Abstract
AIM: To determine the glycemic index (GI), glycemic load (GL) and insulinemic index (II) of five starchy foods that are commonly used in Chinese diets.
METHODS: Ten healthy subjects aged between 20-30 years were recruited. Each subject was asked to consume 50 g of available carbohydrate portions of test foods and reference food. Finger capillary blood samples were collected at the start of eating and 15, 30, 45, 60, 90 and 120 min after consumption. The GI and II of foods were calculated from the ratio of incremental area under the glucose/insulin response curves of test and reference foods. The GL for each test food was determined from its GI value and carbohydrate content.
RESULTS: The results showed that brown rice elicited the highest postprandial glucose and insulin responses, followed by taro, adlay, yam and mung bean noodles, which produced the lowest. Among the five starchy foods, brown rice evoked the highest GI and GL at 82 ± 0.2 and 18 ± 0.2, followed by taro (69 ± 0.4, 12 ± 0.2), adlay (55 ± 0.4, 10 ± 0.2), yam (52 ± 0.3, 9 ± 0.0) and mung bean noodles (28 ± 0.5, 7 ± 0.2), respectively. The II values of the test foods corresponded with GI values. Similarly, brown rice gave the highest II at 81 ± 0.1, followed by taro (73 ± 0.3), adlay (67 ± 0.3), yam (64 ± 0.5) and mung bean noodles (38 ± 0.3). All five starchy foods had lower GI, GL and II than reference bread (P < 0.05).
CONCLUSION: The GI, GL and II values of starchy foods provide important information for the public to manage their diet and could be useful for the prevention of lifestyle-related diseases such as diabetes mellitus.
Keywords: Glycemic response, Glycemic index, Glycemic load, Insulinemic response, Insulinemic index
INTRODUCTION
Insulin resistance increases the risk of type 2 diabetes[1-3]. One characteristic that can be associated with insulin resistance is hyperinsulinemia that may result in deterioration of β-cell function, which is involved in the pathogenic process of diabetes[4]. In the context of current dietary strategies to prevent hyperinsulinemia and insulin resistance, it is imperative to consider diets/foods in terms of their ability to reduce the degree of postprandial glycemia and insulinemia[5]. These issues have important public health implications. Any diet to counteract diabetes should be evaluated for its effects on glucose response and insulin secretion. To do this, it is urgent and necessary to continuously determine the glycemic index (GI) and insulinemic index (II) values of foods in different countries, especially the GI of agricultural foods.
GI was introduced to describe the extent to which different foods elicit varying degrees of postprandial blood glucose. It is defined as the incremental area under the 2 h blood glucose response curve (IAUC) after consuming a test food compared to the corresponding area after a carbohydrate-equivalent amount of a reference food (either glucose or white bread)[6,7]. Expanding this theory to the postprandial insulin levels evoked by foods, the II of foods can also be determined from the corresponding incremental blood insulin areas[8]. Because insulin is the hormone that maintains blood glucose homeostasis, a food or diet high in II could induce a higher degree of postprandial insulin concentration and thus result in higher insulin demand in the long term[9,10]. Therefore, it is compulsory to grade foods based on their GI, along with the II, to prevent both postprandial glycemia and insulinemia in humans. Glycemic load (GL), on the other hand, is a concept that summarizes both GI and the carbohydrate content and is considered to represent the overall glycemic effects of a food[11]. Recent studies have shown that increased dietary GL resulted in predictable increases in glycemia and insulinemia in humans[12,13]. Therefore, it is important to evaluate the concept of GI value of foods together with their concurrent II and GL values.
Tubers and cereals have been considered as the main carbohydrate sources in Chinese diets since the early 1960s. They are not only rich in starch, but also contain vitamins, minerals, phytoestrogens, and trace elements. In the agricultural epoch of Taiwan, where rice and grains are considered rare and expensive, people often consume tubers, such as taro and yam, as a main meal or as a rice substitute to help them harness energy for endurance farm work. In the book, “Ben Chou Gun Mu”[14], a very famous Chinese ancient medical book, they were even described as having medical purposes. With rapid development of the economy, however, eating habits and lifestyle in Taiwan are changing. There is some concern that people think it is detrimental to consume tubers and some cereal products because they are high in starch and regular eating may cause hyperpostprandial glucose responses. Some people even avoid grains or tubers in their diets, particularly diabetic patients. Therefore, it is necessary to evaluate these foods according to their glycemic and insulinemic responses, since they are involved in diet management that helps maintain normoglycemia (possibly also maintaining insulin demand). The five most available starchy foods that are controversial regarding their glycemic effects on humans were chosen for this study. The proximate nutritional components and indigestible starch [dietary fiber (DF) + resistant starch (RS)] were also evaluated in this study.
