Abstract
Background. Health-related quality of life (QOL) is an important outcome for older people who are often on dialysis for life. Little is, however, known about differences in QOL on haemodialysis (HD) and peritoneal dialysis (PD) in older age groups. Randomising patients to either modality to assess outcomes is not feasible.
Methods. In this cross-sectional, multi-centred study we conducted QOL assessments (Short Form-12 Mental and Physical Component Summary scales, Hospital Anxiety and Depression Scale and Illness Intrusiveness Ratings Scale) in 140 people (aged 65 years or older) on PD and HD.
Results. The groups were similar in age, gender, time on dialysis, ethnicity, Index of Deprivation (based on postcode), dialysis adequacy, cognitive function (Mini-Mental State Exam and Trail-Making Test B), nutritional status (Subjective Global Assessment) and social networks. There was a higher comorbidity score in the HD group. Regression analyses were undertaken to ascertain which variables significantly influence each QOL assessment. All were influenced by symptom count highlighting that the patient’s perception of their symptoms is a critical determinant of their mental and physical well being. Modality was found to be an independent predictor of illness intrusion with greater intrusion felt in those on HD.
Conclusions. Overall, in two closely matched demographic groups of older dialysis patients, QOL was similar, if not better, in those on PD. This study strongly supports offering PD to all suitable older people.
Keywords: elderly, haemodialysis, peritoneal dialysis, quality of life
Introduction
There is remarkably little information about how older patients cope with living on dialysis, despite the fact that the incident rate of starting dialysis increases with age, with rates of 113–221 per million age-related population for 45–64 year olds compared to 110–610 for 65–74 year olds and 99–984 for those over 75 years [1]. Not surprisingly therefore, the dialysis population is old with a median age mostly in the 60s and even over 70 years in some European regions [2]. In most countries, older patients are less likely to start on peritoneal dialysis (PD) compared to younger patients, even in countries with a relatively high use of PD. Thus, 13–25% of patients aged 65–74 years and 9–13% for those aged >75 years start on PD in Denmark, Belgium, the Netherlands and the United Kingdom compared to 20–41% of patients aged 45–64 years [2]. This observation is not true for all countries; in Australia, the prevalence of PD in the dialysis population is 20–23% across all age groups, though it does fall to 13% for those aged over 85 years [3]. As patients receive their information about dialysis mainly from their healthcare team, any bias against PD amongst individual doctors and nurses is likely to influence choice of dialysis modality by patients. This is reflected in the large variability seen between individual units within the same country; as an example, in the UK, the percentage of incident patients over 65 years old starting on PD varies from 5 to 55% [4].
PD is a continuous home-based therapy, which offers several potential advantages in older people. It is potentially less disruptive for a patient’s and their family’s lifestyle. Patients also avoid the need for transport (which is often expensive and uncomfortable) to and from a dialysis unit three times a week. Furthermore, the rapid changes in haemodynamic and fluid status associated with haemodialysis (HD) are often poorly tolerated by older patients who often comment on feeling ‘washed out’ after a dialysis session. Indeed, even in younger patients, it takes an average of 6 h to recover from a standard HD session [5].
Many older patients do not have the opportunity for transplantation, so quality of life (QOL) is particularly important. Most studies on QOL have examined the dialysis population as a whole and have not been restricted to specific age groups. These studies have indicated potential advantages of both PD and HD [6–8]. The North Thames Dialysis Study, which was a prospective study of incident and prevalent patients starting on dialysis over the age of 70 years, is remarkably the only study to have focused on older patients. The striking feature of the patients studied was the high proportion on PD which reflected UK practice at the time of the actual study (late 1990s). With the increase in satellite HD, many of these patients would now not receive PD today in the UK, even though the results showed that outcomes—survival and QOL—were not different for patients on HD and PD [9,10].
The largest study looking at choice of dialysis modality when patients are given free choice is the NECOSAD (Netherlands Cooperative Study on the Adequacy of Dialysis) study. About a third of 1347 patients were deemed to have medical or social contraindications to a particular modality, predominantly PD. Of the two-thirds who were given a choice, the principal characteristics associated with not choosing PD were older age, being female and living alone. Patients who were 70 years or older were six times more likely to choose HD than those aged between 18 and 40 years, though patients who had received predialysis care were much more likely to choose PD than those who had not [11].
Older patients with end-stage kidney disease often have considerable comorbidity, not only the vascular disease associated with their renal disease, but also the comorbidity found in many older people, including impaired vision, deafness, poor mobility, arthritis and cognitive dysfunction. They are often socially isolated, live in poor accommodations and may have financial problems. Depression is common among patients on dialysis, occurring in 15–25% patients [12,13], and possibly is even more common in older patients with one small French study suggesting a prevalence of 61% in HD patients aged over 70 years [14]. This compares with <10% in a community-dwelling elderly population [15]. This high rate of depression will impact on QOL and has been shown to be related to functional impairment and life satisfaction [16]. There are no comparative data about depression for older PD patients.
BOLDE (Broadening Options for Long-term Dialysis in the Elderly) is a multicentre study in the UK whose main aim is to enable a higher proportion of older patients to receive the dialysis modality of their choice. The first part of the study, which we report here, is to determine QOL, depression, symptoms and illness intrusion in older (≥65 years) patients on PD compared to HD so that this information can be given to future patients when choosing their dialysis modality. As shown by the NECOSAD study [11], randomised controlled trials to compare outcomes between HD and PD are not feasible. We have therefore aimed to achieve comparable groups by matching HD patients to existing PD patients.
