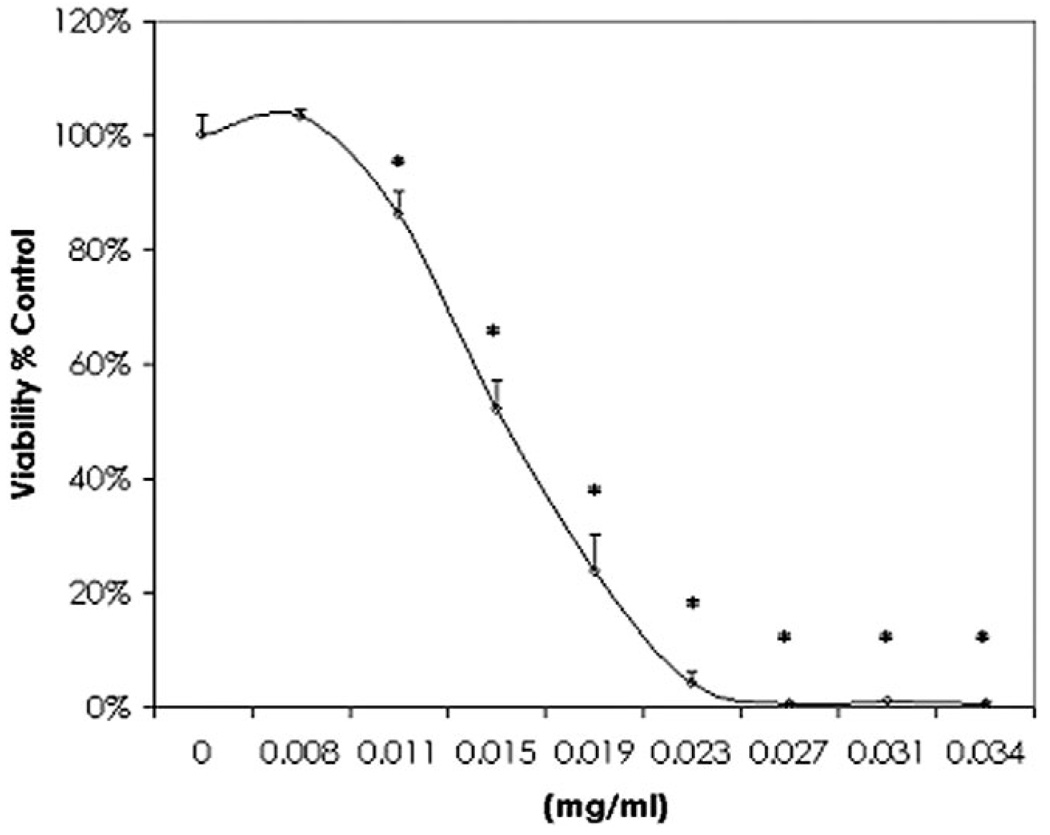
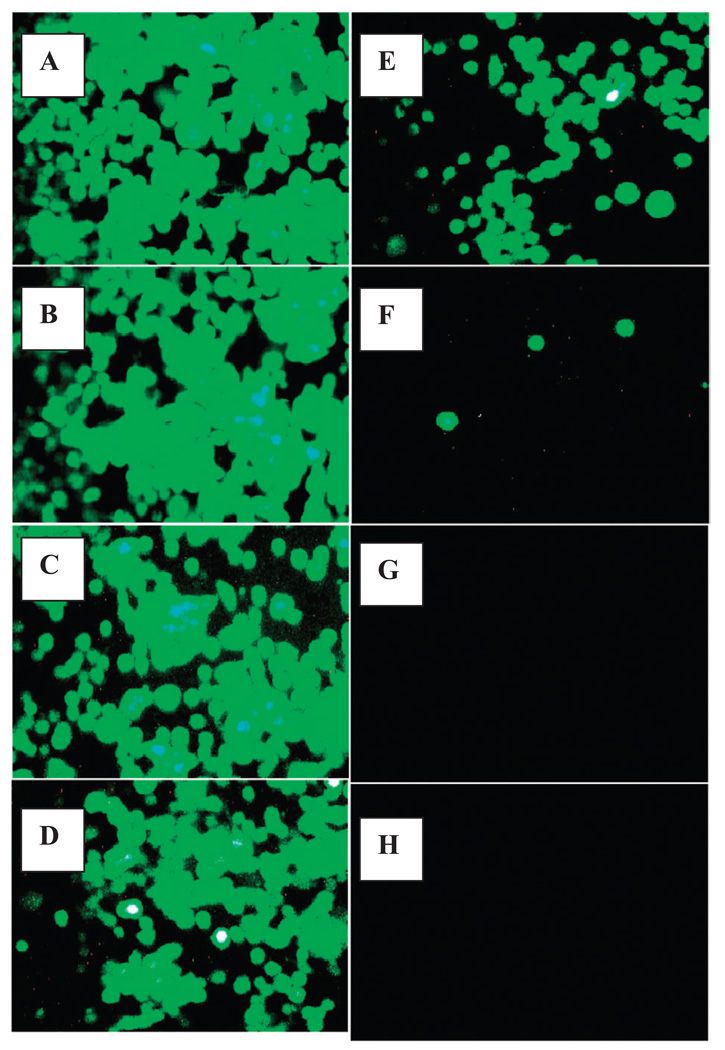
The data obtained show that less than 1% of extracts screened were capable of inducing cell death at <0.1 mg/mL. The most potent plants were ‘Hong Tiao Zi Cao’ (Lithospermum erythrorhizon root) Siebold & Zucc., common name: gromwell root > (Trillium Pendulum) Willd, common name: beth root and (Ferula galbaniflua), common name: galbanum). Figure 1 (Almar blue viability test) and Fig. 2 (FD photographic validation of viability) show that the lethal effects of Lithospermum erythrorhizon root in tumor cells were observed at very low concentration. In order to assess the water soluble fraction of Lithospermum erythrorhizon root due to its general consumer use as a tea, an herbal tea was prepared by boiling powdered root in sterile water for 5 min, then brought to room temperature. The data obtained show that ethanol extracts were identical in strength to the prepared water extract where the LC50 of gromwell root tea was 0.014 mg/mL and the gromwell root extract was 0.015 mg/mL (data not shown).
Figure 1.