MATERIALS AND METHODS
Ethics
The study was approved by the Institutional Review Board of Kaohsiung Medical University. Informed consent was obtained from each subject before the enrollment.
Study subjects
Ten healthy subjects were selected for the study. The subjects were six females and four males, aged between 20-30 years, with a mean body mass index (BMI) of 20.6 ± 0.6 (BMI ± SE, in kg/m2). Subjects were recruited based on the following criteria: (1) healthy weight, stable for 6 mo prior to the study; (2) not being on a diet; (3) non-smoker; (4) not taking prescription medication; (5) normotensive; and (6) normal fasting glucose[7]. All subjects were asked to avoid alcohol, legumes and fried foods, eat a regular meal the night before each test, and refrain from unusual eating habits and activity the day before each test. Subjects were also required to complete a food questionnaire before each test to ensure that they had no irregular eating habits. The procedures of the study were orally explained to the subjects, and by written notification.
Test foods
Five starchy foods and one reference food were tested in 50 g available carbohydrate portions. The test foods examined included adlay (Coix lachryma-jobi L.), brown rice (variety, Tai Ken #9) (Oryza sativa L. japonica), mung bean noodles (glass or cellophane noodles), taro (Colocasia esculenta L. Schott) and yam (Chinese sweet potato) (Ipomoea batatas L. Lam). Brown rice was manufactured by the Union Rice Company (Taipei, Taiwan). Mung bean noodles were produced by the Longkow Company (Taipei, Taiwan). Taro and yam were purchased from a local farm (Kaohsiung County, Taiwan). Regarding food preparation, brown rice was prepared by a preliminary soaking (the ratio of rice to water was 1:1.5) overnight, and cooked by a rice cooker (Tatung Co., Ltd. Taiwan) right before the tests. Mung bean noodles were boiled. Taro and yam were skin peeled, cut into 5 cm cubes and steamed by the rice cooker (Tatung Co., Ltd. Taiwan). The reference food, white bread, was laboratory made the day prior to the tests.
Experimental procedures
This study was conducted using internationally recognized GI methodology[6,7,15,16]. All subjects were blind to the name of the food being tested. White bread was the reference food (GI = 100%) against which all test foods were compared. Subjects arrived at the laboratory at eight to nine o’clock in the morning after 10-12 h overnight fast. Each subject was fed equivalent 50 g available carbohydrate of test foods or reference food in random order. To minimize day to day variation of glucose tolerance, the reference food was tested in triplicate in each subject. All test and reference foods were served with 220 mL of water. An automatic lancet device (Safe-T-Pro; Roche Diagnostics GmbH Mannheim, Germany) was used to collect finger capillary blood samples (1.5 mL). Blood samples were taken immediately before the start of the study (0 min) and 15, 30, 45, 60, 90 and 120 min after the start of eating. The blood samples were collected in heparinized tubes and centrifuged at 10 500 × g for 3 min at 4°C to obtain plasma. Plasma was spotted onto a slide which contained a reagent layer (glucose oxidase and peroxidase) (Fuji Dri-Chem 3000; Fuji Film, Kanagawa, Japan) and analyzed with an automatic biochemistry analyzer (Fuji Dri-Chem 3000s, Fuji Film, Kanagawa, Japan) for glucose concentrations on each test day. Plasma insulin concentrations were analyzed in duplicate using an enzyme-linked immunosorbent assay (ELISA) with immunoassay kit (Insulin ELISA, Mercodia AB, Uppsala, Sweden) and microplate spectrophotometer (PowerWave XS, Bio Tek, Winooski, VT, USA).
