Abstract
AIM: To evaluate long-term endocrine and exocrine pancreatic function, quality of life and health care costs after mild acute pancreatitis and severe acute pancreatitis (SAP).
METHODS: Patients prospectively included in 2001-2005 were followed-up after 42 (36-53) mo. Pancreatic function was evaluated with laboratory tests, the oral glucose tolerance test (OGTT), fecal elastase-1 and a questionnaire. Short Form (SF)-36, was completed.
RESULTS: Fourteen patients with a history of SAP and 26 with mild acute pancreatitis were included. Plasma glucose after OGTT was higher after SAP (9.2 mmol/L vs 7.0 mmol/L, P = 0.044). Diabetes mellitus or impaired glucose tolerance in fasting plasma glucose and/or 120 min plasma glucose were more common in SAP patients (11/14 vs 11/25, P = 0.037). Sick leave, time until the patients could take up recreational activities and time until they had recovered were all longer after SAP (P < 0.001). No significant differences in SF-36 were seen between the groups, or when comparing with age and gender matched reference groups. Total hospital costs, including primary care, follow-up and treatment of complications, were higher after SAP (median €16 572 vs €5000, P < 0.001).
CONCLUSION: Endocrine pancreatic function was affected, especially after severe disease. SAP requires greater resource use with long recovery, but most patients regained a good quality of life.
Keywords: Acute pancreatitis, Endocrine function, Exocrine function, Quality of life, Cost
INTRODUCTION
Acute pancreatitis is associated with a mild and uneventful course in 80% of cases. However, severe acute pancreatitis (SAP) is associated with the risk of complications both in and around the pancreas, and eventually has a fatal outcome in up to 15%-20% of cases[1]. Exocrine and endocrine pancreatic function has mainly been studied after SAP, with divergent results[2-9]. In mild, edematous-interstitial cases of acute pancreatitis, the pancreas can recover completely. After SAP, however, morphological changes may often remain and functional recovery is not always complete[3,4,9]. Impairment of exocrine pancreatic function with steatorrhea has been reported to diminish over time[8,10]. Damaged pancreatic acinar cells may recover with improvement in pancreatic function[4]. However, endocrine dysfunction with diabetes mellitus (DM) seems to be more common over time[9,11].
Until recently, results of treatment strategies were determined only in terms of cure, impairment of pancreatic functions, disability or death. However, there is a need for critical assessment of outcome measures that also include quality of life and health economical aspects. In some studies, SAP patients have been reported to regain a satisfactory quality of life[12-15], while others report a clear impairment[16,17].
Severe disease requires prolonged hospitalization, frequently including a stay in the intensive care unit (ICU). Sometimes there is also a need for multiple radiological and surgical interventions. Long rehabilitation of survivors can be expected, resulting in not only a great challenge for the individual but also a substantial need for resources together with high associated costs for society. There have only been a few previous cost analyses performed for acute pancreatitis[11,15,18-21].
The aim of the present study was to evaluate both pancreatic endocrine and exocrine function, as well as general recovery, quality of life and costs in patients who had recovered from mild acute pancreatitis, as well as from SAP, and to compare these groups at long-term follow-up.
MATERIALS AND METHODS
Study population
Case records from patients with mild acute pancreatitis and SAP, previously evaluated for participation in 2 nutritional studies[22,23], were examined. Severity definition was performed according to the Atlanta classification[24]. Exclusion criteria were dementia, malignancy, an additional history of SAP and chronic pancreatitis. Two out of the 20 SAP patients had died, one had dementia and 2 had developed chronic pancreatitis. In total, 15 patients with SAP, and a group of 30 gender- and age-matched patients from the group with mild disease were asked to participate in this follow-up survey. Invitation was performed by letter followed by a telephone call, offering an appointment at the outpatient clinic. Five patients, one of whom was from the SAP group, declined to participate. Finally, 14 patients with a history of SAP and 26 with a history of mild disease were included. Thus, 40 patients participated in this long-term evaluation for a median of 42 (36-53) mo after the episode of acute pancreatitis. APACHE II, scored in all patients at initial admission to hospital, was a median of 7 (6-10) in patients who later developed severe disease and 6 (5-7) in the group of patients with mild disease (P = 0.012). Five out of 14 patients in the SAP group had an APACHE II < 8 and 3/26 in the group with mild acute pancreatitis had a score ≥ 8.
All patients were seen by the same surgeon at the outpatient clinic. A thorough physical and physiological investigation was performed. Blood samples were taken after fasting and during an oral glucose tolerance test (OGTT), and a fecal sample was collected. All patients completed a questionnaire examining current pancreatic function, medication, abdominal surgical interventions, eating and drinking habits, readmissions for pancreatitis, ability to return to normal daily activity and time until they had recovered from the acute pancreatitis episode. Actual working capacity (including retirement/early retirement/sick leave/still working) was evaluated. Body weight and height was measured. Quality of life forms were completed. Several aspects of the patients’ current condition were evaluated, using a visual analogue scale (VAS: 0-100).
Exocrine pancreatic function
Fecal elastase-1 concentration, a specific human protease synthesized by the acinar cells, was measured in stool samples using a commercial enzyme-linked immunosorbent assay, (ScheBo Biotech, Giessen, Germany). It is non-invasive, stable and correlates well with exocrine pancreatic function tests[25,26]. A value > 200 μg elastase/g stool is considered normal. Subjective pancreatic function was evaluated via a questionnaire, including questions about the incidence of abdominal discomfort, bowel habit including frequency of defecation, presence of diarrhea and steatorrhea, intolerance to fat and other food, unintentional weight loss and use of pancreatic enzyme supplementation.
