Abstract
Aim
The aim of this study was to quantify the reasons and opinions of patients who reported adverse drug reactions (ADRs) in the Netherlands to a pharmacovigilance centre.
Method
A web-based questionnaire was sent to 1370 patients who had previously reported an ADR to a pharmacovigilance centre. The data were analysed using descriptive statistics, χ2 tests and Spearman’s correlation coefficients.
Results
The response rate was 76.5% after one reminder. The main reasons for patients to report ADRs were to share their experiences (89% agreed or strongly agreed), the severity of the reaction (86% agreed or strongly agreed to the statement), worries about their own situation (63.2% agreed or strongly agreed) and the fact the ADR was not mentioned in the patient information leaflet (57.6% agreed or strongly agreed). Of the patient-responders, 93.8% shared the opinion that reporting an ADR can prevent harm to other people, 97.9% believed that reporting contributes to research and knowledge, 90.7% stated that they felt responsible for reporting an ADR and 92.5% stated that they will report a possible ADR once again in the future.
Conclusion
The main motives for patients to report their ADRs to a pharmacovigilance centre were the severity of the ADR and their need to share experiences. The high level of response to the questionnaire shows that patients are involved when it comes to ADRs and that they are also willing to share their motivations for and opinions about the reporting of ADRs with a pharmacovigilance centre.
Keywords: Adverse drug reactions (ADRs), Pharmacovigilance, Patient reporting, Consumer reporting, Web-based questionnaire
Introduction
An increasing number of countries are incorporating the direct reporting of adverse drug reactions (ADRs) by patients into pharmacovigilance systems [1]. Consequently, knowledge of the factors influencing patient reporting to pharmacovigilance systems on a day-to-day basis has been increasing in recent years [1–4].
The organization responsible for the management of the spontaneous reporting system for ADRs in the Netherlands is the Netherlands Pharmacovigilance Centre Lareb, on behalf of the Medicines Evaluation Board. Not only healthcare professionals and marketing authorization holders are able to report ADRs directly to Lareb; since April 2003, patients can also report ADRs directly to Lareb. Patients can report ADRs through an electronic reporting form available on the Lareb website that is identical, in terms of content, to that used by healthcare professionals. Several mandatory fields in this form ensure the completeness of the information before it can be sent to the pharmacovigilance centre. In rare cases where it is not possible for a patient to report electronically, a paper reporting form can be sent upon request. Patients’ and healthcare professionals’ reports are coded according to MedDRA (Medical Dictionary for Regulatory Activities) terminology, individually assessed by trained assessors and stored in the same database. All reporters, both patients and healthcare professionals, receive feedback information on their reported ADR(s) [2].
In 2008, the Netherlands Pharmacovigilance Centre Lareb published their experiences on 3 years of patient reporting [2]. The Centre reported that patients’ reports contained different information than those of healthcare professionals with respect to categories of seriousness and outcome of the reported ADRs [2]. When patients’ and healthcare professionals’ ADR reports on statins were compared following media attention, patient reports were found to provide additional information on the different categories of adverse reactions, the impact of ADRs on daily life and the patient–health professional relationship [3]. In a study carried out in Denmark, patients were also found to report different categories of ADRs for different types of medicines compared to other reporters [4]. Based on these results, the authors of this study concluded that consumers should be actively included in systematic drug surveillance systems. A patient’s own firsthand report of his/her experiences with drugs can capture side effects that clinicians might miss [5, 6]. According to Foster et al. ‘patient-reporting can be an important source of information about side effects in the context of real-life clinical practice’ [7].
The motivations and attitudes of healthcare professionals towards ADR reporting to a pharmacovigilance centre have been studied extensively [8–20]. In contrast, the reasons why patients report ADRs are less well known. As Aagaard et al. [4] noted, consumers’ experiences with and perspectives on ADRs should be further studied. A qualitative study involving guided interviews with 21 patients in the Netherlands was performed to gain insight into the motivations of patients who report ADRs to a pharmacovigilance centre [21]. Most patients expressed altruistic motives, but also the severity of the ADR and the need for extra information about the ADR were mentioned as motives for reporting.
