Abstract
Background. Advanced renal artery stenosis (RAS) may cause progressive deterioration in renal function. We correlated the histopathological findings and clinical characteristics in selected patients with atherosclerotic RAS who underwent nephrectomy of their small kidneys for resistant renovascular hypertension.
Methods. We studied 62 patients who underwent nephrectomy of a small kidney for uncontrolled hypertension between 1990 and 2000.
Results. The mean patient age was 65.4 ± 9.6 years; 28 (45%) were men. Significant tubulointerstitial atrophy with relative glomerular sparing was the predominant pattern of injury in 44 (71%) patients. In 14 (23%) patients, diffuse global glomerulosclerosis was present. The severity of tubulointerstitial atrophy and the extent of glomerulosclerosis were both associated with smaller kidney size (P = 0.002). Three patterns of vascular involvement were present: atheroembolic, atherosclerotic and hypertensive vascular changes, which were documented in 39, 98 and 52% of subjects, respectively. The presence and severity of these vascular changes positively correlated with both atherosclerotic risk factors, such as hypertension, dyslipidaemia and renal insufficiency, and cardiovascular morbidity, including abdominal aortic aneurysm and myocardial infarction. Patients on statin therapy were noted to have less evidence of renal fibrosis as measured by transforming growth factor-beta staining (P = 0.003).
Conclusion. The severity of renal histopathological findings in patients who underwent nephrectomy for resistant hypertension correlated with an increased prevalence of cardiovascular disease, a greater degree of renal dysfunction and more severe dyslipidaemia. Statin therapy may affect development of intra-renal injury by slowing the progression of fibrosis.
Keywords: atheroembolic renal disease, atherosclerotic renal disease, hypertensive renal disease, ischaemic nephropathy, renal artery stenosis
Introduction
Critical renal artery stenosis (RAS), defined as at least a 75% reduction in arterial diameter, can lead to the clinical syndromes of renovascular hypertension and deterioration in renal function, commonly referred to as ischaemic nephropathy. In renovascular hypertension, renal hypoperfusion distal to the stenosis causes activation of the renin-angiotensin-aldosterone system, which stimulates secretion of angiotensin II and aldosterone leading to vasoconstriction and salt retention, respectively.
For deterioration of renal function to occur as a consequence of vascular disease, the entire renal mass must be affected, by either bilateral RAS or stenosis to a solitary kidney. Renal insufficiency does not appear to be simply a function of hypoperfusion and resultant ischaemia, as <10% of normal renal blood flow is required to meet the metabolic needs of renal tissue [1]. Therefore, the term ‘chronic azotemic renovascular disease’ has been suggested by some authors in place of ‘ischaemic nephropathy’ and the terms have been used interchangeably in the current literature [2]. Indeed, experimental studies in a pig model of renovascular hypertension showed that cholesterol feeding can induce reactive oxygen species and stimulate tissue fibrogenic cytokines and apoptotic mechanisms, all of which may contribute to renal injury. These data suggest that complex interactions may exist among vascular injury, blood pressure and target organ damage in humans. However, although clinical studies have indicated that up to 16% of patients on haemodialysis may have atherosclerotic renal artery disease that may contribute to their renal failure [3,4], the exact mechanisms and resultant pathways of renal injury in patients with RAS are not fully understood. Recent studies on renal fibrogenesis in pig models with RAS and high cholesterol diets compared epithelial to mesenchymal cell transition, renal fibrosis and transforming growth factor (TGF)-beta upregulation in pigs with and without statin therapy. The group with statin therapy had diminished epithelial to mesenchymal cell transition, less renal fibrosis and downregulation of TGF-beta [5]. In humans, treatment with statins was shown not only to reduce the risk of progression, but to induce the regression of the anatomical lesion in RAS [6]. However, the effects of statin therapy on signalling pathways that may mediate renal injury remain unknown. In this study, we aimed (i) to investigate the patterns of glomerular, tubulointerstitial and vascular injuries in renal sections of surgically removed kidneys from patients who underwent nephrectomies of their small, poorly functioning kidneys for resistant hypertension and (ii) to correlate the patterns of renal injury and TGF-beta staining with clinical parameters, including lipid abnormalities and statin therapy.
Materials and methods
Clinical data
This study was approved by the Institutional Review Board. The subjects studied had consented to the use of their records for research purposes. A computerized search of our institution’s medical records database was conducted to identify the patients of interest for this study. We identified 62 patients who underwent nephrectomy of a small kidney for uncontrolled hypertension due to RAS secondary to atherosclerosis between 1990 and 2000. Patients requiring nephrectomy for other causes of RAS were excluded. Clinical data were extracted from the medical records, which included inpatient, outpatient, medical provider correspondence and emergency department documentation at the Mayo Clinic Rochester. We have previously reported [7] that nephrectomy in these patients resulted in improved blood pressure control without further loss in renal function. Hypertension in this paper was defined as a persistent systolic blood pressure of >140 mm Hg and diastolic of >90 mm Hg. A favourable blood pressure outcome post-nephrectomy was defined as a systolic blood pressure of <140 mm Hg with a diastolic <90 mm Hg, without the use of any antihypertensive medications, or a reduction in mean arterial pressure [(calculated as systolic + twice diastolic pressure) ÷ 3] >10 mm Hg, or a reduction in the number of antihypertensive medications associated with a >5 mm Hg decrease in mean pressure over a median follow-up of 4 years. Blood pressure recordings were measured on a standard device calibrated at the start of each recording cycle and measured over a 6- or 24-h period. The mean blood pressure value at outpatient visits was used.
