Abstract
In addition to regulating mast cell homeostasis, the activation of KIT following ligation by stem cell factor (SCF) promotes a diversity of mast cell responses including cytokine production and chemotaxis. Although we have previously defined a role for the mTORC1 (mammalian target of rapamycin complex 1) in these responses, it is clear that other signals are also required for maximal KIT-dependent cytokine production and chemotaxis. Here we provide evidence to support a role for glycogen synthase kinase 3β (GSK3β) in such regulation in human mast cells (HuMCs). GSK3β was observed to be constitutively activated in HuMCs. This activity was inhibited by knockdown of GSK3β protein following transduction of these cells with GSK3β-targeted shRNA. This resulted in a marked attenuation in the ability of KIT to promote chemotaxis and, in synergy with FcεRI-mediated signaling, cytokine production. GSK3β regulated KIT-dependent mast cell responses independently of mTOR. However, evidence from the knockdown studies suggested that GSK3β was required for activation of the MAP kinases, p38 and JNK, and downstream phosphorylation of the transcription factors, Jun and ATF2, in addition to activation of the transcription factor NF-κB. These studies provide evidence for a novel pre-requisite priming mechanism for KIT-dependent responses regulated by GSK3β in HuMCs.
Keywords: Mast Cells, GSK3β, SCF, KIT, FcεRI
Introduction
Mast cells are tissue-resident cells of hematopoietic origin that play a role in innate and acquired immune responses (1). Mast cell growth, development, and survival, are driven by stem cell factor (SCF)-dependent activation of its receptor KIT (2). In addition to its role in mast cell homeostasis, however, SCF can also regulate other mast cell functions. In this respect, SCF-mediated activation of KIT potently induces mast cell chemotaxis (3, 4) and adhesion to extracellular matrix (5), supporting a role for SCF in mast cell homing to their tissues of residence in vivo. Furthermore, SCF-mediated KIT activation, particularly in conjunction with aggregation of the high affinity IgE receptor (FcεRI), also promotes the generation of multiple cytokines and chemokines (5–7).
KIT is member of the growth factor receptors with inherent tyrosine kinase activity family (8, 9). Dimerization of KIT, following SCF binding, activates its inherent tyrosine kinase activity resulting in phosphorylation of specific tyrosine residues in the cytoplasmic tail of KIT allowing recruitment of critical adaptor and signaling molecules (10). These receptor-proximal events lead to the initiation of multiple downstream signaling process, eventually culminating in transcriptional regulation (8, 9, 11). Despite a comprehensive understanding of these immediate signaling events elicited by activated KIT, It is unclear how these events subsequently differentially control the diverse group of responses mediated by KIT. In exploring this differential regulation, we recently described, however, that the mTORC1 (mammalian target of rapamycin complex 1) cascade, which is activated downstream of PI3K following challenge of either mouse or human mast cells with SCF (12), contributes to the regulation of SCF-mediated chemotaxis, and SCF/antigen-mediated cytokine production (12). Nevertheless, as a substantial portion of these responses remained following rapamycin-induced inhibition of mTORC1 signaling, it was concluded that other signaling pathways apart from those regulated by mTORC1, participate in the regulation of SCF-mediated chemotaxis and transcriptional activation leading to cytokine and chemokine generation (12).
Here we present evidence to support a role for glycogen synthase kinase 3β (GSK3β) in such regulation. GSK3β is a ubiquitously expressed serine/threonine kinase which has been reported to play a role in the regulation of diverse cellular responses including cell growth, tumorogenesis, cell migration, and cytokine generation (13–15). However, it is not fully understood how GSK3β regulates these responses. In studies employing knockdown of GSK3β expression, we now demonstrate that GSK3β activation is a pre-requisite signal for SCF-mediated chemotaxis and SCF/antigen mediated cytokine generation. Thus, this may constitute a novel priming mechanism for specific mast cell responses. The regulation of the chemotactic response by GSK3β appears dependent on its modulation of JNK and p38 dependent pathways, whereas, the regulation of cytokine generation by GSK3β, may be explained by the differential regulation of transcriptional control downstream of JNK and p38.
Materials and Methods
Mast cell culture
Primary human mast cells (HuMCs) were derived from CD34+ peripheral blood progenitor cells (16) obtained from normal volunteers following informed consent under a protocol approved by the NIH internal review board. The cells were developed in StemPro-34 culture medium containing StemPro-34 supplement (Invitrogen, Carlsbad, CA), L-glutamine (2mM), penicillin (100 U/ml), streptomycin (100 µg/ml), recombinant human (rHu) IL-3 (30 ng/ml, first week only,) rHuIL-6 (100 ng/ml), and rHuSCF (100 ng/ml) (PeproTech Inc, Rocky Hill, NY, USA). Experiments were conducted 7–9 weeks after the initiation of HuMC cultures.
Lentivirus shRNA transfection of 293T cells and transduction of HuMCs
The following GSK3β-targeted shRNA’s were purchased from Sigma (Sigma-Aldrich, Saint Louis, MO, USA):
CCGGGTGTGGATCAGTTGGTAGAAACTCGAGTTTCTACCAACTGATCCACACTTTTT (TRCN0000010552) (Construct A)
CCGGGACACTAAAGTGATTGGAAATCTCGAGATTTCCAATCACTTTAGTGTCTTTTTG (TRCN0000040002) (Construct B)
CCGGCCACTGATTATACCTCTAGTACTCGAGTACTAGAGGTATAATCAGTGGTTTTTG (TRCN0000039998) (Construct C)
CCGGCCCAAACTACACAGAATTTAACTCGAGTTAAATTCTGTGTAGTTTGGGTTTTTG (TRCN0000039999) (Construct D)
CCGGGCAGGACAAGAGATTTAAGAACTCGAGTTCTTAAATCTCTTGTCCTGCTTTTTG (TRCN000040000) (Construct E)
CCGGCAACAAGATGAAGAGCACCAACTCGAGTTGGTGCTCTTCATCTTGTTGTTTTT (SHC002) (Control non target control vector)
The packaging vector (Mission™Lentiviral packaging mix, (Sigma), the pLKO1 transfer vectors with GSK3β shRNA or control shRNA (3.4 µg) were co-transfected into 293T packaging cells (4 × 106 cells) with FuGENE6 transfection reagent (Roche, Indianapolis, IN, USA ) in Opti-MEM medium. The transfected 293T cells were grown in DMEM media containing fetal bovine serum (10 %), L-glutamine (4 mM), penicillin (100 U/ml) and streptomycin (100 µg/ml). Following 16–19 h transfection, media was removed and replaced with fresh DMEM media. After 62–65 h transfection, virus were collected by centrifugation (25,000 rpm, I h 40 min, 4 °C) then the resulting pellet resuspended in 3 ml pre-warmed complete StemPro medium. Transduction of HuMCs was conducted by transfering the 3 ml of the resuspended virus to a T75 culture flask containing 3–4 × 106 HuMCs in 15 ml complete StemPro medium. Two days after the infection, the medium was changed to virus-free complete StemPro medium, and antibiotic selection was initiated (0.2 µg/ml Puromycin (Sigma)). Experiments were conducted on day 7 post transduction. Cytospins of HuMCs transduced with shControl or shGSK3β were stained with toluidine blue as described (17). The cells treated with GSK3β-targeted shRNA are hereafter termed GSK3β knockdown-HuMCs.
