Abstract
Predictors of study retention and scheduled visit attendance in the University of North Carolina Center for AIDS Research (UNC CFAR) prospective clinical cohort of HIV-infected patients enrolled between 1 January 2001 and 1 January 2008 are reported. At study entry, 1636 participants were 32% female, 58% were African-American, 49% had not received HIV care elsewhere, 71% were receiving or initiated combination antiretroviral therapy, and 26% were diagnosed with AIDS, with median (quartiles) age of 40 (34; 47) years, distance to clinic of 45 (21; 70) miles, HIV-1 RNA of 1396 (200; 26,750) copies/ml, and CD4 of 374 (182; 602) cells/mm3. Participants contributed a median of 7 (4; 13) scheduled visits and 2.25 (1.0; 3.9) years alive under follow-up. During 6134 person-years of follow-up, 414 participants dropped out and 145 died. Accounting for differences in death by participant characteristics, the 6-year cumulative probability of retention was 67% [95% confidence limits (CL): 65, 70%], with 6.75 (95% CL: 6.13, 7.43) drop outs per 100 person-years. In a multivariable Cox proportional hazards model, retention was higher among participants who were insured, had not received HIV care elsewhere, had controlled HIV viremia, and were living in nonurban areas or proximate to the clinic. In a multivariable modified Poisson regression model that accounted for differences in drop out and death by participant characteristics, visit attendance was higher among older, AIDS-diagnosed, immune compromised, and cART-initiated participants. The UNC CFAR clinical cohort has ample enrollment with retention and visit attendance modestly influenced by factors such as disease severity.
Introduction
The Center for AIDS Research (CFAR) at the University of North Carolina at Chapel Hill (UNC) initiated a prospective clinic cohort study of HIV-infected patients in 2000. Clinic-based cohorts of HIV-infected patients receiving ongoing medical care in a clinic setting1,2 provide important information about patients receiving HIV care and have contributed substantially to understanding HIV and its clinical management.3
Information available from clinic-based cohorts may not be easily obtained in traditional population-based cohorts. For example, clinic-based cohorts enroll and follow patients as they receive HIV care, thereby reflecting actual care provided. In addition, such cohorts have access to detailed and precise knowledge of received treatments and clinical outcomes.3 However, clinic-based cohorts rely on data obtained through the provision of medical care, and hence may be susceptible to selection bias and confounding based on clinic visit frequency and retention in care. Understanding HIV care access and retention in clinic-based cohorts enables development of strategies for improving HIV care provision and clinical outcomes.4 Identifying patient characteristics associated with retention and attendance also informs the conduct of HIV research by establishing characteristics that should be considered when correcting for confounding and selection bias analytically.5 Here, we describe the UNC CFAR HIV clinical cohort (henceforth, the cohort) and report on predictors of retention and clinic visit attendance.
Materials and Methods
UNC CFAR HIV clinical cohort
Participants
All HIV-infected patients seen at the UNC HIV clinic who are at least 18 years of age are eligible to enroll in the cohort if they are able and choose to provide written informed consent. Of 2221 HIV-infected patients seen for a scheduled visit at the UNC HIV clinic between 1 January 2001 and 1 January 2008, 1889 (85%) were asked to participate in the cohort and 1791 enrolled. The UNC institutional review board approved all study forms and each enrolled participant provided written informed consent in English or Spanish.
Data collection
The cohort receives electronic data from available institutional databases on a daily basis. These data include all clinically obtained demographic characteristics, laboratory values, pathology results, and clinic visit information. The National Death Index and State of North Carolina death certificate data are searched semiannually for mortality. Cause of death is adjudicated based on available information from the medical record, death certificate, and autopsy reports, if available.
Laboratory measurements and clinical care are provided in accordance with HIV treatment guidelines6 and as clinically indicated. At a typical visit, laboratory values at minimum include a complete blood count with differential, CD4 cell count and percent, and HIV-1 RNA viral load. Basic chemistry as well as renal and liver function tests are obtained several times per year. Lipid levels, syphilis serology, and TB skin testing are performed yearly in most participants. Viral load was most commonly measured using standard or ultrasensitive quantitative reverse transcriptase polymerase chain reaction (RT-PCR) (lower limits of detection of 400 and 50 copies/ml, respectively; Amplicor HIV Monitor Test; Roche Diagnostic Systems, Inc., Branchburg, NJ) and more recently real-time PCR assay (TaqMan Assay Roche Diagnostic Systems, Inc., Branchburg, NJ) with a lower limit of detection of 48 copies/ml.
