Abstract
Objectives
Four variants (K60N, Q128R, G202R and A592E) in the nebulette gene (NEBL) were identified in patients with dilated cardiomyopathy (DCM) and endocardial fibroelastosis (EFE). We sought to determine if these mutations cause cardiomyopathy.
Background
Nebulette aligns thin filaments and connects them with the myocardial Z-disk.
Methods
We produced transgenic mice with cardiac-restricted over-expression of human wild-type (WT) or mutant nebulette. Chimera and transgenic mice were examined at 4, 6 and 12 months of age by echocardiography and cardiac MRI. The hearts from embryos and adult mice were assessed by histopathologic, immunohistochemical, ultrastructural and protein analyses. Rat H9C2 cardiomyoblasts with transient expression of nebulette underwent cyclic mechanical strain.
Results
We identified lethal cardiac structural abnormalities in mutant embryonic hearts (K60N and Q128R). Founders of the mutant mice lines developed DCM with severe heart failure. An irregular localization pattern for nebulette and impaired desmin expression was noted in the proband and chimera Q128R mice. Mutant G202R and A592E mice exhibited left ventricular dilation and impaired cardiac function accompanied with the specific changes in I-band or Z-disk proteins by 6 months of age, respectively. The mutations modulated distribution of nebulette in the sarcomere and the Z-disks during stretch of H9C2 cells.
Conclusions
NEBL is a new susceptibility gene for EFE and DCM. Different mutations in nebulette trigger specific mechanisms converging to a common pathological cascade leading to EFE and DCM.
Keywords: nebulette, dilated cardiomyopathy, endocardial fibroelastosis, Z-disk
INTRODUCTION
Dilated cardiomyopathy (DCM) is a myocardial disease characterized by left ventricular (LV) dilation and dysfunction and is believed to be of genetic origin in approximately 35% of all DCM cases.1 Endocardial fibroelastosis (EFE), characterized by diffuse endocardial thickening due to proliferation of fibrous and elastic tissue, is a rare cardiac disorder with poor prognosis that occurs in infants and young children.2 Although virus-induced fetal myocarditis, or X-linked Barth or autosomal recessive inherited genes have been reported to be causative for EFE, most cases are of unknown etiology.3 Various molecular mechanisms are involved in the development of these disorders due to the overlapping functions of cytoskeletal proteins beginning from their involvement in sarcomeric structure, to their functions in cardiac differentiation, myofibrillogenesis and transcriptional regulation.4,5
The nebulin protein family is a group of actin-binding proteins whose universal feature is the presence of “nebulin-repeats” which bind a single actin subunit and is involved in tropomyosin-troponin assembly.6 Moreover, expression of the specific nebulin isoforms with specific numbers of “nebulin-repeats” correlate thin filament length in a striated muscle-specific manner.7 The only cardiac-specific member in the nebulin family is nebulette, a 107kDa protein, that appears to be involved in early events of myogenesis and myofibrillar organization in cardiac muscle.8,9 The “nebulin-repeat”- domain of nebulette tethers it in the I-band, while the C-terminus is embedded inside the Z-line lattice, interacting with α-actinin2,9 the PEVK-domain of titin,7 myopalladin,10 desmin,11 and filamin C.12 The significant increase in the affinity of tropomyosin-troponin for F-actin by nebulette was observed previously.13 Involvement in all these protein-protein interactions suggests the functional importance of nebulette.
Nebulette polymorphism (Asn654Lys) in the actin-binding motif was associated with non-familial DCM.14 To determine if the nebulette gene (NEBL) plays a role in the genetic and cellular basis of cardiac disorders, we screened the nebulette gene in patients with cardiomyopathies. Transgenic (Tg) mice with cardiac-specific over-expression of nebulette were created and functional analysis was pursued to explore the molecular mechanisms of the development and regulation of the clinical phenotypes seen in humans. We investigated the effect of the mutations by applying cyclic mechanical strain in transient nebulette-expressing H9C2 cardiomyoblasts.
METHODS
Mutational Analysis
We assessed genomic DNA from 260 patients with DCM recruited after informed consent in the NIH-funded Pediatric Cardiomyopathy Specimen Registry (PCMR) repository (PCSR; R01 HL087000-01A1, JAT). Cytoskeletal and sarcomeric genes were investigated for mutations as previously described.15 We screened more than 20 candidate genes that met criteria for disease-causation. For each variant identified, available family members, as well as 300 unrelated ethnic matched individuals, were screened.
Human Heart Immunohistochemistry
Paraffin-embedded sample of explanted heart from patient S84-5162 carrying the Q128R variant was available. As control, heart tissue was obtained from normal donor subject after informed consent. The samples were dewaxed and stained with nebulette, desmin, myopalladin and α-actinin2 antibodies using standard techniques.15
Generation and Characterization of α-MyHC-Nebulette Transgenic Embryos and Mice
Full-length 3.1 kb human nebulette wild-type (WT) cDNA (#NM_006393) was amplified by reverse transcription-polymerase chain reaction (RT-PCR) from human heart RNA (Ambion) and ligated into vector containing murine α-myosin heavy chain (α-MyHC) gene promoter with downstream human growth hormone (hGH) gene (Dr. Robbins, University of Cincinnati, OH). Four mutants, K60N, Q128R, G202R, and A592E were created using the QuikChange Site-directed Mutagenesis Kits (Stratagene, La Jolla, CA). The Tg mice were created according to the Baylor College of Medicine animal regulations. Offspring were genotyped using PCR of genomic DNA. Transcription and protein expression levels were confirmed by real-time quantitative RT-PCR and Western blot analysis. Embryos were studied at E7.5, 9.5, 12.5 and 16.5 stages (N>3).
To find the latent intolerance to exercise, Tg and age- and gender-matched non-transgenic (non-Tg) mice underwent graded treadmill testing at four and six months of age (N≥5). The treadmill was set (Exer-6M, Columbus Instruments, OH) at a slope of 15° with a starting speed of 16 m/min. Speed was increased by 2 m/min every 5 min until the mouse exhibited signs of exhaustion, and the total running distance was recorded. Echocardiography and cardiac magnetic resonance imaging (cMRI) were performed as described previously (N=5).15
Assessment of Transgenic Mouse Hearts
The mouse hearts were studied by histology, immunohistochemistry, Masson trichrome or Transmission Electron Microscope (TEM) as described previously.15 Antibodies against nebulette and myopalladin,11 α-actinin (Upstate, Chicago, IL), desmin (Abcam, Cambridge, MA), Z-band alternatively spliced PDZ-motif protein (ZASP; Dr. Georgine Faulkner, ICGEB, Trieste, Italy), tropomyosin, cardiac troponin I (cTnI), cardiac troponin T (cTnT) and filamin C (Santa Cruz Biotechnology) were diluted as recommended by the manufacturer. Multiple sections from at least 5 mice per group were analyzed.