The aim of this study is to quantify both the reasons for reporting ADRs and the opinions of patients regarding the reporting of ADRs to a pharmacovigilance centre in the Netherlands.
Method
Data from interviews investigating patients’ motives for reporting ADRs [21] were used to develop a questionnaire that could be sent to a large group of patient-reporters. Questionnaires were web-based and sent via the online web service SurveyMonkey [22].
Study population
The target population comprised patients who reported an ADR to the Netherlands Pharmacovigilance Centre Lareb between January 1, 2008 and March 1, 2009. A total of 1503 case reports of ADRs were reported by patients during this period. Of these, 1440 patients reported by means of the electronic reporting form on the Lareb website, which is the preferred reporting method of the pharmacovigilance centre, and the e-mail address of each of these 1440 reports was selected. As some patients had reported several times, their e-mail addresses could be present twice or more in the list (but with different report numbers). The final list thus comprised 1370 different e-mail addresses of patient-reporters, who were then approached for inclusion in the study.
Questionnaire
A list of all categories of quotes from an earlier qualitative study [21] was compiled with the support of QSR NVivo ver. 8.0.264.0, a software programme for ordering qualitative data [23]. These categories were rephrased to statements (with rating scales) as questionnaire items. Categories of quotes from the interviews that were much alike were combined in order to reduce the time needed for answering the questionnaire as much as possible. The statements were divided into ‘Reasons’ and ‘Opinions’. The questionnaire items could be rated on a five-point Likert scale (strongly agree to strongly disagree) [24], where the middle position was labelled ‘neutral’ to reflect a neutral position, and not an inability to answer the question. The first theme contained statements relating to reasons for reporting an ADR. Next to the response options on the possible reasons to report an ADR, was an ‘Other’ option along with a free text area for ‘please specify’. The second theme included statements on the opinions of patients with respect to reporting an ADR. Patients were also asked if they would report a possible ADR again in the future. Possible ‘Reasons’ and ‘Opinions’ are given in Table 1
Table 1.
Statements used in the questionnaire to assess the reasons for reporting an adverse drug reaction (ADR) and the opinions of patients regarding the reporting of an ADR
| Reasons |
|---|
| I wanted extra information |
| The adverse drug reaction was severe |
| It was difficult to discuss the adverse drug reaction with my medical practitioner or pharmacist |
| The possibility for reporting an adverse drug reaction just exists |
| I wanted to be heard |
| Someone else pointed the possibility for reporting an adverse drug reaction |
| I was angry about the situation |
| I wanted action to be taken |
| I wanted to share my experiences |
| The adverse drug reaction was not mentioned in the patient information leaflet |
| I was worried about my own situation |
| Opinions |
| Reporting an adverse drug reaction can prevent harm to other people |
| I felt responsible for reporting an adverse drug reaction |
| Reporting an adverse drug reaction that is already mentioned in the patient information leaflet is useless |
| I only report an adverse drug reaction if it is serious |
| Reporting an adverse drug reaction contributes to research and knowledge |
| I report an adverse drug reaction if it is not mentioned in the patient information leaflet |
| I benefit from reporting an adverse drug reaction |
| Reporting an adverse drug reaction contributes to improvement of drugs |
| I report an adverse drug reaction if it is unexpected |
| In the future I will report a possible adverse drug reaction once again |
The questionnaire addressed a number of demographic aspects, including gender, age (<18 years, 18–35 years, 36–64 years, >65 years), level of education (primary school, secondary school, vocational education, higher professional education, academic education) and possible membership in a patients’ association. Patients who are a member of a patient organization may possess more information on the reporting of ADRs to a pharmacovigilance centre and also may have other motives or opinions on this subject.
Sending the questionnaire
The web-based survey was first tested in a small group of field testers and subsequent sent to the selected e-mail addresses on March 23, 2009. Two weeks later (April 6, 2009), a reminder was sent to all non-responders. Collection of responses was finished after 1 month (April 24, 2009). The link in the invitation e-mail was uniquely tied to the survey and the respondent’s e-mail address. Therefore, the message could not be forwarded by respondents, and only one response per e-mail address was allowed. This also implied that random surfers on the Internet could not reach the online survey.