The effects of gromwell root on the loss of cell viability in murine neuroblastoma cells derived from a malignant spontaneous tumor as determined with almar blue. The data are expressed as the mean ± SEM (n =4), and represent viability as % control.
Figure 2.
The effect of gromwell root on the loss of cell viability in murine neuroblastoma cells derived from a malignant spontaneous tumor as determined by photographic acquisition of cells stained with FD. (A) Controls, (B) 0.008 mg/mL, (C) 0.011 mg/mL, (D) 0.015 mg/mL, (E) 0.019 mg/mL, (F) 0.023 mg/mL, (G) 0.027 mg/mL, (H) 0.031 mg/mL.
DISCUSSION
The current study investigates a diverse range of plants for their tumoricidal properties. While in vitro screenings may provide valuable information regarding elucidation of potential chemotherapy agents, it should also be noted that limitations include lack of consideration as to gastrointestinal absorption, kinetics, bioavailability, tissue distribution, route of systemic circulation, catabolism and excretion, all of which contribute to efficacy in vivo (Lin, 1998). With respect to direct tumoricidal properties, the data in this study show the greatest potency for the following three herbs; gromwell root (Lithospermum erythrorhizon Siebold and Zucc.), beth root (Trillium pendulum Willd.) and galbanum (Ferula galbaniflua).