Materials and methods
Patients were recruited from three hospitals in South East England: Hammersmith Hospital in London, St Helier Hospital in Surrey and the Lister Hospital in Stevenage. The three units vary in size with numbers of prevalent HD/PD patients on 31 December 2007 being 1056/67, 561/128 and 329/43, respectively, with 6.3, 13.5 and 19.0% new patients ≥65 years old starting on PD [4].
Ethical approval was obtained from Charing Cross Research Ethics Committee (06/Q0411/137). Data were collected between November 2007 and January 2009. A target recruitment of 70 matched pairs of PD and HD patients was set as this was the maximum number of older PD patients thought to be obtainable from the three renal units.
Inclusion and exclusion criteria
All patients recruited were aged 65 years and over, had been on dialysis for a minimum of 90 days and had not been hospitalised for 30 days. Patients with clinically obvious cognitive impairment (as assessed by either the lead nephrologist or nurse responsible for the patient’s renal care) or with a life expectancy of <6 months were excluded from the study.
Subjects and recruitment
Purposeful recruitment was undertaken to achieve comparable PD and HD groups. The PD patient was recruited first (due to smaller numbers) and matched to an HD patient with similar demographic characteristics: age, gender, time on dialysis (total time excluding episodes of transplantation), ethnicity and socio-economic status as determined by the Index of Deprivation 2007 based on postcode [17]. Where more than one HD patient was identified as a possible match for the PD patient, the lead nephrologist or nurse made a purposive selection. The patient groups were therefore closely matched to each other by characteristics predominantly seen in our PD population.
Baseline demographic and clinical data
Demographic (Table 1) and clinical data (Table 2) were collected from the patients’ medical records and during the assessment. The Stoke–Davies comorbidity score was used. The most recent routine blood test results and dialysis adequacy values were recorded. Adequate dialysis was defined as either an equilibrated Kt/V of >1.2 (two pool), a single pool Kt/V of ≥1.4 and urea reduction ratio of >65% for HD patients or a Kt/V of >1.7 for those on PD [4]. We defined late referrals as patients who commenced dialysis <30 days after being assessed by a nephrologist. This definition aims to identify those patients who were the least likely to be prepared for dialysis. The late referral time span is reported to vary between 30 days and 3–4 months [18], with the UK Renal Registry Report specifying 90 days prior to starting dialysis [4].
Table 1.
Patient characteristics
| Participants |
Difference between PD and HD participants |
Non-participants |
Difference between participants and non-participants | ||||
|---|---|---|---|---|---|---|---|
| PD (n = 70) | HD (n = 70) | P-value | PD (n = 18) | HD (n = 28) | P-value
PD/HD |
||
| Demography | |||||||
| Mean age years (SD) | 73.1 (5.5) | 73.4 (5.1) | 0.812a | 73.8 (5.6) | 73.5 (5.5) | 0.611/0.972c | |
| 65–69 years, n | 21 (30%) | 21 (30%) | 5 (28%) | 7 (25%) | |||
| 70–79 years, n | 41 (59%) | 41 (59%) | 10 (56%) | 17 (61%) | |||
| 80–89 years, n | 8 (11%) | 8 (11%) | 3 (17%) | 4 (14%) | |||
| Male sex, n | 49 (70%) | 49 (70%) | 1.000b | 6 (33%) | 17 (61%) | 0.004/0.376b | |
| Non-Caucasian, n | 3 (4%) | 7 (10%) | 0.189b | 5 (28%) | 3 (11%) | 0.002/0.916b | |
| Mean index of deprivation (SD) | 13.7 (11.3) | 13.7 (8.7) | 0.476c | – | – | – | |
| Mean years of education (SD) | 11.7 (3.5) | 11.7 (3.6) | 0.983c | – | – | – | |
| Living alone, n | 16 (23%) | 18 (26%) | 0.321b | – | – | – | |
| Mean social network score (SD) | 15.2 (4.4) | 14.9 (4.0) | 0.630a | ||||
| Mean time on dialysis months (SD) | 30.5 (28.3) | 31.4 (26.5) | 0.480c | 25.8 (21.3) | 34.1 (31.5) | 0.627/0.820c | |
| 3–12 months on dialysis | 23 (33%) | 18 (26%) | 8 (44%) | 11 (39%) | |||
| >12–36 months on dialysis | 26 (37%) | 31 (44%) | 5 (28%) | 6 (21%) | |||
| >36 months on dialysis | 21 (30%) | 21 (30%) | 5 (28%) | 11 (39%) | |||
Independent t-test.
Pearson chi-square test.
Mann–Whitney test.
Table 2.