At cohort enrollment and prospective 6-month intervals comprehensive and standardized medical record reviews are completed by trained personnel. These data include medications including all antiretroviral and prophylactic agents, illnesses including all AIDS-defining clinical conditions, hospitalizations, and provision of primary HIV care including vaccinations and routine health maintenance.
All cohort participants are eligible to complete a sociodemographic, clinical, and behavioral in-person interview. The data include information on demographics (e.g., education, income, employment, housing), clinical history (e.g., HIV testing, access to care, antiretroviral therapy adherence, comorbidities including mental health and substance abuse), and social and behavioral factors (e.g., social support, quality of life, sexual behaviors). Additional data from external resources are also incorporated into the cohort including nucleotide sequence data from clinically obtained resistance testing, data from clinical trial participation, and available HIV testing data from the State of North Carolina.
Finally, a sample repository of plasma and whole blood cell pellets is maintained. To date, the repository contains over 25,000 plasma samples and 3000 dry pellet cell specimens. Plasma samples are collected and stored on each participant at every visit in which blood is drawn for viral load determination and cell pellets are collected and stored on each participant yearly.
Analysis population
Inclusion criteria
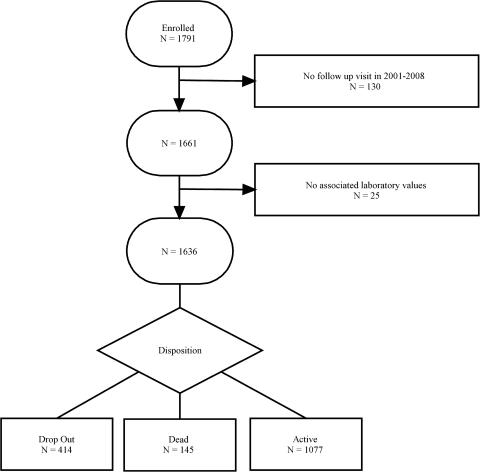
Enrolled participants were considered to be an active participant in the cohort analysis population on being seen at a scheduled follow-up clinic visit subsequent to baseline with laboratory measurements available. Of the 1791 initially enrolled participants 130 did not have a visit subsequent to baseline and 25 did not have laboratory measurements available for any time point during study follow-up. For 98% of the 1636 cohort analysis population participants, the first scheduled follow-up visit with laboratory measurements available was the scheduled visit subsequent to baseline. Participants were no longer considered active when they died or dropped out.
Covariates
Participant characteristics examined in this analysis include age, distance to the clinic, living in an urban area, CD4 cell count, HIV RNA level, AIDS status, therapy use, gender, male homosexual, insurance status (e.g., public, private, and none/other), intravenous drug use, and whether the first HIV care was at UNC. These characteristics were selected based on clinical knowledge or because they have previously been shown to predict cohort participation in the clinic setting.4,7–9 To harmonize all viral load data, values below 400 copies/ml were set to 200 copies/ml. Antiretroviral therapy (ART) use was defined as any use of an antiretroviral agent for HIV infection, and combination ART (cART) as the concurrent use of three or more antiretroviral agents [including triple nucleoside(tide) reverse transcriptase inhibitor use]. Public insurance was defined as receiving Medicare or Medicaid.
Outcomes
There were two primary endpoints of interest in this analysis. The first endpoint was time from study entry to drop out with administrative censoring by study completion on 1 January 2008 as well as censoring at date of death. Participants were considered to have dropped out when they had not been seen for 18 months. Participants who resumed care after an 18 or more month hiatus were not reentered into this analysis.
The second endpoint was attendance at clinic visits. To evaluate clinic attendance we included only attended scheduled clinic visits and excluded walk-in or emergency care visits. Participants who died or dropped out during follow-up were censored at their date of death or drop out.