Endocrine pancreatic function
Fasting plasma (FP) glucose, C-peptide and insulin were measured in all patients. In non-diabetic patients (n = 39), a 75 g, 2 h OGTT was performed to detect impaired glucose tolerance (IGT) and DM. Glucose and C-peptide were measured in venous plasma at 0, 15, 30, 60 and 120 min. Insulin was determined at 0 and 120 min. The guidelines and definitions established by the World Health Organisation were followed[27]. FP glucose ≥ 7.0 mmol/L met the criteria for DM and 6.1-6.9 mmol/L for IGT. OGTT plasma glucose values ≥ 11.1 mmol/L at 2 h were defined as DM and values ≥ 7.8 and < 11.1 mmol/L as IGT. Measurements of baseline and stimulated insulin and C-peptide values allowed the differentiation of DM induced by insulin resistance or beta cell failure. The homeostasis model assessment (HOMA) for evaluating insulin resistance [HOMA IR = fasting insulin (mIE/mL) × FP glucose (mmol/L)/22.5] was calculated[28]. Fasting glycosylated hemoglobin A1c (HBA1c) was measured for assessing long-term glucose homeostasis.
Quality of life
The Swedish version of Standard Short Form 36 (SF-36), a widely used general quality-of-life questionnaire that has been validated in a variety of medical settings, was used[29]. The SF-36 examines 8 areas consisting of social and physical function, physical and emotional well-being, bodily pain, vitality, mental health and overall general health perception. Swedish normative data of age-matched controls were used for comparison.
Costs
Costs were calculated as total hospital costs per patient at the primary hospital stay, including expenses on the ward, ICU stay, anesthesia and operating costs, radiological and clinical physiology expenses, and costs for laboratory analysis and blood products. Subsequent costs, both for in-hospital stay and outpatient care, directly related to the primary acute pancreatitis episode, were also calculated. Sick leave days were retrieved from the patient’s medical records and from the patients at follow-up. All costs are given in 2008 price levels, inflated using the Swedish consumer price index. The costs have been converted from Swedish krona (SEK) to Euros (€) using the yearly average exchange rate for 2008 (9.6055 SEK to €1).
Statistical analysis
Continuous variables are presented as medians with 25th and 75th percentiles. Categorical variables are given as frequencies and percentages. Univariate analysis for continuous variables was conducted with the Wilcoxon test. Categorical variables were analyzed by the χ2-test, except when expected frequencies were less than 5, in which case Fisher’s exact test was used. The Kaplan-Meier estimate was used to calculate time to event. The log-rank test was used to compare the difference between the groups. Data were analyzed using Hmisc, Survival and Design packages of the R software (R Foundation for Statistical Computing, Vienna, Austria), version 2.8.1. The level of significance was set at P < 0.05. The study was approved by the Human Ethics Committee, Lund University.
RESULTS
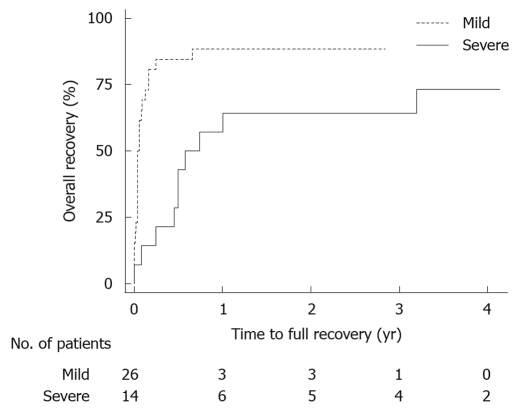
Of the 40 patients finally included in the study, 16 (40%) were men. The median age was 61 (48-68) years at follow-up. Body mass index was 28 (26-31) kg/m2, with no difference between patients with severe or mild acute pancreatitis. Patient data from the episode with acute pancreatitis are presented in Table 1. There was no difference in follow-up time between the groups. Most routine laboratory parameters, such as hemoglobin, creatinine, calcium, bilirubin and lipase showed no difference at follow-up, though pancreas-specific amylase was lower in patients with a history of SAP, and alanine aminotransferase was higher (Table 2). Sick leave, time until the patients could regain recreational activities and time until recovery were all significantly longer after SAP (Table 2). A total of 7 patients from both groups did not feel that they had fully recovered at the time of follow-up, a median 39 (33-49) mo after the episode of acute pancreatitis (Figure 1). There was no difference between the groups when using the subjective VAS for expressing abdominal pain, fatigue, nausea, anxiety, working capacity and energy for recreational activities. The question whether actual difficulties were thought to be late effects of the acute pancreatitis episode also showed no difference comparing SAP and mild acute pancreatitis, 3 (2-43) vs 3 (0-13), P = 0.33. Additional hospital visits, including both scheduled follow-ups and emergency visits due to abdominal pain, excluding any additional acute pancreatitis episodes, were more common after severe disease. In SAP, 12 (86%) patients had one or more visits and in the mild group, 7 (27%) had only one and none any additional visits, P < 0.001. At follow-up, no patient in either the SAP or the mild group used medication regularly for abdominal pain.
