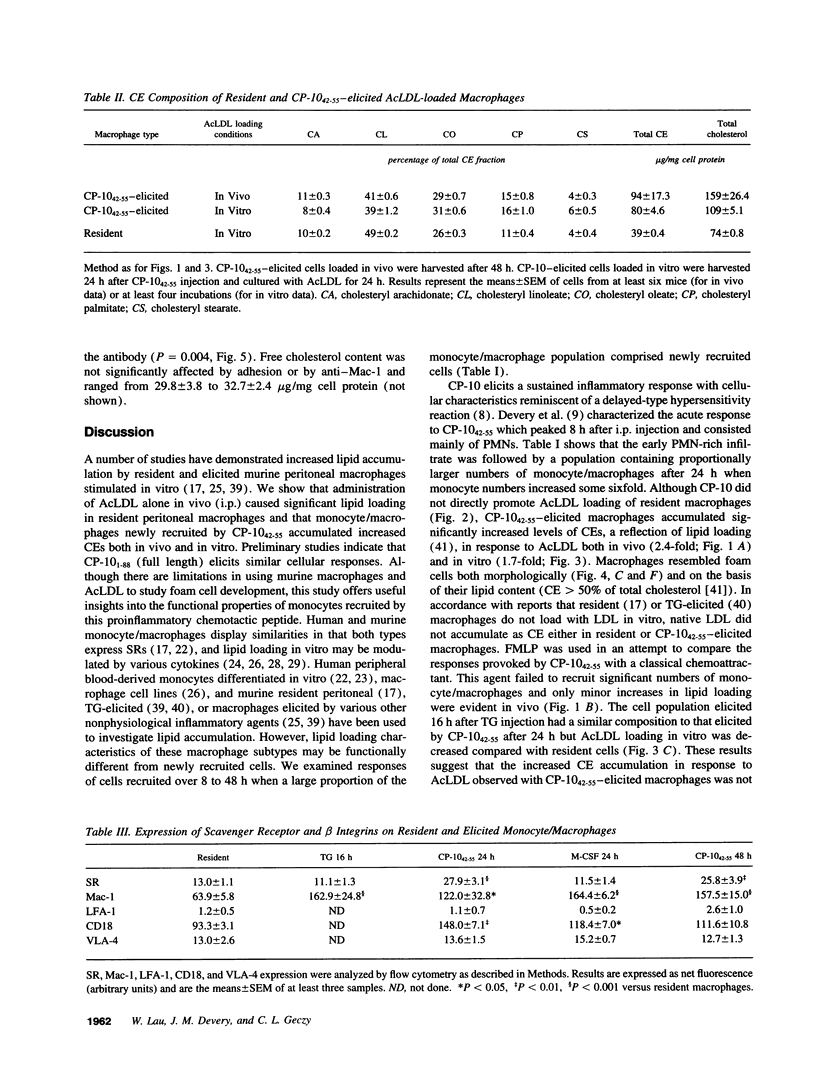
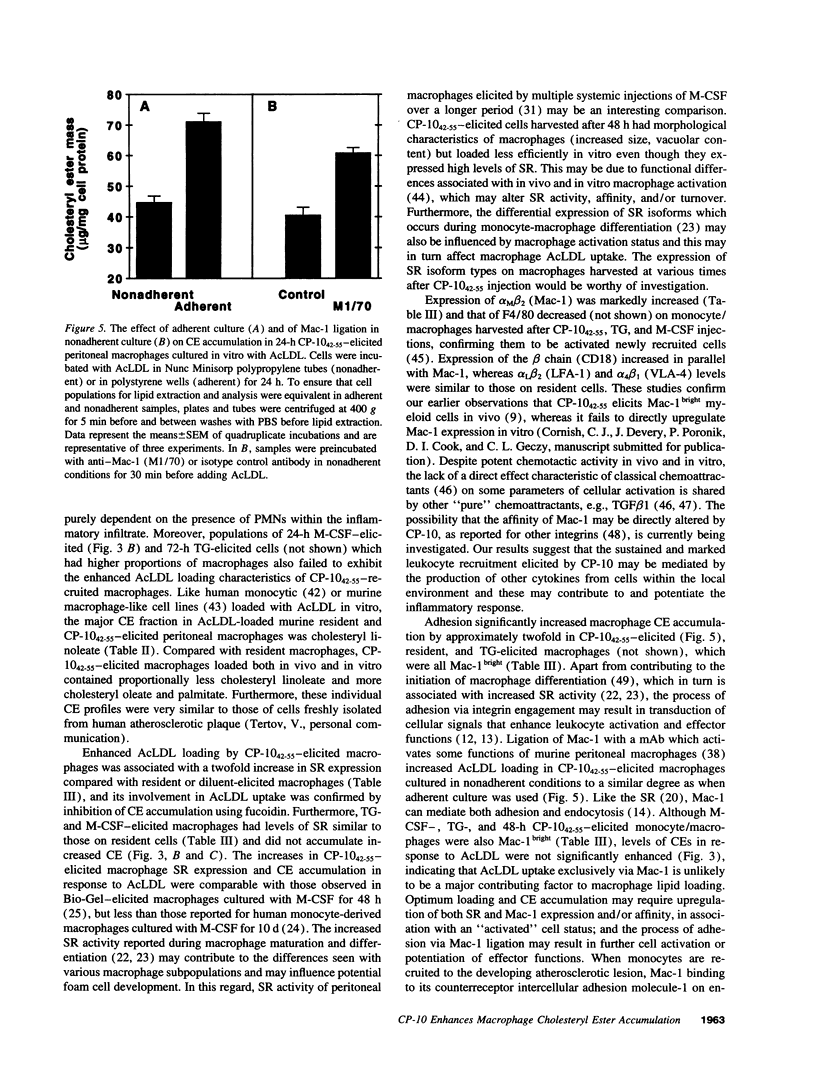
Abstract
In the early development of atherosclerotic plaque, monocytes are recruited to the arterial intima where they accumulate lipid and become foam cells. The recently described murine chemotactic S100 protein, CP-10, may have an important role in this process. Intraperitoneal injection of CP-10(42-55) (chemotactic hinge region peptide) into mice caused a sustained leukocyte recruitment with a sixfold increase in monocyte numbers over 24 h. CP-10(42-55)--elicited monocyte/macrophages accumulated significantly increased cholesteryl esters in response to acetylated LDL, both in vivo and in vitro and this was associated with a twofold increase in scavenger receptor expression. By contrast, thioglycollate- and macrophage colony-stimulating factor-elicited macrophages expressed levels of scavenger receptor similar to those on resident macrophages and did not exhibit enhanced acetylated LDL loading in vitro. The leukocyte integrin Mac-1 (CD11b/CD18) and its beta subunit (CD18), but neither lymphocyte function-associated antigen-1 nor very late activation antigen-4, were upregulated on monocyte/macrophages elicited by CP-10(42-55), thioglycollate, and macrophage colony-stimulating factor. Cholesteryl ester accumulation in vitro was significantly enhanced by adhesion, which appeared to involve macrophage activation via ligation of Mac-1. The initial events of monocyte recruitment and adhesion to the vessel wall may be important in macrophage foam cell development, and CP-10 or related S100 proteins may contribute to the early inflammatory events of atherogenesis by stimulating these events.
