Abstract
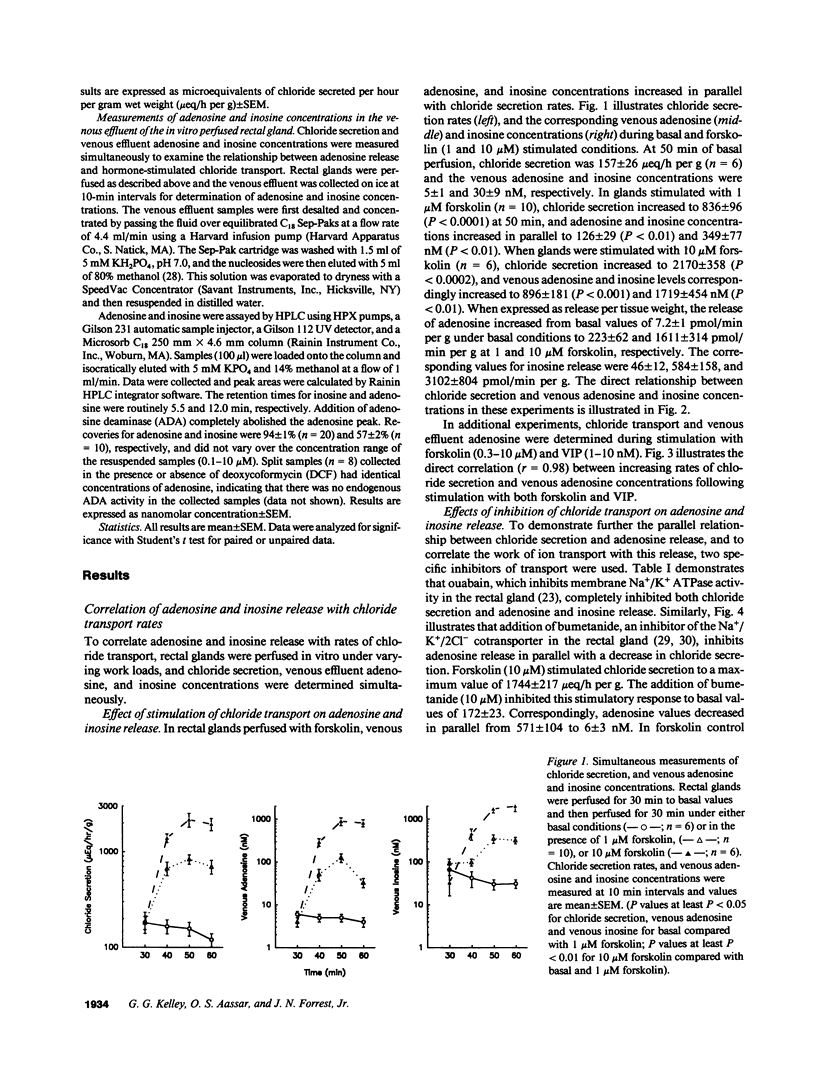
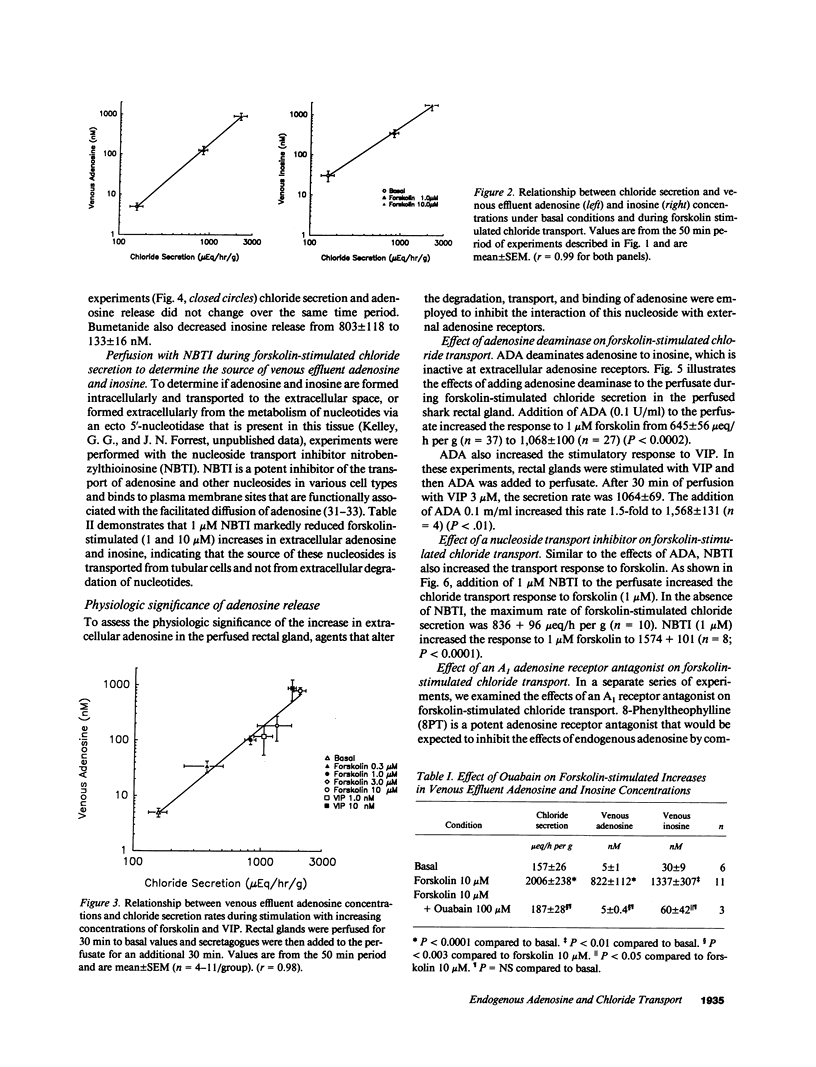
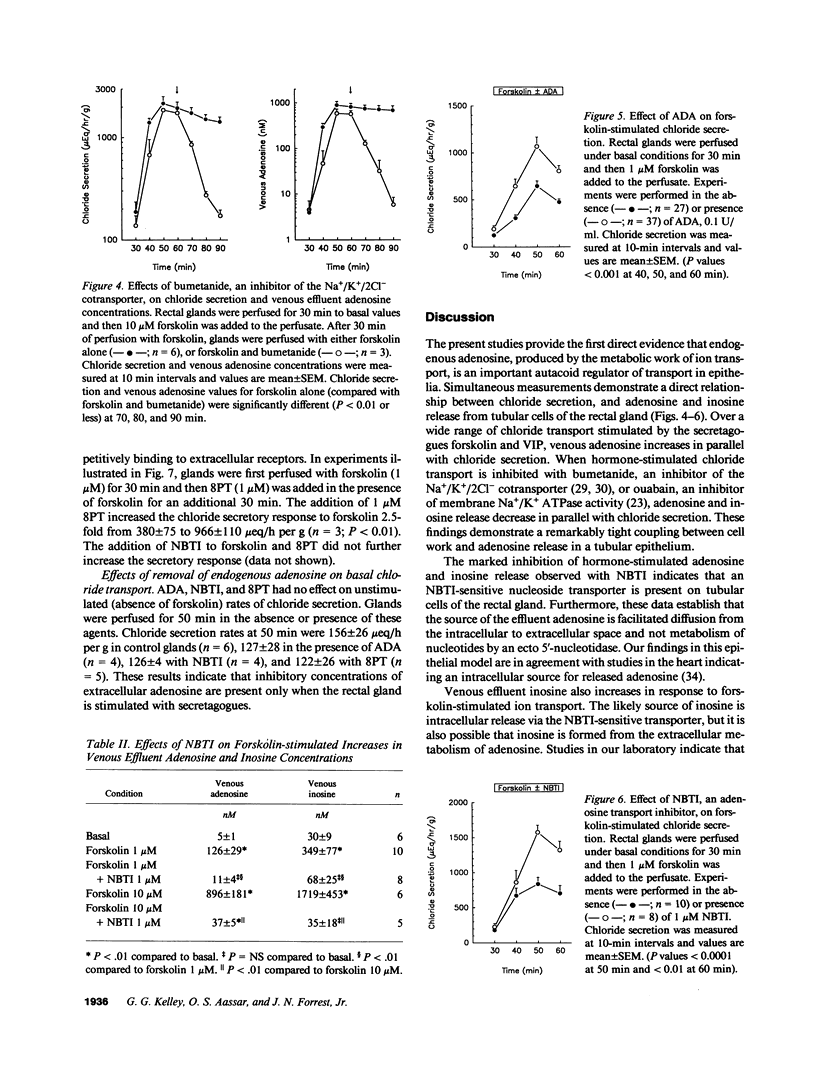
The present studies define the physiologic role of endogenous adenosine in the perfused shark rectal gland, a model epithelia for hormone-stimulated chloride transport. Chloride ion secretion, and venous adenosine and inosine concentrations increased in parallel in response to hormone stimulation. From a basal rate of 157 +/- 26 mu eq/h per g, chloride secretion increased to 836 +/- 96 and 2170 +/- 358 with 1 and 10 microM forskolin, venous adenosine increased from 5.0 +/- 1 to 126 +/- 29 and 896 +/- 181 nM, and inosine increased from 30 +/- 9 to 349 +/- 77 and 1719 +/- 454 nM (all P less than 0.01). Nitrobenzylthioinosine (NBTI), a nucleoside transport inhibitor, completely blocked the release of adenosine and inosine. Inhibition of chloride transport with bumetanide, an inhibitor of the Na+/K+/2Cl- cotransporter, or ouabain, an inhibitor of Na+/K+ ATPase activity, reduced venous adenosine and inosine to basal values. When the interaction of endogenous adenosine with extracellular receptors was prevented by adenosine deaminase, NBTI, or 8-phenyltheophylline, the chloride transport response to secretagogues increased by 1.7-2.3-fold. These studies demonstrate that endogenous adenosine is released in response to hormone-stimulated cellular work and acts at A1 adenosine receptors as a feedback inhibitor of chloride transport.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arch J. R., Newsholme E. A. The control of the metabolism and the hormonal role of adenosine. Essays Biochem. 1978;14:82–123. [PubMed] [Google Scholar]
- Arend L. J., Burnatowska-Hledin M. A., Spielman W. S. Adenosine receptor-mediated calcium mobilization in cortical collecting tubule cells. Am J Physiol. 1988 Nov;255(5 Pt 1):C581–C588. doi: 10.1152/ajpcell.1988.255.5.C581. [DOI] [PubMed] [Google Scholar]
- Arend L. J., Sonnenburg W. K., Smith W. L., Spielman W. S. A1 and A2 adenosine receptors in rabbit cortical collecting tubule cells. Modulation of hormone-stimulated cAMP. J Clin Invest. 1987 Mar;79(3):710–714. doi: 10.1172/JCI112875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERNE R. M. Cardiac nucleotides in hypoxia: possible role in regulation of coronary blood flow. Am J Physiol. 1963 Feb;204:317–322. doi: 10.1152/ajplegacy.1963.204.2.317. [DOI] [PubMed] [Google Scholar]