Flow cytometric analysis for FcεRI and KIT surface expression
Cells were incubated in cytokine-free media for 4 h and washed twice in PBS containing 0.1% BSA. Cells were then stained with CD117-PE (BD Biosciences, San Jose, CA, USA) or PE-conjugated isotype control (BD Biosciences) and FcεRI-APC (eBioscience, San Diego, CA) or APC conjugated isotype control (eBioscience) for 1 h on ice. Cell fluorescence was analyzed (10,000 events) on a gated forward light scatter and side light scatter area previously determined as mast cell-specific using a FACSCaliber flow cytometer (BD Biosciences) and associated CellQuest software.
Cell activation
HuMCs were incubated overnight in complete Stem Pro medium containing human myeloma IgE (100 ng/ml, Calbiochem, EMD Biosciences, La Jolla, CA), biotinylated within the NIAID core facility. The cells were then starved in cytokine-free Stem Pro media for 4 h (for chemotaxis assay and corresponding cell lysate preparations), then the cells were activated by the addition of SCF (30 ng/ml). For cytokine release studies, HuMCs (1×106/ml) were sensitized in complete Stem Pro culture medium overnight and the next day washed in culture medium and triggered concurrently via KIT with SCF (30 ng/ml) and via the FcεRI with streptavidin (SA) (100 ng/ml) for 6 h. In some experiments, cells were pretreated with the mTOR inhibitor, Rapamycin (100 nM) or the PI3K inhibitor, wortmaninn (100 nM), (Calbiochem) for 20 min prior to activation.
Realtime PCR analysis
HuMCs (2–3 × 106/sample) were sensitized overnight with biotinylated human IgE (100 ng/ml) in complete Stem Pro medium. The following day, cells were washed with the same medium three times to remove excess IgE, then the cells were stimulated with SA (100 ng/ml) and SCF (30 ng/ml) for 4 h. Total RNA was isolated from each preparation using the RNeasy Mini KIT (Qiagen, Valencia, CA). One microgram of total cellular RNA was treated for genomic DNA contamination and reverse transcribed using SA Biosciences Reverse Transcription reagents and oligo dT (SA Biosciences, Fredrick, MD). Gene expression was analyzed using real-time PCR on an ABI7500 SDS system (Applied biosystems Inc, Foster City, CA). A common cytokine PCR array was purchased from SA Biosciences and realtime PCR was performed according to manufacture’s instructions. All reactions (two different HuMCs donors) were performed in triplicate for 40 cycles. The relative fold expression levels of cytokines was calculated as follows: for each sample the threshold cycle (Ct) was determined and normalized to the average of five different house keeping genes in the KIT (ΔCt). The ΔCt of treated or untreated cells was then subtracted from untreated control shRNA transduced cells (ΔΔCt) and the relative fold expression was calculated using the formula 2ΔΔCt.
Cytokine quantitation
Cell-free supernatants from activated cells were harvested and cytokine content was measured by using DuoSet enzyme-linked immunosorbent assay system (RnDSystems, Minneapolis, MN)
Chemotaxis
Chemotaxis assays were performed using Transwell polycarbonate membranes (8 µm pore size) (Corning, Corning, NY). HuMCs (1 ×105/well) were incubated in cytokine-free StemPro medium for 4 h and then resuspended in cytokine-free StemPro medium containing 0.5 % BSA. The cell suspension (100 µl) was placed in the upper chamber and pre-incubated in the bottom chamber containing 600 µl of cytokine-free StemPro medium for 30 min at 37 °C. After 30 min, the inserts were replaced into the bottom chambers with or without SCF (30 ng/ml). After 4 h, the migrated cells were collected in the bottom chamber and counted under microscopy.
Immunoblotting
Cell lysates were prepared as described (18). Aliquots of lysates were loaded onto a 4–12 % NuPage BisTris gel (Invitrogen) and following electrophoresis, proteins were transferred onto nitrocellulose membranes. The proteins were probed with following phospho (p)-specific antibodies from Cell Signaling Technology, (Beverley, MA, USA ); p-AKT (S473), p-GSK3β (S9), p-JNK (T183 and Y185), p-MKK3/6 (S189 and S207), p-p38 (T180), p-GS (S641), p-mTOR (S2448), p-p70 S6K (T389), p-4E-BP1 (T37 and T46), p-ATF2 (T71), p-NFκB (S536), p-NFkB (S276), total JNK, total p38 and total NF-κBp65. p-cJun (S73) were from Upstate Inc., (Lake Placid, NY), p-GSK3α/β (Y279/Y216) was from Invitrogen. Total Syk, total KIT, total Lyn and total cJun-antibodies were from Santa Cruz Biotechnology, (Santa Cruz, CA). Immunoreactive proteins were visualized by probing with HRP-conjugated secondary Abs and then by ECL (PerkinElmer). Quantitation of changes in protein phosphorylation, were performed using an Quantity One scanner (Bio-Rad, Hercules, CA).