Glycemic and insulin index determinations
The GI/II was calculated from the ratio of the IAUC of the blood glucose/insulin response curve of test food containing 50 g of available carbohydrate and the same amount of reference food (mean IAUC of three reference white bread samples) expressed as a percentage. Because the GI value of white bread is 71 (measured in advance), therefore, the resulting values need to be multiplied by 0.71 in order to convert them to GI values based on glucose[17-19].
Proximate composition analysis
The fat, protein and carbohydrate contents of test foods were analyzed according to AOAC methods[20]. Crude fat was estimated by solvent extraction in a soxhlet apparatus for 14-16 h with petroleum ether. Crude protein was analyzed by determining the total nitrogen in dried food samples using micro-kjeldahl procedures. A factor of 6.25 was used to convert ‘N’ (nitrogen) value into protein[20]. The analyses of RS + DF were carried out by the method of Onyango and others[21,22] with a slight modification. All measurements were in triplicate.
Statistical analysis
Results are presented as mean ± SE. Insulin concentrations were multiplied by a factor of 6.0 to convert the concentration from mU/L to pmol/L (scientific units). Analysis of variance was performed by using SPSS Windows Release 13.00 (Standard Version, Germany) to determine significant differences. A value of P < 0.05 was considered significant.
RESULTS
Postprandial glucose and insulin responses
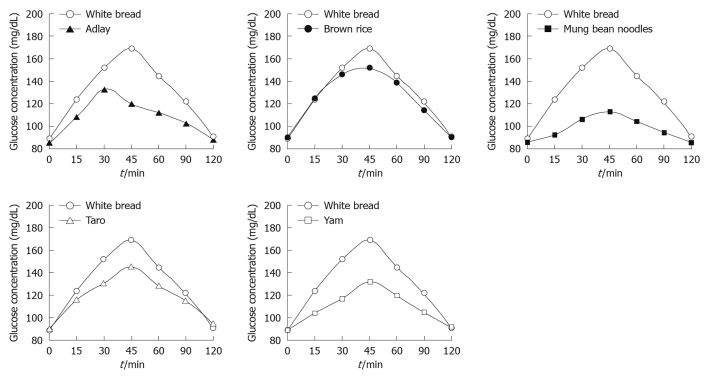
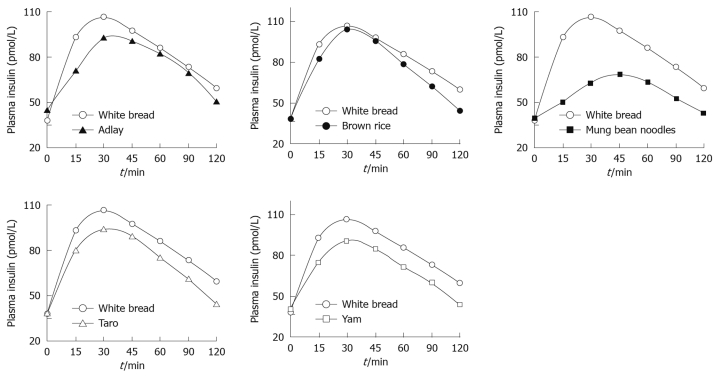
The study protocol was well tolerated. All 10 subjects completed the study. The mean plasma glucose responses curves for the reference and five test foods are displayed in Figure 1. The reference food produced a large rise in blood glucose during the first 45 min and the greatest overall glycemic response. All test foods had similarity in their peak blood glucose concentrations, except adlay which reached a glycemic peak at 30 min. All test foods, however, varied in their overall glycemic responses. Among the test foods, the brown rice elicited the highest glycemic responses followed by the taro, adlay and yam, and the mung bean noodles produced the lowest. Figure 2 shows the mean plasma insulin response curves for the reference and five test foods. The reference food produced the highest peak plasma insulin concentration and the largest overall plasma insulin responses, followed by the brown rice, and the mung bean noodles elicited the lowest plasma insulin responses. All five test foods and the reference food reached their highest response peak at 30 min, except mung bean noodles which reached a peak at 45 min. The plasma insulin responses observed for the five test foods showed a similar profile to their concurrent glycemic responses.
Figure 1.
Mean glucose concentrations elicited by five different starchy foods in healthy subjects. Data are expressed as the change in plasma glucose concentration from the fasting baseline concentration.
Figure 2.
Mean insulin concentrations elicited by five different starchy foods in healthy subjects. Data are expressed as the change in plasma insulin concentration from the fasting baseline concentration.