The level of protein expression was quantified using Western blotting and relative intensity (RI) was carried out using a Kodak Image system. Beta-actin was chosen as a control, since nebulette only interacts with the muscle elements (alpha- and F-actin) and not the nonmuscle elements (beta-actin) in cardiac background.16
Nebulette Expression in H9C2 Cells and Differentiation Under Cyclic Mechanical Stretch
Rat H9C2 cardiomyoblasts were used to study the effects of nebulette mutations on myofibrillar assembly during differentiation under mechanical stress. The human WT and mutant nebulette cDNA ligated into a pcDNA3.1NT-GFP-TOPO vector (Invitrogen, Carlsbad, CA) were prepared and transfected into H9C2 cells plated on BioFlex plates using an Effectine (Qiagen, Valencia, CA). After 48 hours, cells were cultured in DMEM containing 2% of heat-inactivated horse serum, and subjected to 10% one-hour/day cyclic mechanical stretch for 3 days, as previously described.17 The H9C2 cells were fixed, then stained with anti-GFP followed by labeling with Alexa Fluor Phalloidin-594 (Invitrogen, Carlsbad, CA) and visualized using an Olympus confocal microscopy.
Statistical Analysis
Statistical analysis was performed using the Student’s t and ANOVA tests. Data were reported as the mean with standard error mean (SEM). Values of P<0.05 were considered significant.
RESULTS
Mutations and Clinical Correlation
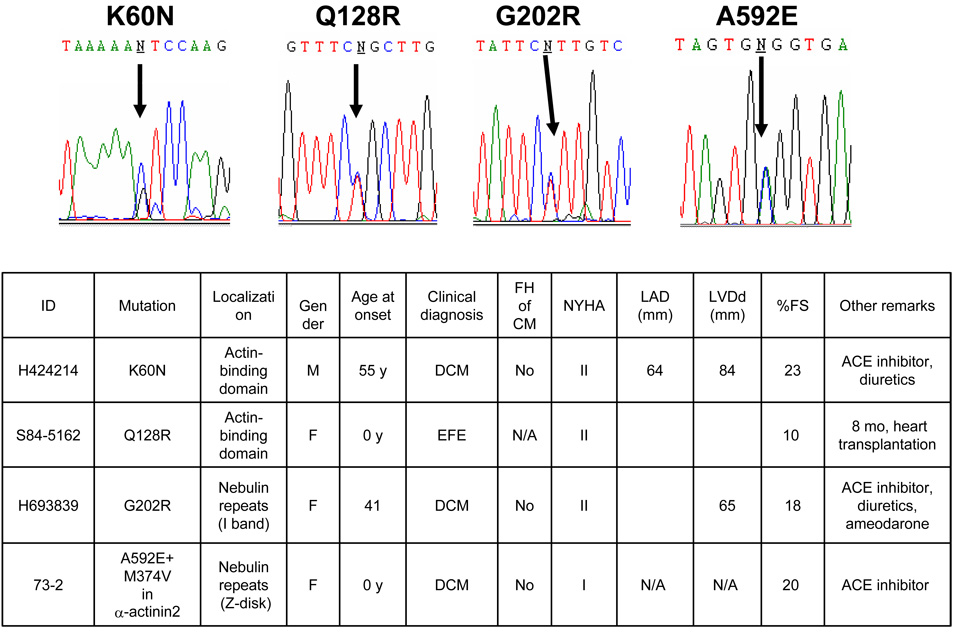
We identified four variants in NEBL: c.180G> C (exon 3; p.K60N), c.383A>G (exon 5; p.Q128R), c.604G>A (exon 7; p.G202R) and c.1775C>A (exon 17; p.A592E) (Fig.1A). K60N has previously been reported in the dbSNP database (rs41277374), but neither the population frequency nor effect on function has been reported. Variants K60N, Q128R and G202R are located in the “nebulin-repeat”-domain, which binds to F-actin and the tropomyosin-troponin complex, while A592E is located in the Z-disk binding region. None of these variants were identified in 300 race- and ethnicity- matched control subjects (600 alleles). Family analysis was available only for K60N variant, which confirmed de-novo origin. The clinical features of the affected subjects hosting these variants were heterogeneous. The newborn patient (S84-5162) carrying the Q128R variant had DCM and EFE with a clinical phenotype demonstrating endocardial thickening, deposition of elastic tissue and collagen (Fig.3A) along with a severely dilated LV and severely depressed systolic function by echocardiography based on fractional shortening (FS) of 10%. The K60N and G202R variants, however, were identified in patients who developed clinical manifestations of DCM in adulthood. The patient carrying the A592E variant displayed the clinical features of DCM as a newborn; this patient also carried a digenic mutation in the α-actinin2 (ACTN2) gene (Fig.1B).
Figure 1. Nebulette mutations in cardiomyopathy.
Upper Panel. Sequence electropherograms of NEBL showing the nucleotide substitutions. Lower Panel. Genetic and clinical features of patients carrying mutations in NEBL.
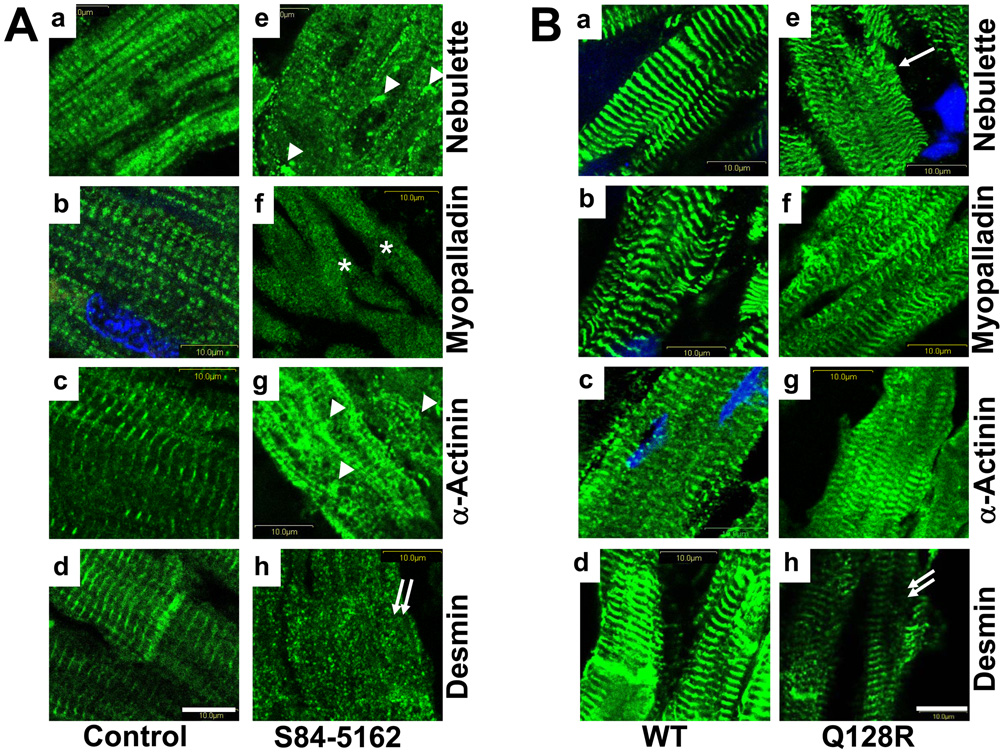
Figure 3. Comparative immunohistochemical analysis of human or mouse hearts.