Full text
PDF

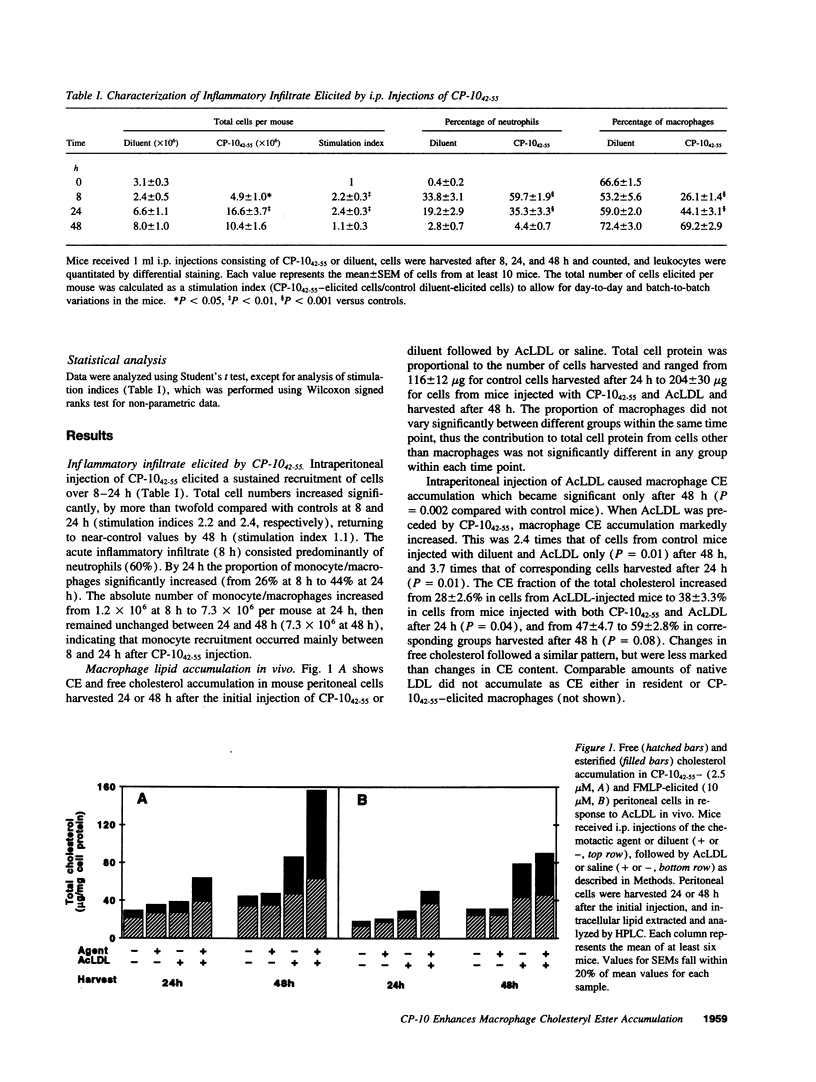
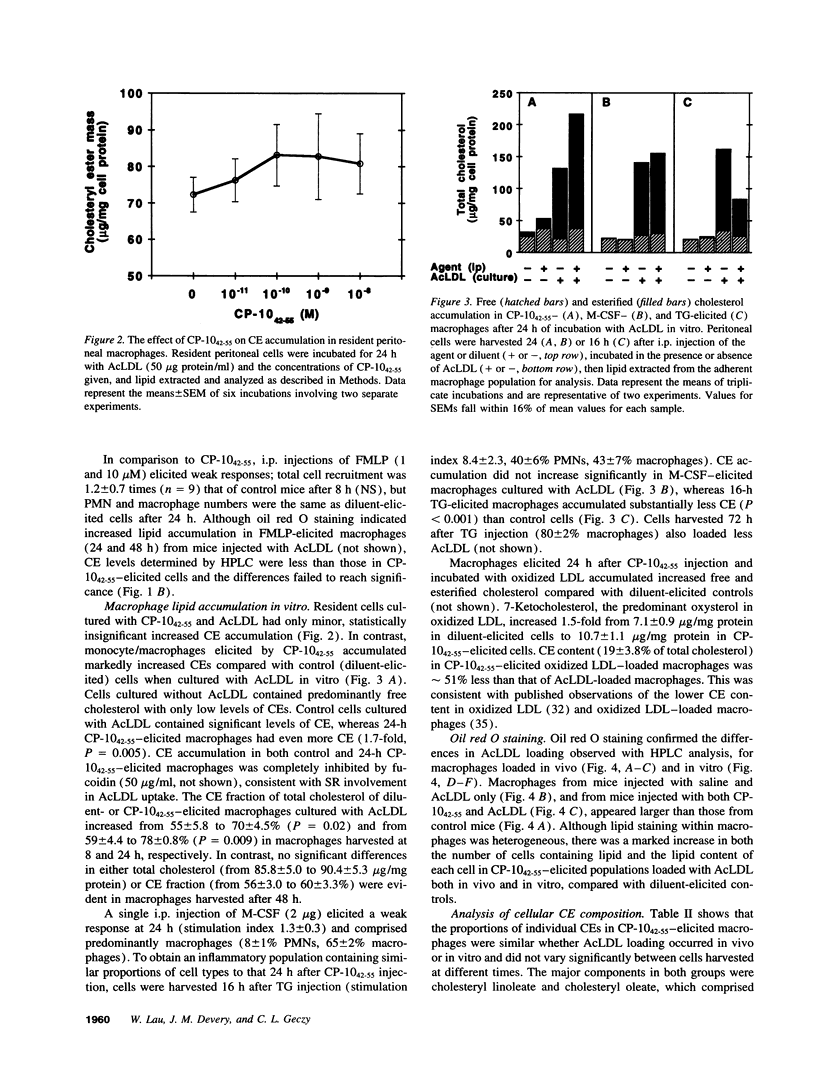
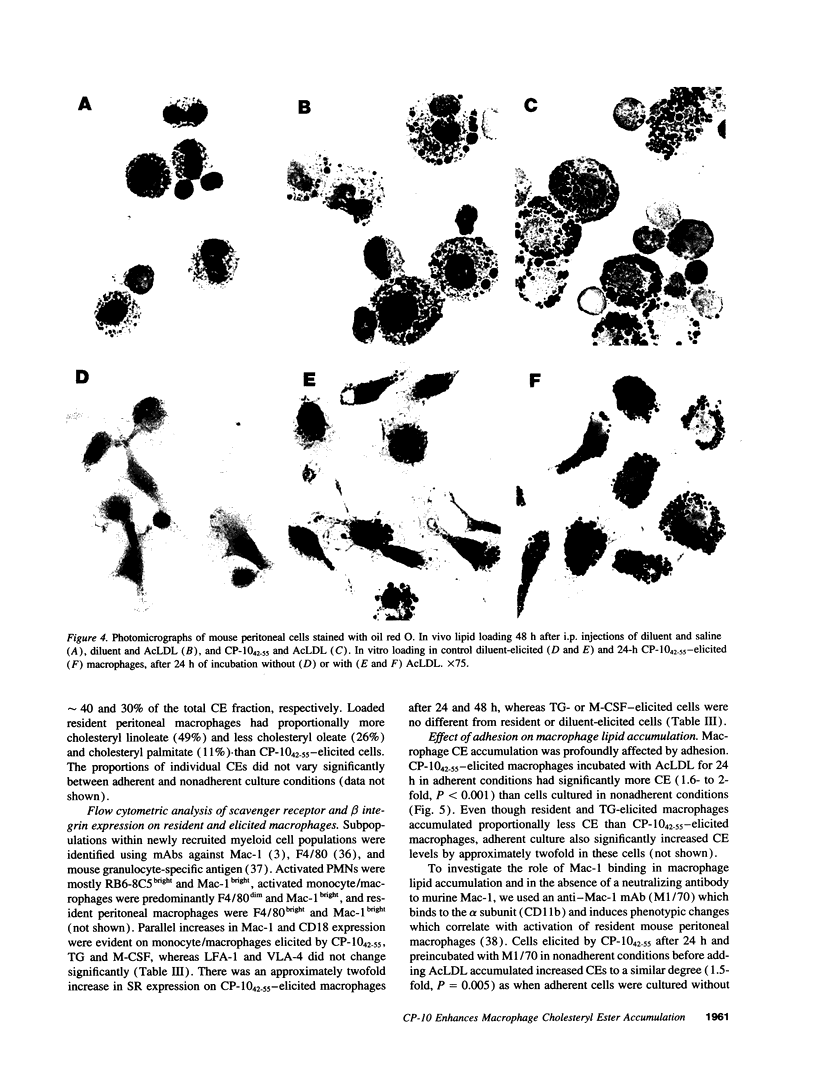


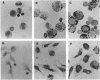
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Hamilton T. A. The cell biology of macrophage activation. Annu Rev Immunol. 1984;2:283–318. doi: 10.1146/annurev.iy.02.040184.001435. [DOI] [PubMed] [Google Scholar]
- Allen J. B., Manthey C. L., Hand A. R., Ohura K., Ellingsworth L., Wahl S. M. Rapid onset synovial inflammation and hyperplasia induced by transforming growth factor beta. J Exp Med. 1990 Jan 1;171(1):231–247. doi: 10.1084/jem.171.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbustini E., Grasso M., Diegoli M., Pucci A., Bramerio M., Ardissino D., Angoli L., de Servi S., Bramucci E., Mussini A. Coronary atherosclerotic plaques with and without thrombus in ischemic heart syndromes: a morphologic, immunohistochemical, and biochemical study. Am J Cardiol. 1991 Sep 3;68(7):36B–50B. doi: 10.1016/0002-9149(91)90383-v. [DOI] [PubMed] [Google Scholar]
- Basu S. K., Goldstein J. L., Anderson G. W., Brown M. S. Degradation of cationized low density lipoprotein and regulation of cholesterol metabolism in homozygous familial hypercholesterolemia fibroblasts. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3178–3182. doi: 10.1073/pnas.73.9.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beekhuizen H., van Furth R. Monocyte adherence to human vascular endothelium. J Leukoc Biol. 1993 Oct;54(4):363–378. [PubMed] [Google Scholar]
- Bottalico L. A., Wager R. E., Agellon L. B., Assoian R. K., Tabas I. Transforming growth factor-beta 1 inhibits scavenger receptor activity in THP-1 human macrophages. J Biol Chem. 1991 Dec 5;266(34):22866–22871. [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L., Krieger M., Ho Y. K., Anderson R. G. Reversible accumulation of cholesteryl esters in macrophages incubated with acetylated lipoproteins. J Cell Biol. 1979 Sep;82(3):597–613. doi: 10.1083/jcb.82.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annu Rev Biochem. 1983;52:223–261. doi: 10.1146/annurev.bi.52.070183.001255. [DOI] [PubMed] [Google Scholar]