Patterns within taxonomical classifications
The results were examined to elucidate for patterns of cytotoxic potency within similar botanical categories. Similar to the results obtained from our previous work (Mazzio and Soliman, 2009), there is an inconsistent nature by which plants exert tumoricidal effects even with similar botanical categories. For example, beth root (Trillium pendulum) falls under the botanical classification: Division Magnoliophyta, Class Liliopsida, Order Liliales, Subclass Lilidae and Family Liliaceae. In this study, a total of 11 plant extracts were assessed under the Liliaceae family, with two extracts ranked in the strongest category (Category 1) including Zhi Mu (Genus Anemarrhena) Bunge., and beth root (Genus Trillium) Willd., with nine extracts falling in the weakest category >5 mg/mL (Category 5). A similar trend was noted for Ferula galbaniflua which is classified under the Division Magnoliophyta, Class Magnoliopsida, Order Apiales, Subclass Rosidae and Family Apiaceae. Of the 17 plants examined in this category, only four were identified as strong (Category 1); F. galbaniflua, F. assafoetida, S. divaricata, A sinensis root-tail, A. gracilistylus root-bark, four were ranked as moderate to strong (Category 2), P. praeruptorum root, N. incisium root, L. chuanxiong, and four as moderate (Category 3), three as weak to moderate (Category 4) and two under the weakest category <5 mg/mL (Category 5).
The data also indicate a non-systematic pattern of cytotoxicity from extracts within the same genus including those derived from Angelica (LC50 2.4–4.1 mg/kg), Citrus (LC50 2.317–>5 mg/kg), Curcuma (LC50 0.27–5 mg/kg), Dioscorea (LC50 0.89–5 mg/kg) and Gleditsia (LC50 0.287–5). Although there was a random nature by which tumoricidal effects were observed amongst botanical classifications, the data did indicate a trend amongst extracts from genus and species under the Division Coniferophyta, Class Pinopsida, Order Pinales, where 4/5 tested had an LC50 < 0.676 mg/kg, including those commonly known as arbovitae, pine and juniper.
Gromwell root
In this study, the most potent plant extract was Lithospermum erythrorhizon Siebold & Zucc. which is classified under the Boraginaceae family (Borage). Its extract yields a red-purple pigment analogous to synthetic dyes purported for use in commercial cosmetics (Lee et al., 2008). These light sensitive pigments are also attributable to the high concentration of shikonin naphthoquinones such as deoxyshikonin, shikonin, acetylshikonin, isobutylshikonin and beta-hydroxyisovalerylshikonin which vary in color according to pH (Cho et al., 1999). A large number of shikonin naphthoquinones are emerging as promising chemotherapy agents with the ability to induce apoptosis in a diverse range of cancer cells (Hou et al., 2006; Cui et al., 2008) also having the capability to inhibit DNA toperisomerase (Ahn et al., 1995) and to protect against UV damage (Ishida and Sakaguchi, 2007). Shikonins also inhibit the proliferation and migration of endothelial cells in culture and block tumor necrosis factor (TNF)-α-induced melanoma in mice (Hisa et al., 1998). It is of interest to note in this study that the tumoricidal effects of Lithospermum erythrorhizon superseded that of other traditional Chinese medicines commonly used for the treatment of cancer, including ‘chuan xin lian’ (andrographis), ‘ya dan zi’ (brucea fruit), ‘ban zhi lian’ (barbat skullcap), ‘shan dou gen’ (bush sophora), ‘shi shang bai’ (selaginella), ‘kuan dong hua’ (colts foot), ‘pai lan’ (eupatorium) and ‘Huang qi’ known as astralagus root (Bensky et al., 2004). According to the literature, the primary use for Lithospermum erythrorhizon root as a traditional Chinese homeopathic medicine is for maintaining the health of the heart and liver, to facilitate the passage of stools and urine and the treatment of skin boils, eczema and burns. The advised oral daily dose of this root is 3–9 g per day, indicating its use at high concentrations as has been used historically and is generally safe (Bensky et al., 2004). This study also examined the water soluble fraction of Lithospermum erythrorhizon root by boiling the powdered root in sterile water for 5 min, and then bringing it to room temperature. The data show that water extracts were near identical in strength yielding an LC50 of 0.014 mg/mL vs the Lithospermum erythrorhizon ethanol extract having an LC50 of 0.015 mg/mL.