Table 1.
Patient characteristics and parameters during the acute care for pancreatitis, divided into mild and severe acute pancreatitis cases
| Parameter | Mild acute pancreatitis | Severe acute pancreatitis | All patients | Difference between groups (P) |
| Gender, male | 10 | 6 | 16 | 0.79 |
| Etiology | ||||
| Gallstone | 16 (62) | 4 (29) | 20 (50) | 0.096 |
| Alcohol | 5 (19) | 5 (36) | 10 (25) | 0.278 |
| Post ERCP | 0 | 3 (21) | 3 (8) | 0.037 |
| Unknown | 5 (19) | 2 (14) | 7 (18) | 1.0 |
| Other diseases | 13 (50) | 5 (36) | 18 (45) | 0.39 |
| ASA class1 | 1.5 (1-2) | 1 (1-1.75) | 1 (1-2) | 0.14 |
| APACHE II1 | 6 (5-7) | 7 (6-10) | 6 (5-7) | 0.012 |
| Weight loss | 13 (50) | 11 (79) | 24 (60) | 0.079 |
| Weight loss in kg1 | 5 (1.5-6) | 9 (7-10) | 6.4 ± 4.7 | 0.003 |
| 6 (3-9) | ||||
| Pancreatic surgery | 0 | 4 (29) | 4 (10) | 0.004 |
| Pancreatic pseudocysts | 0 | 7 (50) | 7 (18) | < 0.001 |
| Hospital stay in days1 | 7.5 (4-9) | 18 (16-24) | 10 (6-16) | < 0.001 |
| ICU stay | 0 | 8 (57) | 8 (20) | < 0.001 |
Values are median and in parentheses are percentages or
interquartile range. ERCP: Endoscopic retrograde cholangiopancreatography; ASA: American Society of Anesthesiologists; APACHE II: Acute Physiology and Chronic Health Evaluation II; ICU: Intensive care unit.
Table 2.
Patient characteristics and parameters at follow-up after mild and severe acute pancreatitis
| Parameter | Mild acute pancreatitis | Severe acute pancreatitis | All patients | Difference between groups (P) |
| Time to follow-up (mo) | 41 (35-50) | 47 (37-63) | 42 (36-53) | 0.14 |
| Age (yr) | 61 (51-70) | 58 (45-67) | 61 (48-68) | 0.72 |
| BMI (kg/m2) | 27 (25-32) | 29 (26-31) | 28 (26-31) | 0.82 |
| ASA | 2 (1-2) | 1 (1-2) | 1.5 (1-2) | 0.68 |
| Serum pancreas amylase (μkat/L) | 0.45 (0.37-0.53) | 0.27 (0.18-0.43) | 0.043 (0.27-0.52) | 0.007 |
| P-ALAT (μkat/L) | 0.30 (0.23-0.47) | 0.39 (0.33-0.54) | 0.34 (0.29-0.50) | 0.035 |
| Sick leave days | 14 (7-30) | 120 (70-165) | 30 (14-97) | < 0.001 |
| Time to activity (d) | 10.5 (0-21) | 90 (60-365) | 21 (2-60) | < 0.001 |
| Time to recovery (d) | 21 (14-60) | 270 (180-1) | 60 (14-365) | 0.005 |
Less than 75% of the patients had recovered at time of follow-up. Values are given as median (interquartile range). BMI: Body mass index; ASA: American Society of Anesthesiologists; P-ALAT: Plasma alanine aminotransferase.
Figure 1.
Log-rank test for time to recovery after acute pancreatitis in mild and severe acute pancreatitis patients.
Exocrine pancreatic function
There were no statistically significant differences between the severe and mild groups concerning incidence of steatorrhea (1/14 vs 2/26), change in bowel habits (4/14 vs 10/25) or the need for pancreatic enzyme supplementation (2/14 vs 1/26). Most patients had weight loss during the acute disease. At the time of follow-up, 4/14 vs 5/26 still had a decreased weight compared to body weight before the disease, with no difference between the groups. A change in diet was more common after SAP (9/14 vs 6/26, P = 0.01). Fecal elastase-1 was found to be decreased only in one of the patients (with SAP). Plasma albumin did not differ between the groups.
