Abstract
Vascular endothelial dysfunction may contribute to the increase in cardiovascular events during HIV-1 infection and its treatment. Antiretroviral therapy (ART), metabolic factors, lipodystrophy, and HIV infection itself may be involved. Ninety-six HIV-infected subjects were evaluated for endothelial function by measurement of brachial artery flow-mediated dilation (FMD) by ultrasound, single-slice CT of the abdomen and mid-thigh, whole-body dual x-ray absorptiomety (DXA) scans, and metabolic evaluations in a cross-sectional study. The median age was 40 years; 28% were female, 38% black, 3% Hispanic, and 59% white. Forty-nine (51%) were receiving ART, which included a PI in 28 (57%) and was non-PI based in 21 (43%). FMD (±SD) in subjects not on ART was 5.5 ± 4.3%, PI-ART 5.3 ± 3.6%, and non-PI-ART 5.5 ± 4.1% (p = 0.9). Age, race, CD4 cell count, and HIV RNA did not correlate significantly with FMD. Among ART-treated subjects in the lowest tertile of thigh subcutaneous fat area (range 3–31 cm2), FMD was 4.4 ± 3.5% and in the highest tertile (range 67–237 cm2) FMD was 6.8 ± 3.6% (p = 0.07, t-test). However, in multivariate analyses, no body composition measure showed a significant association with FMD for either the group as a whole or in ART-treated subjects. ART use, PI use, CD4 cell count, and HIV RNA levels were not associated with endothelial dysfunction by brachial FMD. A definitive association with measures of adiposity was not detected in multivariate analysis, suggesting that lipoatrophy may not be an important contributor to endothelial dysfunction in HIV-infected individuals on ART.
Introduction
Combination antiretroviral therapy for HIV infection is associated with an increased risk of myocardial infarction, particularly with the use of HIV-1 protease inhibitors (PIs)1,2 and possibly also the nucleoside reverse transcriptase inhibitors abacavir3,4 and didanosine.3 Prospective data suggest that PI-associated lipid disorders alone do not explain all of this increased risk.1 Endothelial dysfunction is a critical initial step of atherogenesis that contributes to the progression and clinical manifestations of atherosclerosis.5–7 It is possible that PI-related endothelial dysfunction may be responsible for increased cardiovascular events under PI therapy that is not merely due to PI-associated lipid changes. Long-term use of PI-based antiretroviral regimens has been associated with significant endothelial dysfunction,8 but body composition and insulin resistance variables were not measured in that study. Conversely, in a prospective study of antiretroviral-naive subjects, combination antiretroviral therapy (ART) improved endothelial dysfunction over 24 weeks regardless of the ART regimen used,9 suggesting that in the short term ART improves HIV-associated endothelial dysfunction even when PIs are used.
Four weeks of administration of the HIV-1 PI indinavir significantly impaired endothelial function in healthy HIV-uninfected subjects in three different studies.10–12 However, indinavir is now seldom used in clinical practice and the newer PIs atazanavir and the fixed-dose combination of lopinavir-ritonavir had no effect on endothelial function in healthy, HIV-uninfected subjects.13 Successful treatment of HIV infection, in the short-term, improved endothelial function regardless of whether the PI lopinavir-ritonavir was used.9 Furthermore, more contemporary studies in which few subjects received indinavir have failed to confirm a role for PI-containing antiretroviral regimens in endothelial dysfunction.14
Factors other than PI use that may contribute to endothelial dysfunction in HIV-infected patients include HIV infection itself,9 treatment-associated lipid changes,8,14 and the lipodystrophy syndrome––possibly mediated by its association with insulin resistance,15–17 lipid disorders,16,17 a persistent inflammatory state,15,18 or adipokine alterations.18–23 Physician-defined lipodystrophy was associated with an increased risk of myocardial infarction in a recent large prospective study.3 However, formal body composition measures have not been included in any study of endothelial function in HIV-infected patients. In the present study, our primary objective was to determine the relationship of formal body composition measurements to endothelial dysfunction in a large cross-section of HIV-infected subjects. Metabolic variables, markers of inflammation, HIV disease indicators, and, in particular, ART use and PI use were examined as potential explanatory variables.
Materials and Methods
Subjects
A total of 96 subjects were enrolled between January 2006 and March 2007 (Table 1). Studies were approved by the Indiana University-Purdue University and Clarian Health Partners Institutional Review Board, and all volunteers gave written informed consent. Subjects were recruited from local primary HIV care clinics without regard to antiretroviral use or signs of lipodystrophy. Antiretroviral use was selected by individual practitioners in routine clinical care. Major inclusion criteria included documented HIV infection, age 18–65 years, and fasting serum glucose <126 mg/dl. Major exclusion criteria included a history of diabetes mellitus, active untreated opportunistic infection, known cardiovascular disease, uncontrolled hypertension, serum creatinine more than two times the upper limit of normal, liver aminotransferase level more than five times the upper limit of normal, need for systemic cytotoxic chemotherapy, current use of lipid-lowering medications, chronic systemic glucocorticoids, and active drug or alcohol use or psychiatric illness expected to interfere with adherence to study requirements.
Table 1.