Galbanum
In this study, galbanum (Ferula galbaniflua) was the third most potent extract. Galbanum is a dark brown-yellow sticky resin with a distinct pungent odor classified under the botanical family Apiaceae (carrot family). The gum is derived from cutting the stem of the plant, which upon exposure to the air forms a semi-solid substance. Galbanum has been referenced in historical literature, the bible and by ancient Egyptians as a holy anointing agent and a valuable medicine. Hippocrates described its extraordinary curative powers, and it was one of the earliest drugs known to man as a stimulant, expectorant, diuretic, antispasmodic carminative, antiseptic and antiinflammatory drug. It was commonly used to treat bronchial afflictions and arthritis. The bible in Exodus 30: 34–35 makes reference to the use of galbanum and frankincense as ingredients required in the preparation of holy incense. More recently, its medicinal use was referenced in the British Pharmacopoeia 1898, named ‘Pilula Galbani Composita’ which describes a mixture of galbanum, asafetida, myrrh and glucose. Today, the resin is used primarily as an odorant or flavoring agent associated with the fragrance of must (Bajgrowicz et al., 2003).
In this study, it was found that the extract of F. galbaniflua was ~3.5 fold more toxic to N-2A cells than F. assafoetida L. However, both Ferula species were classified in the strongest category and pre-existing reports also corroborate the substantial antitumor properties for species within this genus. Recently it was reported that the extract of F. vesceritensis Coss. & DR. contains a compound called lapiferin which is responsible for cytotoxic effects on human MCF-7 breast cancer cells (Gamal-Eldeen and Hegazy, 2010). Extracts derived from the roots of F. elaeochytris Korovin contain 6-anthraniloyljaeschkeanadiol which exerts cytotoxic properties on K562R imatinib-resistant human chronic myeloid leukemia and a dasatinib-resistant mouse leukemia cell line (Alkhatib et al., 2008). Similarly, F. szowitsiana DC (umbelliprenin) exerts tumoricidal effects on malignant melanoma, cell lung carcinoma and prostate carcinoma (Barthomeuf et al., 2008), where F. szowitziana DC contains conferone, a sesquiterpene coumarin known to inhibit protein transporter P-glycoprotein indicating potential in treating multidrug resistant carcinoma (Barthomeuf et al., 2006). The present study reports that F. assafoetida L. exerts potent tumoricidal effects. These findings have also been reported where F. assafoetida L is known to contain ferulic acid and farnesiferols, which at very low concentration can prevent vascular endothelial growth factor initiated processes, angiogenesis and the progression of mouse Lewis lung cancer in mice (Lee et al., 2010; Ghosh et al., 2009). In vitro, terpenes and other constituents within extracts of F. assafoetida L. may be responsible for cytotoxic effects which at low concentrations (<4 µg/mL) are induced against cancer cell lines such as HepG2, Hep3B and MCF-7 (Lee et al., 2009).
Beth root
There seems to be no existing research investigating the bio-therapeutic potential for beth root, which only recently was reported to contain steroidal saponins hypothesized to account for therapeutic efficacy in menopausal women (Hayes et al., 2009). It is also likely that the steroidal glycosides within the root may be accountable for cytotoxic effects on tumor cells (Yokosuka and Mimaki, 2008). While there is a lack of existing research on this plant root, historical literature suggests a benefit for the treatment of colds, hemorrhage, diarrhea and dysentery. Future research will be required to investigate constituents in this plant primarily responsible for the lethal effects on malignant cell lines as observed in this study.
In summary, the findings from this study suggest that relative to the hundreds of other plants tested, gromwell root, bethroot and galbanum are the most cytotoxic to tumor cells at low concentrations. These plants should be further explored for anticancer constituents, application to other types of tumor cells, and could be considered for future CAM strategies that apply to suppressing the growth of malignant tumors.
Acknowledgements
This work was supported by a grant from the United States of America National Institute of Health (NIH) National Center for Research Resources NCRR RCMI Program G12RR03020. The authors acknowledge the valuable technical help Ms Kathelene Park.
Footnotes
Conflict of Interest
The authors have declared that there is no conflict of interest.