Multiple clinical factors were collected including demographic, medical and laboratory histories. The medical history included information on prior renal revascularization, duration of hypertension, presence of diabetes, abdominal aortic aneurysm (AAA), cerebrovascular disease, split-function/single-kidney glomerular filtration rate (GFR) of the nephrectomized kidney, history of smoking, myocardial infarction (MI) and medication usage: number of hypertension medications, angiotensin-converting enzyme inhibitor (ACEI)/angiotensin receptor blocker (ARB) use, diuretics and statins. Duration of hypertension represented the length of time the patients were treated for hypertension based on outpatient clinical records. This did not take into account the duration of hypertension prior to diagnosis and treatment. Smoking history was obtained from review of clinical records. Information as to whether patients ever smoked and number of packs per year were collected. Laboratory history included a lipid panel and serum creatinine.
Histopathological data
The blocks of renal tissue from the identified patients were retrieved from our institution’s database. Periodic Acid Schiff (PAS) staining was performed on representative specimens of the nephrectomies. Slides were interpreted using light microscopy by a renal pathologist who was blinded to the subjects’ clinical database and previous renal pathology reports.
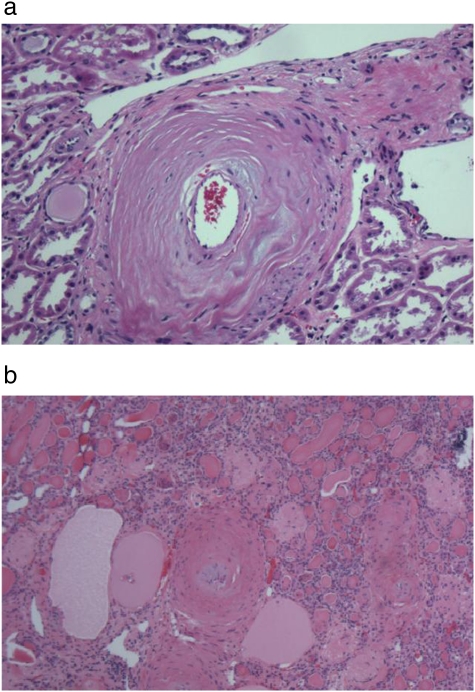
Atherosclerotic vessel disease was defined by the presence of fibro-intimal hyperplasia of the arcuate and interlobular arteries. The severity of atherosclerotic vessel disease was classified by the percentage of intimal plaque thickness; <25% was mild, 25–75% was moderate and >75% was severe. A representative picture is shown in Figure 1a.
Fig. 1.
a. Haematoxylin and eosin (HE) stain of a renal section from a nephrectomized kidney showing severe intimal proliferation of an arcuate renal artery characteristic of atherosclerotic intra-renal vessel disease. b. HE stain of a renal section from a nephrectomized kidney showing medial vessel hypertrophy of an interlobular renal artery, characteristic of hypertensive intra-renal vessel disease.
Hypertensive vessel disease was defined by the presence of hypertrophy/hyperplasia of the arcuate arteries, fibromuscular hyperplasia of the interlobular arteries, with or without hyaline sclerosis of the smaller arteries. A representative picture is shown in Figure 1b.
The definition of advanced glomerulosclerosis was based on the presence of ≥30% of sclerotic glomeruli in 10 different representative fields of view. Less than 30% glomerulosclerosis was considered relative glomerular sparing. When used for statistical analysis, glomerulosclerosis was described as a continuous variable from 0 to 100%, determined by the percentage of glomerulosclerosis present in 10 different representative fields of view. Severe tubulointerstitial atrophy was also defined as the presence of ≥30% tubulointerstitial injury, and mild tubulointerstitial atrophy was defined as the presence of <30% tubulointerstitial injury.
TGF-beta staining was performed using immunohistochemistry with a monoclonal antibody against TGF-beta (anti-TGF-beta1). The distribution of TGF-beta staining was defined as staining within the interstitium of <25%, >25% but <50%, >50% or none. The TGF-beta staining protocol utilized the mouse anti-human TGF-beta antibody from Chemicon International. A dilution of 1:1000 of the mouse anti-human TGF-beta was preheated with Pronase Reagent for 8 min at room temperature and then blocked with Avidin/Biotin blocking kit. The primary reagent was incubated at 4°C overnight. Once the secondary and tertiary reagents (Vectastain Elite Rabbit Kit, Vector Laboratories) were applied, the slides were visualized with NovaRed and counterstained with haematoxylin.