Subject Characteristics
| All | No drug | PI-based regimens | Non-PI-based regimens | p Value | |
|---|---|---|---|---|---|
| N | 96 | 47 | 28 | 21 | |
| Age (years) | 40.4 (9.6) | 38.5 (9.1) | 40.8 (6.7) | 44.2 (12.8) | 0.08 |
| Sex (female) | 27 (28%) | 10 (21%) | 7 (25%) | 10 (48%) | 0.09 |
| Race/ethnicity | 0.71 | ||||
| Hispanic | 3 (3%) | 1 (2%) | 2 (7%) | 0 (0%) | |
| Black | 36 (38%) | 18 (38%) | 11 (39%) | 7 (33%) | |
| White | 57 (59%) | 28 (60%) | 15 (54%) | 14 (67%) | |
| Smoking status (Y/N) | 63 (66%) | 28 (60%) | 20 (71%) | 15 (71%) | 0.48 |
| Family history cardiovascular disease (Y/N) | |||||
| Male relatives | 11/86 (13%) | 2/41 (5%) | 4/25 (16%) | 5/20 (25%) | 0.06 |
| Female relatives | 8/89 (9%) | 5/44 (11%) | 2/26 (8%) | 1/19 (5%) | 0.89 |
| BMI (kg/m2) | 26.0 (5.7) | 26.1 (5.7) | 25.7 (4.5) | 26.1 (7.3) | 0.96 |
| Current CD4 count (cells/mm3) | 478 (3256) | 388 (256) | 507 (419) | 643a (262) | 0.009 |
| CD8 count (cells/mm3) | 901 (494) | 831 (346) | 1005 (729) | 927 (390) | 0.37 |
| CD4/CD8 ratio | 0.57 (0.36) | 0.50 (0.33) | 0.57 (0.42) | 0.75a (0.30) | 0.045 |
| Current HIV RNA (copies/ml) | 64,946 (162,945) | 105,060 (192,558) | 45,149 (153,043) | 400a (0) | 0.04 |
| 77% < 400 | 100% < 400 | ||||
| Nadir CD4 count (cells/mm3) | 275 (238) | 329.0 (217) | 227.8 (302) | 209.4 (156) | 0.08 |
| Total cholesterol (mg/dl) | 171 (40) | 155 (35) | 181b (42) | 194a (31) | 0.001 |
| Triglycerides | 135 (79) | 123 (74) | 156 (74) | 135 (92) | 0.22 |
| HDL cholesterol | 43 (15) | 38 (12) | 43 (14) | 54a,c (18) | 0.001 |
| Non-HDL cholesterol | 128 (35) | 117 (32) | 138b (36) | 139a (32) | 0.007 |
| LDL cholesterol (calculated) | 103 (34) | 93 (31) | 111b (40) | 112a (28) | 0.03 |
| High sensitivity C-reactive protein (mg/liter) | 3.3 (3.4) | 3.1 (2.6) | 3.3 (2.9) | 4.0 (5.3) | 0.62 |
| Interleukin-6 (pg/ml) | 0.59 (1.11) | 0.65 (1.41) | 0.59 (0.85) | 0.47 (0.61) | 0.83 |
| Soluble TNF receptor-2 (ng/ml) | 1.1 (0.5) | 1.3 (0.5) | 0.9b (0.4) | 0.7a (0.4) | 0.001 |
| Plasminogen activator inhibitor-1 (ng/ml) | 58.2 (40.1) | 54.5 (40.9) | 61.3 (37.2) | 61.9 (43.2) | 0.71 |
| Adiponectin (μg/ml) | 5.9 (3.5) | 5.7 (3.2) | 5.7 (3.4) | 6.7 (4.2) | 0.55 |
| Leptin (ng/ml) | 11.3 (21.2) | 9.9 (18.2) | 9.8 (20.4) | 16.2 (27.5) | 0.49 |
| Resistin (ng/ml) | 19.8 (13.7) | 19.9 (13.9) | 22.3 (15.6) | 16.2 (10.1) | 0.31 |
| Soluble intercellular adhesion molecule-1 (ng/ml) | 375 (128) | 386 (106) | 370 (164) | 356 (119) | 0.65 |
| Soluble vascular cell adhesion molecule-1 (ng/ml) | 1057 (1114) | 1397 (1508) | 881b (418) | 583a (222) | 0.01 |
| E-selectin (ng/ml) | 9.1 (4.2) | 9.4 (4.5) | 9.1 (3.9) | 8.6 (4.2) | 0.76 |
| HOMA-IR | 2.8 (1.8) | 3.1 (2.1) | 2.5 (1.5) | 2.8 (1.5) | 0.4 |
| Incremental 2 h insulin AUC on OGTT | 5.3 (4.5) | 5.7 (3.9) | 5.1 (5.5) | 4.7 (4.2) | 0.7 |
| Brachial artery diameter (cm) | 0.40 (0.07) | 0.41 (0.06) | 0.41 (0.07) | 0.38 (0.06) | 0.13 |
| Maximal percent brachial FMD | 6.0 (4.0) | 5.8 (4.3) | 6.0 (3.8) | 6.6 (4.0) | 0.78 |
| Percent nitroglycerin-mediated dilation | 16.6 (14.6) | 18.4 (6.8) | 17.0 (8.3) | 11.7 (28.3) | 0.25 |
p < 0.05 for non-PI regimen vs. not on drug.
p < 0.05 for PI regimen vs. not on drug.
p < 0.05 for non-PI vs. PI regimen.