REFERENCES
- Advance Data from Vital and Health Statistics. CDC. 2004 May 27; [Google Scholar]
- Alkhatib R, Hennebelle T, Joha S, et al. Activity of elaeochytrin A from Ferula elaeochytris on leukemia cell lines. Phytochemistry. 2008;69:2979–2983. doi: 10.1016/j.phytochem.2008.09.019. [DOI] [PubMed] [Google Scholar]
- Ahn BZ, Baik KU, Kweon GR, Lim K, Hwang BD. Acylshikonin analogues: synthesis and inhibition of DNA topoisomerase- I. J Med Chem. 1995;38:1044–1047. doi: 10.1021/jm00006a025. [DOI] [PubMed] [Google Scholar]
- Bajgrowicz JA, Berg-Schultz K, Brunner G. Substituted hepta-1,6-dien-3-ones with green/fruity odours green/galbanum olfactophore model. Bioorg Med Chem. 2003;11:2931–2946. doi: 10.1016/s0968-0896(03)00189-5. [DOI] [PubMed] [Google Scholar]
- Barthomeuf C, Demeule M, Grassi J, Saidkhodjaev A, Beliveau R. Conferone from Ferula schtschurowskiana enhances vinblastine cytotoxicity in MDCK-MDR1 cells by competitively inhibiting P-glycoprotein transport. Planta Med. 2006;72:634–639. doi: 10.1055/s-2006-931574. [DOI] [PubMed] [Google Scholar]
- Barthomeuf C, Lim S, Iranshahi M, Chollet P. Umbelliprenin from Ferula szowitsiana inhibits the growth of human M4Beu metastatic pigmented malignant melanoma cells through cell-cycle arrest in G1 and induction of caspase-dependent apoptosis. Phytomedicine. 2008;15:103–111. doi: 10.1016/j.phymed.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Bensky D, Clavey S, Stoger E, Gamble A, Bensky LL. Chinese Herbal Medicine 3rd edn. Materia Medica. Seattle, WA: Eastland Press; 2004. [Google Scholar]
- Blot WJ. Vitamin/mineral supplementation and cancer risk: international chemoprevention trials. Proc Soc Exp Biol Med. 1997;216:291–296. doi: 10.3181/00379727-216-44180. [DOI] [PubMed] [Google Scholar]
- Boon H, Stewart M, Kennard MA, et al. Use of complementary/alternative medicine by breast cancer survivors in Ontario: prevalence and perceptions. J Clin Oncol. 2000;18:2515–2521. doi: 10.1200/JCO.2000.18.13.2515. [DOI] [PubMed] [Google Scholar]
- Chakraborty S, Roy M, Taraphdar AK, Bhattacharya RK. Cytotoxic effect of root extract of Tiliacora racemosa and oil of Semecarpus anacardium nut in human tumour cells. Phytother Res. 2004;18:595–600. doi: 10.1002/ptr.1501. [DOI] [PubMed] [Google Scholar]
- Cho MH, Paik YS, Hahn TR. Physical stability of shikonin derivatives from the roots of Lithospermum erythrorhizon cultivated in Korea. J Agric Food Chem. 1999;47:4117–4120. doi: 10.1021/jf9902853. [DOI] [PubMed] [Google Scholar]
- Clerici CA, Veneroni L, Giacon B, Mariani L, Fossati-Bellani F. Complementary and alternative medical therapies used by children with cancer treated at an Italian pediatric oncology unit. Pediatr Blood Cancer. 2009;53:599–604. doi: 10.1002/pbc.22093. [DOI] [PubMed] [Google Scholar]
- Conney AH. Enzyme induction and dietary chemicals as approaches to cancer chemoprevention: the Seventh DeWitt S. Goodman Lecture. Cancer Res. 2003;63:7005–7031. [PubMed] [Google Scholar]
- Cui XR, Tsukada M, Suzuki N, et al. Comparison of the cytotoxic activities of naturally occurring hydroxyanthraquinones and hydroxynaphthoquinones. Eur J Med Chem. 2008;43:1206–1215. doi: 10.1016/j.ejmech.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Dy GK, Bekele L, Hanson LJ, et al. Complementary and alternative medicine use by patients enrolled onto phase I clinical trials. J Clin Oncol. 2004;22:4810–4815. doi: 10.1200/JCO.2004.03.121. [DOI] [PubMed] [Google Scholar]