GI, GL and II
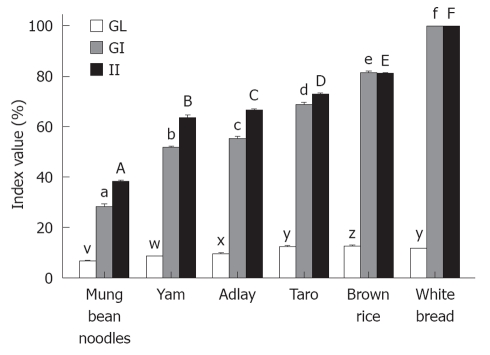
The GIs, GLs and IIs of all the test foods are presented in Figure 3 and the classifications of GIs and GLs are showed in Table 1. The mean GI and GL values of the white bread reference were significantly greater (P < 0.001) than the mean GI and GL values of each of the test foods. The II values of the test foods corresponded with the GIs. The mean II value of the white bread was significantly higher (P < 0.05) than the mean II values of each of the five test foods.
Figure 3.
Glycemic index, glycemic load and insulinemic index values of the starchy foods. The mean glycemic index (GI), glycemic load (GL) and insulinemic index (II) for the reference food (white bread) and the five tested starchy foods. For the GI values, columns with different superscripts (a, b, c, d, e, f) are significantly (P < 0.05) different. Columns representing the GL values with different superscripts (v, w, x, y, z) are significantly different (P < 0.05). Columns representing the II values with different superscripts (A, B, C, D, E, F) are significantly different (P < 0.05).
Table 1.
Glycemic index, glycemic load and insulinemic index of the test foods
|
Glycemic index13 (%) |
Glycemic load23 (g) |
Insulinemic index (%) |
|||
| mean ± SE | Classification | mean ± SE | Classification | mean ± SE | |
| Adlay | 55 ± 0.40 | Low | 9 ± 0.15 | Low | 67 ± 0.27 |
| Brown rice | 82 ± 0.22 | High | 18 ± 0.15 | Medium | 81 ± 0.13 |
| Mung bean noodles | 28 ± 0.50 | Low | 7 ± 0.15 | Low | 38 ± 0.26 |
| Taro | 69 ± 0.35 | Medium | 12 ± 0.16 | Medium | 73 ± 0.30 |
| Yam | 52 ± 0.25 | Low | 9 ± 0.00 | Low | 64 ± 0.45 |
| White bread | 1003 | High | 12 | Medium | 1003 |
Level of glycemic indexs (GIs) were classified according to high (> 69), medium (56-69) and low (< 56) GI;
Level of glycemic loads (GLs) were classified as high (> 20), medium (11-19), and low (< 10) GL;
White bread was used as reference food and was defined as 100.
Proximate nutrition components
The nutrient levels and RS + DF are listed in Table 2. The RS + DF content of the yam, mung bean noodles, and adlay was intermediate (15-20 g), whereas the taro and reference white bread was low (9-10 g). We further estimated the caloric values of the test foods from their carbohydrate, fat and protein contents. All five test foods had caloric values ranging from 330 to 384 kcal (= 1379-1605 kJ) per 100 g.
Table 2.
Major nutrient components and resistant starch content of the test foods (mean ± SE)
| Carbohydrate1 (g/100 g) | Protein1 (g/100 g) | Fat1 (g/100 g) | RS + DF1 (g/100 g) | Calories (kcal/100 g) | |
| Adlay | 85.9 ± 0.5 | 6.7 ± 0.1 | 2.5 ± 0.0 | 15.1 ± 0.5 | 329.9 |
| Brown rice | 86.2 ± 0.1 | 5 ± 0.1 | 1.7 ± 0.1 | 30.8 ± 0.6 | 380.1 |
| Mung bean noodles | 93.5 ± 0.1 | 0.0 ± 0.0 | 0.0 ± 0.1 | 15.7 ± 0.5 | 374.0 |
| Taro | 89.9 ± 0.1 | 3.25 ± 0.0 | 0.1 ± 0.0 | 9.8 ± 0.3 | 373.5 |
| Yam | 89.1 ± 0.1 | 2.12 ± 0.2 | 2.1 ± 0.2 | 20.2 ± 0.5 | 383.8 |
| White bread | 49.9 ± 0.5 | 9.4 ± 0.1 | 7.5 ± 0.0 | 8.8 ± 0.4 | 304.7 |
Analyzed by dry weight. DF: Dietary fiber; RS: Resistant starch.