Clinical demographics
| PD |
HD |
P-value | |||
|---|---|---|---|---|---|
| n | % or Mean (SD) | n | % or Mean (SD) | Difference between PD and HD | |
| Clinical characteristics | |||||
| Stoke–Davies comorbidity count | |||||
| 0 comorbidities | 15 | 21% | 12 | 17% | 0.520 |
| 1–2 comorbidities | 46 | 66% | 34 | 49% | 0.040 |
| ≥3 comorbidities | 9 | 13% | 24 | 34% | 0.003 |
| Median Stoke–Davies comorbidity score (IQR)a | 70 | 1.0 (1.0) | 70 | 2.0 (2.0) | 0.009 |
| Median Stoke–Davies comorbidity score (IQR) <1 yeara | 14 | 0.5 (2.0) | 14 | 1.5 (1.0) | 0.202 |
| Median Stoke–Davies comorbidity score (IQR) >1 yeara | 56 | 1.0 (1.0) | 56 | 2.0 (2.0) | 0.018 |
| Diabetes | 13 | 19% | 25 | 36% | 0.023 |
| Late referral | 9 | 13% | 22 | 32% | 0.007 |
| Symptom counta | 70 | 8.6 (3.1) | 70 | 9.7 (3.2) | 0.039 |
| SGA 3-5 (mild to moderate malnutrition) | 70 | 16% | 70 | 24% | 0.205 |
| Total days in hospital over previous 12 monthsa | 70 | 6.6 (8.8) | 70 | 11.0 (18.1) | 0.069 |
| Adequately dialysed | 70 | 91% | 69 | 86% | 0.274 |
| Pill counta | 70 | 10.6 (5.3) | 65 | 11.1 (5.7) | 0.436 |
| Cognitive function | |||||
| TMT-B within expected range for age and education level | 65 | 58% | 57 | 68% | 0.255 |
| MMSE within expected range for age and education level | 70 | 90% | 68 | 88% | 0.739 |
| Blood values | |||||
| CRP ≤10 mg/L | 45 | 64% | 46 | 63% | 0.889 |
| Haemoglobin (g/dL)a | 70 | 11.6 (1.7) | 70 | 11.7 (1.2) | 0.804 |
Pearson chi-square test used for all other P-values.
Independent t-test.
Mann–Whitney.
Study visit
There was one off-study visit that took ∼1 h and consisted of sequential cognitive, QOL and nutritional assessments (with the exception of one patient who needed to be seen twice due to time constraints). All assessments were conducted by the same researcher thereby eliminating inter-observer variability. All were conducted in English except for one patient where an interpreter was used. For those on HD, assessments were conducted on a non-dialysis day or before dialysis. Each assessment was performed in a private room within a hospital or satellite unit. The researcher had received training on administering the assessments. To minimise bias, the researcher followed a script that ensured consistency of delivery of the assessments to all patients.
Clinical assessments
-
(i) Cognitive function:
Mini-Mental State Examination (MMSE): this test is routinely used in clinical practice [19]. It is effective in detecting cognitive impairment but is not sensitive to small changes in cognitive function. Normative data are available, stratified into age groups and education levels [20]. Scores are measured from 1 to 30, with a score of 23 or below suggesting cognitive impairment. The non-completion rate of the MMSE in this study was 1.5% due to language barriers and poor visual acuity.
Trail-Making Test B (TMT-B): this test assesses areas of executive function and cognition and was selected as it is more discriminatory than the MMSE. Normative data are stratified into age groups and years of education and are used to evaluate performance [21]. The non-completion rate of the TMT-B was 13% in this study due to language barriers (0.7%), poor acuity (4.3%), unable to complete (4.3%), interruption (1.4%), difficulties using pen and paper (0.7%) and refusal (1.4%).
-
(ii) Nutritional assessment:
Subjective Global Assessment (SGA) 7 point: the SGA provides a reliable, easy to use and well-validated measure of nutritional status within the dialysis population [22–24]. In this study, the 7-point scale version [25] was used as it can detect more subtle differences in nutritional status compared to the 3-point scale and yet rates patients within the following categories: well nourished (6–7 points) mildly to moderately malnourished (3–5 points) and severely malnourished (1–2 points). Nobody scored within this latter category.
-
(iii) Social networks:
A questionnaire was designed to provide a measure of the support the patient receives and whether they offer support to anyone within their social sphere [26]. The higher the social network score, the greater the level of support. The questionnaire is in Appendix 1.
QOL outcomes
Short Form-12 (SF-12) version 2: the SF-12 is a self assessment of physical and mental health and has two scores: the Physical Component Summary scale (PCS) and the Mental Component Summary scale (MCS). It is an abbreviated version of the SF-36 and was selected as it minimises the burden of completion in older people. The version used in this study reflects QOL over the previous 4 weeks. As there is over 90% agreement between the SF-12 and the SF-36, population norms for the SF-36 can, following convention, be used to interpret results for the SF-12 [27,28].
Hospital Anxiety and Depression Scale (HADS): HADS is a screening tool with scores ranging from 0 (no symptoms) to 21 for either depression or anxiety. This study focussed on depression scores only. A depression score of 8 and above indicates the possible presence of clinically important depression [29]. Diagnosis using the gold standard for mood disorders (depression) by the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders was shown to be an independent predictor of the HADS depression scale in 70 HD patients in the US [30]. The HADS is somewhat shorter than the widely used Beck Depression Inventory, again minimising burden of completion, making it appropriate for this patient group.
Illness Intrusiveness Ratings Scale (IIRS): the IIRS tool assesses the impact of chronic illness on aspects of life derived from Flanagan’s research into what contributes to QOL in the American population at different ages [31]. The intrusiveness is determined by assessing the extent to which the illness and/or treatment interferes with 13 life domains (health, diet, work, active recreation, passive recreation, financial situation, relationship with partner, sex life, family relations, other social relations, self-expression/self-improvement, religious expression and community involvement). It has been validated in different patient groups including end-stage kidney disease [32]. Scores range from 13 to 91, a higher score indicating a more intrusive effect of the illness and/or treatment.
Symptom prevalence the presence of 16 symptoms that have been shown to be prevalent in older people [33] and in patients on dialysis [34] was assessed by a questionnaire. The symptom questionnaire can be found in Appendix 2.