- Ferrucci LM, McCorkle R, Smith T, Stein KD, Cartmel B. Factors related to the use of dietary supplements by cancer survivors. J Altern Complement Med. 2009;15:673–680. doi: 10.1089/acm.2008.0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finklestein JZ, Tittle K, Meshnik R, Weiner J. Murine neuroblastoma, further evaluation of the C1300 model with single antitumor agents. Cancer Chemother Rep. 1975;59:975–983. [PubMed] [Google Scholar]
- Gamal-Eldeen AM, Hegazy ME. A crystal lapiferin derived from Ferula vesceritensis induces apoptosis pathway in MCF-7 breast cancer cells. Nat Prod Res. 2010;24:246–257. doi: 10.1080/14786410802685398. [DOI] [PubMed] [Google Scholar]
- Genc RE, Senol S, Turgay AS, Kantar M. Complementary and alternative medicine used by pediatric patients with cancer in western Turkey. Oncol Nurs Forum. 2009;36:E159–E164. doi: 10.1188/09.ONF.E159-E164. [DOI] [PubMed] [Google Scholar]
- Gerson-Cwilich R, Serrano-Olvera A, Villalobos-Prieto A. Complementary and alternative medicine (CAM) in Mexican patients with cancer. Clin Transl Oncol. 2006;8:200–207. doi: 10.1007/s12094-006-0011-2. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Banerji A, Mandal S, Banerji J. A new sesquiterpenoid coumarin from Ferula assafoetida. Nat Prod Commun. 2009;4:1023–1024. [PubMed] [Google Scholar]
- Hayes PY, Lehmann R, Penman K, Kitching W, De Voss JJ. Steroidal saponins from the roots of Trillium erectum (Beth root). Phytochemistry. 2009;70:105–113. doi: 10.1016/j.phytochem.2008.10.019. [DOI] [PubMed] [Google Scholar]
- Hisa T, Kimura Y, Takada K, Suzuki F, Takigawa M. Shikonin, an ingredient of Lithospermum erythrorhizon, inhibits angiogenesis in vivo and in vitro. Anticancer Res. 1998;18:783–790. [PubMed] [Google Scholar]
- Hou Y, Guo T, Wu C, He X, Zhao M. Effect of shikonin on human breast cancer cells proliferation and apoptosis in vitro. Yakugaku Zasshi. 2006;126:1383–1386. doi: 10.1248/yakushi.126.1383. [DOI] [PubMed] [Google Scholar]
- Hu Z, Yang X, Ho PC, et al. Herb-drug interactions, a literature review. Drugs. 2005;65:1239–1282. doi: 10.2165/00003495-200565090-00005. [DOI] [PubMed] [Google Scholar]
- Ishida T, Sakaguchi I. Protection of human keratinocytes from UVB-induced inflammation using root extract of Lithospermum erythrorhizon. Biol Pharm Bull. 2007;30:928–934. doi: 10.1248/bpb.30.928. [DOI] [PubMed] [Google Scholar]
- Klebe RJ, Ruddle FH. Neuroblastoma, cell culture analysis of a differentiating stem cell system. J Cell Biol. 1969;43:69A. [Google Scholar]
- Kraft K. Complementary/alternative medicine in the context of prevention of disease and maintenance of health. Prev Med. 2009;49:88–92. doi: 10.1016/j.ypmed.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Kristoffersen AE, Fønnebø V, Norheim AJ. Do cancer patients with a poor prognosis use complementary and alternative medicine more often than others? J Altern Complement Med. 2009;15:35–40. doi: 10.1089/acm.2008.0262. [DOI] [PubMed] [Google Scholar]
- Kumar NB, Allen K, Bell H. Perioperative herbal supplement use in cancer patients, potential implications and recommendations for presurgical screening. Cancer Control. 2005;12:149–157. doi: 10.1177/107327480501200302. [DOI] [PubMed] [Google Scholar]
- Lee CL, Chiang LC, Cheng LH, et al. Influenza A (H (1) N (1)) Antiviral and cytotoxic agents from Ferula assafoetida. J Nat Prod. 2009;72:1568–1572. doi: 10.1021/np900158f. [DOI] [PubMed] [Google Scholar]
- Lee HY, Kim YJ, Kim EJ, Song YK, Byun SY. Red pigment from Lithospermum erythrorhizon by supercritical CO2 extraction. J Cosmet Sci. 2008;59:431–440. [PubMed] [Google Scholar]