DISCUSSION
The present study evaluates the GI, GL and II of five starchy foods that are traditionally used in the Chinese diet. The results suggest that brown rice produces the highest glycemic and insulinemic responses and has a GI lower than white rice cooked in a rice cooker (i.e. GI = 99-156)[11]. This result is surprising as a characteristic of brown rice is that the thick bran layer retained in brown rice is often composed of higher fiber content than white counterparts. As judged by several reports[23,24], the rate of gastric emptying of starch and digestibility of starch influence the glucose responses and GI values. The effects of fiber and RS on gastric emptying and digestibility have been evaluated in previous studies, showing that fiber and RS are indigestible and could delay gastric emptying[25-27]. Therefore, lower blood glucose responses and GIs are expected in brown rice. The present results, however, indicated that the brown rice we tested is considered as high GI and medium GL food. This information appears to coincide with clinical observations of a significant rise in postprandial blood glucose after consuming brown rice in both diabetic patients and healthy consumers. Traditionally, when cooking brown rice, it has often been soaked in cold water before cooking to reduce the hardness and chewy mouthfeel after cooking. A possible explanation for the high GI is that the process of soaking allows starch granule expansion and performance of better gelatinization, leading to improved digestibility and consequently a higher GI level is observed.
The result regarding mung bean noodles showed lower glucose and insulin responses than bread and produced the lowest GI and GL among the five starchy foods, although higher carbohydrate content was observed. Generally, mung bean noodles are made of mung bean or pea starch, high in amylose, which has been reported to have the effect of lowering GI[28]. Taro and yam both have long been used in the Chinese diet. There have been times when rice was considered rare and expensive, so yam has often been eaten as a sweet dessert or used as a rice substitute in traditional diets. The postprandial glucose and insulin responses elicited by yam are slightly lower than taro, and thus gave lower GI values. These properties of yam and taro can be encouraging for people who are concerned about their postprandial blood glucose levels. It is interesting to note that taro and yam both have similar carbohydrate contents, however they produced variable GI and GL values. They also had lower GI and GL than bread (the reference food), despite the fact that carbohydrate level in bread was much lower than in taro and yam. An unexpected observation was the relationship between GI and II (i.e. II has usually been described as lower than the relative GI values). In our results, the IIs observed from the five starchy foods were higher than their relative GIs. For example, the II of adlay was 67 ± 0.3; its GI, however, was 55 ± 0.4. Previous studies indicated co-ingestion of fat and/or protein could increase insulin responses and potentially elicit higher insulinemic responses than relative glycemic responses[29]. In the present study, fat and protein contents were observed among the five test foods and insulin responses are higher than their relative glycemic responses, consequently higher II than the corresponding GI values were found. This result implies that co-ingestion of fat and protein in real foods may influence insulin secretion, despite similar amounts of carbohydrate in their contents. The effect may be viewed as increasing glycemic and insulin responses as higher protein and/or fat contents in the starchy foods are measured. With regard to calorie content, all five starchy foods contained calories of about 368 kcal (per 100 g), which did not reach statistical significance (P < 0.05) when compared with white bread (305 kcal). Accordingly, food with lower GI has better satiety than high GI items. Therefore, the actual calorie input may be much lower in mung bean noodles, adlay, taro and yam than in brown rice and white bread.
Based on the correlation analysis, our results suggested that the RS + DF were negatively correlated with the GI and II values (r2 = -0.66 and -0.10, respectively), and positively correlated with GL (r2 = 0.49). Although this result is in line with previous findings[30,31], showing that indigestible starch reduces postprandial glucose and insulin responses, the study may overestimate the amount of RS and DF in the test foods. All the test foods were served hot (approximately 60°C) to the subjects for GI determination. In the RS + DF analysis, however, all the test foods needed to be cooled and dried before proceeding to analytical procedures. The performance of cooling and drying allows retrogradation to occur in amylose chains and may increase the production of RS (retrograded amylose)[32]; consequently higher RS was observed. In particular, this applied to brown rice and yam.