Statistical methodologies
Frequencies, independent t-tests, Pearson chi-square and Mann–Whitney tests were used to compare the participant characteristics between PD and HD in Tables 1 and 2. Unadjusted QOL outcomes per modality are presented in Table 3. A sub-analysis is presented in Table 4 showing comparisons in those who have been on dialysis for 1 year or less and those who have been dialysing for more than 1 year. If QOL differences are not present between HD and PD patients dialysing for <1 year, it could indicate that QOL is not a factor that influences patient’s choice of dialysis modality. If differences between the two groups only emerge after 1 year, this could be attributable to the effects of the dialysis regime or other factors such as the difference in comorbidity scores which is further explored through regression analyses.
Table 3.
Unadjusted quality of life outcomes in older PD and HD patients
| Quality of life assessments | PD |
HD |
|||
|---|---|---|---|---|---|
| n | n | P-value | |||
| SF-12 PCSa, mean (SD) | 70 | 36 (12.1) | 70 | 34.3 (9.7) | 0.263 |
| SF-12 MCSa, mean (SD) | 70 | 55.0 (8.4) | 70 | 51.3 (12.9) | 0.046 |
| IIRS, median (IQR) | 69 | 22.0 (15.0) | 70 | 26.0 (19.0) | 0.006 |
| HADS: depression, median (IQR) | 70 | 4.0 (5.0) | 70 | 6.0 (5.0) | 0.003 |
| HADS score >8; prevalence of possible depression (%) | 70 | 10 | 70 | 26 | 0.015 |
A higher score indicates better quality of life.
Table 4.
Unadjusted quality of life outcomes for those on dialysis for <1year compared to those on dialysis for >1 year
| Quality of life outcomes | On dialysis for <1 year |
P-value | On dialysis for >1 year |
P-value | ||
|---|---|---|---|---|---|---|
| PD (n = 14) | HD (n = 14) | PD (n = 56) | HD (n = 56) | |||
| SF-12 PCS, mean (SD) | 41.1 (11.0) | 35.1 (11.5) | 0.174 | 35.2 (12.1) | 34.1 (9.3) | 0.587 |
| SF-12 MCS, mean (SD) | 55.1 (7.4) | 58.1 (9.1) | 0.346 | 55.0 (8.7) | 49.6 (13.3) | 0.013 |
| IIRS, median (IQR) | 23.5 (18.5) | 27.0 (20.0) | 0.550 | 22.0 (14.0) | 26.0 (19.0) | 0.005 |
| HADS: depression, median (IQR) | 2.5 (4.0) | 3.5 (4.0) | 0.352 | 4.0 (4.0) | 6.0 (5.0) | 0.008 |
Linear regression analyses were used to ascertain which variables and interactions significantly influence the SF-12 MCS and PCS scales and illness intrusion scores (Table 5). Logistic regression was used to identify the factors associated with having possible clinical depression (HADS score ≥8) which are shown in Table 6. The IIRS model did not follow a normal distribution and was subject to log transformation before analysis. All analyses were undertaken using SPSS 17.0.
Table 5.
Regression analyses for the SF-12 Mental Component Scores, SF-12 Physical Component Scores and Illness Intrusion Ratings Scale (all adjusted for age, time on dialysis, comorbidity scores, symptom count, social network score, gender, modality, nutritional status and cognitive function)
| 95% confidence interval |
|||||
|---|---|---|---|---|---|
| B | Standard error | Lower | Upper | P-value | |
| SF-12 PCS | |||||
| Symptom count | −1.692 | 0.254 | −2.194 | −1.190 | <0.0001 |
| SF-12 MCS | |||||
| Symptom count | −0.948 | 0.273 | −1.489 | −0.407 | 0.001 |
| Males compared to females (when malnourished) | 10.513 | 3.788 | 3.020 | 18.006 | 0.006 |
| Effect of increasing comorbidities (if malnourished) | −3.118 | 1.335 | −5.758 | −0.477 | 0.021 |
| Illness Intrusion Ratings Scale | |||||
| Modality PD compared to HD | −0.881 | – | −0.784 | −0.989 | 0.032 |
| Symptom count | 1.054 | – | 1.036 | 1.074 | <0.0001 |
| Age | −0.986 | – | −0.975 | −0.996 | 0.010 |
Table 6.
Logistic regression for presence of possible depression (HADS ≥8): adjusted for age, time on dialysis, comorbidity scores, symptom count, social network score, gender, modality, nutritional status and cognitive function
| 95% confidence interval |
||||||
|---|---|---|---|---|---|---|
| Parameter | B | Standard error | Exp (B) | Lower | Upper | P-value |
| Symptom count | 0.446 | 0.124 | 1.561 | 1.224 | 1.992 | <0.0001 |
| Gender: female compared to male | 1.773 | 0.724 | 5.888 | 1.424 | 24.339 | 0.014 |
| Stoke–Davies comorbidity score | 0.660 | 0.252 | 1.935 | 1.182 | 3.168 | 0.009 |
Results
Demographics
A total of 140 patients (70 patients each on PD and HD) aged 65 years and above were recruited. Fifty-eight percent of the PD patients in the sample were on automated PD and the remainder was on continuous ambulatory PD. Four out of the 70 HD (6%) patients had been on PD previously. One patient transferred to HD due to poor ultrafiltration after >5 years on PD. The three other patients transferred to HD after 2–6 months due to peritonitis. Their QOL did not affect the overall distribution of the results. Ten out of the 70 PD patients had been on HD previously (five were late referrals who subsequently opted for PD, five were planned referrals who were on HD either due to catheter repositioning or who opted for a home treatment between 4 and 19 months on HD). Twenty-five percent of patients approached declined to participate in the study. Table 1 shows that the non-participants, especially those on PD, were more likely to be from ethnic minority groups (P = 0.041) and to be female (P = 0.014). This partly explains the high male (70%) and white British or European ethnicity (93%) prevalence seen in the study sample. The PD and HD participants were otherwise very comparable in relation to age, gender, time on dialysis, Index of Deprivation, years of education and their social network scores. Other clinical descriptors seen in Table 2 such as dialysis adequacy, nutritional status, cognitive function, C-reactive protein (CRP) and haemoglobin levels were also similar. The main differences between the groups are indicated in Table 2: the PD group had a significantly higher prevalence of patients with 0 to 2 comorbidities, whilst those on HD had a higher prevalence of ≥3 comorbidities (P = 0.003) as well as a higher prevalence of diabetes (P = 0.023) and late referrals (P = 0.007). Significantly more comorbidities were present in the HD group compared to the PD group for those dialysing for over 1 year. No difference in comorbidities was seen in those dialysing for <1 year (Table 2).