Statistical methods
Percentages for discrete characteristics, or medians and quartiles for continuous characteristics, are provided for cohort participants at study entry as well as averaging over the person-time under follow-up. Study retention was illustrated for the cohort using a Kaplan–Meier survival curve.10 Given that the occurrence of death may not be independent of whether a participant is retained in the study (e.g., sicker participants more likely to die as well as be retained9) censoring participants at death may induce selection bias (i.e., informative censoring) and result in an underestimation of retention overall and by participant characteristics. Therefore, the retention survival curve was weighted by the inverse probability-of-censoring due to death to correct for any induced selection bias associated with measured participant characteristics.11–13 In the resulting weighted population, selection bias associated with measured characteristics is removed.
To examine possible predictors of drop out in the cohort we used Cox proportional hazards models.14 Cox models were fit using the previously described inverse probability-of-censoring weights. To determine whether predictors of retention varied by therapy use or after the first year of cohort enrollment, we subsequently stratified the Cox model by time varying initiation of cART and calendar time. The model for the probability of death used to construct weights included the following continuous predictors with four-knot restricted cubic splines with knots placed at the 5, 35, 65, and 95 percentiles: time on study, age, distance to the clinic, and CD4 cell count. Indicators of an AIDS diagnosis, cART initiation, viral load 400–10,000 copies/ml, viral load >10,000 copies/ml, gender, private insurance, public insurance, intravenous drug use, living in an urban area, male homosexual, race, and first HIV care at UNC were also included in this model. The resultant inverse probability weights had a mean (SD) of 1.00 (0.15) with a range from 0.86 to 4.44. Proportional hazards were assessed by statistical tests of the product terms between covariates and time as well as log time.
Scheduled visit attendance was illustrated for the cohort using a plot of the monthly probability of attending a scheduled visit by study follow-up. To examine possible predictors of visit attendance in the cohort we estimated the risk ratio of study attendance using a modified Poisson regression15 where the outcome was an indicator of whether a participant attended a scheduled clinic visit in a given month. We also estimated this risk ratio stratifying the Poisson model by cART use. Given that those most likely to die (or drop out) may be more (or less) likely to attend scheduled clinic visits,9 censoring participants at death or drop out may be informative and result in biased estimates of monthly visit probabilities as well as risk ratios. Therefore, in estimating the probability of and risk ratios for scheduled visit attendance, we accounted for informative losses due to censoring participants at drop out or death by use of inverse probability-of-censoring weights.11,13 The models for the probability of death and drop out used to construct the weights included the same covariates and specifications used to model death in the retention analysis. The resultant inverse probability weights had a mean (SD) of 1.00 (0.18) with a range from 0.68 to 3.98. All analyses were conducted using SAS (SAS Institute, Cary, NC). Statistical significance was based on a two-sided type 1 error of 0.05.
Results
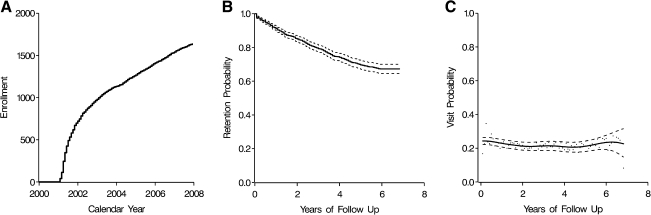
In the cohort of 1636 HIV-infected enrolled participants with at least two attended scheduled visits between 2001 and 2007, 49% (n = 802) first received HIV care at UNC. The total amount of time under HIV care prior to a participant's baseline visit was a median (quartiles) of 3.8 (0.6; 7.2) years, including a median of 1.5 (0.2; 4.9) years under care at UNC prior to baseline. In Fig. 1 we present a chart depicting the flow of patients into the study and the disposition of participants as of 1 January 2008. In Fig. 2A we provide an illustration of the cohort enrollment over calendar years 2001 through 2007. Enrollment was most rapid in 2001 as the prevalent pool of patients was accessed. During 2002 and beyond, approximately 157 ± 58 patients were enrolled per year.
FIG. 1.
Flow chart of HIV-infected men and women, UNC CFAR HIV clinical cohort, 2001–2007.
FIG. 2.
Enrollment by calendar year (A), probability of study retention by years of study follow-up (B), and probability of a scheduled clinic visit in a given month by years of study follow-up, among participants remaining in HIV care (C), among the UNC CFAR HIV clinical cohort, 2001–2007.