Data analysis
Descriptive statistics provided an overview of the patient characteristics, the reasons for reporting ADRs and the opinions of patients on reporting ADRs.
A Pearson Chi-square (χ2) test was performed to detect significant differences in motives and opinions between patients who were members of a patient organization and those who were not and in differences in answers between men and women. Significance was based on a two-sided χ2-test and significance was set at p < 0.05.
Correlations were calculated to measure possible relationships between two or more statements. Because the data were measured at the ordinal level (Likert-scale), Spearman’s correlation coefficient (r) was used in this analysis. In this study, a correlation was considered strong if r > 0.7, moderate if r > 0.4 and < 0.7 and weak if r < 0.4 [25]. Data were analysed using the statistical software programme SPSS Statistics, ver. 17.0 (SPSS, Chicago, IL).
The answers to the open question on the reasons to report (the question ‘Other, please specify…’) were specified by two researchers independently (FvH and CvdW) and categorized. These responses to the open question were then compared with the responses to the statements in the questionnaire to identify possible new motives for reporting that were not covered by the questionnaire.
Results
Response
A total number of 1370 potential participants were approached by e-mail, of which 57 e-mails were undeliverable. Of the remaining 1313 patients who received an e-mail, 1005 completed and returned the questionnaire, which resulted in a 76.5% response rate. Among these 1005 responses were six responses by healthcare professionals whose e-mail addresses had been selected because they had by mistake reported an ADR using the patient reporting form instead of the healthcare professional form. These six responses were not taken into account, which resulted in 999 useful responses for the analysis. The responses to the questionnaire are shown in Fig. 1. The characteristics of the patient-reporters are given in Table 2.
Fig. 1.
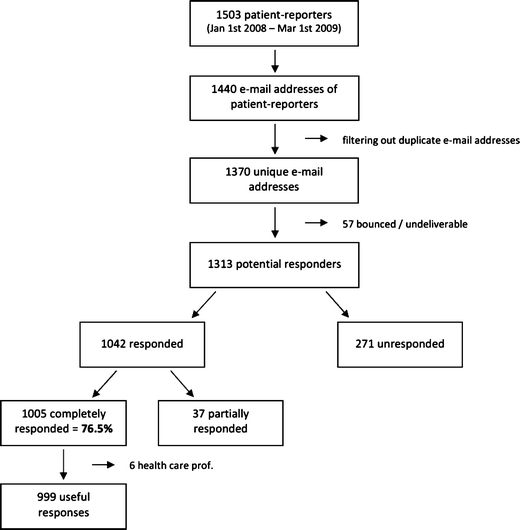
Flowchart of the respondents to the questionnaire
Table 2.
Respondent characteristics
| Variable | Percentage (n) |
|---|---|
| Gender | |
| Male | 33.4% (334) |
| Female | 66.6% (665) |
| Age | |
| <18 years | 0.2% (2) |
| 18-35 year | 16.1% (161) |
| 36-64 year | 69.1% (690) |
| >65 years | 14.6% (146) |
| Education | |
| Primary school | 1.1% (11) |
| Secondary school | 10.8% (108) |
| Vocational education | 33.0% (330) |
| Higher professional education | 37.1% (371) |
| Academic | 17.9% (179) |
| Member of patients associationa | |
| Yes | 24.7% (247) |
| No | 75.1% (750) |
a997 responses, 2 not reported
Reasons to report an ADR
An overview of the responses on the reasons for reporting ADRs is given in Table 3.
Table 3.