Procedures
Subjects were studied as outpatients on the Indiana University General Clinical Research Center after an overnight fast. Fasting blood samples were obtained for HIV disease measures, hepatic function tests, complete blood count, and metabolic and inflammatory variables. Then, a 2-h oral glucose tolerance test (OGTT) was performed using a standard 75 g oral glucose load, with sampling at −10, –5, 30, 60, 90, and 120 min for glucose and insulin levels and to calculate area under the curve (AUC). Blood tests were performed on the day of ultrasound imaging and other imaging studies were accomplished within 2 weeks.
Imaging studies
Single-slice CT of the abdomen at L4-5 was obtained for measurement of subcutaneous and visceral fat areas in addition to psoas muscle attenuation24 using standardized techniques. Single-slice CT of the mid-thigh was obtained at the radiographic midpoint of the femur on the subject's right side for measurement of subcutaneous fat area. Whole-body dual x-ray absorptiometry (DXA) scans were obtained with regional body composition analysis (Lunar DPX-L; Lunar Corp., Madison, WI, system software 4.6b). Total limb fat was the sum of arm and leg fat mass. All imaging studies, measurements, and calculations were performed by technicians who were unaware of subject's antiretroviral treatment status.
Brachial artery ultrasound
The primary outcome measure in this study was brachial flow-mediated dilation (FMD). Brachial artery reactivity testing was performed according to established guidelines.25 Measurements were made after subjects rested supine for at least 10 min in a temperature-controlled (68–71°F) room during the morning of the study visit. Subjects were instructed not to use tobacco-containing products or eat or drink anything besides water for 12 h prior to the study. An Acuson CV70 ultrasound system with a 10-MHz linear-array vascular probe was used to visualize the brachial artery 1–2 cm above the antecubital fossa. FMD was estimated as the larger percent increase in brachial artery diameter measured at 60 and 90 s after release of a blood pressure cuff (which had been inflated to 250 mm Hg for 5 min around the forearm) compared to the resting baseline value. Fifteen minutes after cuff deflation, 0.4 mg of sublingual nitroglycerin (NTG) was administered; NTG-mediated dilation (NTGMD) was estimated as the percent increase in brachial artery diameter 3 min after the administration of NTG compared to the pre-NTG value. Brachial artery diameters were measured in triplicate at end-diastole with digital calipers (AccessPoint 2004 software; Freeland Systems, Inc., Indianapolis, IN). All ultrasound procedures were performed by a single technician (J.S.W.), and all vascular measurements were made by a single investigator (S.K.G.), who was unaware of treatment group or body composition measurements. The intraclass correlations for reproducibility for baseline diameter and FMD measured in 12 healthy volunteers in our laboratory under these conditions were 0.97 and 0.73, respectively.
Metabolic studies
Glucose was assayed in real time by the glucokinase method using a YSI apparatus (Yellow Springs Instruments, Yellow Springs, OH). All other samples were stored at –80°C until assayed in batch. Plasma insulin was measured by a radioimmunoassay insensitive to proinsulin (Linco Research, St. Charles, MO). Total and high-density lipoprotein (HDL) cholesterol and triglycerides were measured by standard enzymatic techniques and low-density lipoprotein (LDL) cholesterol levels were calculated using the Friedewald formula when triglycerides were <400 mg/dl.26 Non-HDL cholesterol was calculated as total cholesterol minus HDL cholesterol. Advanced lipoprotein testing was performed by nuclear magnetic resonance spectroscopy [Otvos, 2000 #827] (NMR Lipoprofile) and high sensitivity C-reactive protein was measured using an immunometric assay (DPC Immulite 2000) on EDTA plasma at LipoScience (Raleigh, NC). Plasma adiponectin, leptin, resistin, interleukin-6, soluble tumor necrosis factor receptor type 2 (sTNFr2), total plasminogen activator inhibitor antigen (PAI-1), soluble intercellular adhesion molecule-1 (sICAM-1), soluble vascular cell adhesion molecule-1 (sVCAM-1), and e-selectin were performed by a multiplex radioimmunoassay (Pierce Biotechnology, Woburn, MA).
Statistical analysis
Continuous variables are summarized by mean and standard deviation and categorical variables are summarized by frequency and percentage. Analysis of variance (ANOVA) was used to compare continuous variables among different groups and the chi-square test was used to compare categorical variables. We also employed the Wilcoxon rank-sum and Fisher's exact test in scenarios when the validity of ANOVA or chi-square test is of concern due to limited sample size. Statistical significance was defined as p < 0.05. Correlations between continuous variables were assessed by Pearson and Spearman's correlation coefficients. Three multiple regression models were constructed to identify factors associated with FMD. In model 1, variables that were found to be statistically significant in the study by Stein et al.,8 namely sex, baseline brachial artery diameter, heart rate, systolic blood pressure, and type of ART (PI, non-PI, or not on drug), were included. In model 2, body fat measures and insulin AUC (variables that were not studied by Stein et al.8) were added to model 1 individually to ascertain the potential association of body composition and insulin sensitivity to FMD. In model 3, all other inflammatory markers, adipokines, and endothelial activation variables that were associated with FMD with a univariate p-value of <0.1 were added individually to model 1. All three models were performed for the study group as a whole and for the group receiving ART. For all three models we included sex, baseline diameter, baseline heart rate, baseline systolic pressure, and type of drug regimen (PI, non-PI, not on drug). All analyses were performed with the SAS version 9.1 (SAS Institute, Cary, NC).