Characteristics of the participants are provided in Table 1. At study entry, the cohort was 32% female, 28% nonurban, 58% African-American (AA), 71% cART initiated before or at study entry, 26% diagnosed with AIDS, with a median (quartiles) age of 40 (34; 47) years, distance to clinic of 45 (21; 70) miles, viral load of 1396 (200; 26,750), and CD4 count of 374 (182; 602) cells/mm3. The 155 participants excluded from the analysis population did not differ from the included participants on most measured characteristics shown in Table 1. However, participants excluded at entry did tend to live further from the clinic, start their HIV care elsewhere, have a lower proportion of homosexual men, and be individuals reporting non-private insurance as compared to included participants (data not shown).
Table 1.
Characteristics of HIV-Infected Men and Women at Study Entry and over Follow-up, UNC CFAR HIV Clinical Cohort, 2001–2007
| Characteristica | Entry N = 1,636 people | Follow-up N = 73,609 person-months |
|---|---|---|
| Age, years | 40 (34; 47) | 43 (37; 49) |
| Female sex, % (n) | 32 (521) | 32 (23,864) |
| African-American race, % (n) | 58 (956) | 59 (43,159) |
| Intravenous drug use, % (n) | 14 (229) | 14 (10,252) |
| Male homosexual, % (n)b | 56 (628) | 56 (27,761) |
| Urban area, % (n)c | 72 (1,179) | 72 (53,060) |
| Distance to clinic, miles | 45 (21; 70) | 44 (22; 69) |
| Insurance, % (n) | ||
| Private | 28 (460) | 32 (23,555) |
| Publicd | 31 (514) | 34 (24,720) |
| None/other | 41 (662) | 34 (25,334) |
| First HIV care at UNC, % (n) | 49 (802) | 51 (37,332) |
| CD4 count, cells/mm3 | 374 (182; 602) | 439 (259; 656) |
| CD4 count, % (n) | ||
| <200 | 27 (446) | 18 (13,534) |
| 200–350 | 20 (330) | 19 (14,027) |
| >350 | 53 (860) | 63 (46,048) |
| Viral load, copies/ml | 1,396 (200; 26,750) | 200 (200; 6,637) |
| Viral load, % (n) | ||
| <400 | 40 (653) | 59 (43,239) |
| 400–10,000 | 25 (413) | 19 (13,797) |
| >10,000 | 35 (570) | 22 (16,573) |
| AIDS, % (n) | 26 (428) | 32 (23,286) |
| ART use, % (n) | 80 (1,307) | 94 (68,972) |
| cART use, % (n) | 71 (1,157) | 87 (64,072) |
Median (quartiles), unless noted otherwise.
Among 1,115 men and 49,745 male person-months.
Urban area defined as >500,000 people in the metropolitan statistical area, compared to less populated areas.
Medicaid or Medicare.
Participants contributed a median of 7 (4; 13) scheduled visits and 2.25 (1.0; 3.9) years alive under follow-up between 1 January 2001 and 1 January 2008. During 6134 person-years of follow-up, 414 participants dropped out of the study {6.75 drop outs per 100 person-years [95% confidence limits (CL): 6.13, 7.43]} and 145 died. Of these 6134 person-years 87% were contributed while a participant was cART initiated and 32% occurred while a participant was AIDS diagnosed. The median (quartiles) age, distance to clinic, viral load, and CD4 count during follow-up were 43 (37; 49) years, 44 (22; 69) miles, 200 (200; 6,637) copies/ml, and 439 (259; 656) cells/mm3. Of the 1636 participants 1416 (87%) initiated cART before or during follow-up.
Figure 2B illustrates study retention over nearly 7 years of follow-up. After accounting for differences in study losses due to death by measured participant characteristics, the 6-year retention was 67% (95% CL: 65, 70%). As shown in Fig. 2B, 80% of the cohort was retained for 2.8 years. Table 2 provides hazard ratios for study retention based on the observed data as well as when ratios were adjusted and inverse probability-of-censoring due to death weighted. Weighted multivariable-adjusted hazard ratios for study retention indicated higher retention among participants who were privately or publicly insured, received their first HIV care at UNC, had controlled HIV-1 viremia, and were living in nonurban areas or proximate to the clinic. A similar pattern was observed when the cohort was restricted to cART users or calendar time after 2002 (data not shown). Initiation of cART (p value = 0.0215), CD4 cell count >350 cells/mm3 (p value = 0.0044), and viral load >10,000 copies/ml (p value = 0.0244) showed evidence of nonproportionality when assessed by log time.