Comparing GI data from other nations, the GI values of starchy foods produced in Taiwan are slightly different to that of counterpart foods produced overseas[9,11,19]. Findings such as this reveal that GI and II values of foods from different countries need to be determined strictly following their own recipes. The GI values of a food could vary when food preparation, cooking methods, food processing, GI testing methods[19] and even geographical location are different. This is more applicable for raw agricultural products. Hence, food with equivalent carbohydrate does not induce similar glycemic and insulinemic responses. This means that GI and GL, along with II values of foods, need to be determined at the same time, in order to provide better understanding as to their postprandial glycemic and insulinemic effects. The present study emphasizes that mung bean noodles, adlay and yam are low GI and GL foods but have variable degrees of II values. The results of this study may provide important information for the public to manage their diet and may prove useful for the prevention of lifestyle-related diseases, such as diabetes mellitus. Continuously evaluating GI values of foods, along with their relative GL and II values, is necessary for individual countries.
COMMENTS
Background
The glycemic and insulinemic effects of foods are relevant to the development of some lifestyle-related diseases and are involved in the therapeutic dietary plan of chronic diseases. However, the glycemic index (GI) and insulinemic index (II) of Chinese traditional starchy foods have not yet been adequately examined.
Research frontiers
GI, one of the most talked about topics in nutrition today, has recently been recommended as a potential tool for both diabetic and individual use. Research has shown that food GI and II values are relevant to the degree of postprandial glycemia and insulinemia and are involved in the therapeutic dietary plan for some lifestyle-related diseases. The need to continuously determine the postprandial glycemic and insulinemic effects of foods is still valuable for health professionals and researchers, and in particular, the GI and II data of agricultural products carried out in individual countries. Although some studies have evaluated the GI of Chinese foods, the glycemic and insulinemic effects of Chinese starchy foods have not yet been examined in parallel. Therefore, it was informative to evaluate the GI, glycemic load (GL) and II of Chinese starchy foods, since they are beneficial for dietary therapy and meal planning.
Innovations and breakthroughs
The present study evaluated the GI and II of five starchy foods that are commonly used in the Chinese traditional diet. The results will provide some preliminary information on both postprandial insulinemic and glycemic effects of Chinese starchy foods and prove useful for consumers to manage their diets, particular for diabetic patients.
Applications
Since a dietary approach is involved in the prevention and management of some chronic diseases, the results of this study will assist the public and health professionals in their meal planning and dietary management.
Terminology
Glycemic effect is expressed as the incremental area under the curve (AUC) of blood glucose response (120 min). Insulinemic effect of food is referring to as the AUC of the blood insulin response. GI is defined as the incremental blood glucose area (120 min) after ingestion of 50 g of available carbohydrates in the test food as a percentage of the corresponding area after an equivalent amount of carbohydrate from a reference food (either white bread or glucose). II is defined as the incremental blood insulin area after eating of 50 g of available carbohydrates in the test food as a percentage of the corresponding area after an equivalent amount of carbohydrate from a reference food. GL is calculated from the GI value of a food multiplied by the amount of carbohydrate in a usual portion size, divided by 100.
Peer review
The authors provided clinically meaningful data for glycemic control of diabetic patients and this reviewer agrees that preventing hyperinsulinemia after feeding would also be important for that.
Acknowledgments
We are grateful to Fiona Atkinson, University of Sydney, for assistance with AUC calculations and Ching-Yun Yu, Kaohsiung Medical University, for her collaboration in ethical approval.