Results
Subject demographics and HIV disease variables are shown in Table 1 for the group as a whole, for those not currently receiving ART (N = 47), for those currently receiving an ART regimen containing a PI (N = 28), and for those currently receiving an ART regimen not containing a PI (N = 21). Among the subjects not receiving ART, 35 (75%) had never received ART and 12 (25%) had prior ART experience (median time off of ART 31 months, range 7–144 months). Current ART use is listed in Table 2. Subjects receiving a PI-based regimen had a significantly shorter total duration of ART than those receiving a non-PI-based regimen (31 months vs. 52 months, respectively, p = 0.002). Brachial artery diameter, NTGMD, and maximal FMD did not differ by treatment group (Table 1). Among the 11 subjects who received abacavir, FMD was not different from the subjects not treated with abacavir. No subject was receiving didanosine.
Table 2.
Current Antiretroviral Treatment
| All treated subjects (N = 49) N (%) | Protease inhibitor-based regimens (N = 28) N (%) | Nonprotease inhibitor-based regimens (N = 21) N (%) | |
|---|---|---|---|
| Nucleoside reverse transcriptase inhibitors | |||
| Abacavir | 11 (22%) | 6 (21%) | 5 (24%) |
| Emtricitabine | 12 (24%) | 9 (32%) | 3 (14%) |
| Lamivudine | 28 (57%) | 15 (54%) | 13 (62%) |
| Stavudine | 2 (4%) | 1 (4%) | 1 (5%) |
| Tenofovir | 20 (41%) | 13 (46%) | 7 (33%) |
| Zidovudine | 21 (43%) | 11 (39%) | 10 (48%) |
| Protease inhibitors | |||
| Atazanavir | 6 (12%) | 6 (21%) | 0 |
| Fosamprenavir | 2 (4%) | 2 (7%) | 0 |
| Lopinavir-ritonavir | 20 (41%) | 20 (71%) | 0 |
| Nelfinavir | 5 (10%) | 5 (18%) | 0 |
| Ritonavir-boosted PI | 20 (41%) | 20 (71%) | 0 |
| Nonnucleoside reverse transciptase inhibitors | |||
| Efavirenz | 19 (39%) | 0 | 19 (91%) |
| Nevirapine | 1 (2%) | 0 | 1 (5%) |
Glucose metabolism
Insulin sensitivity estimated by HOMA-IR and by the incremental AUC for insulin levels during the 2-h OGTT did not differ between treatment groups (Table 1).
Lipids and lipoproteins
Subjects receiving either a PI-based regimen or a non-PI-based regimen had similar total cholesterol, LDL cholesterol, and non-HDL cholesterol levels, but both groups were significantly higher than subjects not receiving drugs (Table 1). Triglyceride levels were not different between the groups. HDL cholesterol levels were higher in the subjects who received non-PI-based regimens compared to those not on drug or on PI-based regimens. Advanced lipoprotein testing by NMR Lipoprofile showed results generally consistent with standard lipid testing (data not shown). The total number of LDL particles was similar in all groups while LDL size was greater in the non-PI group (21.2 ± 1 nm) compared to the untreated and PI-treated groups (20.5 ± 0.7 nm for both, p = 0.003).
Adipokines, vascular, and inflammatory markers
Subjects receiving either a PI-based regimen or a non-PI based regimen had significantly lower levels of sTNFr2 and sVCAM-1 compared to subjects not receiving ART. All other variables were similar between treatment groups (Table 1).
Association of variables with FMD
In univariate analyses shown in Tables 3 and 4, brachial artery diameter correlated negatively with maximal FMD in all subjects (Table 3) and in ART-treated subjects (Table 4), as expected. Height correlated strongly with maximal FMD for the group as a whole. Greater insulin area under the curve (consistent with a greater degree of insulin resistance) unexpectedly correlated with greater FMD in the whole group and in ART-treated subjects.
Table 3.
Univariate Correlations of Maximum Percent Flow-Mediated Dilation (FMmax) with Demographic, Metabolic, Body Composition, and HIV Disease Variables—All Subjects N = 96
| Variablea | N with available data | Pearson r | p Value |
|---|---|---|---|
| Age | 94 | −0.01 | 0.94 |
| Height | 94 | −0.36 | 0.0003 |
| Weight | 94 | −0.05 | 0.60 |
| Body mass index | 94 | 0.14 | 0.19 |
| Subjective loss of facial fat | 88 | 0.04 | 0.69 |
| Subjective gain of belly fat | 89 | 0.19 | 0.07 |
| Current CD4+ cell count | 93 | −0.10 | 0.34 |
| Nadir CD4+ cell count | 91 | −0.16 | 0.14 |
| HIV RNA | 88 | −0.02 | 0.83 |
| Limb fat mass (DXA) | 94 | 0.08 | 0.43 |
| Trunk fat mass (DXA) | 88 | 0.07 | 0.48 |
| Limb fat/total body fat % (DXA) | 94 | −0.11 | 0.30 |
| Total body lean mass (DXA) | 94 | −0.25 | 0.02 |
| Visceral fat area (CT) | 89 | 0.06 | 0.55 |
| Abdominal subcutaneous fat area (CT) | 89 | 0.18 | 0.10 |
| Thigh subcutaneous fat area (CT) | 88 | 0.19 | 0.07 |
| Systolic blood pressure | 63 | 0.14 | 0.26 |
| Diastolic blood pressure | 63 | 0.08 | 0.55 |
| Baseline brachial artery diameter | 94 | −0.41 | <0.0001 |
| Total cholesterol | 88 | −0.02 | 0.87 |
| Triglycerides | 93 | 0.07 | 0.52 |
| LDL cholesterol | 87 | 0.02 | 0.88 |
| HDL cholesterol | 88 | −0.01 | 0.92 |
| LDL particle number | 91 | 0.03 | 0.78 |
| LDL particle size | 91 | −0.08 | 0.47 |
| HDL particle number | 91 | 0.00 | 0.99 |
| HDL particle size | 91 | 0.03 | 0.78 |
| Adiponectin | 91 | −0.07 | 0.49 |
| Leptin | 91 | 0.20 | 0.05 |
| Resistin | 91 | −0.05 | 0.67 |
| Plasminogen activator inhibitor-1 | 91 | 0.03 | 0.55 |
| Soluble tumor necrosis factor receptor-2 | 91 | −0.04 | 0.68 |
| Interleukin-6 | 91 | 0.03 | 0.76 |
| High sensitivity C-reactive protein | 89 | 0.10 | 0.36 |
| Vascular cell adhesion molecule-1 | 91 | −0.13 | 0.20 |
| E-selectin | 91 | −0.10 | 0.33 |
| Fasting plasma glucose | 88 | 0.05 | 0.63 |
| Two-hour insulin area under the curve | 92 | 0.25 | 0.02 |
LDL, low-density lipoprotein; HDL, high-density lipoprotein.