- Butcher E. C. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991 Dec 20;67(6):1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- Carlos T. M., Harlan J. M. Leukocyte-endothelial adhesion molecules. Blood. 1994 Oct 1;84(7):2068–2101. [PubMed] [Google Scholar]
- Colditz I. G., Movat H. Z. Kinetics of neutrophil accumulation in acute inflammatory lesions induced by chemotaxins and chemotaxinigens. J Immunol. 1984 Oct;133(4):2169–2173. [PubMed] [Google Scholar]
- Colditz I., Zwahlen R., Dewald B., Baggiolini M. In vivo inflammatory activity of neutrophil-activating factor, a novel chemotactic peptide derived from human monocytes. Am J Pathol. 1989 Apr;134(4):755–760. [PMC free article] [PubMed] [Google Scholar]
- Davis G. E. The Mac-1 and p150,95 beta 2 integrins bind denatured proteins to mediate leukocyte cell-substrate adhesion. Exp Cell Res. 1992 Jun;200(2):242–252. doi: 10.1016/0014-4827(92)90170-d. [DOI] [PubMed] [Google Scholar]
- Devery J. M., King N. J., Geczy C. L. Acute inflammatory activity of the S100 protein CP-10. Activation of neutrophils in vivo and in vitro. J Immunol. 1994 Feb 15;152(4):1888–1897. [PubMed] [Google Scholar]
- Ding A., Wright S. D., Nathan C. Activation of mouse peritoneal macrophages by monoclonal antibodies to Mac-1 (complement receptor type 3). J Exp Med. 1987 Mar 1;165(3):733–749. doi: 10.1084/jem.165.3.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan S. T., Edgington T. S. Integrin regulation of leukocyte inflammatory functions. CD11b/CD18 enhancement of the tumor necrosis factor-alpha responses of monocytes. J Immunol. 1993 Apr 1;150(7):2972–2980. [PubMed] [Google Scholar]
- Fogelman A. M., Haberland M. E., Seager J., Hokom M., Edwards P. A. Factors regulating the activities of the low density lipoprotein receptor and the scavenger receptor on human monocyte-macrophages. J Lipid Res. 1981 Sep;22(7):1131–1141. [PubMed] [Google Scholar]
- Fong L. G., Fong T. A., Cooper A. D. Inhibition of mouse macrophage degradation of acetyl-low density lipoprotein by interferon-gamma. J Biol Chem. 1990 Jul 15;265(20):11751–11760. [PubMed] [Google Scholar]
- Fraser I., Hughes D., Gordon S. Divalent cation-independent macrophage adhesion inhibited by monoclonal antibody to murine scavenger receptor. Nature. 1993 Jul 22;364(6435):343–346. doi: 10.1038/364343a0. [DOI] [PubMed] [Google Scholar]