QOL outcomes
Table 3 presents the unadjusted QOL outcomes for PD and HD patients. Although there was no significant difference in the SF-12 PCS scores, those on PD had better SF-12 MCS scores (P = 0.046) with significantly less possible depression (P = 0.015) and illness intrusion (P = 0.006). Total number of symptoms was also significantly lower in PD than HD patients (8.6 and 9.7, respectively, P = 0.039). In assessing the individual items on the Illness Intrusion Ratings Scale, HD patients experienced greater intrusion of the illness and/or their treatment in relation to their health (P = 0.001) and diet (P ≤ 0.0001) compared to those on PD. There were not significant differences in the 11 other intrusion scales. Table 4 indicates that there were no differences in the unadjusted values for the SF-12, IIRS or HADS depression scores in those dialysing for <1 year between HD and PD. In contrast, for those on dialysis longer than 12 months, HD patients showed significantly more illness intrusion (P = 0.005), higher depression scores (P = 0.008) and worse SF-12 MCS scores (P = 0.013) than their PD equivalents. To enable a comprehensive comparison between the two modalities, the results were adjusted for multiple factors in regression analyses.
Regression analyses for the SF-12, HADS depression and Illness Intrusion Ratings Scale
For both the linear and logistic regression modelling, the following continuous variables were adjusted for: age, months on dialysis, comorbidity, symptom count and social network score. In addition, the categorical variables adjusted for were: gender, modality, nutritional status and cognitive function. Further modelling (data not presented) found that late referral and dialysis adequacy had no influence on QOL outcomes and therefore were excluded from the final models presented.
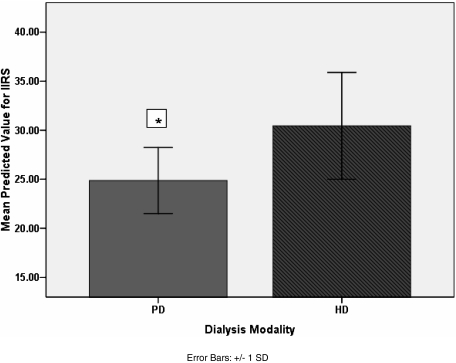
Table 5 summarises the results from the linear regressions. The SF-12 PCS scale was found to be influenced by symptom count alone, with a decrease of 1.7 points for each additional symptom reported (P < 0.0001). Increasing symptom count was also significantly associated with a decline in SF-12 MCS (three outliers were excluded to achieve model of best fit for SF-12 MCS) scores by 0.9 points per symptom (P = 0.001). The SF-12 MCS score was found to be significantly lower in mild to moderately malnourished females compared to equivalent males (P = 0.006). In those who were malnourished, the SF-12 MCS score declined by 3.1 points for each increase in comorbidity score (P = 0.021). Increasing illness intrusion was associated with a greater symptom count (P < 0.0001) and decreasing age (P = 0.01). The effect of modality is illustrated in Figure 1, as it was found to independently contribute to illness intrusion with poorer scores in those on HD than in PD (P = 0.032).
Fig. 1.
Illness Intrusion Ratings Scores (IIRS) for PD and HD adjusted for age, time on dialysis, comorbidity scores, symptom count, social network score, gender, modality, nutritional status and cognitive function. *Significantly less illness intrusion in PD group, P = 0.032.
Logistic regression was used to establish the variables that are associated with those that have a HADS score of ≥8 (possible depression). Table 6 illustrates that the odds of having possible depression were found to be 5.9 times higher for women than for men (P = 0.014). For each additional symptom, the odds of having possible depression increased by a factor of 1.561 (P < 0.0001). Similarly for each additional comorbidity, the odds of having depression increased by a factor of 1.935 (P = 0.009).
Discussion
The results of this study support the use of PD in older patients. The ultimate study design to establish differences in the QOL of older patients on PD compared to HD would be a prospective randomised study. Such a study, however, is not feasible as it is well established that patients cannot be randomised between the two dialysis modalities. We have therefore performed a cross-sectional study and attempted to match HD to existing PD patients. The results show that this matching process was successful and the patients were well matched for age, sex, ethnicity and social status as measured by index of deprivation. HD patients, however, did have a higher comorbidity score than patients on PD. Increasing comorbidity did negatively impact the SF-12 MCS scores (only when malnutrition was present) and increased the odds of having possible depression. This study did not aim to address which individual comorbidities may have the greatest impact on QOL. Comorbidity did not, however, impact on the illness intrusion scores, whereas modality and age did with less illness intrusion in older patients and those on PD. Comorbidity also did not influence the SF-12 PCS scores. Gender was found to play a role in QOL with possible depression (HADS score ≥8) being more common in women than men on dialysis. Overall, symptom count was the single variable that appeared to influence all QOL measures studied and had more of an influence than comorbidity alone.