Bold represents those variables with p value of <0.1 that are used in multivariable models.
Table 4.
Univariate Correlations of Maximum Percent Flow-Mediated Dilation (FMDmax) with Demographic, Metabolic, Body Composition, and HIV Disease Variables—Subjects Receiving ART, N = 49
| Variablea | N with available data | Pearson r | p Value |
|---|---|---|---|
| Age | 48 | −0.05 | 0.73 |
| Height | 48 | −0.26 | 0.08 |
| Weight | 48 | 0.07 | 0.62 |
| Body mass index | 48 | 0.24 | 0.11 |
| Duration on ART | 48 | 0.15 | 0.29 |
| Cumulative time on thymidine analogs | 48 | −0.05 | 0.74 |
| Subjective loss of facial fat | 48 | −0.04 | 0.80 |
| Subjective gain of belly fat | 45 | 0.09 | 0.56 |
| Current CD4+ cell count | 47 | 0.20 | 0.18 |
| Nadir CD4+ cell count | 45 | −0.29 | 0.05 |
| HIV RNA | 44 | −0.12 | 0.46 |
| Limb fat mass (DXA) | 48 | 0.27 | 0.06 |
| Trunk fat mass (DXA) | 46 | 0.09 | 0.21 |
| Limb fat/total body fat % (DXA) | 48 | 0.18 | 0.22 |
| Total body lean mass (DXA) | 48 | −0.19 | 0.21 |
| Visceral fat area (CT) | 45 | 0.13 | 0.39 |
| Abdominal subcutaneous fat area (CT) | 45 | 0.23 | 0.13 |
| Thigh subcutaneous fat area (CT) | 44 | 0.29 | 0.06 |
| Systolic blood pressure | 37 | 0.35 | 0.03 |
| Diastolic blood pressure | 37 | 0.14 | 0.42 |
| Baseline brachial artery diameter | 48 | −0.33 | 0.02 |
| Total cholesterol | 44 | −0.11 | 0.48 |
| Triglycerides | 47 | 0.02 | 0.90 |
| LDL cholesterol | 44 | 0.02 | 0.92 |
| HDL cholesterol | 44 | 0.02 | 0.92 |
| LDL particle number | 48 | −0.02 | 0.92 |
| LDL particle size | 48 | 0.00 | 0.99 |
| HDL particle number | 48 | −0.03 | 0.85 |
| HDL particle size | 48 | 0.10 | 0.48 |
| Adiponectin | 48 | 0.10 | 0.48 |
| Leptin | 48 | 0.28 | 0.06 |
| Resistin | 48 | 0.13 | 0.39 |
| Plasminogen activator inhibitor-1 | 48 | 0.21 | 0.15 |
| Soluble tumor necrosis factor receptor-2 | 48 | 0.19 | 0.20 |
| Interleukin-6 | 48 | −0.08 | 0.57 |
| High sensitivity C-reactive protein | 47 | −0.01 | 0.95 |
| Vascular cell adhesion molecule-1 | 48 | −0.02 | 0.90 |
| E-selectin | 48 | 0.13 | 0.39 |
| Fasting plasma glucose | 44 | 0.22 | 0.16 |
| Two-hour insulin area under the curve | 47 | 0.38 | 0.07 |
LDL, low-density lipoprotein; HDL, high-density lipoprotein.
Bold represents those variables with p value of <0.1 that are used in multivariable models.
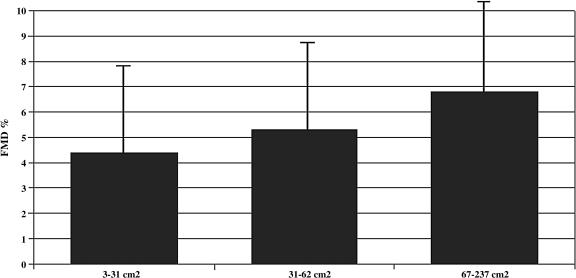
In ART-treated subjects, thigh subcutaneous fat area and DXA limb fat mass had borderline univariate correlations with maximal FMD (Pearson r = 0.29 and 0.27, respectively, p = 0.06 for each). Among ART-treated subjects in the lowest tertile of thigh subcutaneous fat area (range 3–31 cm2), FMD was 4.4 ± 3.5% and in the highest tertile (range 67–237 cm2) FMD was 6.8 ± 3.6% (p = 0.07, t-test, Fig. 1).