Panels a–d are sections from human (A) or WT-Tg mouse (B) control hearts. Panels e–h are sections from the S84-5162 proband’ heart (A) or Q128R-Tg chimera heart (B), respectively. Nebulette (a, e); myopalladin (b, f); α- actinin2 in (c, g); and desmin (d, h) are shown in green, nuclei are blue (DAPI). Bar=10 µm.
Mutations, K60N and Q128R, Cause Embryonic Lethality and DCM in Chimera Mice
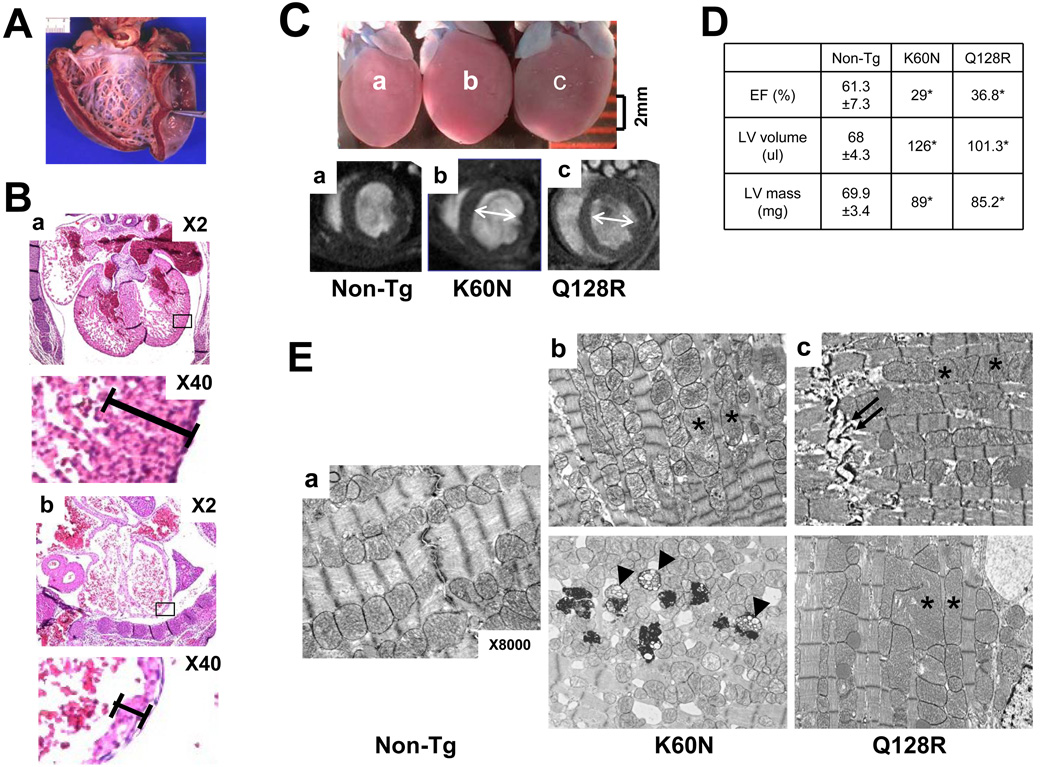
WT, G202R, and A592E mice showed 45%, 40% and 35% transmission frequencies, respectively, however, we were unable to identify positive livable offspring from K60N and Q128R founders. No mutant embryos were harvested from the K60N line at E7.5 or later, while mutant Q128R embryos were harvested up to age E12.5. Since K60N Tg embryos numbers were 50–70% less than expected, the possibility of embryonic lethality at an earlier stage of development could not be excluded. Histology of the Q128R embryonic hearts revealed severe cardiac changes including biventricular dilation (Fig.2B-b, X2) and wall thinning (X40). Such cardiac structural changes are potential causes of embryonic death.
Figure 2. Morphohistologic, functional and ultrastructural features of Q128R hearts of the proband, Tg embryos and chimera mice.
Panel A. Gross morphology of the Q128R proband heart. Panel B. Representative H&E sections of the hearts from (a) WT-Tg and (b) Q128R-Tg embryos at embryonic stage E12.5. Upper panel represents X2; lower panel represents X40 magnification. Panel C. Representative gross morphology and short axis LV end-diastolic cMRI images of mouse hearts. Heart enlargement and LV dilation (arrows) in K60N-Tg (b), and Q128R-Tg (c) chimera mouse hearts compared to non-Tg (a). of non-Tg (a), Bar-0.2cm. Panel C. cMRI analysis of mouse hearts. (*−p<0.05). Panel E. TEM analysis of WT-Tg (a), K60N (b) and Q128RTg (c) mouse hearts (X8000).
Founders of the K60N and Q128R lines died at 1 year of age with severe heart failure. Enlargement of the heart was evident (Fig.2C). cMRI of hearts revealed mild increase in LV mass but remarkable dilation in mutants. Ejection fraction (EF) was decreased (p<0.05) in compared to control mice (Fig.2D). TEM of the K60N and Q128R hearts revealed enlarged and deformed mitochondria (Fig.2E, asterisks) as well as abnormal lysosomes and mitochondrial remnants (arrowheads). Localized intercalated disc disruption and accumulation of lipids in cardiomyocytes were noted in Q128R (arrows).