- Geng Y. J., Hansson G. K. Interferon-gamma inhibits scavenger receptor expression and foam cell formation in human monocyte-derived macrophages. J Clin Invest. 1992 Apr;89(4):1322–1330. doi: 10.1172/JCI115718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Y., Kodama T., Hansson G. K. Differential expression of scavenger receptor isoforms during monocyte-macrophage differentiation and foam cell formation. Arterioscler Thromb. 1994 May;14(5):798–806. doi: 10.1161/01.atv.14.5.798. [DOI] [PubMed] [Google Scholar]
- Gerrity R. G. The role of the monocyte in atherogenesis: I. Transition of blood-borne monocytes into foam cells in fatty lesions. Am J Pathol. 1981 May;103(2):181–190. [PMC free article] [PubMed] [Google Scholar]
- Goebeler M., Roth J., Teigelkamp S., Sorg C. The monoclonal antibody MAC387 detects an epitope on the calcium-binding protein MRP14. J Leukoc Biol. 1994 Feb;55(2):259–261. doi: 10.1002/jlb.55.2.259. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Ho Y. K., Basu S. K., Brown M. S. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci U S A. 1979 Jan;76(1):333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D., Reidy M. A., Benditt E. P., Schwartz S. M. Cell proliferation in human coronary arteries. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4600–4604. doi: 10.1073/pnas.87.12.4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines K. A., Kolasinski S. L., Cronstein B. N., Reibman J., Gold L. I., Weissmann G. Chemoattraction of neutrophils by substance P and transforming growth factor-beta 1 is inadequately explained by current models of lipid remodeling. J Immunol. 1993 Aug 1;151(3):1491–1499. [PubMed] [Google Scholar]
- Hampton R. Y., Golenbock D. T., Penman M., Krieger M., Raetz C. R. Recognition and plasma clearance of endotoxin by scavenger receptors. Nature. 1991 Jul 25;352(6333):342–344. doi: 10.1038/352342a0. [DOI] [PubMed] [Google Scholar]
- Hessian P. A., Edgeworth J., Hogg N. MRP-8 and MRP-14, two abundant Ca(2+)-binding proteins of neutrophils and monocytes. J Leukoc Biol. 1993 Feb;53(2):197–204. [PubMed] [Google Scholar]
- Hestdal K., Ruscetti F. W., Ihle J. N., Jacobsen S. E., Dubois C. M., Kopp W. C., Longo D. L., Keller J. R. Characterization and regulation of RB6-8C5 antigen expression on murine bone marrow cells. J Immunol. 1991 Jul 1;147(1):22–28. [PubMed] [Google Scholar]