Motives for reporting ADRs
| Motive | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
|---|---|---|---|---|---|
| I wanted extra information | 19.6% (196) | 25.2% (252) | 22.2% (222) | 17.9% (179) | 15.0% (150) |
| The adverse drug reaction was severe | 53.7% (536) | 32.5% (325) | 8.2% (82) | 3.5% (35) | 2.1% (21) |
| It was difficult to discuss the adverse drug reaction with my medical practitioner or pharmacist | 6.0% (60) | 9.3% (93) | 15.8% (158) | 34.5% (345) | 34.3% (343) |
| The possibility for reporting an adverse drug reaction just exists | 34.7% (347) | 38.0% (380) | 17.9% (179) | 5.6% (56) | 3.7% (37) |
| I wanted to be heard | 25.1% (251) | 28.4% (284) | 26.4% (264) |
12.7% (127) |
7.3% (73) |
| Someone else pointed the possibility for reporting an adverse drug reaction | 14.9% (149) | 17.1% (171) | 14.2% (142) | 28.7% (287) | 25.0% (250) |
| I was angry about the situation | 20.7% (207) | 18.9% (189) | 20.5% (205) | 22.7% (227) | 17.1% (171) |
| I wanted action to be taken | 27.3% (273) | 31.0% (310) | 23.6% (236) | 11.0% (110) | 7.0% (70) |
| I wanted to share my experiences | 48.1% (481) | 40.9% (409) | 6.3% (63) | 2.8% (28) | 1.8% (18) |
| The adverse drug reaction was not mentioned in the patient information leaflet | 33.2% (332) | 24.4% (244) | 17.5% (175) | 17.1% (171) | 7.7% (77) |
| I was worried about my own situation | 32.5% (325) | 30.7% (307) | 17.5% (175) | 12.2% (122) | 7.0% (70) |
Data on motives are given as the percentage of total responses, with the frequency (n) of the response given in parenthesis
Most frequently reported response is given in bold
The main reasons for patients to report ADRs were to share their experiences (89% agreed or strongly agreed), the severity of the reaction (86% agreed or strongly agreed to the statement), worries about their own situation (63.2% agreed or strongly agreed) and the fact the ADR was not mentioned in the patient information leaflet (57.6% agreed or strongly agreed).
Responses to the open question
The open text field in the questionnaire for ‘Other reasons for reporting’ was filled in by 228 respondents. The reasons to report an ADR which were most frequently mentioned in this open question are ‘For other people’ (n = 33), ‘Medical practitioner or pharmacist does not acknowledge the problem or take it seriously’ (n = 27), ‘ADR was severe’ (n = 21) and ‘Comments on patient information leaflet’ (n = 20). The other responses to the open question (n = 127) could not be grouped together easily because of a large variety of subjects.
The responses to the open question were compared to the statements in the questionnaire. The reason ‘Medical practitioner or pharmacist does not acknowledge the problem or take it seriously’ was not included as a statement in this survey. Apparently, the respondents considered this a different reason than the statement ‘It was difficult to discuss the adverse drug reaction with my medical practitioner or pharmacist’.
Other findings
Among the patient-responders, 93.8% shared the opinion that reporting an ADR can prevent harm to other people, 97.9% believed that reporting contributes to research and knowledge, 90.7% stated that they felt responsible for reporting an ADR and 92.5% patients stated that they will report a possible ADR once again in the future. The opinions of the patient-responders on reporting ADRs are shown in Table 4.
Table 4.
Opinions on reporting ADRs
| Opinions | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
|---|---|---|---|---|---|
| Reporting an adverse drug reaction can prevent harm to other people | 61.1% (610) | 32.7% (327) | 4.1% (41) | 1.6% (16) | 0.5% (5) |
| I felt responsible for reporting an adverse drug reaction | 50.5% (504) | 40.2% (402) | 8.1% (81) | 1.0% (10) | 0.2% (2) |
| Reporting an adverse drug reaction that is already mentioned in the patient information leaflet is useless | 13.9% (139) | 20.1% (201) | 18.8% (188) | 31.7% (317) | 15.4% (154) |
| I only report an adverse drug reaction if it is serious | 24.6% (246) | 34.5% (345) | 14.9% (149) | 20.5% (205) | 5.4% (54) |
| Reporting an adverse drug reaction contributes to research and knowledge | 60.9% (608) | 37.0% (370) | 1.8% (18) | 0 | 0.3% (3) |
| I report an adverse drug reaction if it is not mentioned in the patient information leaflet | 39.5% (395) | 35.2% (352) | 14.8% (148) | 8.7% (87) | 1.7% (17) |
| I benefit from reporting an adverse drug reaction | 11.7% (117) | 23.3% (233) | 36.2% (362) | 19.8% (198) | 8.9% (89) |
| Reporting an adverse drug reaction contributes to improvement of drugs | 41.4% (414) | 43.7% (437) | 13.2% (132) | 1.2% (12) | 0.4% (4) |
| I report an adverse drug reaction if it is unexpected | 30.2% (302) | 41.1% (411) | 17.0% (170) | 9.0% (90) | 2.6% (26) |
| In the future I will report a possible adverse drug reaction once again | 47.9% (479) | 44.4% (444) | 6.7% (67) | 0.3% (3) | 0.6% (6) |
Data on motives are given as the percentage of total responses, with the frequency (n) of the response given in parenthesis
Most frequently reported response is given in bold
There was no statistically significant differences in the answers between men and women for all statements (χ2 test p > 0.05), with the exception of the motive ‘I was worried about my own situation’ (χ2 test p = 0.022): 70% of the men were worried about their situation in comparison with 59.8% of the women.