Comparative immunohistochemistry of human (control and S84-5162) and transgenic mouse hearts (non-Tg and Q128R) revealed that nebulette was seen disassociated with Z-lines, probably, depositing as aggregates throughout the sarcoplasm in the S84-5162 heart (Fig.3A-e, arrowheads); the Q128R mutant mice exhibited punctate foci and more diffuse fluorescence for nebulette than the controls (Fig.3B-e, arrows); myopalladin (f) and α-actinin2 (g) were smeared in S84-5162 (asterisks), whereas loss of desmin association with Z-disks in both S84-5162 and Q128R-Tg hearts was notable (h, double arrows). In Q128R-Tg mouse hearts, (Fig.3B-e), myopalladin (f), and α-actinin2 (g) were not altered.
Transgenic G202R and A592E Mice Exhibit Signs of DCM
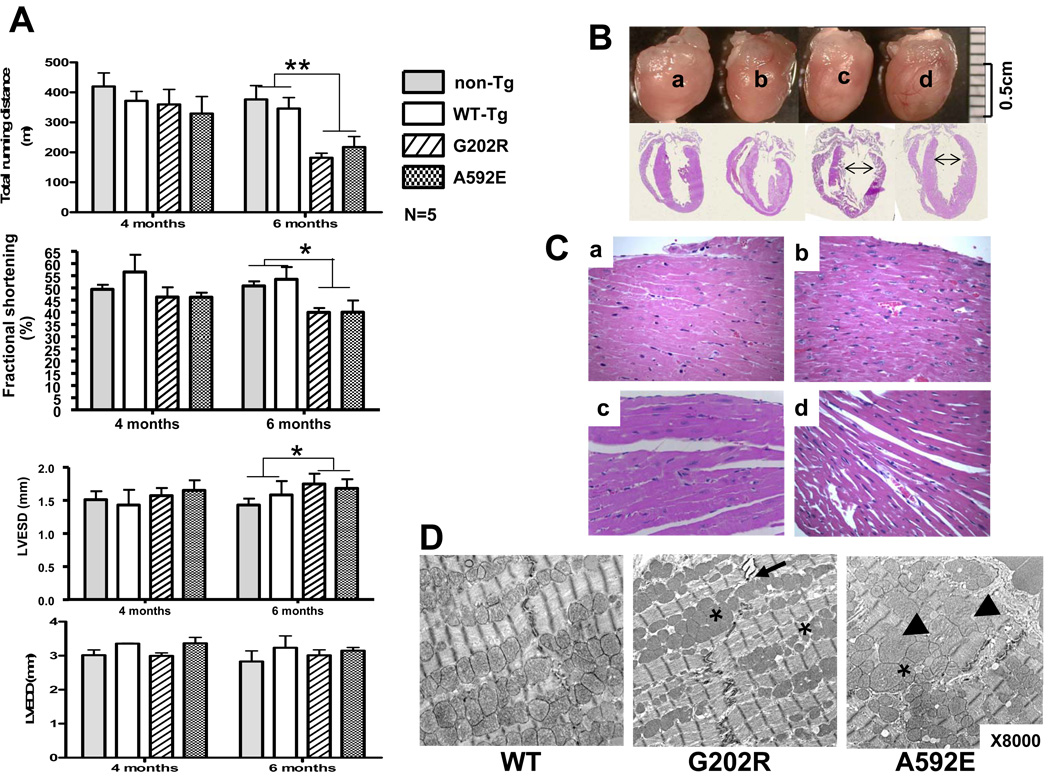
WT, G202R and A592E Tg mice were born and initially developed normally. By 6 months of age, marked reduction in the tolerance against acute stress was identified in G202R and A592E mice (p<0.01). This was evidenced by decreased running distances (270.7±89.0 m and 273.4±55.77 m, respectively) compared to non-Tg (398.2±21.54) and WT-Tg mice (358.0±13.00) (Fig.4A, upper panel). Decreased FS in mutant mice was noted: 50.37±0.7% and 55.24±1.5% in non-Tg and WT-Tg, respectively, versus 43.36±3.1% in G202R-Tg and 43.14±3.1% in A592E-Tg (p<0.05). Significant dilation of the LV, as evidenced by increased LV end-systolic dimensions (LVESD) in G202R-Tg (1.66±0.04 mm) and A592E-Tg (1.67±0.02 mm) compared to non-Tg (1.47±0.04 mm) and WT-Tg (1.51±0.07 mm) mice without changes in LV end-diastolic dimensions (LVEDD) were detected (Fig.4, lower panels). The LV mass-body weight ratio was unchanged compared to normal (data not shown).
Figure 4. Cardiac function and morphology in transgenic mice.
Panel A. Graded treadmill testing and echocardiography in 4- and 6-month-old mice (N=5). Mutant G202R (stripe) and A592E (dots) mice exhibited a marked reduction in exercise tolerance (p<0.01**) compared to non-Tg (grey) and WT-Tg (white) mice. Decrease of FS (p<0.05*) and increase of LVESD (p<0.05*) was evident in mutants. Panel B. Morphology and long-axis four-chamber images of mouse hearts. Bar=0.5 cm. Panel C. Cardiac histology with H&E (X40). Representative sections from non-Tg (a), WT-Tg (b), G202R (c), and A592E (d) are shown. Panel D. Ultrastructural analysis of mouse hearts at X8000.