Statistical analysis
Continuous variables were described by the mean ± standard deviation or median (range), as appropriate. Categorical variables were described using counts (percent). The univariate and multiple variable models for a discrete outcome, such as intra-renal hypertensive vessel disease (IRHVD), were created using logistic regression to find the odds ratio and P-value. The univariate and multiple variable models of atherosclerotic intra-renal vessel disease and TGF-beta were created using ordinal logistic regression. Continuous variable outcomes, such as glomerulosclerosis, were assessed using linear regression. Covariates examined included demographic factors, such as age, sex and body mass index, medical history, vital signs, lab values and medication usage. One or two of the demographic or medication variables, which were statistically significant in univariate analyses, were adjusted for in the multiple variable models. Additionally, the associations of the vascular pathologies were investigated using chi-square tests, ordered contingency tables and linear regression. All statistical tests were two-sided, and P-values of <0.05 were considered statistically significant. SAS v9.1 (SAS Institute Inc, Cary, NC) was used.
Results
Clinical characteristics
Baseline characteristics of the selected patients with resistant hypertension who underwent nephrectomy secondary to atherosclerotic RAS are summarized in Table 1. The diagnosis of RAS was made based on an angiogram in 52 (84%) of the patients. Of the remaining 10 (16%), the diagnosis was made based on magnetic resonance angiography in seven patients, and on renogram and Doppler ultrasound in three. In the 62 patients who underwent nephrectomy for uncontrolled hypertension, renal revascularization was not considered technically feasible or beneficial due to the extent of renal atrophy of the affected side and loss of renal function. Of the 62 patients, only 13 (21%) underwent renal revascularization prior to nephrectomy. Of the 13 (21%) patients, 2 had two prior renal endovascular revascularizations, and 1 had three prior renal endovascular revascularizations. Prior revascularization of the affected side was deemed a failure and hence the patients were referred for nephrectomy. Of note, five patients had received bilateral renal revascularization prior to unilateral nephrectomy.
Table 1.
Baseline clinical characteristics of 62 patients prior to undergoing nephrectomy for small kidneys due to uncontrolled hypertension
| Characteristic | Number (%), mean ± SD or median |
|---|---|
| Age (year) | 65.4 ± 9.6 |
| Gender (men) | 28 (45) |
| Prior renal revascularization | 13 (21) |
| Duration of hypertension (year) | 11.1 ± 9.0 |
| Number of hypertension medications | 3.3 ± 1.1 |
| Systolic blood pressure (mm Hg) | 171.0 ± 18.7 |
| Diastolic blood pressure (mm Hg) | 87.2 ± 10.6 |
| ACEI/ARB therapy | 31 (50) |
| Diabetes | 10 (16) |
| Low-density lipoprotein (mg/dL) | 128.7 ± 35.0 |
| High-density lipoprotein (mg/dL) | 44.2 ± 15.2 |
| Statin therapy | 16 (26) |
| Abdominal aortic aneurysm | 21 (34) |
| Cerebrovascular diseasea | 34 (55) |
| Serum creatinine (mg/dL) | 1.88 ± 0.59 |
| Smoking history (yes) | 47 (76) |
| History of myocardial infarction | 14 (23) |
ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker.
Cerebrovascular disease is defined as a history of cerebrovascular accident, transient ischaemic attack and/or carotid vessel disease.