- Lee JH, Choi S, Lee Y, et al. Herbal compound farnesiferol C exerts antiangiogenic and antitumor activity and targets multiple aspects of VEGFR1 (Flt1) or VEGFR2 (Flk1) signaling cascades. Mol Cancer Ther. 2010;9:389–399. doi: 10.1158/1535-7163.MCT-09-0775. [DOI] [PubMed] [Google Scholar]
- Lin JH. Applications and limitations of interspecies scaling and in vitro extrapolation in pharmacokinetics. Drug Metab Dispos. 1998;26:1202–1212. [PubMed] [Google Scholar]
- Mazzio EA, Soliman KF. In vitro screening for the tumoricidal properties of international medicinal herbs. Phytother Res. 2009;23:385–398. doi: 10.1002/ptr.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzio E, Yoon KJ, Soliman KF. Acetyl-l-carnitine cytoprotection against 1-methyl-4-phenylpyridinium toxicity in neuroblastoma cells. Biochem Pharmacol. 2003;66:297–306. doi: 10.1016/s0006-2952(03)00261-2. [DOI] [PubMed] [Google Scholar]
- Melnick SJ. Developmental therapeutics, review of biologically based CAM therapies for potential application in children with cancer, part I. J Pediatr Hematol Oncol. 2006;28:221–230. doi: 10.1097/01.mph.0000212922.16427.04. [DOI] [PubMed] [Google Scholar]
- Molassiotis A, Browall M, Milovics L, Panteli V, Patiraki E, Fernandez-Ortega P. Complementary and alternative medicine use in patients with gynecological cancers in Europe. Int J Gynecol Cancer. 2006;16 Suppl 1:219–224. doi: 10.1111/j.1525-1438.2006.00309.x. [DOI] [PubMed] [Google Scholar]
- Ohno S, Ohno Y, Suzuki N, Soma G, Inoue M. High-dose vitamin C (ascorbic acid) therapy in the treatment of patients with advanced cancer. Anticancer Res. 2009;29:809–815. [PubMed] [Google Scholar]
- Owens B, Jackson M, Berndt A. Complementary therapy used by Hispanic women during treatment for breast cancer. J Holist Nurs. 2009;27:167–176. doi: 10.1177/0898010108330801. [DOI] [PubMed] [Google Scholar]
- Richardson MA, Sanders T, Palmer JL, Greisinger A, Singletary SE. Complementary/alternative medicine use in a comprehensive cancer center and the implications for oncology. J Clin Oncol. 2000;18:2505–2514. doi: 10.1200/JCO.2000.18.13.2505. [DOI] [PubMed] [Google Scholar]
- Rosenberg Zand RS, Jenkins DJ, Diamandis EP. Flavonoids and steroid hormone-dependent cancers. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;777:219–232. doi: 10.1016/s1570-0232(02)00213-1. [DOI] [PubMed] [Google Scholar]
- Scott JA, Kearney N, Hummerston S, Molassiotis A. Use of complementary and alternative medicine in patients with cancer: a UK survey. Eur J Oncol Nurs. 2005;9:131–137. doi: 10.1016/j.ejon.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Tarhan O, Alacacioglu A, Somali I, et al. Complementary-alternative medicine among cancer patients in the western region of Turkey. J Buon. 2009;14:265–269. [PubMed] [Google Scholar]
- van Tonder E, Herselman MG, Visser J. The prevalence of dietary-related complementary and alternative therapies and their perceived usefulness among cancer patients. J Hum Nutr Diet. 2009;22:528–535. doi: 10.1111/j.1365-277X.2009.00986.x. [DOI] [PubMed] [Google Scholar]
- Yates JS, Mustian KM, Morrow GR, et al. Prevalence of complementary and alternative medicine use in cancer patients during treatment. Support Care Cancer. 2005;13:806–811. doi: 10.1007/s00520-004-0770-7. [DOI] [PubMed] [Google Scholar]
- Yokosuka A, Mimaki Y. Steroidal glycosides from the underground parts of Trillium erectum and their cytotoxic activity. Phytochemistry. 2008;69:2724–2730. doi: 10.1016/j.phytochem.2008.08.004. [DOI] [PubMed] [Google Scholar]