Footnotes
Peer reviewer: Akio Inui, MD, PhD, Professor, Department of Behavioral Medicine, Kagoshima University Graduate School of Medical and Dental Sciences, 8-35-1 Sakuragaoka, Kagoshima 890-8520, Japan
S- Editor Tian L L- Editor Logan S E- Editor Zheng XM
References
- 1.Foster GD, Wyatt HR, Hill JO, McGuckin BG, Brill C, Mohammed BS, Szapary PO, Rader DJ, Edman JS, Klein S. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med. 2003;348:2082–2090. doi: 10.1056/NEJMoa022207. [DOI] [PubMed] [Google Scholar]
- 2.Jones CN, Abbasi F, Carantoni M, Polonsky KS, Reaven GM. Roles of insulin resistance and obesity in regulation of plasma insulin concentrations. Am J Physiol Endocrinol Metab. 2000;278:E501–E508. doi: 10.1152/ajpendo.2000.278.3.E501. [DOI] [PubMed] [Google Scholar]
- 3.Aarsland A, Chinkes D, Wolfe RR. Contributions of de novo synthesis of fatty acids to total VLDL-triglyceride secretion during prolonged hyperglycemia/hyperinsulinemia in normal man. J Clin Invest. 1996;98:2008–2017. doi: 10.1172/JCI119005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kahn SE. The importance of the beta-cell in the pathogenesis of type 2 diabetes mellitus. Am J Med. 2000;108 Suppl 6a:2S–8S. doi: 10.1016/s0002-9343(00)00336-3. [DOI] [PubMed] [Google Scholar]
- 5.Brand-Miller JC. Postprandial glycemia, glycemic index, and the prevention of type 2 diabetes. Am J Clin Nutr. 2004;80:243–244. doi: 10.1093/ajcn/80.2.243. [DOI] [PubMed] [Google Scholar]
- 6.Carbohydrates in human nutrition. Report of a Joint FAO/WHO Expert Consultation. FAO Food Nutr Pap. 1998;66:1–140. [PubMed] [Google Scholar]
- 7.Brand-Miller J, Holt S. Testing the glycaemic index of foods: in vivo, not in vitro. Eur J Clin Nutr. 2004;58:700–701. doi: 10.1038/sj.ejcn.1601856. [DOI] [PubMed] [Google Scholar]
- 8.Ostman EM, Liljeberg Elmståhl HG, Björck IM. Inconsistency between glycemic and insulinemic responses to regular and fermented milk products. Am J Clin Nutr. 2001;74:96–100. doi: 10.1093/ajcn/74.1.96. [DOI] [PubMed] [Google Scholar]
- 9.Choi K, Kim YB. Molecular mechanism of insulin resistance in obesity and type 2 diabetes. Korean J Intern Med. 2010;25:119–129. doi: 10.3904/kjim.2010.25.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han TS, Williams K, Sattar N, Hunt KJ, Lean ME, Haffner SM. Analysis of obesity and hyperinsulinemia in the development of metabolic syndrome: San Antonio Heart Study. Obes Res. 2002;10:923–931. doi: 10.1038/oby.2002.126. [DOI] [PubMed] [Google Scholar]
- 11.Foster-Powell K, Holt SH, Brand-Miller JC. International table of glycemic index and glycemic load values: 2002. Am J Clin Nutr. 2002;76:5–56. doi: 10.1093/ajcn/76.1.5. [DOI] [PubMed] [Google Scholar]
- 12.Schulze MB, Liu S, Rimm EB, Manson JE, Willett WC, Hu FB. Glycemic index, glycemic load, and dietary fiber intake and incidence of type 2 diabetes in younger and middle-aged women. Am J Clin Nutr. 2004;80:348–356. doi: 10.1093/ajcn/80.2.348. [DOI] [PubMed] [Google Scholar]
- 13.Salmerón J, Ascherio A, Rimm EB, Colditz GA, Spiegelman D, Jenkins DJ, Stampfer MJ, Wing AL, Willett WC. Dietary fiber, glycemic load, and risk of NIDDM in men. Diabetes Care. 1997;20:545–550. doi: 10.2337/diacare.20.4.545. [DOI] [PubMed] [Google Scholar]
- 14.Li SC. Pen Tsao Kang Mu (Systematic Pharmacopoeia) 1997. China, 1596: 100-2826. [Google Scholar]
- 15.Heilbronn LK, Noakes M, Clifton PM. The effect of high- and low-glycemic index energy restricted diets on plasma lipid and glucose profiles in type 2 diabetic subjects with varying glycemic control. J Am Coll Nutr. 2002;21:120–127. doi: 10.1080/07315724.2002.10719204. [DOI] [PubMed] [Google Scholar]