Some of the clinical variables were not present or lacking in the medical records, including the number of years and packs per year smoking history, the use of ACEI/ARB and left ventricular ejection fraction. Since these variables were missing in up to 50% of the patients, they were excluded from the statistical analysis. Duration of statin therapy was not collected.
Histopathological characteristics
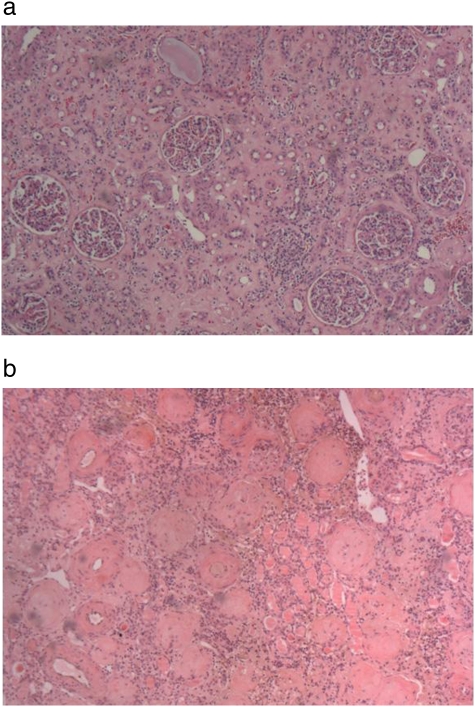
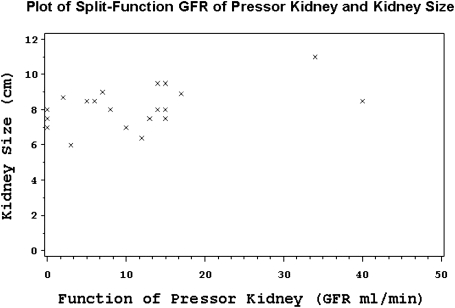
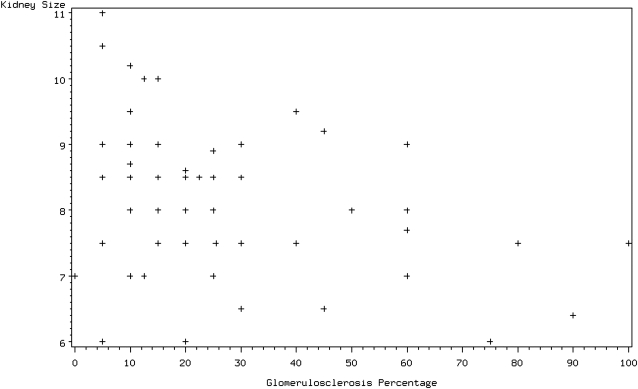
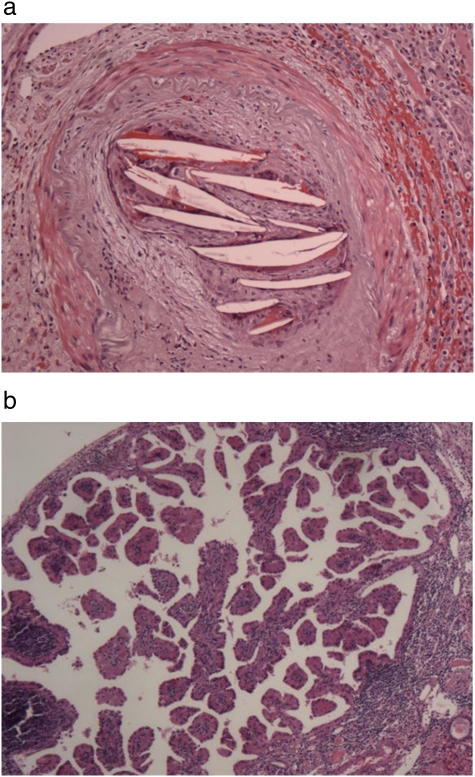
Severe tubulointerstitial atrophy was present in 58 (93.6%) of the 62 nephrectomy slides. In 53 cases (85.5%), there was evidence of moderate to severe medullary disease, of which 36% displayed thyroidization (atrophic tubules containing proteinaceous casts). Fourteen cases showed advanced glomerulosclerosis (23%) and only one case had 100% sclerosis of the glomeruli. Two major patterns of renal atrophy were present: severe tubulointerstitial atrophy with glomerular sparing, defined as global glomerulosclerosis of <30%, and tubulointerstitial atrophy with glomerular injury of similar severity (Figure 2a, b). The most common finding was severe tubulointerstitial atrophy with global glomerulosclerosis of <30% in 44 (71%) of the cases. The mean size of the affected kidney based on imaging studies was 7.91 ± 1.57 centimetres (cm) and 8.17 ± 1.10 cm based on the pathologic report. The kidney size of nephrectomized kidneys closely correlated with their respective GFRs, as determined by a nuclear renogram: every 0.5 cm increase in kidney size was associated with a 10% increase in GFR, P = 0.03 (Figure 3). The presence of advanced glomerulosclerosis was associated with smaller kidney size, P = 0.002. Specifically, an increase of glomerulosclerosis of 5% was associated with a 0.09 cm decrease in kidney size, with a Spearman correlation coefficient of 0.34 (Figure 4). Similarly, the severity of tubulointerstitial atrophy correlated with a decrease in kidney size: mild tubulointerstitial atrophy was associated with a mean affected kidney size of 9.7 ± 1.2 cm, compared to 8.0 ± 1.0 cm in patients with severe tubulointerstitial atrophy (P = 0.002). Atheroembolic vessel disease was found at an incidence of 24 (39%) (Figure 5a), while a microscopic microadenoma was present in 6 (10%) (Figure 5b). There were two cases of almost complete obstruction of the affected renal artery due to atherosclerotic plaque and hypertensive vessel disease.
Fig. 2.
a. HE stain of a renal section from a nephrectomized kidney showing severe tubulointerstitial atrophy with glomerular sparing. b. HE stain of a renal section from a nephrectomized kidney showing severe tubulointerstitial atrophy with advanced glomerulosclerosis.
Fig. 3.
Plot of split-function GFR of pressor kidneys and kidney size.
Fig. 4.
Correlation graph of the extent of glomerulosclerosis with kidney size.
Fig. 5.
a. HE stain of a renal section from a nephrectomized kidney showing multiple cholesterol crystals occluding an arcuate renal artery, characteristic of atheroembolic intra-renal vessel disease. b. HE stain of a renal section showing an example of an intra-renal microadenoma.
Vascular findings
Three types of vessel disease were identified: atherosclerotic, hypertensive and atheroembolic.