Endocrine pancreatic function
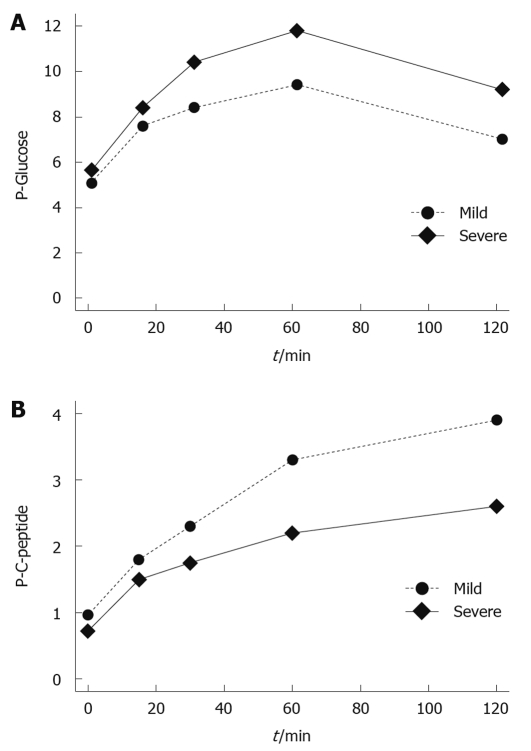
One patient with a history of mild acute pancreatitis was excluded from this part of the follow-up because of type 2 DM, already treated with insulin before the acute pancreatitis episode. Another patient had a history of insulin treatment starting immediately after the SAP episode, but had no treatment for DM for the 9 mo prior to the acute episode. FP glucose was 5.2 mmol/L and the patient was included in the OGTT. FP glucose had a tendency to be higher and plasma glucose was higher after the OGTT in patients with a history of SAP as compared to those with mild acute pancreatitis (P = 0.055 and P = 0.044, respectively; Figure 2). A higher level was also registered for HBA1C (P = 0.041). Patients with a history of SAP more frequently fulfilled the criteria for DM and/or IGT in either FP glucose or 120 min plasma glucose, or both, (11/14 vs 11/25, P = 0.037). There was no significant difference in the incidence of DM when comparing different etiologies of acute pancreatitis; 4/10 with alcohol as the etiological factor and 3/19 with underlying gallstone disease had DM (P = 0.193). The 4 patients subjected to pancreatic surgery all fulfilled the criteria for DM or IGT. Plasma C-peptide was higher in patients fulfilling the criteria for DM, both fasting and after the OGTT, and a significant difference was also seen for serum insulin (Table 3). Fasting C-peptide as well as HOMA-IR had a tendency, although not significant, to be lower in patients with DM and/or IGT after severe as compared to mild disease.
Figure 2.
Relationship between mild and severe acute pancreatitis patients in the oral glucose tolerance test evaluating plasma glucose (mmol/L, A) and C-peptide values (nmol/L, B).
Table 3.
Endocrine parameters in patients classified as having diabetes according to the World Health Organization definition and patients not fulfilling the criteria
| Parameter | Diabetic patients (n = 9) | Non-diabetic patients (n = 30) | Difference between groups (P) |
| Fasting plasma glucose, 0 min1 | 6.9 (6.0-7.3) | 5.1 (4.6-5.5) | < 0.001 |
| Plasma glucose 120 min1 | 13 (12-14) | 6.8 (5.5-8.2) | < 0.001 |
| Plasma C-peptide 0 min2 | 1.7 (1.3-2) | 0.72 (0.6-1.0) | < 0.001 |
| Plasma C-peptide 120 min2 | 4.8 (4.1-5.9) | 2.9 (2.2-4.4) | 0.024 |
| Serum insulin 0 min3 | 16 (13-17) | 6 (4-9) | 0.001 |
| Serum insulin 120 min3 | 103 (79-126) | 42 (28-60) | 0.001 |
| HOMA-insulin resistance | 4.2 (3.7-5.4) | 1.3 (0.9-2.2) | < 0.001 |
| HBA1c | 5.3 (5.0- 5.6) | 4.6 (4.5-4.8) | < 0.001 |
mmol/L;
nmol/L;
mIE/L; Values are given in median (interquartile range). HOMA: Homeostasis model assessment; HBA1c: Fasting glycosylated hemoglobin.
Quality of life
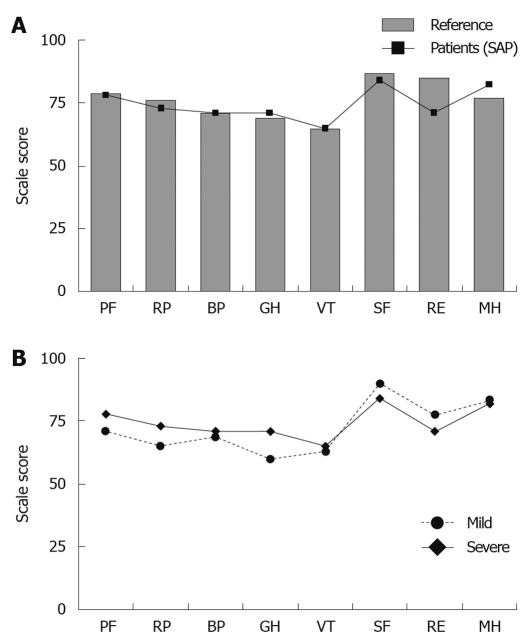
An exact gender- and age-matched reference group (n = 84) was randomly selected for the SAP group from the Swedish SF-36 norm database (n = 8930). Six control subjects were used for each patient (quota = 6:1). The numbers of reference subjects were decided from the lowest number representing one study patient (female, 83 years old). The corresponding figures for mild acute pancreatitis had a reference group of 182 persons, and a quota = 7:1 decided from the lowest number representing one study patient (male, 79 years old). No significant differences were seen between the groups with a history of severe and mild disease in the 8 SF-36 domains (Figure 3A). When comparing patients with SAP with their respective reference group, no significant differences could be found (Figure 3B).
Figure 3.
The graphs shows quality of life, measured by Short Form-36, in mild and severe acute pancreatitis patients, comparing age- and gender-matched control group. A: Mean values for patients after mild (black squares) acute pancreatitis and severe (black circles) acute pancreatitis in the 8 Short Form-36 domains; B: Mean value for patients after severe (black squares) acute pancreatitis compared with an age- and gender-matched control group (grey bars) in the 8 SF-36 domains. SAP: Severe acute pancreatitis; PF: Physical function; RP: Role physical; BP: Bodily pain; GH: General health; VT: Vitality; SF: Social functioning; RE: Role emotional; MH: Mental health.