FIG. 1.
Percentage flow-mediated dilation (±SD) by tertile of mid-thigh subcutaneous fat area among ART-treated subjects (N = 44). p = 0.07 across groups, t-test.
In multivariate analysis model 1, only baseline brachial artery diameter was significantly associated (positive correlation), whereas sex, heart rate, systolic blood pressure, and type of drug (PI, non-PI, not on drug) were not associated with FMD. After adding the nadir CD4 cell count to model 1, nadir CD4 is not significant (p = 0.35). In model 2, limb fat mass, trunk fat mass, limb fat/total body fat percentage, total body lean mass, visceral fat area, thigh subcutaneous fat area, and insulin AUC from OGTT were each added to the model individually. No body composition measures were associated with FMD. Limb fat/total body fat percentage (p = 0.09, negative correlation) and insulin AUC (p = 0.05, positive correlation) were marginally associated with FMD. In model 3, no other metabolic, inflammatory, vascular, or HIV disease variables were associated with FMD.
Discussion
Recent data from the large multinational cohort D:A:D study suggest that a physician diagnosis of lipodystrophy was associated with an increased incidence of myocardial infarction.3 Detailed measurements of regional body fat, however, were not performed in that study. Our data suggested a possible relationship between fat distribution and endothelial function measured by FMD, but on multivariate analysis no measure of body composition by CT or DXA was independently associated. It is possible that a significant association of limb fat loss and endothelial dysfunction would have been detected with a greater sample size or with measurement of longitudinal changes over time. Fat distribution pattern may present an avenue for further exploration in HIV lipodystrophy-associated cardiovascular risk.
A large variety of metabolic, inflammatory, lipid, and HIV disease variables were examined in the current study and similar to the results of others9,14 no single laboratory measure was strongly correlated with endothelial function. A recent study found a potential association between heightened circulating inflammatory markers and markers of endothelial activation,27 but our study using brachial FMD showed no such associations with inflammatory markers. Indeed, we did not show a correlation of circulating markers of endothelial activation with our physiologic measure of endothelial function. We speculate that circulating markers may not reliably reflect physiologic function in HIV-infected patients. It is possible that the local inflammatory processes in the vessel wall that lead to impaired endothelial function in HIV are associated with circulating marker changes that are overwhelmed by systemic production of inflammatory markers. Indeed, our group demonstrated that brachial FMD improved significantly in HIV-infected subjects treated with the antiinflammatory agent salsalate, in spite of no improvement in circulating inflammatory markers.28
We were unable to confirm a role for the PI class in inducing endothelial dysfunction. Human data8,10–12 and experimental models29–33 have implicated PIs as a cause of endothelial dysfunction. The mechanism appears to include impaired nitric oxide bioavailability.12,30 Stein and colleagues8 documented dyslipidemia and severe endothelial dysfunction in a cross-sectional observational study of subjects who received long-term PI-based ART (mean 70 total months, which included 31 months on a PI), but not in those treated for HIV without a PI. Importantly, half of their PI-treated subjects received the PI indinavir.8 Interestingly the PI-treated subjects in that study tended to have greater waist-to-hip ratios and BMI (p = 0.09 and p = 0.07, respectively), suggesting that lipodystrophy may have been more prevalent among the PI-treated subjects, although formal measurements of adiposity were not performed.8 More contemporary studies in which few subjects received indinavir have failed to confirm a role for PI-containing antiretroviral regimens in endothelial dysfunction14 or endothelial activation.27 In fact, there was a nonsignificant trend for better endothelial function measured by brachial FMD among subjects receiving PIs (predominantly nelfinavir and lopinavir-ritonavir) in the study of Solages and colleagues.14 Similarly, use of the PI lopinavir-ritonavir was the strongest predictor of better endothelial function by brachial FMD in a small cross-sectional study.34
Although not all PIs have been examined in this manner, in studies in healthy subjects only indinavir has been implicated as a cause of endothelial dysfunction10–12 and thus this effect may be agent specific. When studied in a manner similar to indinavir in healthy subjects, the PIs atazanavir and lopinavir-ritonavir do not cause endothelial dysfunction.13 Consistent with our results, lopinavir-ritonavir administration to six healthy subjects for 4 weeks actually led to nonsignificant percentage increases in endothelium-dependent vasodilation.35 In experimental models only indinavir and ritonavir have been consistently implicated in endothelial dysfunction. As is the case with glucose and lipid metabolism effects,17 different PIs appear to have divergent effects on endothelial function. As such, any potential effects of antiretroviral medications on endothelial function must be individually assessed rather than attributed as a class effect.
In conclusion, we did not find that measures of fat distribution, measures of insulin resistance, adipokines, or circulating markers of inflammation and endothelial activation were associated with physiologically measured endothelial function in HIV-infected patients. Additional study is needed to understand the underlying mechanisms for HIV-related endothelial dysfunction.