- Hirsch S., Austyn J. M., Gordon S. Expression of the macrophage-specific antigen F4/80 during differentiation of mouse bone marrow cells in culture. J Exp Med. 1981 Sep 1;154(3):713–725. doi: 10.1084/jem.154.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg N., Allen C., Edgeworth J. Monoclonal antibody 5.5 reacts with p8,14, a myeloid molecule associated with some vascular endothelium. Eur J Immunol. 1989 Jun;19(6):1053–1061. doi: 10.1002/eji.1830190615. [DOI] [PubMed] [Google Scholar]
- Hume D. A., Pavli P., Donahue R. E., Fidler I. J. The effect of human recombinant macrophage colony-stimulating factor (CSF-1) on the murine mononuclear phagocyte system in vivo. J Immunol. 1988 Nov 15;141(10):3405–3409. [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Iismaa S. E., Hu S., Kocher M., Lackmann M., Harrison C. A., Thliveris S., Geczy C. L. Recombinant and cellular expression of the murine chemotactic protein, CP-10. DNA Cell Biol. 1994 Feb;13(2):183–192. doi: 10.1089/dna.1994.13.183. [DOI] [PubMed] [Google Scholar]
- Ishibashi S., Inaba T., Shimano H., Harada K., Inoue I., Mokuno H., Mori N., Gotoda T., Takaku F., Yamada N. Monocyte colony-stimulating factor enhances uptake and degradation of acetylated low density lipoproteins and cholesterol esterification in human monocyte-derived macrophages. J Biol Chem. 1990 Aug 25;265(24):14109–14117. [PubMed] [Google Scholar]
- Khoo J. C., Miller E., Pio F., Steinberg D., Witztum J. L. Monoclonal antibodies against LDL further enhance macrophage uptake of LDL aggregates. Arterioscler Thromb. 1992 Nov;12(11):1258–1266. doi: 10.1161/01.atv.12.11.1258. [DOI] [PubMed] [Google Scholar]
- Kraemer F. B., Tavangar K., Gandjei R. K., Kirlew K., Behr S. R. Effects of activation on lipid and lipoprotein metabolism in murine macrophages. Arteriosclerosis. 1990 Jan-Feb;10(1):8–16. doi: 10.1161/01.atv.10.1.8. [DOI] [PubMed] [Google Scholar]
- Kritharides L., Jessup W., Gifford J., Dean R. T. A method for defining the stages of low-density lipoprotein oxidation by the separation of cholesterol- and cholesteryl ester-oxidation products using HPLC. Anal Biochem. 1993 Aug 15;213(1):79–89. doi: 10.1006/abio.1993.1389. [DOI] [PubMed] [Google Scholar]