Statistical analysis
Data are represented as the mean ± SE. The statistical analyses were performed by unpaired Student’s t test. Differences were considered significant when p < 0.05. The n values represent experiments from multiple preparations.
Results
Expression and knockdown of GSK3β in human mast cells
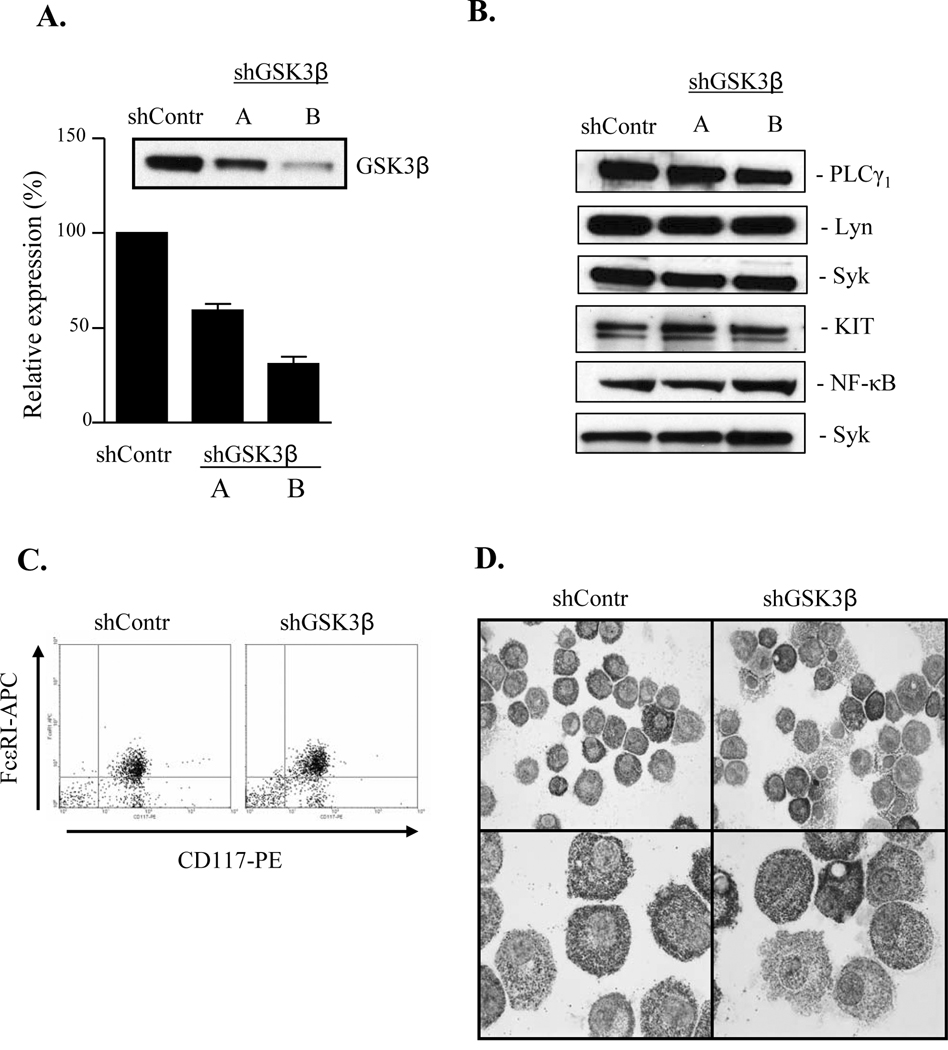
Disruption of the GSK-3β gene in mice leads to an embryonically lethal phenotype (19), therefore, to explore the role of GSK3β in human mast cell function, we elected to utilize a gene knockdown approach. To achieve this, HuMCs were stably transduced with GSK3β-targetted shRNA, using a lentivirus system. Five (A–E) different constructs were examined for their ability to selectively knockdown GSK3β expression. As a control, we used a scrambled shRNA construct purchased from Sigma. The level of expression of GSK3β in the cells treated with the control scrambled shRNA was not substantially different from that observed in untreated cells (data not shown). Of the shRNA’s targeting GSK3β, 4 decreased GSK3β levels to varying degrees within the cells following transduction. Of these, 2 (A and B) were selected for further characterization based on their differential abilities to reduce GSK3β expression in HuMCs (A: 41±3 %; B: 69±4%) (Fig.1A). These constructs had little effect on the expression of other molecules examined including PLCγ1, Lyn, Syk, KIT and NF−κB (Fig. 1B). In addition, no marked differences were seen in surface expression of KIT and FcεRI (Fig. 1C) in cells transduced with GSK3β-targetted shRNA (86.7±1.7 % double positive cells compared to the control cells (91.6±2.9 % double positive cells, n=3). There was also little change in the gross morphology of the cells treated with the GSK3β-targeted shRNA compared to control cells (Fig. 1D) or non-transduced cells (data not shown). In subsequent studies, responses observed in GSK3β knockdown HuMCs are compared to those observed in HuMCs incubated with scrambled control shRNA.
Figure 1. shRNA-mediated knockdown of GSK3β in quiescent HuMCs.
HuMCs transduced with scrambled shRNA (shContr) or shRNA for GSK3β (two different constructs; shGSK3β-A and shGSK3β-B). Whole-cell extracts were prepared and immunoblotted with anti-GSK3β, anti-PLCγ1, anti-Lyn, anti-Syk, anti-KIT, anti-NF-κB antibodies (panel A and B). FcεRI and KIT surface expression on HuMCs transduced with scrambled shRNA (shContr) or shRNA for GSK3β (shGSK3β) analyzed by flow cytometry (C). HuMCs transduced with scrambled shRNA (shContr) or shRNA for GSK3β (shGSK3β) stained with toluidine blue (D), (n=6 in panel A and n=2–3 in panel B–D).
Knockdown of GSK3β phosphorylation in quiescent and activated mast cells
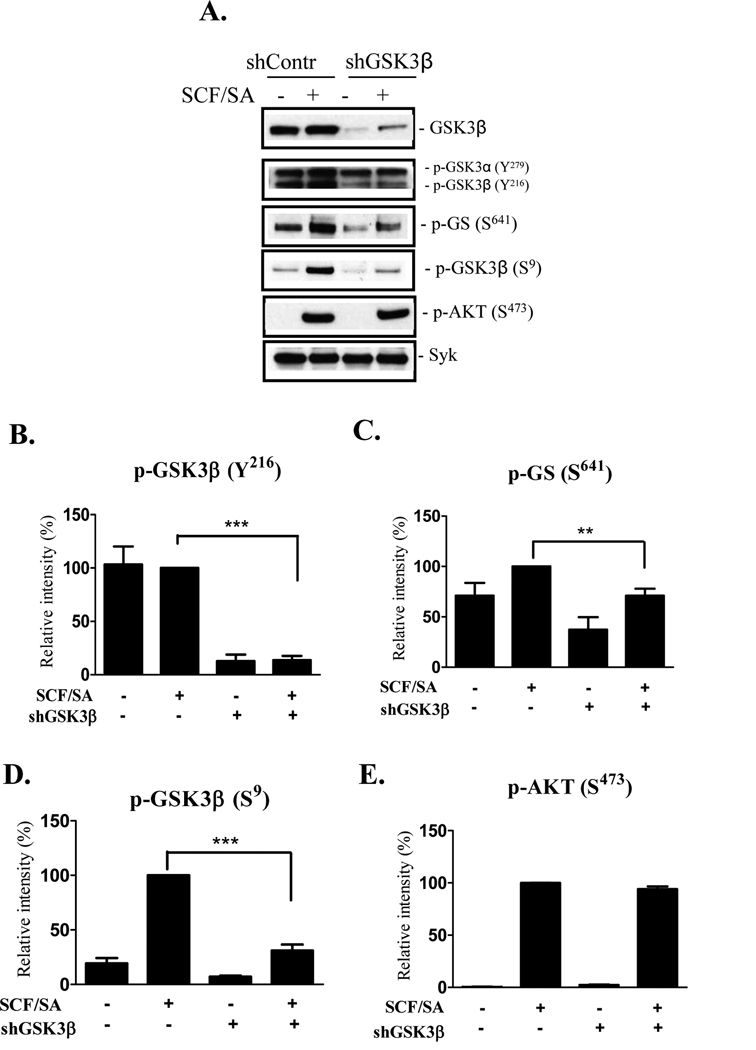
Optimal cytokine generation in HuMCs requires that the cells be concurrently activated through KIT and the FcεRI (6, 20). We therefore examined GSK3β phosphorylation under these conditions following GSK3β knockdown. GSK3β activity is regulated by the phosphorylation status of Y216 and S9. Y216 is reported to be constitutively phosphorylated in resting cells thus maintaining GSK3β in an active state (14, 21, 22). However, the ability of GSK3β to phosphorylate its specific substrates, for example glycogen synthase (GS), requires priming of the substrate by means of prior phosphorylation by an additional kinase (23, 24). In resting HuMCs we similarly observed constitutive phosphorylation of Y216 (Fig. 2A, B). When the cells were activated through KIT and FcεRI, we observed no consistent increase in the phosphorylation of this residue. Similarly the GSK3β substrate, GS was constitutively phosphorylated at S641 (Fig. 2A, C). However, in contrast to the phosphorylation of GSK3β, this phosphorylation was slightly elevated in cells activated through KIT and FcεRI. Surprisingly, in light of this observation, we also observed that the phosphorylation of the inhibitory S9 residue of GSK3β was enhanced in the activated HuMCs (Fig. 2 A, D). Similar responses to the above were observed in HuMCs activated via either FcεRI or KIT alone (data not shown).
Figure 2. Knockdown of GSK3β phosphorylation in quiescent and activated HuMCs.