Acknowledgments
This work was supported by Grants HL72711, HL73682, and M01-RR00750 from the NIH. We are indebted to the subjects who volunteered for this study, to the staff of the Indiana University General Clinical Research Center, and to Kathy L. Clayton and Gina-Bob Dubé for managing the references. Results were presented in part at the 9th International Workshop on Lipodystrophy and Adverse Drug Reactions in HIV, Sydney, Australia, July 2007.
Author Disclosure Statement
M.P.D. has served as a consultant or received research support from Abbott, Bristol-Myers Squibb, GlaxoSmithKline, and Merck. S.K.G. has served as a consultant or received research support from Tibotec Therapeutics and Gilead Sciences, Inc.
References
- 1.D. A. D. Study Group. Friis-Moller N. Reiss P, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356:1723–1735. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 2.Mary-Krause M. Cotte L. Simon A. Partisani M. Costagliola D. Increased risk of myocardial infarction with duration of protease inhibitor therapy in HIV-infected men. AIDS. 2003;17:2479–2486. doi: 10.1097/00002030-200311210-00010. [DOI] [PubMed] [Google Scholar]
- 3.D. A. D. Study Group. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients enrolled in the D:A:D study: A multi-cohort collaboration. Lancet. 2008;371:1417–1426. doi: 10.1016/S0140-6736(08)60423-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strategies for Management of Anti-Retroviral Therapy, Insight and D. A. D. Study Groups. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients. AIDS. 2008;22:F17–F24. doi: 10.1097/QAD.0b013e32830fe35e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Celermajer DS. Endothelial dysfunction: Does it matter? Is it reversible? J Am Coll Cardiol. 1997;30:325–333. doi: 10.1016/s0735-1097(97)00189-7. [DOI] [PubMed] [Google Scholar]
- 6.Kinlay S. Ganz P. Role of endothelial dysfunction in coronary artery disease and implications for therapy. Am J Cardiol. 1997;80:11I–16I. doi: 10.1016/s0002-9149(97)00793-5. [DOI] [PubMed] [Google Scholar]
- 7.Anderson TJ. Prognostic significance of brachial flow-mediated vasodilation. Circulation. 2007;115:2373–2375. doi: 10.1161/CIRCULATIONAHA.107.697045. [DOI] [PubMed] [Google Scholar]
- 8.Stein JH. Klein MA. Bellehumeur JL, et al. Use of human immunodeficiency virus-1 protease inhibitors is associated with atherogenic lipoprotein changes and endothelial dysfunction. Circulation. 2001;104:257–262. doi: 10.1161/01.cir.104.3.257. [DOI] [PubMed] [Google Scholar]
- 9.Torriani FJ. Komarow L. Parker RA, et al. Endothelial function in human immunodeficiency virus-infected antiretroviral-naive subjects before and after starting potent antiretroviral therapy: The ACTG (AIDS Clinical Trials Group) Study 5152s. J Am College Cardiol. 2008;52:569–576. doi: 10.1016/j.jacc.2008.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dubé MP. Gorski JC. Shen C. Severe impairment of endothelial function with the HIV-1 protease inhibitor indinavir is not mediated by insulin resistance in healthy subjects. Cardiovasc Toxicol. 2008;8:15–22. doi: 10.1007/s12012-007-9010-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shankar SS. Considine RV. Gorski JC. Steinberg HO. Insulin sensitivity is preserved despite disrupted endothelial function. Am J Physiol Endocrinol Metab. 2006;291:E691–E696. doi: 10.1152/ajpendo.00006.2006. [DOI] [PubMed] [Google Scholar]
- 12.Shankar SS. Dubé MP. Gorski JC. Klaunig JE. Steinberg HO. Indinavir impairs endothelial function in healthy HIV-negative men. Am Heart J. 2005;150:933. doi: 10.1016/j.ahj.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Dubé MP. Shen C. Greenwald M. Mather KJ. No impairment of endothelial function or insulin sensitivity with 4 weeks of the HIV protease inhibitors atazanavir or lopinavir-ritonavir in healthy subjects without HIV infection: A placebo-controlled trial. Clin Infect Dis. 2008;47:567–574. doi: 10.1086/590154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Solages A. Vita JA. Thornton DJ, et al. Endothelial function in HIV-infected persons. Clin Infect Dis. 2006;42:1325–1332. doi: 10.1086/503261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mynarcik DC. McNurlan MA. Steigbigel RT. Fuhrer J. Gelato MC. Association of severe insulin resistance with both loss of limb fat and elevated serum tumor necrosis factor receptor levels in HIV lipodystrophy. J Acquir Immune Defic Syndr. 2000;25:312–321. doi: 10.1097/00042560-200012010-00004. [DOI] [PubMed] [Google Scholar]
- 16.Hadigan C. Meigs JB. Corcoran C, et al. Metabolic abnormalities and cardiovascular disease risk factors in adults with human immunodeficiency virus infection and lipodystrophy. Clin Infect Dis. 2001;32:130–139. doi: 10.1086/317541. [DOI] [PubMed] [Google Scholar]