- Bardenheuer H., Schrader J. Relationship between myocardial oxygen consumption, coronary flow, and adenosine release in an improved isolated working heart preparation of guinea pigs. Circ Res. 1983 Mar;52(3):263–271. doi: 10.1161/01.res.52.3.263. [DOI] [PubMed] [Google Scholar]
- Bardenheuer H., Schrader J. Supply-to-demand ratio for oxygen determines formation of adenosine by the heart. Am J Physiol. 1986 Feb;250(2 Pt 2):H173–H180. doi: 10.1152/ajpheart.1986.250.2.H173. [DOI] [PubMed] [Google Scholar]
- Bockman E. L., Berne R. M., Rubio R. Adenosine and active hyperemia in dog skeletal muscle. Am J Physiol. 1976 Jun;230(6):1531–1537. doi: 10.1152/ajplegacy.1976.230.6.1531. [DOI] [PubMed] [Google Scholar]
- Bockman E. L., Berne R. M., Rubio R. Release of adenosine and lack of release of ATP from contracting skeletal muscle. Pflugers Arch. 1975 Mar 26;355(3):229–241. doi: 10.1007/BF00583686. [DOI] [PubMed] [Google Scholar]
- Bockman E. L., Berne R. M., Rubio R. Release of adenosine and lack of release of ATP from contracting skeletal muscle. Pflugers Arch. 1975 Mar 26;355(3):229–241. doi: 10.1007/BF00583686. [DOI] [PubMed] [Google Scholar]
- Bünger R., Soboll S. Cytosolic adenylates and adenosine release in perfused working heart. Comparison of whole tissue with cytosolic non-aqueous fractionation analyses. Eur J Biochem. 1986 Aug 15;159(1):203–213. doi: 10.1111/j.1432-1033.1986.tb09854.x. [DOI] [PubMed] [Google Scholar]
- Cass C. E., Gaudette L. A., Paterson A. R. Mediated transport of nucleosides in human erythrocytes. Specific binding of the inhibitor nitrobenzylthioinosine to nucleoside transport sites in the erythrocyte membrane. Biochim Biophys Acta. 1974 Apr 12;345(1):1–10. doi: 10.1016/0005-2736(74)90239-9. [DOI] [PubMed] [Google Scholar]
- Daly J. W. Adenosine receptors: targets for future drugs. J Med Chem. 1982 Mar;25(3):197–207. doi: 10.1021/jm00345a001. [DOI] [PubMed] [Google Scholar]
- Dobson J. G., Jr, Schrader J. Role of extracellular and intracellular adenosine in the attenuation of catecholamine evoked responses in guinea pig heart. J Mol Cell Cardiol. 1984 Sep;16(9):813–822. doi: 10.1016/s0022-2828(84)80005-x. [DOI] [PubMed] [Google Scholar]