Membership in a patient organization was compared with the answers on the statements. There was no statistically significant difference in the answers (agree, neutral, disagree) between members or non-members for all statements (χ2 tests p > 0.05), except for the opinion ‘In the future I will report a possible adverse drug reaction once again’ (χ2 test p = 0.036): 95.5% of the members of a patient organization agreed to this statement compared to 91.3% of non-members.
The correlation coefficients between the reasons for reporting ADRs, between the opinions and between the reasons and opinions were mostly moderate or weak (r > 0.4 < 0.7 or r < 0.4). The highest correlation coefficient (r) was 0. 616 (between Reason 7 ‘I was angry about the situation’ and Reason 8 ‘I wanted action to be taken’).
Discussion
Findings
This study confirms the findings of the previous qualitative interviews [21].Based on the results of the completed questionnaires, patients report ADRs for various reasons, of which the most important are a severe ADR, wanting to share experiences, worry about the ADR in a personal context and the ADR not being mentioned in the patient information leaflet. Patients also believe that reporting an ADR can prevent harm to other people and that reporting contributes to research and knowledge. Most patients stated that they felt responsible for reporting an ADR.
An additional motive for reporting ADRs, derived from the open question, was ‘My medical practitioner or pharmacist does not acknowledge the problem or take it seriously’. This motive was mentioned during the qualitative interviews as well [21]. It is likely that the wording of the statement in the questionnaire about communication with the healthcare professional (reason 3) was not clear enough in emphasizing the acknowledgement of the medical practitioner or pharmacist instead of the difficulty in discussing the ADR. The severity of an ADR as a motive for reporting does not necessarily mean that the patient suffered from an ADR that was serious according to international criteria [26]. Medical seriousness may differ from patients’ views on what constitutes a serious problem [27].
In 2009, 27.0% of the Dutch population between the age of 15 and– 65 years had achieved an educational level of higher professional education/academic [28]. In comparison, 55% of the participants in this study had a high level of education. The high level of education among the respondents is not surprising considering that this group may be better informed about healthcare in general and the possibility to report ADRs.
Two-thirds of the respondents to the questionnaire were female. This is almost the same proportion as in the whole population of patient-reporters in our research period (1440 in total, 63.6% women). This is also in line with a previous study by De Langen et al. [2], who found that 63% of the patient-reporters were female. In 2009, 50.5% of the Dutch population were female. This difference in the percentages could possibly be a result of women experiencing a higher incidence of ADRs than men [29] and/or of women being more eager to report ADRs to a pharmacovigilance centre. However, there were no differences in answers between men and women, except for the answers to the reason ‘Being worried about own situation’.
Elderly people are more at risk of developing an ADR [30], so it is important that the motives and opinions of this group are also taken into account. Of the respondents to the questionnaire, 14.6% were older than 65 years; of this latter group, less then 2% of the respondents were older than 80 years. This corresponds fairly well to the demographic structure of the Dutch population in 2009: 11.2% were aged 65–80 years and 3.8% were older than 80 years [28].