HuMCs, transduced with scrambled shRNA (shContr) or shRNA for GSK3β (shGSK3β-B), were sensitized overnight and then stimulated with SA (100 ng/ml) and SCF (30 ng/ml) for 2 min as described in materials and methods. Whole-cell extracts were prepared and immunoblotted with anti-p-GSK3β(Y216), anti-p-Glycogen Synthase (p-GS(S641)), anti-p- GSK3β(S9), or anti-p-AKT(S473) antibodies (A). Protein loading of the samples was normalized by probing for Syk. Data were generated by scanning the blots from 3–5 independent experiments, and normalized to the response obtained at 2 min with SCF/SA stimulation (B–E) (n=3–5, **p < 0.001 and *** p < 0.0001, by Student’s t test).
As expected, due to the effective reduction in total GSK3β protein in the GSK3β knockdown-HuMCs, the phosphorylation of GSK3β-Y216 and GSK3β-S9 was substantially reduced in both quiescent and activated HuMCs (Fig. 2). The phosphorylation of GS at S641, however, was also reduced in these cells. In contrast, and by means of a negative control, we observed no decrease in the SCF/SA dependent phosphorylation of AKT, a surrogate marker for PI3K activation, in these cells (Fig. 2 A, E). Taken together, these data suggest that, in HuMCs, GSK3β is active under resting conditions and that, upon cell activation through FcεRI and KIT, this permits an increase in phosphorylation of the GSK3β substrate GS. These responses can be effectively reduced in the HuMCs following shRNA-induced GSK3β knockdown.
Effect of shRNA-induced knockdown of GSK3β on mast cell cytokine production
Having successfully established knockdown of GSK3β activity in HuMCs, we next investigated the role of GSK3β in mast cell cytokine generation. For these studies, the cells were again co-stimulated through KIT, via SCF, and the FcεRI, through sensitizing with biotinylated IgE and challenging with SA as the response to either stimulus alone is not marked (20). To initially screen the effect of GSK3β knockdown on multiple cytokines, cells were stimulated for an optimal period of 4 h, based on previous kinetic studies (25), then cytokine gene expression was determined by a commercially available array. In these studies, we observed a marked increase in mRNA’s for multiple cytokines including those for GM-CSF, IL-8 and IL-13 (Fig 3A–C and supplemental data). These responses were markedly attenuated in GSK3β -knockdown cells compared to the control cells (Fig. 3A–C). Based on this initial screen, we examined the amounts of GM-CSF and IL-8 protein present in the supernatants of control and GSK3β knockdown-HuMCs challenged with SCF in the presence of SA. As demonstrated in Figure 3D and E, there was a marked reduction in SCF/SA-induced generation of GM-CSF and IL-8 in the GSK3β knockdown-HuMCs compared to controls. The relative inhibition produced by the two constructs (Fig 3D and E) correlated with their relative abilities to knockdown GSK3β protein levels (Fig. 1A). This close correlation was further illustrated by plotting the relative expression of GSK3β protein levels to that of IL-8 production (Fig. 3F).
Figure 3. GSK3β regulates SCF/SA-mediated GM-CSF, IL-8 production and SCF-mediated chemotaxis in HuMCs.
HuMCs transduced with scrambled shRNA (shControl) or shRNA for GSK3β (shGSK3β B) were sensitized overnight and then stimulated with SA (100 ng/ml) and SCF (30 ng/ml) for 4h. Total RNA was isolated from the indicated samples, reverse transcribed and quantified by real-time PCR. Relative expression of GM-SCF, IL-8 and IL-13 mRNA was calculated as described in materials and methods (A–C). Data are shown from two independent experiments. HuMCs, transduced with scrambled shRNA (shContr) or shRNA for GSK3β (two different constructs; construct A or B) were sensitized overnight and then stimulated with SA (100 ng/ml) and SCF (30 ng/ml) for 6h (D, E). Supernatants were collected and ELISA was performed for human GM-SCF and IL-8 (D and E) (n=6–9, * p < 0.05, ** p < 0.001 and *** p < 0.0001, by Student’s t test). The degree of GSK3β knockdown inversely correlates with SCF/SA-mediated IL-8 release (F) (n=6, Spearman correlation test). The diamond symbol represents the means, and shown standard errors, of the values obtained from control cells (n=6). These data were not included in the statistical analysis of the correlation. HuMCs, transduced with scrambled shRNA (shContr) or shRNA for GSK3β (two different constructs; construct A or B), were starved for 4 h and then stimulated with SCF (30 ng/ml) for 4 h in a transwell chemotaxis assay (G), (n=3–5, ** p < 0.001, by Student’s t test).
Effect of shRNA-induced knockdown of GSK3β on mast cell chemotaxis
In addition to stimulating cytokine generation in mast cells, SCF is a potent chemotactic agent for HuMCs (4). To thus explore whether SCF-mediated chemotaxis was similarly dependent on GSK3β, we next examined the relative ability of HuMCs to migrate in response to SCF following GSK3β knockdown. From Figure 3G, it can be seen that GSK3β-knockdown HuMCs displayed a reduced capacity to migrate towards SCF compared to control cells. The extent of inhibition of migration again correlated to the extent of knockdown of GSK3β observed in these studies, with GSK3β shRNA B producing a greater knockdown of GSK3β and migration than that produced by GSK3β shRNA A (Fig. 3G).
GSK3β regulates mast cell responses independently of mTOR
We next examined how GSK3β may regulate mast cell cytokine production and chemotaxis. GSK3β has been reported to phosphorylate the mTOR regulator, tuberin (TSC2) in HEK293T cells (26), thereby influencing the activation of the mTORC1 and mTORC2 cascades. Our previous studies in HuMCs demonstrated that the mTORC1 cascade, downstream of PI3K, contributes to SCF/SA-mediated cytokine production and SCF-mediated chemotaxis in human mast cells (12). The mTORC1 cascade is initiated by the PI3K-dependent activation of AKT which phosphorylates and inactivates TSC1 and TSC2 (tuberin), negative regulators of mTOR activation. This results in the sequential phosphorylation and activation of mTOR and its downstream substrates: the transcriptional regulators p70S6K (p70 ribosomal S6 kinase) and 4E-BP1 (eukaryotic initiation factor 4E binding protein 1) (27, 28). In contrast, the mTORC2 cascade, leads to the phosphorylation (S473) and activation of AKT (29–31). We therefore investigated whether the reduced cytokine production in the GSK3β knockdown-HuMCs may be a consequence of inhibition of the mTORC1 or mTORC2-regulated signaling cascades.
As before, GSK3β knockdown significantly reduced GSK3β expression in HuMCs (Fig. 4A, B). However, there were minimal differences in the basal and SCF/SA-induced phosphorylation of mTOR, p70S6K and 4E-BP1 in the GSK3β knockdown-HuMCs cells compared to controls (Fig. 4A–E). Furthermore, as discussed earlier, there was also no difference in the resting or SCF/SA induced, mTORC2-dependent phosphorylation of AKT(S473) in the GSK3β knockdown-HuMCs when compared to control cells (Fig. 2A, E). Similarly, the mTORC1 inhibitor, rapamycin, failed to block the phosphorylation of GSK3β (S9, Y216) (Fig. 4F) despite a marked inhibition of GSK3β (S9 only) by the PI3K inhibitor wortmannin. Taken together, these data support the conclusion that GSK3β regulates SCF/SA-induced mast cell responses independently of both the mTORC1 and mTORC2 cascades. We therefore next examined whether GSK3β regulated human mast cell cytokine production and chemotaxis by an alternative mechanism.