- Drury A. N., Szent-Györgyi A. The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. J Physiol. 1929 Nov 25;68(3):213–237. doi: 10.1113/jphysiol.1929.sp002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein F. H., Rosen S., Galicka-Piskorska G., Spokes K., Brezis M., Silva P. Relation of adenosine to medullary injury in the perfused rat kidney. Miner Electrolyte Metab. 1990;16(4):185–190. [PubMed] [Google Scholar]
- Forrest J. N., Jr, Wang F., Beyenbach K. W. Perfusion of isolated tubules of the shark rectal gland. Electrical characteristics and response to hormones. J Clin Invest. 1983 Sep;72(3):1163–1167. doi: 10.1172/JCI111041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco M., Bell P. D., Navar L. G. Effect of adenosine A1 analogue on tubuloglomerular feedback mechanism. Am J Physiol. 1989 Aug;257(2 Pt 2):F231–F236. doi: 10.1152/ajprenal.1989.257.2.F231. [DOI] [PubMed] [Google Scholar]
- Fuchs B. D., Gorman M. W., Sparks H. V. Adenosine release into venous plasma during free flow exercise. Proc Soc Exp Biol Med. 1986 Mar;181(3):364–370. doi: 10.3181/00379727-181-42266. [DOI] [PubMed] [Google Scholar]
- Greger R., Schlatter E., Gögelein H. Cl- -channels in the apical cell membrane of the rectal gland "induced" by cAMP. Pflugers Arch. 1985 Apr;403(4):446–448. doi: 10.1007/BF00589260. [DOI] [PubMed] [Google Scholar]
- Greger R., Schlatter E., Wang F., Forrest J. N., Jr Mechanism of NaCl secretion in rectal gland tubules of spiny dogfish (Squalus acanthias). III. Effects of stimulation of secretion by cyclic AMP. Pflugers Arch. 1984 Dec;402(4):376–384. doi: 10.1007/BF00583938. [DOI] [PubMed] [Google Scholar]
- Kelley G. G., Poeschla E. M., Barron H. V., Forrest J. N., Jr A1 adenosine receptors inhibit chloride transport in the shark rectal gland. Dissociation of inhibition and cyclic AMP. J Clin Invest. 1990 May;85(5):1629–1636. doi: 10.1172/JCI114614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller W. L., Thomas R. A., Berne R. M., Rubio R. Adenosine production in the ischemic kidney. Circ Res. 1978 Sep;43(3):390–397. doi: 10.1161/01.res.43.3.390. [DOI] [PubMed] [Google Scholar]
- Murray R. D., Churchill P. C. Concentration dependency of the renal vascular and renin secretory responses to adenosine receptor agonists. J Pharmacol Exp Ther. 1985 Jan;232(1):189–193. [PubMed] [Google Scholar]