The main weakness of the current study is that the patients studied are not representative of the dialysis population as a whole. In particular, mainly White Europeans were recruited. In relation to selection and recruitment of patients, there may have been a positive selection bias within the HD population as the most suitable HD patient was selected to participate if there was more than one appropriate HD match. This selection was determined by the lead nurse or nephrologist caring for the patient. Another problem is the cross-sectional design of the study so the patients studied are survivors and therefore fitter. This would have a greater apparent effect in the PD population as sicker PD patients could have transferred to HD, whereas sick HD patients are unlikely to transfer to PD. The study design also makes it difficult to answer the question as to whether fitter and less depressed patients choose PD rather than HD. An argument against this is the observation that QOL measurements in patients who had been on dialysis for <1 year were the same in the HD and PD groups; the differences only emerged in patients who had been on dialysis for more than 1 year (Table 4). In linear regression analysis in those on dialysis for more than 1 year (n = 112), comorbidity level did not significantly influence the IIRS and HADS depression scores, whereas modality (PD) was found to influence these outcomes positively (data not shown).
The North Thames Dialysis Study, which has been the only other study exclusively in older people on dialysis [10], found very similar SF-36 PCS (33.2–34.0) and MCS (50.7–51.3) results to our study; as in the present study, these were no different between the HD and PD patient groups in adjusted analyses. Approximately 18% of patients in the current study had an HADS score ≥8 (possible depression). This is of the same order as found in other studies of patients on dialysis [12,13]. We found that the significant predictors of possible depression were symptom count, comorbidity and female sex. None of these are surprising; a recent meta-analysis has found that poor self-rated health status and the presence of chronic disease are the predominant risk factors for depression among the elderly [35]. The adverse relationship between being female and QOL has been described in other HD-related studies [36,37], and the overall population prevalence of depression is higher in women than men [38]. As found in the NECOSAD study, symptom score had an adverse effect on all measures of QOL in the current study [39].
The choice of dialysis modality has a major impact on many aspects of an individual’s life. Therefore lifestyle, social environment and personality, as well as medical factors, need to be considered in the decision-making process. Providing patients with a choice of dialysis modality in itself appears to improve the QOL [40]. The principal determinants of QOL, as rated by the older person, are the value of being independent and being in control of one’s own life [41]. In light of this, PD should be ideally suited to the elderly. Interestingly, data from the NECOSAD study [11] show that where patients are given appropriate education to make a choice of dialysis modality, 50% chose PD, though the elderly were less likely to do so. The proportion of patients >65 years old starting on PD in the three centres taking part in the study was much lower than this and ranged from 6 to 19%. It is therefore possible that some of the HD patients in our study would have been suitable for PD, particularly as there was no difference in the cognitive function, nutritional status or social network scores between the HD and PD groups. This potentially has added to the strength of this study by having patients that possibly would have been suitable for either modality which may not have occurred in units that have much higher quotas of patients on PD.
The perception that older patients are more likely to have barriers to PD related to physical problems, social circumstances and cognitive dysfunction [42] can result in the healthcare team believing that PD at home is not feasible in this patient group. This may lead to inappropriate information being given to the patient which may preclude further discussion or assistance on how to overcome these barriers. Indeed, Oliver and Quinn, in a recent review, have argued that low rates of PD utilisation reflect a breakdown in the process of care [43]. In a previous study, Oliver et al. showed that with appropriate multidisciplinary team support and education, over 50% of an elderly population deemed eligible for PD chose PD [44].
Conclusion
The findings from this study support the greater use of PD in older people, and suggest that there may be substantial under-utilisation in many centres in the UK. The fact that QOL may well be better on PD due to its potentially lower intrusion into older peoples’ lives should influence the content of predialysis education. Improved education would enable patients to choose dialysis modality based on how it is going to affect their ability to maintain the aspects of life they value.
Appendix 1: Social networks questionnaire
• Please answer all the questions below, by placing an ‘X’ in the box beside the answer that best describes your response.
• There are no ‘right’ or ‘wrong’ answers, so please just choose the answer that best fits your own personal experience.
-
Who normally lives at home with you?
A. I live with my spouse
B. I live with my family
C. I live with a carer
D. I live alone
-
Does anyone else in the family live close to your home?
A. My children
B. Another relative
C. None of the family
-
Do you have a circle of friends?
A. Yes, a large circle
B. Yes, a small circle
C. No, not many friends
-
Considering the people living with you, do you think any of them understand your illness and the dialysis?
A. Yes, they understand well
B. Yes, they understand a little
C. No, they don’t understand
D. I live on my own
-
If you have to come into hospital (other than to dialyse), who would you normally expect to visit you?
A. Several family members and/or friends
B. One or two family members and/or friends
C. Nobody
-
When you have a problem with your illness, who can you turn to for help or support?
A. Several family members and/or friends
B. One or two family members and/or friends
C. A doctor or nurse
D. Nobody
-
Who would turn to you for help or support if they became ill?
A. Several family members and/or friends
B. One or two family members and/or friends
C. Nobody
-
If you receive some good news, with whom would you normally share this?
A. Several family members and/or friends
B. One or two family members and/or friends
C. Nobody
-
Who would come to you with their good news?
A. Several family members and/or friends
B. One or two family members and/or friends
C. Nobody
-
If you felt generally fed up and miserable, who would you turn to for support?
A. Several family members and/or friends
B. One or two family members and/or friends
C. Nobody
-
Who would turn to you for support if they felt fed up and miserable?