Intra-renal atherosclerotic vessel disease
There were three grades of severity of intra-renal atherosclerotic vessel disease (IRAVD): mild (n = 12), moderate (n = 39) and severe (n = 10). All patients had atherosclerotic RAS as the underlying aetiology of renovascular hypertension. Of the 62 patients, 61 (98%) had evidence of IRAVD. One case showed pure hypertensive vessel disease. Multiple clinical variables including age, duration of hypertension, elevated low-density lipoprotein (LDL) and cerebral vessel disease correlated with the degree of IRAVD severity. For example, an increase of 10 years in age was associated with an increase in severity of IRAVD, with an odds ratio of 1.83 (P = 0.03), and there was a strong association with a longer hypertension history (OR = 2.66, P = 0.003). An elevation in LDL by 10 points was associated with 1.22 times the odds of more severe IRAVD (P = 0.048). However, when adjusted for age, the association of an elevated LDL with more severe IRAVD became statistically not significant (P = 0.18). In addition, cerebrovascular disease was found to be weakly associated with increased IRAVD severity (OR = 2.60, P = 0.08). After adjusting for age, this association was no longer statistically significant (P = 0.14), Table 2.
Table 2.
Factors associated with mild, moderate and severe atherosclerotic intra-renal vessel disease
| Characteristics | Mild | Moderate | Severe | Odds ratio | P-value | Age-adjusted P-value |
|---|---|---|---|---|---|---|
| n = 12 | n = 39 | n = 10 | ||||
| Age (in years) | 60.2 ± 9.5 | 66.1 ± 10.0 | 69.2 ± 5.8 | 1.83 | 0.033 | |
| History of hypertension (in years) | 6.3 ± 4.8 | 10.9 ± 8.4 | 18.3 ± 11.4 | 2.66 | 0.003 | 0.008 |
| LDL (mg/dL) | 101.3 ± 29.8 | 133.6 ± 37.8 | 141.0 ± 17.2 | 1.22 | 0.048 | 0.18 |
| Cerebrovascular disease | 5 (42%) | 21 (54%) | 8 (80%) | 2.60 | 0.083 | 0.14 |
| Serum creatinine (mg/dL) | 1.82 ± 0.50 | 1.80 ± 0.58 | 2.24 ± 0.67 | 1.07 | 0.123 | 0.06 |
Intra-renal hypertensive vessel disease
There were 30 cases of IRAVD only, 31 cases of both atherosclerotic and hypertensive vessel disease and one case of IRHVD only. Males had higher odds of having IRHVD than females (OR = 4.58, P = 0.006). There was an association of MI with IRHVD (OR = 4.71, P = 0.03). Moreover, a 10-point drop in high-density lipoprotein (HDL) was associated with 2.20 times the odds of having IRHVD (P = 0.005), while each 0.1 mg/dL increase in serum creatinine was associated with 1.1 times greater odds of having IRHVD (P = 0.01). When adjusted for gender, all other associations with IRHVD became statistically not significant, except for the decreased HDL which remained statistically significant at P = 0.017. Interestingly, having IRHVD was found to be associated with having a more diffuse pattern of global glomerulosclerosis, P = 0.047. When the risk of hypertensive vessel disease was assessed with other clinical variables, such as the duration of hypertension history, pre-nephrectomy blood pressure, number of hypertension medications and smoking history, none were found to have statistical significance, Table 3.
Table 3.
Factors associated with the presence and absence of hypertensive intra-renal vessel disease
| Characteristics | IRHVD changes | No IRHVD changes | Odds ratio | P-value | Gender-adjusted P-value |
|---|---|---|---|---|---|
| n = 32 | n = 30 | ||||
| Male gender | 20 (71%) | 8 (29%) | 4.58 | 0.006 | |
| History of myocardial infarction | 11 (79%) | 3 (21%) | 4.71 | 0.030 | 0.07 |
| HDL (mg/dL) | 37.7 ± 10.1 | 51. ± 16.8 | 2.20 | 0.005 | 0.02 |
| Serum creatinine (mg/dL) | 2.07 ± 0.617 | 1.67 ± 0.49 | 1.14 | 0.01 | 0.051 |
Atheroembolic vessel disease
Atheroembolic intra-renal vessel disease was present in 39% (24) of the cases. As atheroembolism is commonly precipitated by endovascular interventions, we reviewed all vascular procedures and renal angiograms up to 3 years prior to nephrectomy for our cohort. We found that 93% (57) of all patients in the cohort, and 92% (22) of those with signs of atheroembolic renal disease had at least one intravascular procedure up to 3 years prior to nephrectomy. AAA (OR = 3.22, P = 0.04), elevated serum creatinine (OR = 1.14 for each 0.1 mg/dL increase, P = 0.01) and increased LDL (OR = 1.37 for each 10 mg/dL increase, P = 0.02) were associated with higher odds of having atheroembolic vessel disease. After adjusting for age, the association of atheroembolic intra-renal vessel disease with elevated LDL (OR = 1.42 for each 10 mg/dL increase, P = 0.02), serum creatinine (OR = 1.14 for each 0.1 mg/dL increase, P = 0.009) and having an AAA (OR = 3.37, P = 0.032) remained statistically significant. Furthermore, atheroembolic intra-renal vessel disease was associated with worse renal function (−7.9 mL/min/1.73 m2, P = 0.02), Table 4.