- Olsson R. A. Changes in content of purine nucleoside in canine myocardium during coronary occlusion. Circ Res. 1970 Mar;26(3):301–306. doi: 10.1161/01.res.26.3.301. [DOI] [PubMed] [Google Scholar]
- Olsson R. A., Pearson J. D. Cardiovascular purinoceptors. Physiol Rev. 1990 Jul;70(3):761–845. doi: 10.1152/physrev.1990.70.3.761. [DOI] [PubMed] [Google Scholar]
- Osswald H., Hermes H. H., Nabakowski G. Role of adenosine in signal transmission of tubuloglomerular feedback. Kidney Int Suppl. 1982 Aug;12:S136–S142. [PubMed] [Google Scholar]
- Osswald H., Nabakowski G., Hermes H. Adenosine as a possible mediator of metabolic control of glomerular filtration rate. Int J Biochem. 1980;12(1-2):263–267. doi: 10.1016/0020-711x(80)90082-8. [DOI] [PubMed] [Google Scholar]
- Osswald H., Spielman W. S., Knox F. G. Mechanism of adenosine-mediated decreases in glomerular filtration rate in dogs. Circ Res. 1978 Sep;43(3):465–469. doi: 10.1161/01.res.43.3.465. [DOI] [PubMed] [Google Scholar]
- Palfrey H. C., Silva P., Epstein F. H. Sensitivity of cAMP-stimulated salt secretion in shark rectal gland to "loop" diuretics. Am J Physiol. 1984 Mar;246(3 Pt 1):C242–C246. doi: 10.1152/ajpcell.1984.246.3.C242. [DOI] [PubMed] [Google Scholar]
- Phair R. D., Sparks H. V. Adenosine content of skeletal muscle during active hyperemia and ischemic contraction. Am J Physiol. 1979 Jul;237(1):H1–H9. doi: 10.1152/ajpheart.1979.237.1.H1. [DOI] [PubMed] [Google Scholar]
- Ramos-Salazar A., Baines A. D. Role of 5'-nucleotidase in adenosine-mediated renal vasoconstriction during hypoxia. J Pharmacol Exp Ther. 1986 Feb;236(2):494–499. [PubMed] [Google Scholar]
- Rossi N. F., Churchill P. C., Jacobson K. A., Leahy A. E. Further characterization of the renovascular effects of N6-cyclohexyladenosine in the isolated perfused rat kidney. J Pharmacol Exp Ther. 1987 Mar;240(3):911–915. [PMC free article] [PubMed] [Google Scholar]
- Rossi N., Churchill P., Ellis V., Amore B. Mechanism of adenosine receptor-induced renal vasoconstriction in rats. Am J Physiol. 1988 Oct;255(4 Pt 2):H885–H890. doi: 10.1152/ajpheart.1988.255.4.H885. [DOI] [PubMed] [Google Scholar]