A. Several family members and/or friends
B. One or two family members and/or friends
C. Nobody
Appendix 2: Symptom included in questionnaire
Pain in joints
Blurred vision
Weakness of limbs
Headaches
Light-headedness or dizziness
Cold hands and feet
Cramps in legs
Shortness of breath
Unsteadiness
Dry mouth
Sleep problems
Swelling of legs
Nausea
Itching
Lack of energy
Taste changes
Conflict of interest statement
Edwina Brown receives speaker fees from Baxter Healthcare and Gambro.
References
- 1.Stel VS, Kramer A, Zoccali C, et al. The 2006 ERA-EDTA Registry annual report: a precis. J Nephrol. 2009;22:1–12. [PubMed] [Google Scholar]
- 2. ERA-EDTA: 2007 ERA-EDTA Annual Report. http://www.era-edta-reg.org/files/annualreports/pdf/AnnRep2007.pdf (21 September 2009, date last accessed)
- 3. ANZDATA. 31st Annual Report, 2008. Chapter 6. http://www.anzdata.org.au/anzdata/AnzdataReport/31stReport/Ch06PD.pdf (21 September 2009, date last accessed) [Google Scholar]
- 4.Ansell D, Feehally J, Fogarty D, et al. UK Renal Registry Report 2008. 2009 The Renal Association. [Google Scholar]
- 5.Lindsay RM, Heidenheim PA, Nesrallah G, et al. Daily Hemodialysis Study Group London Health Sciences Centre. Minutes to recovery after a hemodialysis session: a simple health-related quality of life question that is reliable, valid, and sensitive to change. Clin J Am Soc Nephrol. 2006;1:952–959. doi: 10.2215/CJN.00040106. [DOI] [PubMed] [Google Scholar]
- 6.Juergensen E, Wuerth D, Finkelstein S, et al. Hemodialysis and peritoneal dialysis: patients’ assessment of their satisfaction with therapy and the impact of the therapy on their lives. Clin J Am Soc Nephrol. 2006;1:1191–1196. doi: 10.2215/CJN.01220406. [DOI] [PubMed] [Google Scholar]
- 7.Rubin HR, Fink NE, Plantinga LC, et al. Patient ratings of dialysis care with peritoneal dialysis vs hemodialysis. JAMA. 2004;291:697–703. doi: 10.1001/jama.291.6.697. [DOI] [PubMed] [Google Scholar]
- 8.Wu A, Fink N, Marsh M, et al. Changes in quality of life during hemodialysis and peritoneal dialysis treatment: generic and disease specific measures. J Am Soc Nephrol. 2004;15:743–753. doi: 10.1097/01.asn.0000113315.81448.ca. [DOI] [PubMed] [Google Scholar]
- 9.Harris SA, Lamping DL, Brown EA, et al. Clinical outcomes and quality of life in elderly patients on peritoneal dialysis versus hemodialysis. PeritDialInt. 2002;22:463–470. [PubMed] [Google Scholar]
- 10.Lamping DL, Constantinovici N, Roderick P, et al. Clinical outcomes, quality of life, and costs in the North Thames Dialysis Study of elderly people on dialysis: a prospective cohort study. Lancet. 2000;356:1543–1550. doi: 10.1016/S0140-6736(00)03123-8. [DOI] [PubMed] [Google Scholar]
- 11.Jager KJ, Korevaar JC, Dekker FW, et al. The effect of contraindications and patient preference on dialysis modality selection in ESRD patients in The Netherlands. Am J Kidney Dis. 2004;43:891–899. doi: 10.1053/j.ajkd.2003.12.051. [DOI] [PubMed] [Google Scholar]
- 12.Hedayati SS, Bosworth HB, Briley LP, et al. Death or hospitalization of patients on chronic hemodialysis is associated with a physician-based diagnosis of depression. Kidney Int. 2008;74:930–936. doi: 10.1038/ki.2008.311. [DOI] [PubMed] [Google Scholar]
- 13.Abdel-Kader K, Unruh ML, Weisbord SD. Symptom burden, depression, and quality of life in chronic and end-stage kidney disease. Clin J Am Soc Nephrol. 2009;4:1057–1064. doi: 10.2215/CJN.00430109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tyrrell J, Paturel L, Cadec B, et al. Older patients undergoing dialysis treatment: cognitive functioning, depressive mood and health-related quality of life. Aging Ment Health. 2005;9:374–379. doi: 10.1080/13607860500089518. [DOI] [PubMed] [Google Scholar]
- 15.Graham JE, Rockwood K, Beattie BL, et al. Prevalence and severity of cognitive impairment with and without dementia in an elderly population. Lancet. 1997;349:1793–1796. doi: 10.1016/S0140-6736(97)01007-6. [DOI] [PubMed] [Google Scholar]
- 16.Kutner NG, Brogan D, Dallas Hall W, et al. Functional impairment, depression, and life satisfaction among older hemodialysis patients and age-matched controls: a prospective study. ArchPhysMedRehabil. 2000;8:453–459. doi: 10.1053/mr.2000.3878. [DOI] [PubMed] [Google Scholar]
- 17. Office for National Statistics 2007. Neighbourhood Statistics http://www.neighbourhood.statistics.gov.uk/dissemination/ (1 November 2009, date last accessed) [Google Scholar]
- 18.Roderick P, Jones C, Drey N, et al. Late referral for end-stage renal disease: a region-wide survey in the south west of England. Nephrol Dial Transplant. 2002;17:1252–1259. doi: 10.1093/ndt/17.7.1252. [DOI] [PubMed] [Google Scholar]
- 19.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 20.Crum RM, Anthony JC, Bassett SS, et al. Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA. 1993;269:2386–2391. [PubMed] [Google Scholar]