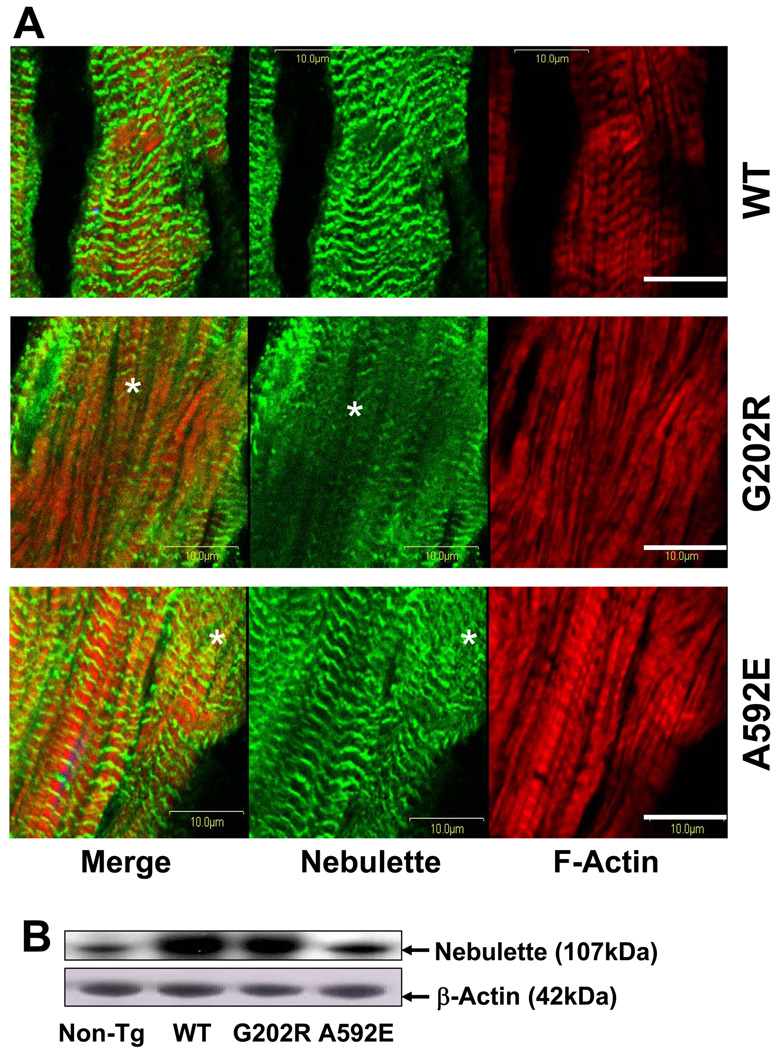
Cardiac enlargement due to LV dilation (Fig.4B, arrows) along with myocyte disarray and interstitial cell infiltration was evident in the hearts of the G202R and A592E mice (Fig.4C, c and d, respectively). TEM revealed focal separation of intercalated disks (Fig.4D, arrow), increased residual body liposome and mild variation in mitochondrial shape in the G202R mutants (asterisks), while the A592E-Tg hearts had mild accumulation of lipids (arrowheads) and abnormalities of mitochondrial shape and size. Disorganization and disarray of over-expressed nebulette in G202R-Tg and A592E-Tg hearts were detected by immunochemical analysis (Fig.5A, asterisks). Protein analysis (Fig.5B) revealed over-expressed nebulette in Tg mouse hearts (RI=1.9 to 3.4) compared to non-Tg animals (RI=1.0).
Figure 5. Immunohistochemical analysis of nebulette in mouse hearts.
Panel A. Representative cardiac sections stained for nebulette (green), F-actin (red), and DAPI (blue). Bar=10µm. Panel B: Level of expression of nebulette in mouse hearts. Loading control is beta-Actin.
Effects of Nebulette Mutations on I-band and Z-disk Composition
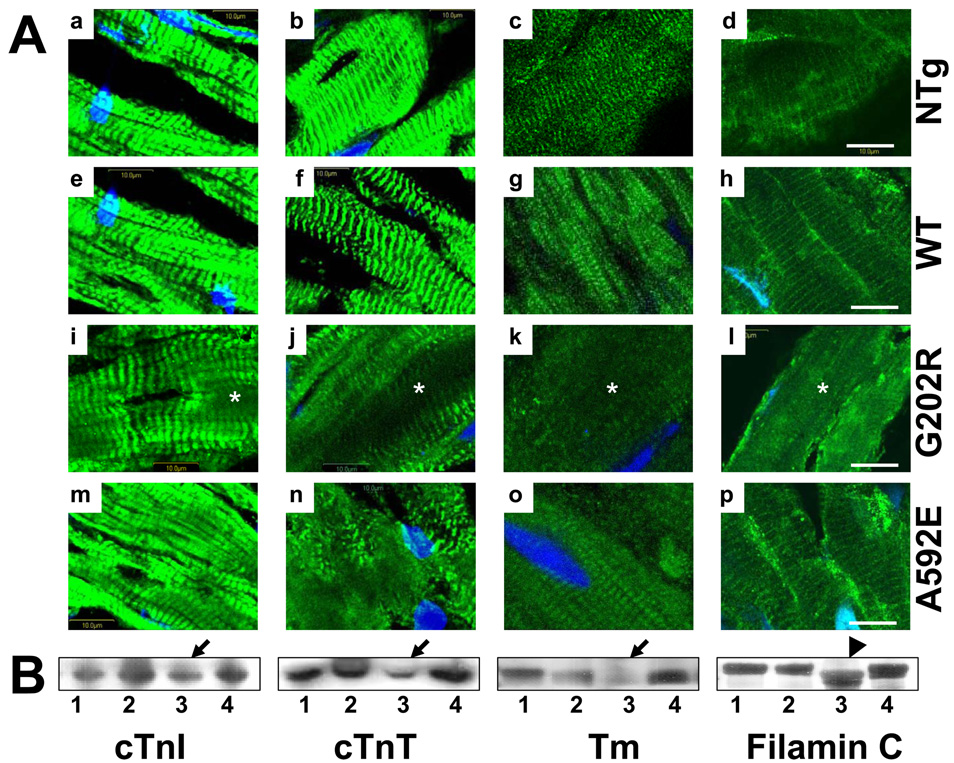
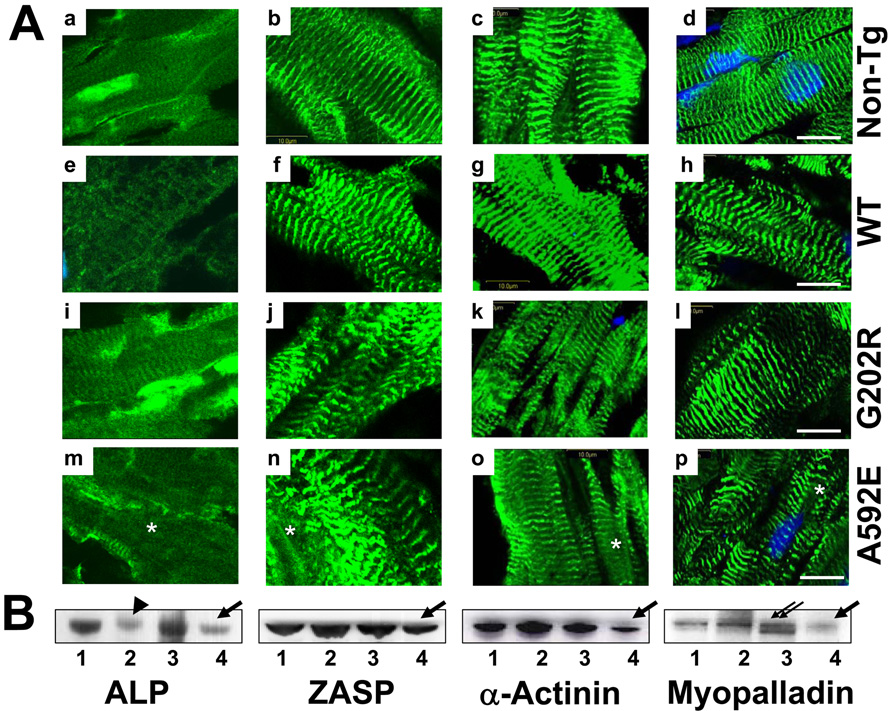
Proteins expressed mainly in the I-band, such as cTnI (RI=0.5), cTnT (RI=0.3), and tropomyosin (RI=0.2) were significantly downregulated (Fig.6B, arrows) and disrupted in G202R-Tg mutants (Fig.6A, i–l, asterisks). The mobility of filamin C by Western blot analysis was abnormal suggesting cleavage (Fig.6B, arrowhead). In contrast, Z-disk-associated proteins, such as α-actinin-associated LIM protein (ALP) (RI=0.2), ZASP (RI=0.7), α-actinin2 (RI=0.4), and myopalladin (RI=0.3) appeared to be downregulated (Fig.7B, arrows) and more disrupted (Fig.7A, m–p, asterisks) in the A592E-Tg hearts. Localization of myopalladin in Z-disks in the G202R- and A592E-Tg mouse hearts appeared normal; however, Western blotting revealed a second lower molecular weight band in the G202R mice, suggesting cleavage (Fig.7B, double arrows). We also noted that ALP expression was downregulated in WT (RI=0.2, arrowhead) mouse hearts compared to non-Tg (RI=1.0) and G202R-Tg mice.