- 16.Nilsson A, Granfeldt Y, Ostman E, Preston T, Björck I. Effects of GI and content of indigestible carbohydrates of cereal-based evening meals on glucose tolerance at a subsequent standardised breakfast. Eur J Clin Nutr. 2006;60:1092–1099. doi: 10.1038/sj.ejcn.1602423. [DOI] [PubMed] [Google Scholar]
- 17.Velangi A, Fernandes G, Wolever TM. Evaluation of a glucose meter for determining the glycemic responses of foods. Clin Chim Acta. 2005;356:191–198. doi: 10.1016/j.cccn.2005.01.025. [DOI] [PubMed] [Google Scholar]
- 18.Wolever TM, Vorster HH, Björck I, Brand-Miller J, Brighenti F, Mann JI, Ramdath DD, Granfeldt Y, Holt S, Perry TL, et al. Determination of the glycaemic index of foods: interlaboratory study. Eur J Clin Nutr. 2003;57:475–482. doi: 10.1038/sj.ejcn.1601551. [DOI] [PubMed] [Google Scholar]
- 19.Lin MHA, Wu MC, Lin J. Variable classifications of glycemic index determined by glucose meters. J Clin Biochem Nutr. 2010;47:45–52. doi: 10.3164/jcbn.10-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin MHA AOAC. Official methods of analysis. 17th ed. Washington, DC: Association of Official Analytical Chemists; 2000. [Google Scholar]
- 21.Onyango C, Bley T, Jacob A, Henle T, Rohm H. Influence of incubation temperature and time on resistant starch type III formation from autoclaved and acid-hydrolysed cassava starch. Carbohydr Polym. 2006;66:494–499. [Google Scholar]
- 22.Rosin PM, Lajolo FM, Menezes EW. Measurement and characterization of dietary starches. J Food Compost Anal. 2002;15:367–377. [Google Scholar]
- 23.Nugent AP. Health properties of resistant starch. Nutr Bull. 2005;30:27–54. [Google Scholar]
- 24.Chung HJ, Shin DH, Lim ST. In vitro starch digestibility and estimated glycemic index of chemically modified corn starches. Food Res Int. 2008;41:579–585. [Google Scholar]
- 25.Vonk RJ, Hagedoorn RE, de Graaff R, Elzinga H, Tabak S, Yang YX, Stellaard F. Digestion of so-called resistant starch sources in the human small intestine. Am J Clin Nutr. 2000;72:432–438. doi: 10.1093/ajcn/72.2.432. [DOI] [PubMed] [Google Scholar]
- 26.Silvester KR, Englyst HN, Cummings JH. Ileal recovery of starch from whole diets containing resistant starch measured in vitro and fermentation of ileal effluent. Am J Clin Nutr. 1995;62:403–411. doi: 10.1093/ajcn/62.2.403. [DOI] [PubMed] [Google Scholar]
- 27.Liljeberg H, Björck I. Delayed gastric emptying rate may explain improved glycaemia in healthy subjects to a starchy meal with added vinegar. Eur J Clin Nutr. 1998;52:368–371. doi: 10.1038/sj.ejcn.1600572. [DOI] [PubMed] [Google Scholar]
- 28.Denardin CC, Walter M, da Silva LP, Souto GD, Fagundes CAA. Effect of amylose content of rice varieties on glycemic metabolism and biological responses in rats. Food Chem. 2007;105:1474–1479. [Google Scholar]
- 29.Nuttall FQ, Mooradian AD, Gannon MC, Billington C, Krezowski P. Effect of protein ingestion on the glucose and insulin response to a standardized oral glucose load. Diabetes Care. 1984;7:465–470. doi: 10.2337/diacare.7.5.465. [DOI] [PubMed] [Google Scholar]
- 30.Scazzina F, Del Rio D, Pellegrini N, Brighenti F. Sourdough bread: Starch digestibility and postprandial glycemic response. J Cereal Sci. 2009;49:419–421. [Google Scholar]
- 31.Nilsson AC, Ostman EM, Holst JJ, Björck IM. Including indigestible carbohydrates in the evening meal of healthy subjects improves glucose tolerance, lowers inflammatory markers, and increases satiety after a subsequent standardized breakfast. J Nutr. 2008;138:732–739. doi: 10.1093/jn/138.4.732. [DOI] [PubMed] [Google Scholar]
- 32.Han JA, BeMiller JN. Preparation and physical characteristics of slowly digesting modified food starches. Carbohydr Polym. 2007;67:366–374. [Google Scholar]