Figure 4. GSK3β regulates SCF/SA-mediated mast cell responses independently of mTOR.
HuMCs, transduced with scrambled shRNA (shContr) or shRNA for GSK3β (shGSK3β-B), were sensitized overnight and then stimulated with SA (100 ng/ml) and SCF (30 ng/ml) for 10 min as described in materials and methods. Whole-cell extracts were prepared and immunoblotted with anti-GSK3β anti-p-mTOR(S2448), anti-p-p70S6K(T389), anti-p-4E-BP1(T37, T46) antibodies (A). Data in B–E were generated by scanning the blots from 3–5 independent experiments, and normalized to the response obtained at 10 min with SCF/SA stimulation (n=3–5, **p < 0.001 for comparison with SCF/SA response in shContr transduced HuMCs by Student’s t test). In (F), HuMCs were pretreated with Rapamycin (100 nM) or Wortmaninn (100 nM) 20 min prior stimulation with SCF or SA at the time indicated. Whole-cell extracts were prepared and immunoblotted with anti-p-AKT(S473), anti-p-GSK3β(Y216), or anti-p-GSK3β(S9) antibodies. Protein loading of the samples was normalized by probing for Syk.
Regulation of the MAPK activation by GSK3β in human mast cells
The MAP kinases (MAPK), p38, and JNK, regulate transcriptional activation pathways which contribute to mast cell function including cytokine generation (32). We, thus, next investigated whether the MAPK cascade represented a key intermediary step in the regulation of GSK3β–regulated mast cell cytokine production. As can be seen in Figure 5A, in contrast to the lack of effect on the mTOR cascades, the increase in both JNK (Fig.5A and B) and p-38 (Fig.5A and D) phosphorylation in response to SCF/SA was reduced in the GSK3β knockdown-HuMCs compared to the control treated cells. There was no effect on total p38 or JNK protein levels under these conditions (Fig. 5A). The SCF/SA-induced phosphorylation of MKK3/6, upstream of p38, was however also markedly reduced in the GSK3β knockdown-HuMCs (Fig 5A, D).
Figure 5. GSK3β regulates SCF/SA and SCF-mediated MAPK activity in HuMCs.
HuMCs, transduced with scrambled shRNA (shContr) or shRNA for GSK3β (shGSK3β-B), were sensitized overnight and then (A) stimulated with SA (100 ng/ml) and SCF (30 ng/ml), or (E) starved for 4 h then stimulated with SCF (30 ng/ml), for 2 min as described in materials and methods. Whole-cell extracts were prepared and immunoblotted (A, E) with anti-p-JNK (T183, T185), total JNK, anti-p-MKK3/6 (S189, S207), anti-p-p38 (T180), total p-38 or p-AKT (S473) antibodies. Protein loading of the samples was normalized by probing for Syk (A,E). Data in B–D,F–I were generated by scanning the blots in 3–5 independent experiments and normalized to the response obtained with SCF/SA stimulation (B–D), or SCF response (F–I), in shContr transduced HuMCs (n=3–5, * p < 0.05, ** p < 0.001 and *** p < 0.0001, by Student’s t test).
Since the MAPKs JNK and p38 have also been shown to regulate SCF/KIT induced mast cell migration (33–35), we next determined the phosphorylation status of these proteins under the conditions utilized for the chemotaxis studies, i.e. stimulation with SCF after a 4 h period of starvation in SCF-depleted media. As shown in Figure 5E–I, SCF-mediated MKK3/6, JNK and p38 activation, but not total p38 or JNK protein, were significantly reduced in the GSK3β knockdown cells, compared to control treated cells under these conditions. In contrast, SCF-induced AKT activation was unaffected compared to control treated cells (Fig. 5E and I). From these data, we conclude that GSK3β regulates the activation of the MAP kinases, p38 and JNK, thus providing an explanation for the reduced SCF induced chemotaxis observed in the GSK3β knockdown-HuMCs. Furthermore GSK3β-dependent, p38- and JNK-mediated transcription activation would also provide an explanation for the reduced SCF/SA induced cytokine production of served in the GSK3β knockdown-HuMCs. We thus next examined the SCF/SA-induced phosphorylation of specific transcription factors in the GSK3β knockdown-HuMCs.
Transcription factors regulation by GSK3β
The MAPK pathway leads to the phosphorylation and, thus, regulation of multiple transcription factors including Jun and ATF2. These, and potentially other transcription factors which are known to be phosphorylated in mast cells, may contribute to mast cell cytokine production and other transcriptional-dependent processes. We therefore examined whether GSK3β regulated the phosphorylation of Jun, ATF2, and NF-κB. As can be seen in Figure 6, the phosphorylation of the AP-1 transcription factors c-Jun (Fig. 6A,B) and ATF2 (Fig. 6A,C) in response to SCF/SA stimulation was greatly reduced in GSK3β knockdown-HuMCs compared to control treated cells. Furthermore, the phosphorylation of the p65 NF-κB subunit was also significantly reduced in the GSK3β knockdown cells compared to control treated cells (Fig. 6A,D). Taken together, these data support the conclusion that GSK3β activation is a pre-requisite signal for the MAPK-dependent activation of c-Jun and ATF2 and also NF-κB p65 activity which in turn regulates SCF/SA induced cytokine production.
Figure 6. GSK3β regulates SCF/SA-mediated AP1 transcription factors and NF-κB activity in HuMCs.
HuMCs, transduced with scrambled shRNA (shContr) or shRNA for GSK3β (shGSK3β-B), were sensitized overnight and then stimulated with SA (100 ng/ml) and SCF (30 ng/ml) for 30 min as described in materials and methods. Whole-cell extracts were prepared and immunoblotted with anti-phospho-cJun (S73), total cJun, anti-phospho-ATF2(T71), or anti-phospho-NF-κB(S536) antibodies (A). Protein loading of the samples was normalized by probing for Syk (A). Data in B–D were generated by scanning in 3–4 independent experiments and normalizing to the responses obtained with SA/SCF stimulation ( *p < 0.05 **p < 0.001 and ***p < 0.0001, by Student’s t test).
Discussion
In this study, we present evidence that supports a pre-requisite role for GSK3β in KIT-mediated mast cell chemotaxis and KIT/FcεRI-mediated enhanced gene expression leading to cytokine production in HuMCs. As reported in other cell types (14, 21, 22), in quiescent HuMCs, GSK3β was determined to be constitutively activated. This conclusion was supported by the observed basal phosphorylation of the activating tyrosine residue (Y216) in GSK3β, the phosphorylation of its substrate GS at S641 and the reduction of these phosphorylation states in the GSK3β knockdown cells (Fig 2). Although we did not observe a consistent increase in phosphorylation of GSK3β at the Y216 position in response to SCF and/or SA, under the conditions utilized to examine chemotaxis and cytokine production, there was an apparent increase in the phosphorylation of GS at S641 under these conditions, which was reduced in the GSK3β knockdown. The constitutive Y216 phosphorylation of GSK3β may be due to the cells being maintained in SCF. Indeed, when the cells were starved of SCF for a prolonged period of time (overnight) prior to stimulation, we were able to observe an SCF-dependent increase in the phosphorylation of this residue. Thus the phosphorylation of this residue may be directly dependent on Kit. Regardless, our results suggest that triggering of mast cells through KIT and/or FcεRI facilitates the ability of GSK3β to phosphorylate its substrate(s) without necessarily increasing its constitutive activity; a potential mechanism of action that is elaborated upon below.