Comparison to other studies
To the best of our knowledge, the Netherlands pharmacovigilance centre is the only such centre to have published studies on patient’s motives for reporting ADRs to a pharmacovigilance centre [21, 31]. During the first 1-year trial period of patient reporting in the Netherlands, patients were asked why they reported an ADR to the pharmacovigilance centre instead of to their healthcare professional. Of the respondents, 23% stated that they felt their complaints would not be taken seriously elsewhere, and 22% had already reported the ADR to a healthcare professional—with no result [31]. In a qualitative study with 21 patients, almost all patients had multiple motives for reporting. Among the altruistic motives were preventing harm to other patients, making the ADR publicly known, increasing medical knowledge and wanting to improve the patient information leaflet. Personal motives to report an ADR included wanting more information about the ADR, indicating that the ADR was too severe not to report, being angry or wanting confirmation of their ADR [21]. These results have been generalized and quantified in a large group in the present study.
Several studies have been conducted with the aim of investigating their motivations of healthcare professionals for (not) reporting ADRs to a pharmacovigilance centre [8–20]. Hasford et al. [12] and Ekman et al. [9] indicated that the severity of the reaction, unusual reaction of the reporter to the drug and a reaction caused by a new drug were the main reasons motivating healthcare professionals to report ADRs. In the study by Bäckström et al. [20], three-quarters of the respondents stated that the severity of the reaction was the main factor determining whether a suspected ADR was reported or not. Biriell et al. [16] found the following motives as positive reasons for physicians and pharmacists to report an ADR: desire to contribute to medical knowledge, reaction previously unknown to the reporter, reaction to new drug, desire to report all significant reactions, known association between drug and reaction and severity of reaction.
Some of the motives found for healthcare professionals are also important reasons for patients to report, such as severity of the reaction and wanting to contribute to medical knowledge.
Validity of the study design
The statements in the questionnaire were based upon qualitative interviews with patients that were aimed at increasing the validity of the survey instrument [32]. The response rate to the questionnaire was more than 76.5%, which is high compared to some other studies on motivations for (not) reporting ADRs [9, 20]. The high response rate increases the validity of the results found in this study.
There are also some limitations to this study. The study population comprised patients who had already reported an ADR to the pharmacovigilance centre in the Netherlands, which is a selected population. One could assume that patients who are interested in their drug treatment and especially those who had already experienced an ADR would be more willing to answer this type of questionnaire. We did not include the motives and opinions of patients who experienced an ADR but who choose not report this to the pharmacovigilance centre.
Because 96% of our target study population consisted of patients who had previously reported an ADR through the online reporting form, it was possible to use a web-based questionnaire. Most people in the Netherlands have access to the Internet: in 2009, 91% of the Dutch population younger than 65 years had such access according to Statistics Netherlands [28]. However, selection bias cannot be excluded. In the age category 65–75 years, Statistics Netherlands reported that in 2009 only 64% of the population had Internet access, and it had no specific data on Internet access for age groups older than 75 years. Of the responders in the 2009 survey, 91% up to the age of 55 years had used the Internet in the 3 months preceding the survey. In comparison, for people aged 55–65 years this was 82% and for people aged 65–75 years, 53% [28]. Patients who did not use the electronic reporting form (n = 63) were excluded from this study, but it is possible that other motives were present in this group. The pharmacovigilance centre accepts consumers reporting for relatives; for example, children with access to the Internet could report for an elderly parent.
The use of the Internet is also correlated to the level of education [28], which may also have increased selection bias. The level of education in the Netherlands is higher than the average level in the European Union (EU-27 countries), with 27% of the Dutch population educated to the highest level versus 19.1% of the EU-27 population [33]. In 2009, an average of 65% of the households of the EU-27 countries had access to the Internet, which is considerably lower than the 90% in the Netherlands [33]. In fact, the percentage of access to the Internet in the Netherlands is the highest of all European countries [33]. Therefore, the use of a web-bases questionnaire might not be feasible in all countries in the same manner as in the Netherlands.
Conclusions
Based on the results of our questionnaire survey, the main motives for patients to report an ADRs to a national pharmacovigilance centre were the severity of the ADR and the need to share experiences. Altruistic motives are reflected by the high responses to the statements ‘Reporting an adverse drug reaction can prevent harm to other people’, ‘I felt responsible for reporting an adverse drug reaction’ and ‘Reporting an adverse drug reaction contributes to research and knowledge’. There was only a small group of patients who felt that they reported an ADR for personal benefit. The high level of response to the questionnaire shows that patients are involved when it comes to ADRs and also willing to share their motivations for and opinions about the reporting of ADRs with the pharmacovigilance centre. More than 90% of the patients who had already reported an ADR once agreed with the statement ‘In the future I will report a possible adverse drug reaction once again’.