Figure 6. Comparative analysis of I-band proteins in mouse hearts.
Panel A. Representative cardiac sections stained for cTnI (a, e, i and m), cTnT (b, f, j and n), Tm (c, g, k and o), and filamin C (d, h, l and p) from non-Tg (a–d), WT- (e–h), G202R (i–l) and A592E (m–p) mice. Proteins are green and nuclei are blue (DAPI). Bar=10µm. Panel B. Western blot analysis of I-band proteins in non-Tg (1), WT- (2), G202R- (3) and A592E-Tg (4) hearts. Beta-Actin is used as a loading control (Figure 5B). Downregulation is indicated by arrows and cleavage is indicated by arrowhead.
Figure 7. Comparative analysis of Z-disk proteins in mouse hearts.
Panel A. Representative cardiac sections stained for ALP (a, e, i and m), ZASP (b, f, j and n), α-actinin-2 (c, g, k and o), and myopalladin (d, h, l and p) from a non-Tg (a–d), WT (e–h), G202R (i–l) and A592E (m–p) mouse. Proteins are green and nuclei are blue (DAPI). Bar=10µm. Panel B. Western blotting of Z-disk proteins in non-Tg (1), WT (2), G202R (3) and A592E (4) hearts. Beta-Actin is used as a loading control (Figure 5B). Arrows and arrowhead indicate downregulation, asterisk indicates cleavage.
Abnormal Distribution of Mutated Nebulette Under Cyclic Mechanical Stretch
We have chosen rat H9C2 embryonic cardiomyoblasts (ATCC, Manassas, VA) as a model of cardiomyocytes to investigate nebulette expression pattern during differentiation. Transfection efficiency of WT and mutant nebulette-GFP in H9C2 cells was approximately 10%. Perinuclear cytoplasmic localization of nebulette-GFP was observed in differentiating WT and mutant cells (data not shown).
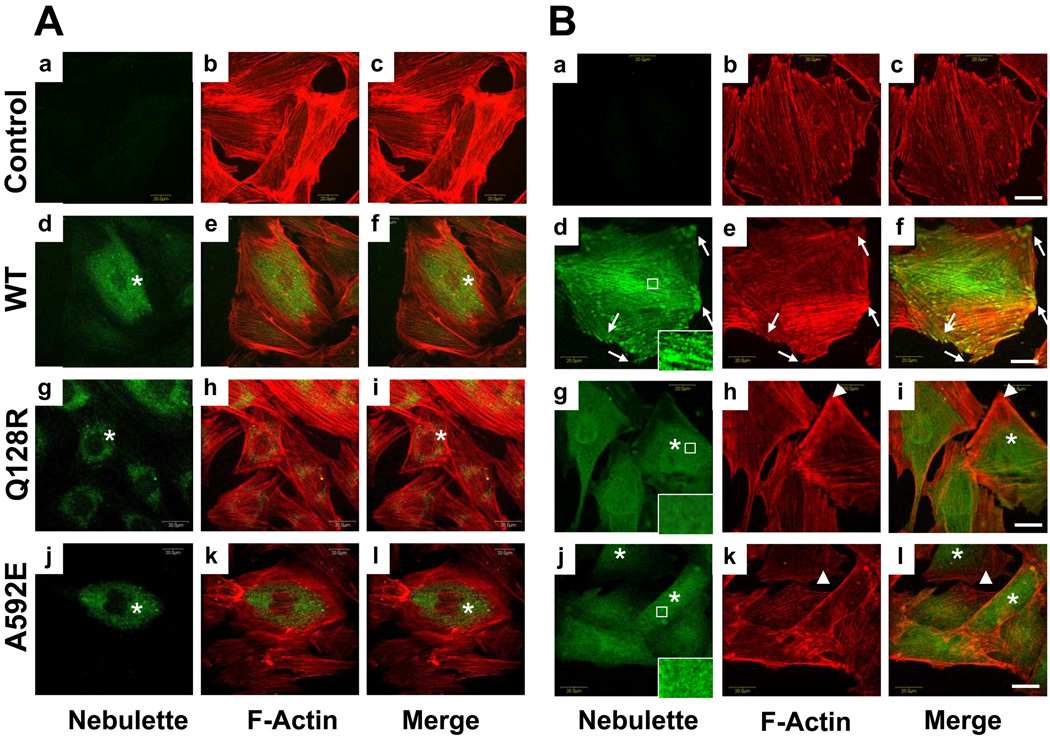
Further, we hypothesized whether nebulette can act as a stretch-induced mechanosensor in cardiomyoblasts.16 To test this hypothesis as well as to investigate expression of mutated nebulette in differentiating cardiomyoblasts during mechanical stretch we performed cyclic mechanical strain of H9C2 cardiomyoblasts. On day 1 of cyclic mechanical stretch, both WT and mutant nebulette-GFP were localized uniformly to the perinuclear region in differentiating cells (Fig.8A, asterisks). By day 3, WT nebulette was distributed throughout the cytoplasm along with F-actin filaments reaching the periphery of cells (Fig.8B, d–f, arrows) and tended to form lines, presumably, assembling into the maturating Z-lines (higher resolution, right corner). In contrast, despite of normally distributed F-actin filaments extended to periphery of sarcomere (Fig.8B, g–l, arrowheads), sustained perinuclear nebulette-GFP localization in all mutants was evident (asterisks, Q128R and A592E mutants representing I band or Z-disk mutations, respectively, are shown).