In addition to the phosphorylation of (Y216) in GSK3β, however, we observed that the inhibitory S9 residue GSK3β was also phosphorylated in a PI3K-dependent manner following SCF/ SA challenge (Fig 2D and 4F). This phenomenon has also been reported in monocytes, dendritic cells, and T cells, following exposure to TLR2-, TLR4-, TLR5-, and TLR9-agonists, E coli, and viral peptide respectively (36–38). In our study, however, the observed increased phosphorylation of GS, at least at early time points, would suggest that downregulation of GSK3β activation may occur latently to the constitutive activation. We have previously demonstrated that PI3K, and signals dependent upon PI3K activity, are delayed responses compared to other signals initiated upon FcεRI or Kit activation (12). Thus it is likely that any response due to downregulating GSK3β activity would be chronologically secondary to those regulated by GSK3β activation. Nevertheless, these data do suggest that the ability of GSK3β to phosphorylate its substrates may depend upon the net balance between positive and negative regulation of GSK3β activity.
The marked reduction in the ability of SCF/SA to enhance IL-8, IL-13, and GM-CSF mRNA levels and IL-8 and GM-CSF secretion, associated with the diminution of GSK3β activity in the GSK3β knockdown-HuMCs (Fig 3F), strongly supports a requirement for GSK3β activity in the regulation of KIT/FcεRI-mediated cytokine production. This conclusion is further supported by the close statistical correlation between the degree of GSK3β knockdown and IL-8 secretion. Similarly, the close correlation between GSK3β knockdown and reduction in SCF-induced chemotaxis in the GSK3β knockdown-HuMCs, also provides evidence for a pre-requisite role for GSK3β in the SCF-induced chemotactic response.
There are conflicting reports regarding the role of GSK3β in cytokine production in other cells of hematopoietic lineage. Treatment of monocytes with GSK3β inhibitors such as LiCl and/or SB216763, or with GSK3β–targeted siRNA, has been reported to inhibit TLR2-, 4-, 5-, and 9-dependent release of IL-1β, IL-6, TNF-α, IL-12, and IFN-γ but to enhance TLR-dependent production of IL-10 (36). GSK3β inhibitors were also reported to inhibit E coli-induced IL-12, IL-6 and TNF-α, but not IL-10, release from dendritic cells (37). In contrast, in T cells, GSK3β inhibitors were observed to enhance viral peptide-induced IL-2 production, whereas over-expression of GSK3β in T cells down-regulated the response (38). This apparent dichotomy in the GSK3β-dependent regulation of cytokine generation in the various cell types may reflect the potential for GSK3β to both negatively and positively regulate transcriptional signaling pathways for cytokine production. Indeed, it is possible that, in addition to regulating positive signals, negative signaling pathways may also be regulated by GSK3β in mast cells. In this respect, it has been suggested that the ability of AKT to enhance cytokine generation through NF-AT activation in mouse mast cells may be due to downregulation of GSK3β activity (39). Whether this may also be true for HuMCs is unclear from the present study, however the induced phosphorylation of the inhibitory GSK3β S9 residue in response to SCF/SA in HuMCs was reduced by the PI3K inhibitor wortmannin (Fig. 4F).
There has emerged no common mechanistic explanation as to how GSK3β may be exerting its regulatory influence on cytokine generation and other process in hematopoietic cells. As we have previously demonstrated that the mTORC1 cascade contributes to KIT/FcεRI-mediated cytokine production and KIT-mediated mast cell chemotaxis; (12) and as GSK3β has been proposed to regulate the mTOR pathway through phosphorylation of tuberin (26), the scenario existed that, in the HuMCs, GSK3β may be acting via regulation of mTOR pathways. However, the observations that the KIT/FcεRI-mediated phosphorylation of components of the mTORC1 and mTORC2 cascades was not reduced in the GSK3β knockdown-HuMC, (Fig. 4), argues against this possibility. It has been proposed, that the contrasting roles for GSK3β in TLR cytokine production in monocytes may be explained by opposing regulation of the transcription factors CREB and NF-κB through competition for binding to a common co-activator protein CBP (CREB binding protein) (36). According to this model, GSK3β inhibition would increase CREB activation allowing CREB to compete with the p65 subunit of NF-κB for binding to CBP. In our present study, however, although SCF/SA-induced phosphorylation of the p65 subunit of NF-κB was observed to be significantly reduced in the GSK3β knockdown-HuMCs (Fig. 6 A and D), we did not consistently observe an increase in CREB activity in these cells (data not shown). Regardless, these data are in agreement with other studies showing that GSK3β is required for NF-κB activation (19).
The most remarkable defects that we observed however in the GSK3β knockdown-HuMCs were in the p38 and JNK MAPK pathways and, particularly, in the respective downstream transcription factors ATF2 and c-Jun. JNK activity has previously been shown to regulate cytokine production mediated by AP1 transcription factors in both mouse bone marrow-derived mast cells (32) and HuMCs (6) Similarly, both JNK and p38 have been previously described to regulate mast cell chemotaxis (33–35). Therefore, the reduced SCF/SA-induced cytokine production and SCF-induced chemotaxis observed in the GSK3β knockdown-HuMCs may be explained by defective JNK and p38 signaling in these cells (Fig. 5A–D).