- Lackmann M., Cornish C. J., Simpson R. J., Moritz R. L., Geczy C. L. Purification and structural analysis of a murine chemotactic cytokine (CP-10) with sequence homology to S100 proteins. J Biol Chem. 1992 Apr 15;267(11):7499–7504. [PubMed] [Google Scholar]
- Lackmann M., Rajasekariah P., Iismaa S. E., Jones G., Cornish C. J., Hu S., Simpson R. J., Moritz R. L., Geczy C. L. Identification of a chemotactic domain of the pro-inflammatory S100 protein CP-10. J Immunol. 1993 Apr 1;150(7):2981–2991. [PubMed] [Google Scholar]
- Lagasse E., Weissman I. L. Mouse MRP8 and MRP14, two intracellular calcium-binding proteins associated with the development of the myeloid lineage. Blood. 1992 Apr 15;79(8):1907–1915. [PubMed] [Google Scholar]
- Miller L. J., Bainton D. F., Borregaard N., Springer T. A. Stimulated mobilization of monocyte Mac-1 and p150,95 adhesion proteins from an intracellular vesicular compartment to the cell surface. J Clin Invest. 1987 Aug;80(2):535–544. doi: 10.1172/JCI113102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibbering P. H., Van de Gevel J. S., Van Furth R. A cell-ELISA for the quantification of adherent murine macrophages and the surface expression of antigens. J Immunol Methods. 1990 Jul 20;131(1):25–32. doi: 10.1016/0022-1759(90)90228-n. [DOI] [PubMed] [Google Scholar]
- O'Brien K. D., Allen M. D., McDonald T. O., Chait A., Harlan J. M., Fishbein D., McCarty J., Ferguson M., Hudkins K., Benjamin C. D. Vascular cell adhesion molecule-1 is expressed in human coronary atherosclerotic plaques. Implications for the mode of progression of advanced coronary atherosclerosis. J Clin Invest. 1993 Aug;92(2):945–951. doi: 10.1172/JCI116670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn M. T., Parthasarathy S., Fong L. G., Steinberg D. Oxidatively modified low density lipoproteins: a potential role in recruitment and retention of monocyte/macrophages during atherogenesis. Proc Natl Acad Sci U S A. 1987 May;84(9):2995–2998. doi: 10.1073/pnas.84.9.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld M. E., Ylä-Herttuala S., Lipton B. A., Ord V. A., Witztum J. L., Steinberg D. Macrophage colony-stimulating factor mRNA and protein in atherosclerotic lesions of rabbits and humans. Am J Pathol. 