Table 4.
Factors associated with the presence of atheroembolic intra-renal vessel disease
| Characteristics | Atheroemboli | No atheroemboli | Odds ratio | P-value |
|---|---|---|---|---|
| n = 24 | n = 38 | |||
| Abdominal aortic aneurysm | 12 (57%) | 9 (29%) | 3.22 | 0.04 |
| Serum creatinine (mg/dL) | 2.129 ± 0.57 | 1.72 ± 0.55 | 1.14 | 0.01 |
| LDL (mg/dL) | 150.7 ± 28.3 | 119.8 ± 33.9 | 1.37 | 0.02 |
TGF-beta and renal fibrosis
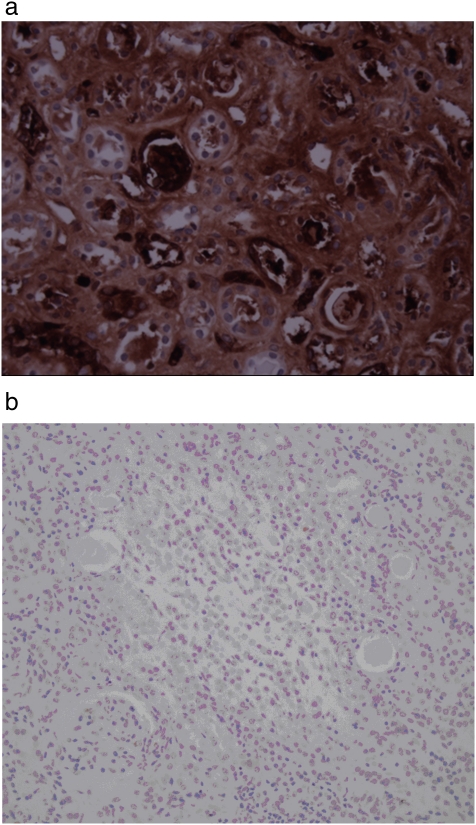
Two main variables were found to have statistically significant associations with the presence of increased TGF-beta staining: no prior history of renal revascularization (OR = 3.23, P = 0.045) and lack of statin use (OR = 5.56, P = 0.003). When adjusted for statin use, the association of prior renal revascularization with TGF-beta was no longer statistically significant. Of note, TGF-beta staining did not correlate with kidney size, and this did not change after adjusting for statin use. Correlation with duration of statin therapy was not performed, as this information was not available. Representative pictures are shown in Figure 6a, b.
Fig. 6.
a. Immunocytochemistry of TGF-beta of a renal section from a nephrectomized kidney showing increased uptake within the interstitium. b. Immunocytochemistry of TGF-beta of a renal section from a normal kidney showing no TGF-beta uptake.
Discussion
Our results demonstrate that severe tubulointerstitial atrophy with mild global glomerulosclerosis is the most common finding in kidney sections from patients who underwent nephrectomy for resistant hypertension and concomitant renovascular disease that led to kidney atrophy. Atherosclerotic RAS was associated with advanced IRAVD, which, in turn, positively correlated with the overall atherosclerotic burden, as manifested by older age, longer duration of hypertension, increased LDL and presence of cerebrovascular disease. In half of our cases, hypertensive vessel disease was present and was associated with an increased prevalence of MI. Moreover, an unexpectedly high rate of atheroembolic disease was recorded prior to nephrectomy, affecting ∼1/3 of the kidneys. This likely contributes to patients’ renal injury, subsequent atrophy and deterioration in renal function.
Atherosclerotic vascular occlusive disease in renal arteries, similar to other vascular beds, tends to progress [8,9]. In a study by Caps et al., a total of 295 kidneys in 170 patients with atherosclerotic RAS were monitored with serial renal artery duplex scans for a mean of 33 months. The cumulative incidence of progression was 35% at 3 years and 51% at 5 years, while a total occlusion affected nine renal arteries, all of which had a ≥60% stenosis upon initial examination [10]. The risk of progression was highest in the presence of high-grade stenoses, elevated systolic blood pressure and diabetes mellitus. However, little is known regarding the histopathological changes in renal tissue that accompany RAS and its progression to occlusion. Most of the current data have come from animal studies, which used acute ligation of the renal artery as a model of RAS and related syndromes, i.e. renovascular hypertension and ischaemic renal atrophy. These studies identified tubular cells with active solute transport in the outer medulla as the major target of hypoperfusion injury [11,12]. In the outer medulla, oxygenation is relatively poor, even under normal conditions, due to the vascular anatomy of the kidney that provides a countercurrent mechanism. Therefore, these cells may be particularly sensitive to renal hypoperfusion and impaired oxygenation that occurs in RAS. Tubular cell dysfunction has been shown to be one of the earliest features of hypertensive vascular disease [13]. These studies further indicated that glomerulosclerosis was rarely present and reported no significant vascular changes in the ligated kidneys. In a recent study by Wright et al., patients with biopsy-proven atherosclerotic nephropathy were followed for about 2 years [14]. In the group that progressed to worsening renal function, there was histological evidence suggestive of more extensive interstitial atrophy and glomerulosclerosis. These findings correlate with our results and emphasize the important role of histological findings in predicting clinical renal outcome.