- 17.Grinspoon S. Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med. 2005;352:48–62. doi: 10.1056/NEJMra041811. [DOI] [PubMed] [Google Scholar]
- 18.Vigouroux C. Maachi M. Nguyen TH, et al. Serum adipocytokines are related to lipodystrophy and metabolic disorders in HIV-infected men under antiretroviral therapy. AIDS. 2003;17:1503–1511. doi: 10.1097/00002030-200307040-00011. [DOI] [PubMed] [Google Scholar]
- 19.Mynarcik DC. Combs T. McNurlan MA. Scherer PE. Komaroff E. Gelato MC. Adiponectin and leptin levels in HIV-infected subjects with insulin resistance and body fat redistribution. J Acquir Immune Defic Syndr. 2002;31:514–520. doi: 10.1097/00126334-200212150-00009. [DOI] [PubMed] [Google Scholar]
- 20.Tong Q. Sankal AJ. Hadigan CM, et al. Regulation of adiponectin in human immunodeficiency virus-infected patients: Relationship to body composition and metabolic indices. J Clin Endocrinol Metab. 2003;88:1559–1564. doi: 10.1210/jc.2002-021600. [DOI] [PubMed] [Google Scholar]
- 21.Addy CL. Gavrila A. Tsiodras S. Brodovicz K. Karchmer AW. Mantzoros CS. Hypoadiponectinemia is associated with insulin resistance, hypertriglyceridemia, and fat redistribution in human immunodeficiency virus-infected patients treated with highly active antiretroviral therapy. J Clin Endocrinol Metab. 2003;88:627–636. doi: 10.1210/jc.2002-020795. [DOI] [PubMed] [Google Scholar]
- 22.Sutinen J. Korsheninnikova E. Funahashi T. Matsuzawa Y. Nyman T. Yki-Jarvinen H. Circulating concentration of adiponectin and its expression in subcutaneous adipose tissue in patients with highly active antiretroviral therapy-associated lipodystrophy. J Clin Endocrinol Metab. 2003;88:1907–1910. doi: 10.1210/jc.2002-021922. [DOI] [PubMed] [Google Scholar]
- 23.Kosmiski L. Kuritzkes D. Lichtenstein K. Eckel R. Adipocyte-derived hormone levels in HIV lipodystrophy. Antiviral Ther. 2003;8:9–15. [PubMed] [Google Scholar]
- 24.Torriani M. Hadigan C. Jensen ME. Grinspoon S. Psoas muscle attenuation measurement with computed tomography indicates intramuscular fat accumulation in patients with the HIV-lipodystrophy syndrome. J Appl Physiol. 2003;95:1005–1010. doi: 10.1152/japplphysiol.00366.2003. [DOI] [PubMed] [Google Scholar]
- 25.Corretti MC. Anderson TJ. Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. . [Erratum appears in J Am Coll Cardiol 2002;39(6):1082.] [DOI] [PubMed] [Google Scholar]
- 26.Friedewald WT. Levy RI. Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 27.Ross AC. Armentrout R. O'Riordan MA, et al. Endothelial activation markers are linked to HIV status and are independent of antiretroviral therapy and lipoatrophy. J Acquir Immune Defic Syndr. 2008;49(5):499–506. doi: 10.1097/QAI.0b013e318189a794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta SK. Johnson RM. Saha C, et al. Improvement in HIV-related endothelial dysfunction using the anti-inflammatory agent salsalate: A pilot study. AIDS. 2008;22:653–655. doi: 10.1097/QAD.0b013e3282f470d2. [DOI] [PubMed] [Google Scholar]
- 29.Jiang B. Hebert VY. Zavecz JH. Dugas TR. Antiretrovirals induce direct endothelial dysfunction in vivo. J Acquir Immune Defic Syndr. 2006;42:391–395. doi: 10.1097/01.qai.0000228790.40235.0c. [DOI] [PubMed] [Google Scholar]
- 30.Fu W. Chai H. Yao Q. Chen C. Effects of HIV protease inhibitor ritonavir on vasomotor function and endothelial nitric oxide synthase expression. J Acquir Immune Defic Syndr. 2005;39:152–158. [PubMed] [Google Scholar]
- 31.Conklin BS. Fu W. Lin PH. Lumsden AB. Yao Q. Chen C. HIV protease inhibitor ritonavir decreases endothelium-dependent vasorelaxation and increases superoxide in porcine arteries. Cardiovasc Res. 2004;63:168–175. doi: 10.1016/j.cardiores.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 32.Mondal D. Pradhan L. Ali M. Agrawal KC. HAART drugs induce oxidative stress in human endothelial cells and increase endothelial recruitment of mononuclear cells: Exacerbation by inflammatory cytokines and amelioration by antioxidants. Cardiovasc Toxicol. 2004;4:287–302. doi: 10.1385/ct:4:3:287. [DOI] [PubMed] [Google Scholar]
- 33.Chen C. Lu XH. Yan S. Chai H. Yao Q. HIV protease inhibitor ritonavir increases endothelial monolayer permeability. Biochem Biophys Res Commun. 2005;335:874–882. doi: 10.1016/j.bbrc.2005.07.155. [DOI] [PubMed] [Google Scholar]
- 34.Gupta SK. Mather KJ. Agarwal R. Saha CK. Considine RV. Dubé MP. Proteinuria and endothelial dysfunction in stable HIV-infected patients. A pilot study. J Acquir Immune Defic Syndr. 2007;45:596–598. doi: 10.1097/QAI.0b013e318061d2fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grubb JR. Dejam A. Voell J, et al. Lopinavir-ritonavir: Effects on endothelial cell function in healthy subjects. J Infect Dis. 2006;193:1516–1519. doi: 10.1086/503807. [DOI] [PubMed] [Google Scholar]