Table 2.
Characteristics Associated with Study Retention among 1,636 Participants over 73,609 Person-Months, UNC CFAR HIV Clinical Cohort, 2001–2007a
| Characteristic | Crude hazard ratio for study retention | 95% CLb | Adjusted hazard ratiocfor study retention | 95% CLb | Adjusted, weighted hazard ratioc,dfor study retention | 95% CLc |
|---|---|---|---|---|---|---|
| Age, per 10 years | 1.14 | 1.03, 1.27 | 1.12 | 1.00, 1.25 | 1.12 | 1.00, 1.26 |
| Female sex | 1.02 | 0.83, 1.26 | 1.15 | 0.89, 1.48 | 1.15 | 0.88, 1.48 |
| African-American race | 0.99 | 0.82, 1.21 | 1.04 | 0.84, 1.28 | 1.06 | 0.86, 1.31 |
| Intravenous drug use | 1.00 | 0.76, 1.32 | 1.04 | 0.77, 1.41 | 1.01 | 0.75, 1.37 |
| Male homosexuale | 1.05 | 0.86, 1.28 | 1.21 | 0.94, 1.55 | 1.23 | 0.96, 1.58 |
| Urban areaf | 0.83 | 0.67, 1.04 | 0.70 | 0.55, 0.88 | 0.70 | 0.55, 0.89 |
| Distance to clinic, per 50 miles | 0.79 | 0.70, 0.89 | 0.74 | 0.66, 0.84 | 0.73 | 0.65, 0.83 |
| Insurance | ||||||
| Private vs. none/other | 1.52 | 1.20, 1.94 | 1.46 | 1.14, 1.86 | 1.47 | 1.15, 1.89 |
| Publicg vs. none/other | 1.26 | 1.01, 1.59 | 1.31 | 1.03, 1.66 | 1.31 | 1.04, 1.67 |
| First HIV care at UNC | 1.41 | 1.16, 1.72 | 1.49 | 1.22, 1.83 | 1.49 | 1.21, 1.82 |
| CD4 count, cells/mm3 | ||||||
| >350 vs. <200 | 0.98 | 0.75, 1.27 | 0.86 | 0.64, 1.15 | 0.86 | 0.64, 1.16 |
| 200–350 vs. <200 | 0.80 | 0.59 1.09 | 0.74 | 0.54, 1.02 | 0.74 | 0.54, 1.03 |
| Viral load, copies per ml | ||||||
| >10,000 vs. <400 | 0.51 | 0.41, 0.64 | 0.52 | 0.40, 0.66 | 0.52 | 0.40, 0.67 |
| 400–10,000 vs. <400 | 0.56 | 0.43, 0.71 | 0.58 | 0.45 0.75 | 0.58 | 0.45, 0.75 |
| AIDS | 1.41 | 1.13, 1.76 | 1.30 | 1.03, 1.64 | 1.27 | 1.00, 1.61 |
| cART use | 1.39 | 1.08, 1.80 | 1.20 | 0.91, 1.58 | 1.21 | 0.92, 1.59 |
Hazard ratios and confidence limits for study drop out were inverted.
CL, confidence limits.
Adjusted for variables present in the table.
Weighted by the inverse probability-of-censoring due to death.
Compared to male heterosexuals.
Urban area defined as >500,000 people in the metropolitan statistical area, compared to less populated areas.
Medicaid or Medicare.
Figure 2C illustrates the probability of attending a scheduled clinic visit over nearly 7 years of study follow-up. After accounting for differences in study losses due to drop out or death by measured participant characteristics, the probability of attending a scheduled visit in any given month was 0.24 (95% CL: 0.24, 0.25) or 2.90 (95% CL: 2.83, 2.97) visits per year. Table 3 provides risk ratios for scheduled visit attendance based on the observed data as well as when ratios were adjusted and inverse probability-of-censoring due to drop out or death weighted. Weighted multivariable-adjusted risk ratios for scheduled visit attendance indicated attendance was higher among participants who were older, AIDS diagnosed, immune compromised, and cART initiated. A similar pattern was observed when the cohort was restricted to cART users (data not shown).