Animal models of RAS have several limitations as to their applicability to human disease. First, the natural history of RAS is characteristic of a gradual progression, with haemodynamically significant stenosis developing over a long period of time. In contrast, reductions in renal blood flow that are brought on by acute ligations in animal models are abrupt and almost instantaneous. Second, patients with RAS commonly suffer from several co-morbid conditions (such as hyperlipidaemia and preexisting essential hypertension) that previously have been shown to affect their risk for RAS and affect the course, progression and intervention outcomes in these patients.
Our data extend previous observations arising from animal studies to human disease, as data are limited with respect to histopathological findings in human kidneys that have undergone atrophy secondary to advanced atherosclerotic renovascular disease. Similar to animal studies of experimental ischaemic renal atrophy, we report advanced tubulointerstitial atrophy in the sections of human atrophic kidneys. However, unlike those from animal models, these sections demonstrate the presence of glomerulosclerosis and extensive vascular pathology. The chronicity of RAS progression in human disease allows for different protective mechanisms to develop that, on one side, may help preserve renal blood flow and glomerular filtration, while leading to functional and morphological changes on the other side, and which are different from those observed after acute ischaemic injury. For example, upregulation of angiotensin II, although critical in maintaining kidney function distal to a stenosis, simultaneously may lead to cellular proliferation and stimulation of TGF-beta, which contribute to renal fibrogenesis [15], endothelial dysfunction, accelerated atherosclerosis and progressive glomerular sclerosis. Likewise, renal underperfusion may lead to alterations in vasoactive pathways, including oxidative stress, activation of endothelin and reduced nitric oxide, all of which may modulate cytokines and inflammatory mediators leading to irreversible interstitial fibrosis [1]. Therefore, in human disease, tissue hypoxia is not the only stimulus but likely is one of several stimuli by which underperfusion activates progressive fibrogenesis in the kidney. In addition, comorbid states in these patients may contribute to renal injury, similar to animal studies showing that cholesterol feeding in a pig model of renovascular hypertension stimulates tissue fibrosis [16]. Likewise, in our study, we were able to show that the use of statins markedly decreased the amount of renal fibrosis in patients with atherosclerotic renovascular hypertension.
There are several limitations in our study. First, the length of the kidneys that were used in this study was consistent with advanced atrophy, raising the possibility that some of the findings may be due to end-stage kidneys, rather than underperfusion itself. However, all of these patients had undergone renal imaging prior to nephrectomy that unequivocally confirmed the presence of RAS of more than 75%. Second, as resistant hypertension was an indication for nephrectomy, a preselection bias for extensive hypertensive changes might have occurred. Our study is the first to describe the patterns of injury associated with atherosclerotic RAS and atrophic kidneys in patients with resistant hypertension. A larger study with a control group of patients with the same degree of renal atrophy and resistant hypertension who are treated with medical therapy or stenting may help eliminate some of the bias, but will not allow for clinicopathological correlations, as patients who undergo stenting or medical therapy alone are less likely to have a renal biopsy. Third, we assessed the use of ACEI/ARB as an important variable, but only 50% of our study patients were on these medications. We hypothesized that patients on ACEI/ARB would have less renal damage and potentially less TGF-beta staining, but we did not detect any statistically significant differences. A larger study focusing on the effects of ACEI/ARB on renal pathology of patients with atherosclerotic RAS over the last decade will likely show an increased use of ACEI/ARB and will be better powered to make conclusions about the effects of these medications.
Despite these limitations, our data clearly show extensive tubular atrophy that has been previously described in animal models and demonstrate the additional findings of the combination of glomerulosclerosis and vessel changes (hypertensive, atherosclerotic and atheroembolic disease) that may be characteristic of human disease. We also demonstrate that statin therapy may downregulate TGF-beta expression and signalling, thus limiting renal fibrosis and injury in atherosclerotic RAS. The benefits of the anti-inflammatory effects of statins in preventing cardiovascular disease are well documented [17], while their anti-fibrotic effects in preventing progression of renal disease [18] and their role in reducing the rate of progression of atherosclerotic RAS [6,19] are increasingly recognized. Our data suggest that the anti-fibrotic effects of statin therapy in renal disease may be, at least partially, mediated through TGF-beta downregulation.
Conclusion
In summary, our study provides a comprehensive analysis of the histopathological changes in kidneys that were subject to long-standing, chronic underperfusion due to advanced RAS and provides the clinical correlates related to these histopathological findings. The effects of renal underperfusion appear to be mediated by dysregulation of pro-fibrotic pathways and further potentiated by patients’ comorbidities, ultimately leading to renal atrophy. In future studies, we are planning to study the relative contributions of different signalling pathways to progressive renal injury under conditions of decreased renal perfusion. These studies may facilitate identification of possible targets for medical therapy that may delay kidney atrophy and deterioration in renal function.