Costs
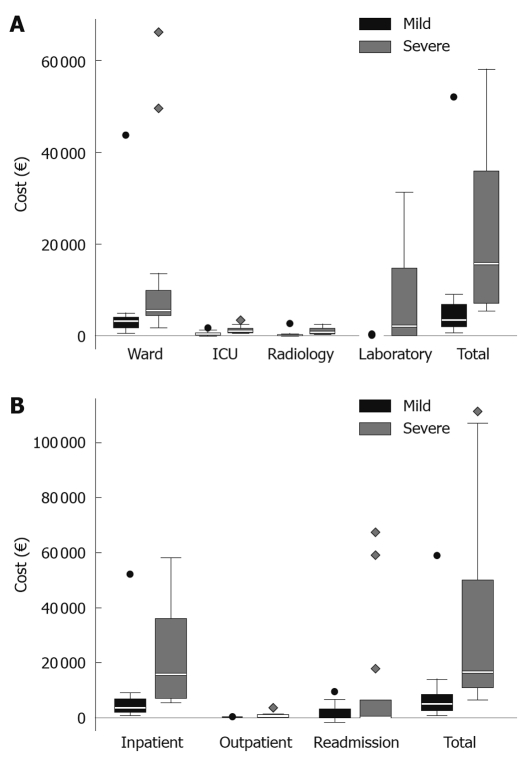
There was a pronounced difference between severe and mild acute pancreatitis when comparing costs for the primary hospital stay, a median of €15 774 (€7455-€35 960) in SAP vs €3480 (€2049-€6662) in mild acute pancreatitis and a mean of €23 592 ± €18 821 vs €5908 ± €9740, (P < 0.001, Figure 4A). When including total hospital costs, adding costs for follow-up, both including in-hospital stay and outpatient care, the difference between severe and mild disease was a median of €16 572 (€11 017-€45 619) vs €5000 (€2562-€8384) and a mean of €35 427 ± €36 790 vs €7536 ± €11 228 (P < 0.001, Figure 4B). This means that the severe cases were about 3.3 times more expensive regarding the hospital costs. For the subgroup of SAP patients requiring stay in the ICU (n = 8), the total hospital costs, including care for late complications was a median of €30 026 (€17 636-€84 323) and a mean of €49 894 ± €41 905.
Figure 4.
Box-plots showing the specified hospital costs in acute pancreatitis patients. A: In-hospital costs during primary care for acute pancreatitis, in mild and severe acute pancreatitis patients; B: Costs at follow-up, including cost for primary care and all subsequent inpatient and outpatient costs, in mild and severe acute pancreatitis patients. ICU: Intensive care unit.
DISCUSSION
At the Marseilles symposium on pancreatitis in 1963 it was stated that after recovery complete restitution of the pancreas was the norm[30]. Since then several studies have been performed, mostly on SAP patients, with different follow-up times and measured parameters, with somewhat divergent results. In 1983 Mitchell and co-workers published a study including patients with both mild acute pancreatitis and SAP. All had initial exocrine insufficiency and 80% recovered after 12 mo[8]. The 1984 Marseilles Symposium acknowledged that both exocrine and endocrine function of the pancreas can be disrupted to varying degrees and duration following an acute attack of pancreatitis[31]. There is thus a dysfunction during the initial stage after acute pancreatitis, but functional recovery of the gland is controversial. Full recovery has been described[6], but some dysfunction is the usual scenario, especially after severe disease with necrosis[2,4,9,15].
After surgical treatment, a persistent exocrine insufficiency has been noted in up to 80%-85% of patients[3,4]. Acute pancreatitis with alcohol as an etiological factor may more likely be associated with exocrine impairment[32], though this has not been confirmed by others[9]. Exocrine insufficiency may not necessarily be clinically relevant and evaluation is difficult, particularly when non-invasive methods are used. Invasive tests also have their drawbacks. Furthermore, pancreatic insufficiency is often not obvious until 90% of the gland has been destroyed. In the present study, only one patient had an objective exocrine insufficiency, as measured by fecal elastase-1. The evaluation of this test has been proven as a highly sensitive and specific pancreatic function tests, even comparable with oral pancreatic function tests, such as the pancreaolauryl test[26,33]. When analyzing the patient’s answers regarding change in stool habits, including frequency, mild impairment was common, with a fluctuation over time, and constant steatorrhea at follow-up was uncommon. Medication with pancreatic enzyme supplements was used by a very limited number of patients. Change in diet was also a common answer, which can be due to a number of factors. Thus, there is a grey area concerning when and to what extent an exocrine dysfunction is present and of clinical relevance.