- Schnermann J., Weihprecht H., Briggs J. P. Inhibition of tubuloglomerular feedback during adenosine1 receptor blockade. Am J Physiol. 1990 Mar;258(3 Pt 2):F553–F561. doi: 10.1152/ajprenal.1990.258.3.F553. [DOI] [PubMed] [Google Scholar]
- Silva P., Epstein J. A., Stevens A., Spokes K., Epstein F. H. Ouabain binding in rectal gland of Squalus acanthias. J Membr Biol. 1983;75(2):105–114. doi: 10.1007/BF01995630. [DOI] [PubMed] [Google Scholar]
- Silva P., Stoff J., Epstein F. H. Indirect evidence for enhancement of Na-K-ATPase activity with stimulation of rectal gland secretion. Am J Physiol. 1979 Dec;237(6):F468–F472. doi: 10.1152/ajprenal.1979.237.6.F468. [DOI] [PubMed] [Google Scholar]
- Silva P., Stoff J., Field M., Fine L., Forrest J. N., Epstein F. H. Mechanism of active chloride secretion by shark rectal gland: role of Na-K-ATPase in chloride transport. Am J Physiol. 1977 Oct;233(4):F298–F306. doi: 10.1152/ajprenal.1977.233.4.F298. [DOI] [PubMed] [Google Scholar]
- Spielman W. S., Arend L. J. Adenosine receptors and signaling in the kidney. Hypertension. 1991 Feb;17(2):117–130. doi: 10.1161/01.hyp.17.2.117. [DOI] [PubMed] [Google Scholar]
- Spielman W. S., Thompson C. I. A proposed role for adenosine in the regulation of renal hemodynamics and renin release. Am J Physiol. 1982 May;242(5):F423–F435. doi: 10.1152/ajprenal.1982.242.5.F423. [DOI] [PubMed] [Google Scholar]
- Thompson C. I., Sparks H. V., Spielman W. S. Renal handling and production of plasma and urinary adenosine. Am J Physiol. 1985 Apr;248(4 Pt 2):F545–F551. doi: 10.1152/ajprenal.1985.248.4.F545. [DOI] [PubMed] [Google Scholar]
- Watt A. H., Routledge P. A. Adenosine: an importance beyond ATP. Br Med J (Clin Res Ed) 1986 Dec 6;293(6560):1455–1456. doi: 10.1136/bmj.293.6560.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn H. R., Welsh J. E., Rubio R., Berne R. M. Brain adenosine production in rat during sustained alteration in systemic blood pressure. Am J Physiol. 1980 Nov;239(5):H636–H641. doi: 10.1152/ajpheart.1980.239.5.H636. [DOI] [PubMed] [Google Scholar]
- Winn H. R., Welsh J. E., Rubio R., Berne R. M. Changes in brain adenosine during bicuculline-induced seizures in rats. Effects of hypoxia and altered systemic blood pressure. Circ Res. 1980 Oct;47(4):568–577. doi: 10.1161/01.res.47.4.568. [DOI] [PubMed] [Google Scholar]