Acknowledgments
Conflicts of interest
Nothing to declare
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Blenkinsopp A, Wilkie P, Wang M, et al. Patient reporting of suspected adverse drug reactions: a review of published literature and international experience. Br J Clin Pharmacol. 2007;63:148–156. doi: 10.1111/j.1365-2125.2006.02746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Langen J, van Hunsel F, Passier A, et al. Adverse drug reaction reporting by patients in the Netherlands: three years of experience. Drug Saf. 2008;31:515–524. doi: 10.2165/00002018-200831060-00006. [DOI] [PubMed] [Google Scholar]
- 3.van Hunsel F, Passier A, van Grootheest AC. Comparing patients' and healthcare professionals' ADR reports after media attention: the broadcast of a Dutch television programme about the benefits and risks of statins as an example. Br J Clin Pharmacol. 2009;67:558–564. doi: 10.1111/j.1365-2125.2009.03400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aagaard L, Nielsen LH, Hansen EH. Consumer reporting of adverse drug reactions: a retrospective analysis of the Danish adverse drug reaction database from 2004 to 2006. Drug Saf. 2009;32:1067–1074. doi: 10.2165/11316680-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 5.Basch E. The missing voice of patients in drug-safety reporting. N Engl J Med. 2010;362:865–869. doi: 10.1056/NEJMp0911494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Grootheest K, de Jong-van den Berg L. Patients' role in reporting adverse drug reactions. Expert Opin Drug Saf. 2004;3:363–368. doi: 10.1517/14740338.3.4.363. [DOI] [PubMed] [Google Scholar]
- 7.Foster JM, van der Molen T, de Jong-van den Berg L. Patient-reporting of side effects may provide an important source of information in clinical practice. Eur J Clin Pharmacol. 2007;63:979–980. doi: 10.1007/s00228-007-0339-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belton KJ. Attitude survey of adverse drug-reaction reporting by health care professionals across the European Union. The European Pharmacovigilance Research Group. Eur J Clin Pharmacol. 1997;52:423–427. doi: 10.1007/s002280050314. [DOI] [PubMed] [Google Scholar]
- 9.Ekman E, Backstrom M. Attitudes among hospital physicians to the reporting of adverse drug reactions in Sweden. Eur J Clin Pharmacol. 2009;65:43–46. doi: 10.1007/s00228-008-0564-9. [DOI] [PubMed] [Google Scholar]
- 10.Herdeiro MT, Figueiras A, Polonia J, et al. Influence of pharmacists' attitudes on adverse drug reaction reporting : a case-control study in Portugal. Drug Saf. 2006;29:331–340. doi: 10.2165/00002018-200629040-00004. [DOI] [PubMed] [Google Scholar]
- 11.Herdeiro MT, Figueiras A, Polonia J, et al. Physicians' attitudes and adverse drug reaction reporting: a case-control study in Portugal. Drug Saf. 2005;28:825–833. doi: 10.2165/00002018-200528090-00007. [DOI] [PubMed] [Google Scholar]
- 12.Hasford J, Goettler M, Munter KH, et al. Physicians' knowledge and attitudes regarding the spontaneous reporting system for adverse drug reactions. J Clin Epidemiol. 2002;55:945–950. doi: 10.1016/S0895-4356(02)00450-X. [DOI] [PubMed] [Google Scholar]
- 13.Eland IA, Belton KJ, van Grootheest AC, et al. Attitudinal survey of voluntary reporting of adverse drug reactions. Br J Clin Pharmacol. 1999;48:623–627. doi: 10.1046/j.1365-2125.1999.00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belton KJ, Lewis SC, Payne S, et al. Attitudinal survey of adverse drug reaction reporting by medical practitioners in the United Kingdom. Br J Clin Pharmacol. 1995;39:223–226. doi: 10.1111/j.1365-2125.1995.tb04440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Granas AG, Buajordet M, Stenberg-Nilsen H, et al. Pharmacists' attitudes towards the reporting of suspected adverse drug reactions in Norway. Pharmacoepidemiol Drug Saf. 2007;16:429–434. doi: 10.1002/pds.1298. [DOI] [PubMed] [Google Scholar]