Table 3.
Characteristics Associated with Scheduled Visit Attendance among 1,636 Participants Remaining in HIV Care over 73,609 Person-Months, UNC CFAR HIV Clinical Cohort, 2001–2007a
| Characteristic | Crude risk ratio for visit attendance | 95% CLb | Adjusted risk ratiocfor visit attendance | 95% CLb | Adjusted, weighted risk ratioc,dfor visit attendance | 95% CLb |
|---|---|---|---|---|---|---|
| Age, per 10 years | 1.07 | 1.05, 1.09 | 1.06 | 1.04, 1.09 | 1.06 | 1.04, 1.09 |
| Female sex | 0.96 | 0.92, 1.00 | 1.02 | 0.97, 1.08 | 1.01 | 0.96, 1.07 |
| African-American race | 0.95 | 0.91, 0.99 | 0.95 | 0.91, 0.99 | 0.96 | 0.92, 1.00 |
| Intravenous drug use | 1.05 | 0.99, 1.12 | 1.03 | 0.97, 1.10 | 1.03 | 0.96, 1.11 |
| Male homosexuale | 1.04 | 1.00, 1.08 | 1.05 | 1.00, 1.10 | 1.05 | 1.00, 1.11 |
| Urban areaf | 1.03 | 0.98, 1.08 | 1.02 | 0.97, 1.07 | 1.02 | 0.97, 1.07 |
| Distance to clinic, per 50 miles Insurance | 0.97 | 0.94, 1.00 | 0.97 | 0.94, 1.00 | 0.97 | 0.94, 1.01 |
| Private vs. none/other | 1.07 | 1.02, 1.12 | 1.04 | 0.99, 1.09 | 1.04 | 0.99, 1.09 |
| Publicg vs. none/other | 1.06 | 1.00, 1.11 | 1.05 | 1.00, 1.10 | 1.05 | 1.00, 1.11 |
| First HIV care at UNC | 1.00 | 0.96, 1.05 | 1.03 | 0.99, 1.07 | 1.03 | 0.98, 1.07 |
| CD4 count, cells/mm3 | ||||||
| >350 vs. <200 | 0.90 | 0.86, 0.95 | 0.95 | 0.90 0.99 | 0.93 | 0.88, 0.98 |
| 200–350 vs. <200 | 0.97 | 0.92, 1.03 | 0.99 | 0.93, 1.04 | 0.97 | 0.92, 1.04 |
| Viral load, copies per ml | ||||||
| >10,000 vs. <400 | 0.99 | 0.94, 1.04 | 1.01 | 0.96, 1.06 | 0.99 | 0.94, 1.04 |
| 400–10,000 vs. <400 | 0.97 | 0.92, 1.01 | 1.00 | 0.96, 1.05 | 0.99 | 0.95, 1.04 |
| AIDS | 1.17 | 1.12, 1.22 | 1.12 | 1.07, 1.17 | 1.13 | 1.08, 1.18 |
| cART use | 1.22 | 1.15, 1.28 | 1.16 | 1.10, 1.22 | 1.14 | 1.08, 1.20 |
Risk ratios and confidence limits conditional on remaining alive and under follow-up.
CL, confidence limits.
Adjusted for variables present in the table.
Weighted by the inverse probability-of-censoring of drop out and death.
Compared to male heterosexuals.
Urban area defined as >500,000 people in the metropolitan statistical area, compared to less populated areas.
Medicaid or Medicare.
Discussion
During nearly 7 years of follow-up, the UNC CFAR HIV clinical cohort has demonstrated ample ability to enroll and retain participants with only approximately 6.75% of the participants dropping out per year. This retention estimate is on par with prospective cohort studies in challenging populations (e.g., about 10% annual drop out among intravenous drug users in the AIDS Link to Intravenous Experience study16) as well as other clinic-based cohorts (e.g., 10% annual drop out among participants in the Centers for AIDS Research Network of Integrated Clinical Systems17).