Figure 8. Effects of cyclic mechanical stretch of H9C2 cells.
Panel A. Cells after cyclic strain without differentiation. Panel B. Differentiating H9C2 cells after cyclic mechanical strain. Expression of nebulette (green) and/or actin (red) are shown in control rat H9C2 cardiomyoblasts (a–b), H9C2 cells expressing WT-nebulette-GFP (c–e), Q128R (g–i) and A592E (j–l) representative cells with transient expression of each mutant nebulette-GFP, respectively. Bar=20 µm. Experiments were repeated three times to confirm stability of results.
DISCUSSION
Over the past decade, variants in a many genes encoding structural proteins have been reported in patients with cardiomyopathies.1 In particular, genes encoding cytoskeletal and sarcomeric proteins, including Z-disk proteins, have been found to be the “final common pathway” of DCM.18 The events leading to EFE are not well understood, but could result from an environmental insult, such as a virus or chemical exposure, in a genetically susceptible individual.2,3 The goal of this study was to uncover the molecular basis of cardiac dysfunction in subjects with DCM. We focused in the sarcomere and Z-disk and identified NEBL gene variants (K60N, Q128R, G202R and A592E) in patients with DCM and EFE. We demonstrated that the mutations lead to a variety of cardiac phenotypes and levels of severity in vivo, from embryonic lethality due to a lethal cardiac abnormality (K60N and Q128R), to DCM with clinical signs of impairment in cardiac function (G202R and A592E) as seen in humans recapitulating the human disease phenotype, with or without EFE. Interestingly, G202R and A592E mutations were found to result in cardiac dysfunction despite causing different ultrastructural changes. I-band proteins were significantly disrupted in G202R mouse hearts, whereas Z-disk associated proteins appeared to be more disrupted in the A592E hearts. These facts led us to hypothesize that NEBL mutations have different effects on protein function depending on the location of the mutation in specific portions of the “nebulin-repeats”.
Nebulette is a component of the early dense body structures involved in myofibrillogenesis of developing cardiac muscle.8,9 We demonstrated that cyclic mechanical strain appeared to be the factor which initiated the distribution of nebulette through the sarcomere and, probably, could be important determinant in stimulating formation of Z-lines in differentiating H9C2 cardiomyoblasts. To support this, we found that nebulette mutations did not affect nebulette distribution and differentiation of cardiomyoblasts. When H9C2 cells subjected to cyclic strain, we demonstrated that nebulette mutations perturbed the co-distribution of nebulette-GFP throughout the sarcomere with the actin filaments and delayed expression of nebulette-GFP in the maturating Z-lines under cyclic mechanical strain. Taken together, these data support our hypothesis that nebulette may function as a mechanosensor and may have signaling properties in stretch-induced activation of specific mechanotransduction pathways in cardiomyoblasts during myogenesis. These data also support our in vivo results that demonstrate that expression of mutant Q128R nebulette leads to embryonic lethality due to severe structural abnormalities in the embryonic heart. Moreover, an abnormal expression pattern for nebulette, myopalladin and desmin was discovered in the heart of patient carrying the Q128R mutation. It is possible that interruption of these proteins could cause the specific phenotype of EFE in humans. We speculate that nebulette-desmin chain transmits information from the Z-disks to the intermediate filaments maintaining the structural and functional integrity of myocytes.12 Based on its timing during embryogenesis, as well as its functional effects on key binding partners, it appears likely that Q128R mutation alters cardiac development, specifically affecting myofibrillogenesis through the early nebulette-actin interactions, later nebulette-desmin association. We assume that disruption in nebulette-actin co-distribution and/or delay in nebulette expression in the maturating Z-disks at the earliest stages of myogenesis may be present in K60N mice.
In contrast to K60N and Q128R, the effect of the G202R mutation, which resulted in downregulated tropomyosin and troponins in the mutant mice, suggests that this mutation may cause changes in force generation in mature cardiomyocytes.19 Our data was consistent with Bonzo et al reported that overexpression of nebulette fragments was associated with loss of endogenous nebulette tropomyosin and troponins accompanied with decrease in thin filament length and concomitant loss of F-actin, but not overall actin staining. 7 In addition, “nebulin-repeats” was found to contribute to the assembly and the allosteric property of the striated muscle thin filament.14 Although, we did not measure actin-filaments length in G202R mice, it is likelihood dysregulated tropomyosin, troponins and filamin C may cause development of LV dilation.
On the other hand, the A592E mutation led to cytoarchitectural changes with different patterns in expression of Z-disk proteins. It is believed that myofibrillar functional integrity is regulated by the Z-disk, network linking the sarcomere, cytoskeleton, sarcoplasmic reticulum and sarcolemma.4 Dysregulation of Z-disk proteins in A592E-Tg hearts suggests that A592E may disturb force transmission. Mature cardiomyocytes respond to mechanical stretch with an immediate increase in contractility as well as long-term changes in gene expression resulting in myocyte hypertrophy.5 Orchestrated cross-talking between multiple independent and mechanosensitive signaling pathways such as MAPK, PI3K/Akt, Ras, JAK/STAT and Ca2+ signaling determines the final phenotype of the cardiomyopathies. The desmin-based unit is one of the major contributors in mechanotransduction12,16 and it appears that I-band-nebulette-Z-disk connection may play a crucial role in transmitting information from the Z-disks to the intermediate filaments.
We identified novel nebulette mutations (K60N, Q128R, G202R and A592E) in patients with DCM, EFE and cardiac failure. We propose that NEBL is a new susceptibility gene for EFE and DCM and these mutations lead to cardiac remodeling and dysfunction. In summary, we suggest that disruption of the proposed signaling pathways leads to unfavorable cardiac remodeling and consequently cardiac dysfunction. Furthermore, mutations in certain domains of nebulette will alter the function of it’s interacting proteins in distinct, domain-defined manner and ultimately will result in activation and/or disruption of signaling cascades, culminating in structural remodeling of cardiomyocytes.