Endocrine dysfunction with glucosuria and elevated blood sugar levels is common during the acute phase of pancreatitis, but is usually self-limiting and resolves. Endocrine dysfunction with DM and IGT is, however, more common over time[9]. DM overall is also known to occur more often after operative treatment[11]. The 4 patients subjected to pancreatic surgery in the present survey all fulfilled the criteria for DM or IGT. We further found DM or IGT in 79% after SAP and in 42% after mild acute pancreatitis. Loss of β-cell function is expected after severe necrotizing disease, and the patients in the present study also had lower C-peptide and insulin levels after SAP as compared with mild disease. Fasting C-peptide and insulin levels were, however, higher in the patients with DM, as compared with the other patients, indicating that insulin resistance is an additional and important explanation for the development of DM. The insulin resistance was obvious, not only with hyperinsulinemia, but also with an elevated HOMA-IR in both groups, with a tendency to be more pronounced after mild disease. The present rate of DM and IGT found at follow-up in acute pancreatitis patients is much higher than expected in the population. Insulin resistance is furthermore associated with different conditions such as obesity, liver failure and inflammatory conditions, though patients in the present survey did not fulfill any of these criteria. Insulin resistance is a prominent feature in patients after pancreatic resection[34], implying the coexistence of pancreatic damage and hyperinsulinemia[5,35]. The pathophysiological mechanisms involved are, however, not clarified. The result in the present study indicates that the insufficiency after acute pancreatitis may be an underestimated problem. When taking the risk for untreated DM patients with poor metabolic control into consideration, a follow-up of these patients may be more important than hitherto proposed.
In follow-up studies, the focus has been on pancreatic dysfunction and less attention has been paid to other aspects of recovery and long-term detriment. With an increasing number of patients surviving SAP, more attention has been directed towards quality of life and long-term outcomes. Until recently, results of treatment strategies were determined only in terms of death, disability and cure. Quality of life as an outcome measure has only sporadically been reported, with a tendency for, or a statistically significant, reduced quality of life after acute pancreatitis[12,15-17]. In the present study, SF-36 was used, which in 36 questions, divided into 8 scales, estimates both function and wellbeing, and can be summarized as health-related quality of life. In a Finnish study, a difference was seen in the SF-36 general health domain, but the conclusion was that there were no clinically significant differences in quality of life compared with that of the normal population[14]. This is in accordance with our results, where no difference was noted between groups, nor between these and the normal reference population. After debridement for pancreatic necrosis, quality of life has been shown to far exceed that noted in patients with other severe medical diseases, such as congestive heart failure and severe hypertension[12]. It may be that experiencing critical illness, such as SAP, might change the opinion about what is really important in life, contributing to explaining why some patients feel well, despite possible persisting restrictions in daily life activities and not being entirely recovered.
Only a few reports on cost analyses in acute pancreatitis have been made, mainly focusing on severe cases[15,20]. One study has estimated hospital costs for acute pancreatitis patients, regardless of severity grade, with figures obtained from a large national hospital database, reporting a cost of $9870 per hospitalization and a good long-term outcome[19]. In the present study, we calculated not only costs for the primary admission but also additional hospital costs directly related to the pancreatitis episode. Data on total hospital costs has not previously been presented. Expressed as percentages, the increase was highest in the mild group due to subsequent treatment of gallstone disease. This is an important aspect that can possibly be optimized with less resource use. The additional costs after SAP showed a wide spread with a few patients requiring a great deal of resources. Reports on when the patients return to daily activity and work are also limited. Doepel et al[11] found that 84% of patients who were working the year before the onset of SAP returned to work. In the present study, corresponding figures are higher, but still the return to work in many cases took a long time after SAP, with the possibility that some patients, despite surviving the acute disease, are never able to return to a normal life and work again. The time until patients felt recovered varied between patients and groups, but overall it took a long time, with several patients not being subjectively recovered, but back in full time work. Patients not recovered at the time of follow-up were mainly, but not exclusively, found in the group recovering from SAP.
Generally, reports in the literature concerning follow-up after acute pancreatitis have limitations and have led to contradictory results. This is due to a number of factors existing in almost all studies, e.g. a limited total number of patients, absence of agreement on a classification system describing the severity of the disease, differences in the proportions concerning e.g. etiology, different criteria for IGT and DM, different tests used to evaluate exocrine insufficiency and different instruments to evaluate quality of life. The follow-up time also varied widely[36].
In the present study, most of the patients with a history of SAP had a major need to talk and discuss what actually had happened during their hospital stay and during the follow-up period. In the group with mild acute pancreatitis, a number of patients even had to remind themselves that they really had been ill a few years previously. A structured follow-up plan for patients that have undergone SAP, dealing with physiological aspects, including information on signs of exocrine insufficiency and the possible benefits of controlling blood glucose levels, could be of great benefit.
A weakness in the present study is the limited number of patients. A strength, however, is the attempt to make a complete follow-up including several important factors, and that a strict definition of SAP has been used. Another strength is that the same surgeon evaluated all patients at the follow-up in the outpatient clinic.
In conclusion, the present study presents a thorough long-term evaluation concerning several aspects associated with severe and mild acute pancreatitis. The results point at an impairment in endocrine function and also a subtle exocrine dysfunction. Sick leave and time until the patients recover can be long and the disease is associated with high costs for society, especially after SAP. The quality of life both after severe and mild acute pancreatitis is, however, as good as in the normal population years after the disease.
COMMENT
Background
Acute pancreatitis is associated with a mild and uneventful course in 80% of patients. However, severe acute pancreatitis is associated with the risk of complications both in and around the pancreas. Long-term exocrine and endocrine pancreatic function has been studied with divergent results. Morphological changes may often remain and functional recovery is not always complete. Additional important outcome measures are quality of life and health economical aspects.