- 16.Biriell C, Edwards IR. Reasons for reporting adverse drug reactions–some thoughts based on an international review. Pharmacoepidemiol Drug Saf. 1997;6:21–26. doi: 10.1002/(SICI)1099-1557(199701)6:1<21::AID-PDS259>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 17.van Grootheest AC, Mes K, de Jong-van den Berg LTW (2002) Attitudes of community pharmacists in the Netherlands towards ADR reporting. Int J Pharm Pract 10:267–72
- 18.Lopez-Gonzalez E, Herdeiro MT, Figueiras A. Determinants of under-reporting of adverse drug reactions: a systematic review. Drug Saf. 2009;32:19–31. doi: 10.2165/00002018-200932010-00002. [DOI] [PubMed] [Google Scholar]
- 19.Passier A, ten Napel M, van Grootheest K, et al. Reporting of adverse drug reactions by general practitioners: a questionnaire-based study in the Netherlands. Drug Saf. 2009;32:851–858. doi: 10.2165/11314490-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 20.Backstrom M, Mjorndal T, Dahlqvist R, et al. Attitudes to reporting adverse drug reactions in northern Sweden. Eur J Clin Pharmacol. 2000;56:729–732. doi: 10.1007/s002280000202. [DOI] [PubMed] [Google Scholar]
- 21.van Hunsel FPAM, ten Berge EAAM, Borgsteedse SD, van Grootheest AC. What motivates patients to report an adverse drug reaction? Ann Pharmacother. 2010;44:936–937. doi: 10.1345/aph.1M632. [DOI] [PubMed] [Google Scholar]
- 22.SurveyMonkey (2009) Available at: www.SurveyMonkey.com (access date 01-04-2010)
- 23.QSR International (2008) NVivo 8 Help. Using the software. QSR International, Melbourne. Accessed:01 April 2010
- 24.Streiner DL, Norman GR. Health measurement scales. A practical guide to their development and use. 3. Oxford: Oxford University Press; 2003. [Google Scholar]
- 25.Cohen J. Statistical power analysis for the behavioral sciences. 2. New Jersey: Hillsdale; 1988. [Google Scholar]
- 26.CIOMS Working Group IV (1998). Benefit–risk balance for marketed drugs: evaluating safety signals. World Health Organisation, Geneva
- 27.Frankenfeld C. "Serious" and "severe" adverse drug reactions need defining. Basic Mus J. 2004;329:573. doi: 10.1136/bmj.329.7465.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Statistics Netherlands (2009) Available at: www.cbs.nl. Accessed: 10 June 2010
- 29.Zopf Y, Rabe C, Neubert A, et al. Women encounter ADRs more often than do men. Eur J Clin Pharmacol. 2008;64:999–1004. doi: 10.1007/s00228-008-0494-6. [DOI] [PubMed] [Google Scholar]
- 30.van der Hooft CS, Sturkenboom MC, van Grootheest K, Kingma HJ, Stricker BH. Adverse drug reaction-related hospitalisations: a nationwide study in The Netherlands. Drug Saf. 2006;29(2):161–168. doi: 10.2165/00002018-200629020-00006. [DOI] [PubMed] [Google Scholar]
- 31.van Grootheest AC, Passier JL, van Puijenbroek EP. Direct reporting of side effects by the patient: favourable experience in the first year. Ned Tijdschr Geneeskd. 2005;149:529–533. [PubMed] [Google Scholar]
- 32.O'Cathain A, Murphy E, Nicholl J. Why, and how, mixed methods research is undertaken in health services research in England: a mixed methods study. BMC Health Serv Res. 2007;7:85. doi: 10.1186/1472-6963-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eurostat (2010) Available at: http://epp.eurostat.ec.europa.eu. Accessed 10 June 2010