- 21.Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19:203–214. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 22.Visser R, Dekker FW, Boeschoten EW, et al. Reliability of the 7-point subjective global assessment scale in assessing nutritional status of dialysis patients. Adv Perit Dial. 1999;15:222–225. [PubMed] [Google Scholar]
- 23.Steiber A, Leon JB, Secker D, et al. Multicenter study of the validity and reliability of subjective global assessment in the hemodialysis population. JRenNutr. 2007;17:336–342. doi: 10.1053/j.jrn.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Enia G, Sicuso C, Alati G, et al. Subjective global assessment of nutrition in dialysis patients. NephrolDialTransplant. 1993;8:1094–1098. [PubMed] [Google Scholar]
- 25.Churchill DN. Nutrition and adequacy of dialysis. PeritDialInt. 1997;17:S60–S62. [PubMed] [Google Scholar]
- 26.Sensky T, Leger C, Gilmour S. Psychosocial and cognitive factors associated with adherence to dietary and fluid restriction regimens by people on chronic haemodialysis. Psychother Psychosom. 1996;65:36–42. doi: 10.1159/000289029. [DOI] [PubMed] [Google Scholar]
- 27.Nortvedt MW, Riise T, Myhr KM, et al. Performance of the SF-36, SF-12, and RAND-36 summary scales in a multiple sclerosis population. Med Care. 2000;38:1022–1028. doi: 10.1097/00005650-200010000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 30.Cukor D, Coplan J, Brown C, et al. Anxiety disorders in adults treated by hemodialysis: a single-center study. Am J Kidney Dis. 2008;52:128–136. doi: 10.1053/j.ajkd.2008.02.300. [DOI] [PubMed] [Google Scholar]
- 31.Flanagan JC. Measurement of quality of life: current state of the art. Arch Phys Med Rehabil. 1982;63:56–59. [PubMed] [Google Scholar]
- 32.Devins GM, Edworthy SM, Seland TP, et al. Differences in illness intrusiveness across rheumatoid arthritis, end-stage renal disease, and multiple sclerosis. J Nerv Ment Dis. 1993;181:377–381. doi: 10.1097/00005053-199306000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Bulpitt CJ, Fletcher AE, Thijs L, et al. Symptoms reported by elderly patients with isolated systolic hypertension: baseline data from the SYST-EUR trial. Systolic Hypertension in Europe. Age Ageing. 1999;28:15–22. doi: 10.1093/ageing/28.1.15. [DOI] [PubMed] [Google Scholar]
- 34.Weisbord SD, Fried LF, Arnold RM, et al. Development of a symptom assessment instrument for chronic hemodialysis patients: the Dialysis Symptom Index. J Pain Symptom Manage. 27:226–240. doi: 10.1016/j.jpainsymman.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 35.Chang-Quan H, Xue-Mei Z, Bi-Rong D, et al. Health status and risk for depression among the elderly: a meta-analysis of published literature. AgeAgeing. 2010;39:23–30. doi: 10.1093/ageing/afp187. [DOI] [PubMed] [Google Scholar]
- 36.Seica A, Segall L, Verzan C, et al. Factors affecting the quality of life of haemodialysis patients from Romania: a multicentric study. Nephrol Dial Transplant. 2009;24:626–629. doi: 10.1093/ndt/gfn506. [DOI] [PubMed] [Google Scholar]
- 37.Rebollo P, Ortega F, Baltar JM, et al. Health-related quality of life HRQOL in end stage renal disease ESRD patients over 65 years. GeriatrNephrolUrol. 1998;8:85–94. doi: 10.1023/a:1008338802209. [DOI] [PubMed] [Google Scholar]
- 38.Kuehner C. Gender differences in unipolar depression: an update of epidemiological findings and possible explanations. Acta Psychiatr Scand. 2003;108:163–174. doi: 10.1034/j.1600-0447.2003.00204.x. [DOI] [PubMed] [Google Scholar]
- 39.Merkus MP, Jager KJ, Dekker FW, et al. Physical symptoms and quality of life in patients on chronic dialysis: results of The Netherlands Cooperative Study on Adequacy of Dialysis (NECOSAD) Nephrol Dial Transplant. 1999;14:1163–1170. doi: 10.1093/ndt/14.5.1163. [DOI] [PubMed] [Google Scholar]
- 40.Szabo E, Moody H, Hamilton T, et al. Choice of treatment improves quality of life: a study on patients undergoing dialysis. ArchInternMed. 1997;157:1352–1356. [PubMed] [Google Scholar]
- 41.Ahmed S, Addicott C, Qureshi M, et al. Opinions of elderly people on treatment for end-stage renal disease. Gerontology. 1999;45:156–159. doi: 10.1159/000022078. [DOI] [PubMed] [Google Scholar]
- 42.Finkelstein FO, Afolalu B, Wuerth D, et al. The elderly patient on CAPD: helping patients cope with peritoneal dialysis. PeritDialInt. 2008;28:449–451. [PubMed] [Google Scholar]
- 43.Oliver MJ, Quinn RR. Is the decline of peritoneal dialysis in the elderly a breakdown in the process of care? PeritDialInt. 2008;28:452–456. [PubMed] [Google Scholar]
- 44.Oliver MJ, Quinn RR, Richardson EP, et al. Home care assistance and the utilization of peritoneal dialysis. Kidney Int. 2007;71:673–678. doi: 10.1038/sj.ki.5002107. [DOI] [PubMed] [Google Scholar]