Research frontiers
Long-term evaluation of patients after acute pancreatitis has mainly been focused on the severe disease with divergent results concerning pancreatic function and quality of life. Endocrine and exocrine dysfunction usually occurs. Only a few cost analyses have been published hitherto.
Innovations and breakthroughs
In this study, the authors found that the incidence of endocrine dysfunction was high but clinically important exocrine dysfunction limited after both severe and mild acute pancreatitis. The recovery was long especially after the severe form, but the quality of life was excellent. The hospital costs were high after severe acute pancreatitis, with a large spread within the group.
Applications
It is important to be aware of the high incidence of endocrine dysfunction, and a structured follow-up plan can be of importance. A good long time quality of life is important information for the patients and relatives during the demanding severe disease.
Peer review
The present paper is interesting and well written. Number of admitted patients are not so numerous but well selected and stratified.
Acknowledgments
The authors like to thank professor Mona Landin-Ohlsson for help with interpretation of the results from the endocrine function test.
Footnotes
Supported by Skane county council research and development foundation
Peer reviewer: Claudio Bassi, MD, Professor, Department of Surgery and Gastroenterology, Hospital GB Rossi, University of Verona, Piazza LA Scuro, 37134 Verona, Italy
S- Editor Wang YR L- Editor Cant MR E- Editor Lin YP
References
- 1.Beger HG, Rau BM. Severe acute pancreatitis: Clinical course and management. World J Gastroenterol. 2007;13:5043–5051. doi: 10.3748/wjg.v13.i38.5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appelros S, Lindgren S, Borgström A. Short and long term outcome of severe acute pancreatitis. Eur J Surg. 2001;167:281–286. doi: 10.1080/110241501300091462. [DOI] [PubMed] [Google Scholar]
- 3.Boreham B, Ammori BJ. A prospective evaluation of pancreatic exocrine function in patients with acute pancreatitis: correlation with extent of necrosis and pancreatic endocrine insufficiency. Pancreatology. 2003;3:303–308. doi: 10.1159/000071768. [DOI] [PubMed] [Google Scholar]
- 4.Bozkurt T, Maroske D, Adler G. Exocrine pancreatic function after recovery from necrotizing pancreatitis. Hepatogastroenterology. 1995;42:55–58. [PubMed] [Google Scholar]
- 5.Buscher HC, Jacobs ML, Ong GL, van Goor H, Weber RF, Bruining HA. Beta-cell function of the pancreas after necrotizing pancreatitis. Dig Surg. 1999;16:496–500. doi: 10.1159/000018775. [DOI] [PubMed] [Google Scholar]
- 6.Ibars EP, Sánchez de Rojas EA, Quereda LA, Ramis RF, Sanjuan VM, Peris RT. Pancreatic function after acute biliary pancreatitis: does it change? World J Surg. 2002;26:479–486. doi: 10.1007/s00268-001-0253-7. [DOI] [PubMed] [Google Scholar]
- 7.Malecka-Panas E, Gasiorowska A, Kropiwnicka A, Zlobinska A, Drzewoski J. Endocrine pancreatic function in patients after acute pancreatitis. Hepatogastroenterology. 2002;49:1707–1712. [PubMed] [Google Scholar]
- 8.Mitchell CJ, Playforth MJ, Kelleher J, McMahon MJ. Functional recovery of the exocrine pancreas after acute pancreatitis. Scand J Gastroenterol. 1983;18:5–8. doi: 10.3109/00365528309181549. [DOI] [PubMed] [Google Scholar]
- 9.Tsiotos GG, Luque-de León E, Sarr MG. Long-term outcome of necrotizing pancreatitis treated by necrosectomy. Br J Surg. 1998;85:1650–1653. doi: 10.1046/j.1365-2168.1998.00950.x. [DOI] [PubMed] [Google Scholar]
- 10.Angelini G, Pederzoli P, Caliari S, Fratton S, Brocco G, Marzoli G, Bovo P, Cavallini G, Scuro LA. Long-term outcome of acute necrohemorrhagic pancreatitis. A 4-year follow-up. Digestion. 1984;30:131–137. doi: 10.1159/000199097. [DOI] [PubMed] [Google Scholar]
- 11.Doepel M, Eriksson J, Halme L, Kumpulainen T, Höckerstedt K. Good long-term results in patients surviving severe acute pancreatitis. Br J Surg. 1993;80:1583–6. doi: 10.1002/bjs.1800801229. [DOI] [PubMed] [Google Scholar]
- 12.Broome AH, Eisen GM, Harland RC, Collins BH, Meyers WC, Pappas TN. Quality of life after treatment for pancreatitis. Ann Surg. 1996;223:665–670; discussion 670-672. doi: 10.1097/00000658-199606000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cinquepalmi L, Boni L, Dionigi G, Rovera F, Diurni M, Benevento A, Dionigi R. Long-term results and quality of life of patients undergoing sequential surgical treatment for severe acute pancreatitis complicated by infected pancreatic necrosis. Surg Infect (Larchmt) 2006;7 Suppl 2:S113–S116. doi: 10.1089/sur.2006.7.s2-113. [DOI] [PubMed] [Google Scholar]