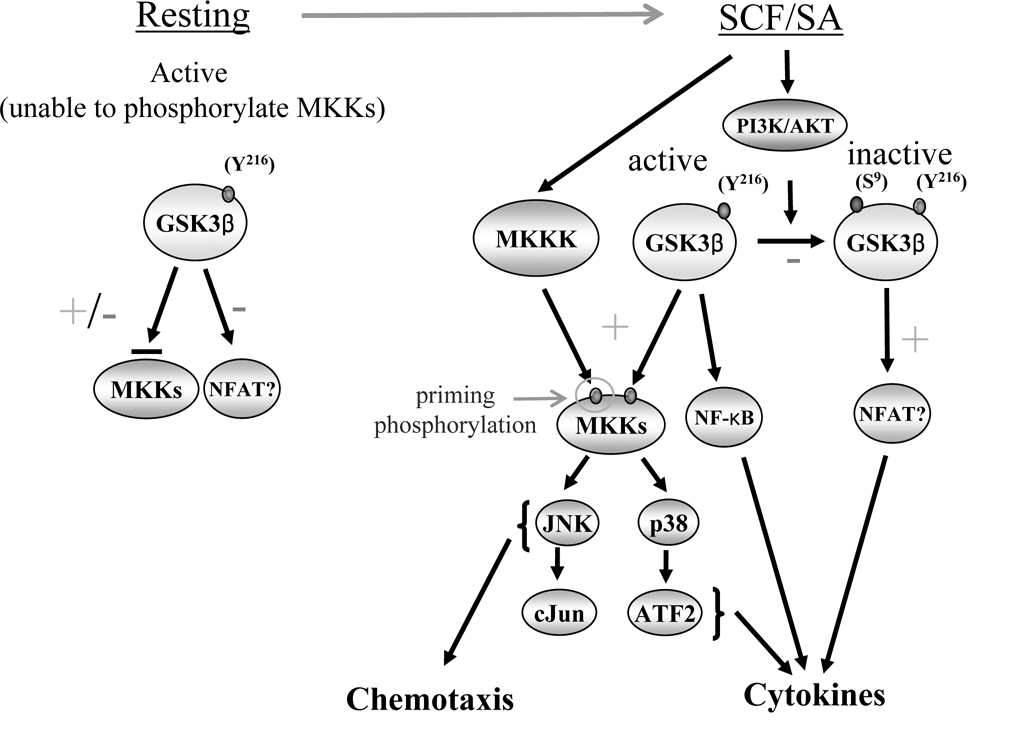
How GSK3β may act as a pre-requisite signal for the regulation of these pathways may be explained by the unique manner in which GSKβ phosphorylates its substrates. As discussed, GSK3β substrates require prior phosphorylation by a secondary kinase at amino acids 4–5 COOH-termini to the GSK3β phosphorylation sites for optimal GSK3β-mediated phosphorylation. Thus, although, GSK3β is active in resting conditions, it cannot optimally phosphorylate its substrates, until upon FcεRI or KIT kinase activation, the GSK3β substrates become phosphorylated as a consequence of the activation of one of the kinases downstream of these receptors. This would then allow GSK3β to optimally phosphorylate its target signaling proteins and hence transduce the signals required for gene expression leading to cytokine production, and the processes required for chemotaxis (Fig. 7). Of potential relevance may be the presence of two highly conserved SxxxS/T sequences in MKK3 and MKK6 which are responsible for the phosphorylation and activation of p38, and in MKK4 and MKK7 which are responsible for the phosphorylation and activation of JNK. Multiple such sequences are also found in MEKK1 and MEKK4, upstream kinases of MKK4 and MKK7. Thus, phosphorylation of these sites by GSK3β following initial phosphorylation by a “priming” serine/threonine kinase may provide a mechanism by which constitutive activation of GSK3β may regulate the activation of p38 and JNK and subsequent downstream transcription factors. In support of this conclusion, we observed that SCF and SA/SCF-mediated MKK3/6 phosphorylation was markedly reduced in the GSK3β knockdown-HuMCs (Fig. 5).
Figure 7. Potential model by which constitutively activated GSK3β may regulate HuMC cytokine production and chemotaxis.
Under resting conditions, GSK3β is constitutively active, due to phosphorylation of the Y216 residue, but is unable to optimally phosphorylate its substrates as they require initial phosphorylation by a “priming” kinase to allow these reactions to take place. Upon SCF/SA challenge, GSK3β substrates are phosphorylated by priming kinases thus allowing GSK3β to phosphorylate and activate these substrates. This leads to activation of the JNK and p38 MAPK pathways thereby initiation of to chemotaxis, and downstream transcription factors and NF-κB leading to cytokine production. GSK3β activity is terminated upon phosphorylation at the S9 position as a consequence of activation of PI3K. Based on other reports, it is possible that GSK3β may also regulate an inhibitory pathway for NF-AT activation (39) leading to cytokine production. Based on other reports, it is possible that GSK3β may also regulate an inhibitory pathway for NF-AT activation (39).
In summary, here we have presented evidence to support the conclusion that GSK3β is a pre-requisite signal for KIT-mediated chemotaxis and KIT/FcεRI-mediated cytokine production in human mast cells. The regulation of cytokine generation by GSK3β could be explained by the differential regulation of transcriptional control downstream of JNK and p38, as well as transcriptional control of NF-κB p65 subunit, whereas the regulation of the chemotactic response by GSK3β may be explained by its modulation of JNK- and p38-dependent pathways. As with other cells types, it is however possible, that, as yet undefined, inhibitory pathways both regulating GSK3β activation and regulated by GSK3β activity may play a role in human mast cell biology. Thus GSK3β may be act as a central regulator for the precise control of the signaling processes required for mast cell chemotaxis and cytokine production.
Supplementary Material
Abbreviations
- SCF
Stem cell factor
- FcεRI
high affinity receptor for IgE
- mTORC
mammalian target of rapamycin complex
- PI3K
phosphoinositide 3-kinase
- GSK3β
glycogen synthase kinase 3β
- HuMCs
human mast cells
- SA
streptavidin
- GS
glycogen synthase
Footnotes
This work was supported by the National Institute of Allergy And Infectious Diseases, Division of Intramural Research within the National Institutes of Health. M.R. is grateful for support from the Swedish Heart Lung Foundation.
References
- 1.Mekori YA, Metcalfe DD. Mast cells in innate immunity. Immunol Rev. 2000;173:131–140. doi: 10.1034/j.1600-065x.2000.917305.x. [DOI] [PubMed] [Google Scholar]
- 2.Metcalfe DD. Mast cells and mastocytosis. Blood. 2008;112:946–956. doi: 10.1182/blood-2007-11-078097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okayama Y, Kawakami T. Development, migration, and survival of mast cells. Immunol Res. 2006;34:97–115. doi: 10.1385/IR:34:2:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nilsson G, Butterfield JH, Nilsson K, Siegbahn A. Stem cell factor is a chemotactic factor for human mast cells. J Immunol. 1994;153:3717–3723. [PubMed] [Google Scholar]
- 5.Ali K, Bilancio A, Thomas M, Pearce W, Gilfillan AM, Tkaczyk C, Kuehn N, Gray A, Giddings J, Peskett E, Fox R, Bruce I, Walker C, Sawyer C, Okkenhaug K, Finan P, Vanhaesebroeck B. Essential role for the p110δ phosphoinositide 3-kinase in the allergic response. Nature. 2004;431:1007–1011. doi: 10.1038/nature02991. [DOI] [PubMed] [Google Scholar]
- 6.Hundley TR, Gilfillan AM, Tkaczyk C, Andrade MV, Metcalfe DD, Beaven MA. Kit and FcεRI mediate unique and convergent signals for release of inflammatory mediators from human mast cells. Blood. 2004;104:2410–2417. doi: 10.1182/blood-2004-02-0631. [DOI] [PubMed] [Google Scholar]
- 7.Vosseller K, Stella G, Yee NS, Besmer P. c-kit receptor signaling through its phosphatidylinositide-3'-kinase-binding site and protein kinase C: role in mast cell enhancement of degranulation, adhesion, and membrane ruffling. Mol Biol Cell. 1997;8:909–922. doi: 10.1091/mbc.8.5.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roskoski R., Jr Structure and regulation of Kit protein-tyrosine kinase--the stem cell factor receptor. Biochem Biophys Res Commun. 2005;338:1307–1315. doi: 10.1016/j.bbrc.2005.09.150. [DOI] [PubMed] [Google Scholar]
- 9.Roskoski R., Jr Signaling by Kit protein-tyrosine kinase--the stem cell factor receptor. Biochem Biophys Res Commun. 2005;337:1–13. doi: 10.1016/j.bbrc.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 10.Jensen BM, Akin C, Gilfillan AM. Pharmacological targeting of the KIT growth factor receptor: a therapeutic consideration for mast cell disorders. British journal of pharmacology. 2008;154:1572–1582. doi: 10.1038/bjp.2008.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Linnekin D. Early signaling pathways activated by c-Kit in hematopoietic cells. Int J Biochem Cell Biol. 1999;31:1053–1074. doi: 10.1016/s1357-2725(99)00078-3. [DOI] [PubMed] [Google Scholar]