1992 Feb;140(2):291–300. [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Sakata N., Kitani A., Hara M., Hirose T., Hirose W., Norioka K., Harigai M., Kawagoe M., Nakamura H. Characterization of human monocytic cell line, U937, in taking up acetylated low-density lipoprotein and cholesteryl ester accumulation. A flow cytometric and HPLC study. Biochim Biophys Acta. 1990 Feb 6;1042(2):210–216. doi: 10.1016/0005-2760(90)90010-u. [DOI] [PubMed] [Google Scholar]
- Vercaemst R., Union A., Rosseneu M. Separation and quantitation of free cholesterol and cholesteryl esters in a macrophage cell line by high-performance liquid chromatography. J Chromatogr. 1989 Sep 29;494:43–52. doi: 10.1016/s0378-4347(00)82655-9. [DOI] [PubMed] [Google Scholar]
- Wang J. M., Griffin J. D., Rambaldi A., Chen Z. G., Mantovani A. Induction of monocyte migration by recombinant macrophage colony-stimulating factor. J Immunol. 1988 Jul 15;141(2):575–579. [PubMed] [Google Scholar]
- Ylä-Herttuala S., Rosenfeld M. E., Parthasarathy S., Sigal E., Särkioja T., Witztum J. L., Steinberg D. Gene expression in macrophage-rich human atherosclerotic lesions. 15-lipoxygenase and acetyl low density lipoprotein receptor messenger RNA colocalize with oxidation specific lipid-protein adducts. J Clin Invest. 1991 Apr;87(4):1146–1152. doi: 10.1172/JCI115111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H. F., Basra H. J., Steinbrecher U. P. Effects of oxidatively modified LDL on cholesterol esterification in cultured macrophages. J Lipid Res. 1990 Aug;31(8):1361–1369. [PubMed] [Google Scholar]
- de Villiers W. J., Fraser I. P., Hughes D. A., Doyle A. G., Gordon S. Macrophage-colony-stimulating factor selectively enhances macrophage scavenger receptor expression and function. J Exp Med. 1994 Aug 1;180(2):705–709. doi: 10.1084/jem.180.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lenten B. J., Fogelman A. M. Lipopolysaccharide-induced inhibition of scavenger receptor expression in human monocyte-macrophages is mediated through tumor necrosis factor-alpha. J Immunol. 1992 Jan 1;148(1):112–116. [PubMed] [Google Scholar]
- van der Wal A. C., Das P. K., Tigges A. J., Becker A. E. Adhesion molecules on the endothelium and mononuclear cells in human atherosclerotic lesions. Am J Pathol. 1992 Dec;141(6):1427–1433. [PMC free article] [PubMed] [Google Scholar]