- 14.Halonen KI, Pettilä V, Leppäniemi AK, Kemppainen EA, Puolakkainen PA, Haapiainen RK. Long-term health-related quality of life in survivors of severe acute pancreatitis. Intensive Care Med. 2003;29:782–786. doi: 10.1007/s00134-003-1700-8. [DOI] [PubMed] [Google Scholar]
- 15.Soran A, Chelluri L, Lee KK, Tisherman SA. Outcome and quality of life of patients with acute pancreatitis requiring intensive care. J Surg Res. 2000;91:89–94. doi: 10.1006/jsre.2000.5925. [DOI] [PubMed] [Google Scholar]
- 16.Hochman D, Louie B, Bailey R. Determination of patient quality of life following severe acute pancreatitis. Can J Surg. 2006;49:101–106. [PMC free article] [PubMed] [Google Scholar]
- 17.Symersky T, van Hoorn B, Masclee AA. The outcome of a long-term follow-up of pancreatic function after recovery from acute pancreatitis. JOP. 2006;7:447–453. [PubMed] [Google Scholar]
- 18.Lilja HE, Leppäniemi A, Kemppainen E. Utilization of intensive care unit resources in severe acute pancreatitis. JOP. 2008;9:179–184. [PubMed] [Google Scholar]
- 19.Fagenholz PJ, Fernández-del Castillo C, Harris NS, Pelletier AJ, Camargo CA Jr. Direct medical costs of acute pancreatitis hospitalizations in the United States. Pancreas. 2007;35:302–307. doi: 10.1097/MPA.0b013e3180cac24b. [DOI] [PubMed] [Google Scholar]
- 20.Fenton-Lee D, Imrie CW. Pancreatic necrosis: assessment of outcome related to quality of life and cost of management. Br J Surg. 1993;80:1579–1582. doi: 10.1002/bjs.1800801228. [DOI] [PubMed] [Google Scholar]
- 21.Neoptolemos JP, Raraty M, Finch M, Sutton R. Acute pancreatitis: the substantial human and financial costs. Gut. 1998;42:886–891. doi: 10.1136/gut.42.6.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eckerwall GE, Axelsson JB, Andersson RG. Early nasogastric feeding in predicted severe acute pancreatitis: A clinical, randomized study. Ann Surg. 2006;244:959–965; discussion 965-967. doi: 10.1097/01.sla.0000246866.01930.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eckerwall GE, Tingstedt BB, Bergenzaun PE, Andersson RG. Immediate oral feeding in patients with mild acute pancreatitis is safe and may accelerate recovery--a randomized clinical study. Clin Nutr. 2007;26:758–763. doi: 10.1016/j.clnu.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Bradley EL 3rd. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg. 1993;128:586–590. doi: 10.1001/archsurg.1993.01420170122019. [DOI] [PubMed] [Google Scholar]
- 25.Domínguez-Muñoz JE, Hieronymus C, Sauerbruch T, Malfertheiner P. Fecal elastase test: evaluation of a new noninvasive pancreatic function test. Am J Gastroenterol. 1995;90:1834–1837. [PubMed] [Google Scholar]
- 26.Dominici R, Franzini C. Fecal elastase-1 as a test for pancreatic function: a review. Clin Chem Lab Med. 2002;40:325–332. doi: 10.1515/CCLM.2002.051. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia:report of a WHO/IDF consultation. Geneva: World Health Organization; 2006. [Google Scholar]
- 28.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 29.Sullivan M, Karlsson J, Taft C. SF-36 Hälsoenkät: Svensk Manual och Tolkningsguide, 2:a upplagan (Swedish Manual and Interpretation Guide, 2nd edition) Gothenburg: Sahlgrenska University Hospital; 2002. [Google Scholar]
- 30.Sarles H. Proposal adopted unanimously by the participants of the Symposium on Pancreatitis at Marseilles; In: Sarles H, editor. Pancreatitis at Marseilles. Basel: Bibl Gastroenterol; 1965. [Google Scholar]
- 31.Singer MV, Gyr K, Sarles H. Revised classification of pancreatitis. Report of the Second International Symposium on the Classification of Pancreatitis in Marseille, France, March 28-30, 1984. Gastroenterology. 1985;89:683–685. [PubMed] [Google Scholar]
- 32.Migliori M, Pezzilli R, Tomassetti P, Gullo L. Exocrine pancreatic function after alcoholic or biliary acute pancreatitis. Pancreas. 2004;28:359–363. doi: 10.1097/00006676-200405000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Löser C, Möllgaard A, Fölsch UR. Faecal elastase 1: a novel, highly sensitive, and specific tubeless pancreatic function test. Gut. 1996;39:580–586. doi: 10.1136/gut.39.4.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yki-Järvinen H, Kiviluoto T, Taskinen MR. Insulin resistance is a prominent feature of patients with pancreatogenic diabetes. Metabolism. 1986;35:718–727. doi: 10.1016/0026-0495(86)90239-8. [DOI] [PubMed] [Google Scholar]
- 35.Eriksson J, Doepel M, Widén E, Halme L, Ekstrand A, Groop L, Höckerstedt K. Pancreatic surgery, not pancreatitis, is the primary cause of diabetes after acute fulminant pancreatitis. Gut. 1992;33:843–847. doi: 10.1136/gut.33.6.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nordback IH, Auvinen OA. Long-term results after pancreas resection for acute necrotizing pancreatitis. Br J Surg. 1985;72:687–689. doi: 10.1002/bjs.1800720905. [DOI] [PubMed] [Google Scholar]