ACKNOWLEDGMENTS
We thank the participating clinicians who contributed to the clinical evaluation and blood sampling of the patients, as well as the subjects and families themselves. This work was supported in part by a Postdoctoral Fellowship and Beginning-Grant-in-Aid from the American Heart Association (AHA; EP), the Children’s Cardiomyopathy Foundation (EP, JAT), the Vivian L. Smith Foundation, TexGen, the John Patrick Albright Foundation, and NIH-NHLBI (R01 HL53392, The Pediatric Cardiomyopathy Registry and R01 HL087000, The Pediatric Cardiomyopathy Specimen Repository; JAT); Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology, Japan, grants for Japan–France collaboration research and Japan-Korea collaboration research from the Japan Society for the Promotion of Science, and research grants from the Ministry of Health, Labour and Welfare, Japan, Seizon Kagaku Foundation, and Association Française contre les Myopathies, France (AK, TA); the Mayo Clinic Windland Smith Rice Comprehensive Sudden Cardiac Death Program (MJA).
Abbreviations
- EFE
endocardial fibroelastosis
- DCM
dilated cardiomyopathy
- PCMR
Pediatric Cardiomyopathy Registry
Footnotes
No conflict of interest
REFERENCES
- 1.Towbin JA, Lowe AM, Colan SD, et al. Incidence, causes, and outcomes of dilated cardiomyopathy in children. JAMA. 2006;296(15):1867–1876. doi: 10.1001/jama.296.15.1867. [DOI] [PubMed] [Google Scholar]
- 2.Kamisago M, Schmitt JP, McNamara D, et al. Sarcomere protein gene mutations and inherited heart disease: a beta-cardiac myosin heavy chain mutation causing endocardial fibroelastosis and heart failure. Novartis Found Symp. 2006;274:176–189. 272–276. [PubMed] [Google Scholar]
- 3.Ni J, Bowles NE, Kim YH, Demmler G, et al. Viral infection of the myocardium in endocardial fibroelastosis. Molecular evidence for the role of mumps virus as an etiologic agent. Circulation. 1997;95(1):133–139. doi: 10.1161/01.cir.95.1.133. [DOI] [PubMed] [Google Scholar]
- 4.Pyle WG, Solaro RJ. At the crossroads of myocardial signaling: the role of Z-discs in intracellular signaling and cardiac function. Circ Res. 2004;94(3):296–305. doi: 10.1161/01.RES.0000116143.74830.A9. 20. [DOI] [PubMed] [Google Scholar]
- 5.Towbin JA, Bowles NE. The failing heart. Nature. 2002 Jan 10;415(6868):227–233. doi: 10.1038/415227a. [DOI] [PubMed] [Google Scholar]
- 6.Bonzo JR, Norris AA, Esham M, Moncman CL. The nebulette repeat domain is necessary for proper maintenance of tropomyosin with the cardiac sarcomere. Exp Cell Res. 2008 Nov 15;314(19):3519–3530. doi: 10.1016/j.yexcr.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Grunewald TG, Butt E. The LIM and SH3 domain protein family: structural proteins or signal transducers or both? Mol Cancer. 2008 Apr 17;7:31. doi: 10.1186/1476-4598-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moncman CL, Wang K. Nebulette: a 107 kD nebulin-like protein in cardiac muscle. Cell Motil Cytoskeleton. 1995;32(3):205–225. doi: 10.1002/cm.970320305. [DOI] [PubMed] [Google Scholar]
- 9.Esham M, Bryan K, Milnes J, et al. Expression of nebulette during early cardiac development. Cell Motil Cytoskeleton. 2007 Apr;64(4):258–273. doi: 10.1002/cm.20180. [DOI] [PubMed] [Google Scholar]
- 10.Bang ML, Mudry RE, McElhinny AS, et al. Myopalladin, a novel 145-kilodalton sarcomeric protein with multiple roles in Z-disc and I-band protein assemblies. J. Cell Biol. 2001;153:413–427. doi: 10.1083/jcb.153.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X, Osinska H, Gerdes AM, Robbins J. Desmin filaments and cardiac disease: establishing causality. J Card Fail. 2002 Dec;8(6 Suppl):S287–S292. doi: 10.1054/jcaf.2002.129279. [DOI] [PubMed] [Google Scholar]
- 12.Holmes WB, Moncman CL. Nebulette interacts with filamin C. Cell Motil Cytoskeleton. 2008 Feb;65(2):130–142. doi: 10.1002/cm.20249. [DOI] [PubMed] [Google Scholar]
- 13.Ogut O, Hossain MM, Jin JP. Interactions between nebulin-like motifs and thin filament regulatory proteins. J Biol Chem. 2003;278(5):3089–3097. doi: 10.1074/jbc.M205853200. [DOI] [PubMed] [Google Scholar]
- 14.Arimura T, Nakamura T, Hiroi S, et al. Characterization of the human NEBL gene: a polymorphism in an actin-binding motif is associated with nonfamilial idiopathic dilated cardiomyopathy. Hum Genet. 2000;107(5):440–451. doi: 10.1007/s004390000389. [DOI] [PubMed] [Google Scholar]
- 15.Yang Z, Bowles NE, Scherer SE, et al. Desmosomal dysfunction due to mutations in desmoplakin causes Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy. Circ. Res. 2006;99(6):646–655. doi: 10.1161/01.RES.0000241482.19382.c6. [DOI] [PubMed] [Google Scholar]
- 16.Moncman CL, Wang K. Targeted disruption of nebulette protein expression alters cardiac myofibril assembly and function. Exp Cell Res. 2002 Feb 15;273(2):204–218. doi: 10.1006/excr.2001.5423. [DOI] [PubMed] [Google Scholar]
- 17.Kumar A, Murphy R, Robinson P, Wei L, Boriek AM. Cyclic mechanical strain inhibits skeletal myogenesis through activation of focal adhesion kinase, Rac-1 GTPase, and NF kappaB transcription factor. FASEB J. 2004;18(13):1524–1535. doi: 10.1096/fj.04-2414com. [DOI] [PubMed] [Google Scholar]
- 18.Bowles NE, Bowles KR, Towbin JA. The "final common pathway" hypothesis and inherited cardiovascular disease. The role of cytoskeletal proteins in dilated cardiomyopathy. Herz. 2000;25(3):168–175. doi: 10.1007/s000590050003. [DOI] [PubMed] [Google Scholar]
- 19.Lammerding JAN, Kamm RD, Lee RT. Mechanotransduction in Cardiac Myocytes. Ann NY Acad Sci. 2004 May 1;1015(1):53–70. doi: 10.1196/annals.1302.005. [DOI] [PubMed] [Google Scholar]