Compared to other clinic-based cohorts,17 the UNC clinic cohort was older at enrollment with a greater proportion of females and African-Americans. Among participants retained in the cohort, the majority of the person-time was contributed while participants were on cART. Scheduled visit attendance did not appear to be strongly related to any of the examined participant characteristics. Associations were modest for those factors that reached statistical significance (e.g., hazard ratios of 0.93 to 1.14). Therefore, participants who attend scheduled visits do not appear to be a highly selected subset of clinic patients.
However, factors that were associated with retention in the cohort may help predict participant behavior as well as inform retention strategies and the conduct of HIV research. The fact that private or public insurance and success on antiretroviral therapy were associated with retention is not surprising. The association of nonurban residence, proximal distance, and receiving first HIV care at UNC with retention merits further evaluation and may suggest that the converse groups be targeted by retention efforts. Specific reasons for loss to follow-up are unknown and demand further attention. Over one-half of patients at UNC enter a clinical trial for ART at some point during their care and may therefore not attend UNC HIV clinic visits during this interval though laboratory and treatment data are collected for participants in research studies. Of the 414 participants who were lost to follow-up, 76 (18%) entered a clinical trial at some point during their care. Therefore, the number of participants entering a clinical trial coincident with drop out is likely low. A proportion of patients is imprisoned after initiating HIV care at UNC, and although UNC clinicians provide all HIV care in the North Carolina prison system these patients would be considered lost to follow-up in these analyses. Moreover it is possible that patients transfer their HIV care to another provider. Although possible this is less likely in central-eastern North Carolina where the number of HIV care providers and clinics is relatively limited in comparison to urban centers. Finally, the majority (over 80%) of cohort participants are seen within 12 months of death, suggesting that many participants who leave HIV care for 18 or more months eventually return to reinitiate care.
Higher visit attendance among older and immune compromised participants is consistent with observations in the UAB 1917 clinic cohort. Mugavero and co-authors9 showed that older participants were less likely to miss one or more clinic visits during their first year in care. Participants with a CD4 cell count of 200–350 cells/mm3 were similarly less likely to miss visits as compared to participants with a CD4 cell count of ≥350 cells/mm3. African-Americans as well as participants with public rather than private insurance were also more likely to miss visits. Although possibly due to chance, in the UNC clinic cohort African-Americans were less likely to attend clinic visits, whereas those with private or public insurance were more likely to attend visits.
There are limitations to the present work. First, the reported hazard and risk ratios are vulnerable to confounding by unmeasured factors. Second, the reported hazard and risk ratios are vulnerable to selection bias due to informative censoring by unmeasured factors. Third, we do not account for the fact that variables as assessed are typically imperfect measures of the true underlying characteristic (e.g., reported versus actual injection drug use). Fourth, there was evidence for violation of the proportional hazards assumption on the log time scale in the retention analysis. Therefore, reported hazard ratios for the effect of cART initiation, CD4 cell count, and viral load on retention represent time-weighted averages of the changing association between these characteristics and retention.
Despite these limitations, included cohort participants did not appear to substantially differ from those who were excluded. Use of inverse probability weights to recover the missed visits for individuals who died or dropped out during follow-up reduces the potential impact of these missed visits on study results. Specifically, observed visits are reweighted, conditional on observed characteristics, to reflect the number of attended visits that would have occurred in the absence of deaths or drop outs while respecting the amount of information contained in the observed data.13 Informally, a participant at a high risk of death or drop out is up-weighted to account for their peers who died or dropped out. In this study, there was little difference between the weighted and unweighted results. This lack of a difference suggests that the observed data were not highly selected. Alternatively, the observed data may be highly selected based on unmeasured characteristics.
The present results demonstrate that a relatively large and well-defined clinical cohort in the epicenter of the U.S. epidemic, the southeast, has adequate retention with limited selection bias apparent in the observed population over nearly 7 years of follow-up. In conclusion, the UNC CFAR HIV clinical cohort is a viable national resource for the conduct of HIV-related biomedical research.