- 12.Kim MS, Kuehn HS, Metcalfe DD, Gilfillan AM. Activation and function of the mTORC1 pathway in mast cells. J Immunol. 2008;180:4586–4595. doi: 10.4049/jimmunol.180.7.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen P, Goedert M. GSK3 inhibitors: development and therapeutic potential. Nat Rev Drug Discov. 2004;3:479–487. doi: 10.1038/nrd1415. [DOI] [PubMed] [Google Scholar]
- 14.Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci. 2003;116:1175–1186. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu X, Paik PK, Chen J, Yarilina A, Kockeritz L, Lu TT, Woodgett JR, Ivashkiv LB. IFN-γ suppresses IL-10 production and synergizes with TLR2 by regulating GSK3 and CREB/AP-1 proteins. Immunity. 2006;24:563–574. doi: 10.1016/j.immuni.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 16.Kirshenbaum AS, Goff JP, Semere T, Foster B, Scott LM, Metcalfe DD. Demonstration that human mast cells arise from a progenitor cell population that is CD34(+), c-kit(+), and expresses aminopeptidase N (CD13) Blood. 1999;94:2333–2342. [PubMed] [Google Scholar]
- 17.Kirshenbaum AS, Metcalfe DD. Growth of human mast cells from bone marrow and peripheral blood-derived CD34+ pluripotent progenitor cells. Methods Mol Biol. 2006;315:105–112. doi: 10.1385/1-59259-967-2:105. [DOI] [PubMed] [Google Scholar]
- 18.Tkaczyk C, Metcalfe DD, Gilfillan AM. Determination of protein phosphorylation in FcεRI-activated human mast cells by immunoblot analysis requires protein extraction under denaturing conditions. J Immunol Methods. 2002;268:239–243. doi: 10.1016/s0022-1759(02)00210-7. [DOI] [PubMed] [Google Scholar]
- 19.Hoeflich KP, Luo J, Rubie EA, Tsao MS, Jin O, Woodgett JR. Requirement for glycogen synthase kinase-3β in cell survival and NF-κB activation. Nature. 2000;406:86–90. doi: 10.1038/35017574. [DOI] [PubMed] [Google Scholar]
- 20.Jensen BM, Beaven MA, Iwaki S, Metcalfe DD, Gilfillan AM. Concurrent inhibition of kit- and FcεRI-mediated signaling: coordinated suppression of mast cell activation. The Journal of pharmacology and experimental therapeutics. 2008;324:128–138. doi: 10.1124/jpet.107.125237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hughes K, Nikolakaki E, Plyte SE, Totty NF, Woodgett JR. Modulation of the glycogen synthase kinase-3 family by tyrosine phosphorylation. Embo J. 1993;12:803–808. doi: 10.1002/j.1460-2075.1993.tb05715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harwood AJ. Regulation of GSK-3: a cellular multiprocessor. Cell. 2001;105:821–824. doi: 10.1016/s0092-8674(01)00412-3. [DOI] [PubMed] [Google Scholar]
- 23.Fiol CJ, Mahrenholz AM, Wang Y, Roeske RW, Roach PJ. Formation of protein kinase recognition sites by covalent modification of the substrate. Molecular mechanism for the synergistic action of casein kinase II and glycogen synthase kinase 3. The Journal of biological chemistry. 1987;262:14042–14048. [PubMed] [Google Scholar]
- 24.Frame S, Cohen P, Biondi RM. A common phosphate binding site explains the unique substrate specificity of GSK3 and its inactivation by phosphorylation. Mol Cell. 2001;7:1321–1327. doi: 10.1016/s1097-2765(01)00253-2. [DOI] [PubMed] [Google Scholar]
- 25.Okayama Y, Hagaman DD, Metcalfe DD. A comparison of mediators released or generated by IFN-γ-treated human mast cells following aggregation of FcγRI or FcεRI. J Immunol. 2001;166:4705–4712. doi: 10.4049/jimmunol.166.7.4705. [DOI] [PubMed] [Google Scholar]
- 26.Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, Yang Q, Bennett C, Harada Y, Stankunas K, Wang CY, He X, MacDougald OA, You M, Williams BO, Guan KL. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126:955–968. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 27.Dann SG, Selvaraj A, Thomas G. mTOR Complex1-S6K1 signaling: at the crossroads of obesity, diabetes and cancer. Trends Mol Med. 2007;13:252–259. doi: 10.1016/j.molmed.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Shah OJ, Anthony JC, Kimball SR, Jefferson LS. 4E-BP1 and S6K1: translational integration sites for nutritional and hormonal information in muscle. Am J Physiol Endocrinol Metab. 2000;279:E715–E729. doi: 10.1152/ajpendo.2000.279.4.E715. [DOI] [PubMed] [Google Scholar]
- 29.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 30.Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, Huang Q, Qin J, Su B. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–137. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 31.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 32.Hata D, Kitaura J, Hartman SE, Kawakami Y, Yokota T, Kawakami T. Bruton's tyrosine kinase-mediated interleukin-2 gene activation in mast cells. Dependence on the c-Jun N-terminal kinase activation pathway. The Journal of biological chemistry. 1998;273:10979–10987. doi: 10.1074/jbc.273.18.10979. [DOI] [PubMed] [Google Scholar]
- 33.Samayawardhena LA, Pallen CJ. Protein-tyrosine phosphatase alpha regulates stem cell factor-dependent c-Kit activation and migration of mast cells. The Journal of biological chemistry. 2008;283:29175–29185. doi: 10.1074/jbc.M804077200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Samayawardhena LA, Hu J, Stein PL, Craig AW. Fyn kinase acts upstream of Shp2 and p38 mitogen-activated protein kinase to promote chemotaxis of mast cells towards stem cell factor. Cellular signalling. 2006;18:1447–1454. doi: 10.1016/j.cellsig.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 35.Sundstrom M, Alfredsson J, Olsson N, Nilsson G. Stem cell factor-induced migration of mast cells requires p38 mitogen-activated protein kinase activity. Experimental cell research. 2001;267:144–151. doi: 10.1006/excr.2001.5239. [DOI] [PubMed] [Google Scholar]
- 36.Martin M, Rehani K, Jope RS, Michalek SM. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nature immunology. 2005;6:777–784. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodionova E, Conzelmann M, Maraskovsky E, Hess M, Kirsch M, Giese T, Ho AD, Zoller M, Dreger P, Luft T. GSK-3 mediates differentiation and activation of proinflammatory dendritic cells. Blood. 2007;109:1584–1592. doi: 10.1182/blood-2006-06-028951. [DOI] [PubMed] [Google Scholar]
- 38.Ohteki T, Parsons M, Zakarian A, Jones RG, Nguyen LT, Woodgett JR, Ohashi PS. Negative regulation of T cell proliferation and interleukin 2 production by the serine threonine kinase GSK-3. The Journal of experimental medicine. 2000;192:99–104. doi: 10.1084/jem.192.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kitaura J, Asai K, Maeda-Yamamoto M, Kawakami Y, Kikkawa U, Kawakami T. Akt-dependent cytokine production in mast cells. The Journal of experimental medicine. 2000;192:729–740. doi: 10.1084/jem.192.5.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.