Acknowledgments
This project was supported by a National Institute of Health Grant (P01 HL85307).
Conflict of interest statement. None declared.
References
- 1.Lerman L, Textor SC. Pathophysiology of ischemic nephropathy. Urol Clin North Am. 2001;28:793–803. doi: 10.1016/s0094-0143(01)80034-3. [DOI] [PubMed] [Google Scholar]
- 2.Textor SC, Wilcox CS. Renal artery stenosis: a common, treatable cause of renal failure? Annu Rev Med. 2001;52:421–442. doi: 10.1146/annurev.med.52.1.421. [DOI] [PubMed] [Google Scholar]
- 3.Guo H, Kalra PA, Gilbertson DT, et al. Atherosclerotic renovascular disease in older US patients starting dialysis, 1996 to 2001. Circulation. 2007;115:50–58. doi: 10.1161/CIRCULATIONAHA.106.637751. [DOI] [PubMed] [Google Scholar]
- 4.Greco BA, Breyer JA. The natural history of renal artery stenosis: who should be evaluated for suspected ischemic nephropathy? Semin Nephrol. 1996;16:2–11. [PubMed] [Google Scholar]
- 5.Chade AR, Zhu XY, Grande JP, et al. Simvastatin abates development of renal fibrosis in experimental renovascular disease. J Hypertens. 2008;26:1651–1660. doi: 10.1097/HJH.0b013e328302833a. [DOI] [PubMed] [Google Scholar]
- 6.Cheung CM, Patel A, Shaheen N, et al. The effects of statins on the progression of atherosclerotic renovascular disease. Nephron. 2007;107:35–42. doi: 10.1159/000107552. [DOI] [PubMed] [Google Scholar]
- 7.Kane GC, Textor SC, Schirger A, et al. Revisiting the role of nephrectomy for advanced renovascular disease. Am J Med. 2003;114:729–735. doi: 10.1016/s0002-9343(03)00161-x. [DOI] [PubMed] [Google Scholar]
- 8.Schreiber MJ, Pohl MA, Novick AC. The natural history of atherosclerotic and fibrous renal artery disease. Urol Clin North Am. 1984;11:383–392. [PubMed] [Google Scholar]
- 9.Zierler RE, Bergelin RO, Davidson RC, et al. A prospective study of disease progression in patients with atherosclerotic renal artery stenosis. Am J Hypertens. 1996;9:1055–1061. doi: 10.1016/0895-7061(96)00196-3. [DOI] [PubMed] [Google Scholar]
- 10.Caps MT, Perissinotto C, Zierler RE, et al. Prospective study of atherosclerotic disease progression in the renal artery. Circulation. 1998;98:2866–2872. doi: 10.1161/01.cir.98.25.2866. [DOI] [PubMed] [Google Scholar]
- 11.Gobe GC, Axelsen RA, Searle JW. Cellular events in experimental unilateral ischemic renal atrophy and in regeneration after contralateral nephrectomy. Lab Invest. 1990;63:770–779. [PubMed] [Google Scholar]
- 12.Truong LD, Farhood A, Tasby J, et al. Experimental chronic renal ischemia: morphologic and immunologic studies. Kidney Int. 1992;41:1676–1689. doi: 10.1038/ki.1992.241. [DOI] [PubMed] [Google Scholar]
- 13.Smith HW. Unilateral nephrectomy in hypertensive disease. J Urol. 1956;76:685–701. doi: 10.1016/S0022-5347(17)66752-1. [DOI] [PubMed] [Google Scholar]
- 14.Wright JR, Duggal A, Thomas R, et al. Clinicopathological correlation in biopsy-proven atherosclerotic nephropathy: implications for renal functional outcome in atherosclerotic renovascular disease. Nephrol Dial Transplant. 2001;16:765–770. doi: 10.1093/ndt/16.4.765. [DOI] [PubMed] [Google Scholar]
- 15.Grande JP. Role of transforming growth factor-beta in tissue injury and repair. Proc Soc Exp Biol Med. 1997;214:27–40. doi: 10.3181/00379727-214-44066. [DOI] [PubMed] [Google Scholar]
- 16.Chade AR, Rodriguez-Porcel M, Grande JP, et al. Mechanisms of renal structural alterations in combined hypercholesterolemia and renal artery stenosis. Arterioscler Thromb Vasc Biol. 2003;23:1295–1301. doi: 10.1161/01.ATV.0000077477.40824.52. [DOI] [PubMed] [Google Scholar]
- 17.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 18.Fried LF. Effects of HMG-CoA reductase inhibitors (statins) on progression of kidney disease. Kidney Int. 2008;74:571–576. doi: 10.1038/ki.2008.231. [DOI] [PubMed] [Google Scholar]
- 19.Khong TK, Missouris CG, Belli AM, et al. Regression of atherosclerotic renal artery stenosis with aggressive lipid lowering therapy. J Hum Hypertens. 2001;15:431–433. doi: 10.1038/sj.jhh.1001196. [DOI] [PubMed] [Google Scholar]