Acknowledgments
This research was supported by National Institutes of Health Grant P30 AI50410. The authors thank all participants, clinicians, investigators, and staff involved with the UNC CFAR HIV clinical cohort.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Moore RD. Understanding the clinical and economic outcomes of HIV therapy: The Johns Hopkins HIV clinical practice cohort. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17(Suppl 1):S38–S41. doi: 10.1097/00042560-199801001-00011. [DOI] [PubMed] [Google Scholar]
- 2.Chen RY. Accortt NA. Westfall AO, et al. Distribution of health care expenditures for HIV-infected patients. Clin Infect Dis. 2006;42(7):1003–1010. doi: 10.1086/500453. [DOI] [PubMed] [Google Scholar]
- 3.Lau B. Gange SJ. Moore RD. Interval and clinical cohort studies: Epidemiological issues. AIDS Res Hum Retroviruses. 2007;23(6):769–776. doi: 10.1089/aid.2006.0171. [DOI] [PubMed] [Google Scholar]
- 4.Mugavero MJ. Lin HY. Allison JJ, et al. Racial disparities in HIV virologic failure: Do missed visits matter? J Acquir Immune Defic Syndr. 2009;50(1):100–108. doi: 10.1097/QAI.0b013e31818d5c37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hernan MA. McAdams M. McGrath N. Lanoy E. Costagliola D. Observation plans in longitudinal studies with time-varying treatments. Stat Methods Med Res. 2009;18(1):27–52. doi: 10.1177/0962280208092345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panel on Antiretroviral Guidelines for Adults and Adolescents: Guidelines for the use of antiretroviral agents in HIV-1-infected adults, adolescents. Department of Health and Human Services. Nov 3, 2008. http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. [Oct 19;2009 ]. pp. 1–139.http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf
- 7.Catz SL. McClure JB. Jones GN. Brantley PJ. Predictors of outpatient medical appointment attendance among persons with HIV. AIDS Care. 1999;11(3):361–373. doi: 10.1080/09540129947983. [DOI] [PubMed] [Google Scholar]
- 8.Mugavero MJ. Lin HY. Allison JJ, et al. Failure to establish HIV care: Characterizing the “no show” phenomenon. Clin Infect Dis. 2007;45(1):127–130. doi: 10.1086/518587. [DOI] [PubMed] [Google Scholar]
- 9.Mugavero MJ. Lin HY. Willig JH, et al. Missed visits and mortality among patients establishing initial outpatient HIV treatment. Clin Infect Dis. 2009;48(2):248–256. doi: 10.1086/595705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaplan EL. Meier P. Nonparametric-estimation from incomplete observations. J Am Statist Assoc. 1958;53(282):457–481. [Google Scholar]
- 11.Cain LE. Cole SR. Inverse probability-of-censoring weights for the correction of time-varying noncompliance in the effect of randomized highly active antiretroviral therapy on incident AIDS or death. Statist Med. 2009;28(12):1725–1738. doi: 10.1002/sim.3585. [DOI] [PubMed] [Google Scholar]
- 12.Cole SR. Hernan MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed. 2004;75(1):45–49. doi: 10.1016/j.cmpb.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Robins JM. Finkelstein DM. Correcting for noncompliance and dependent censoring in an AIDS clinical trial with inverse probability of censoring weighted (IPCW) log-rank tests. Biometrics. 2000;56(3):779–788. doi: 10.1111/j.0006-341x.2000.00779.x. [DOI] [PubMed] [Google Scholar]
- 14.Cox DR. Regression models and life-tables. J R Statist Soc Ser B-Statist Methodol. 1972;34(2):187. [Google Scholar]
- 15.Zou GY. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 16.Nelson KE. Galai N. Safaeian M. Strathdee SA. Celentano DD. Vlahov D. Temporal trends in the incidence of human immunodeficiency virus infection and risk behavior among injection drug users in Baltimore, Maryland, 1988–1998. Am J Epidemiol. 2002;156(7):641–653. doi: 10.1093/aje/kwf086. [DOI] [PubMed] [Google Scholar]
- 17.Kitahata MM. Rodriguez B. Haubrich R, et al. Cohort profile: The Centers for AIDS Research Network of Integrated Clinical Systems. Int J Epidemiol. 2008;37(5):948–955. doi: 10.1093/ije/dym231. [DOI] [PMC free article] [PubMed] [Google Scholar]