Abstract
The antithrombotic effects of bovine activated protein C (APC) and protein S were investigated in a rabbit model of microarterial thrombosis. Because of the species specificity of the APC-protein S interaction, bovine APC expresses potent anticoagulant activity in rabbit plasma only when bovine protein S is also present. This provided a way to assess the contribution of bovine protein S to the antithrombotic effect of bovine APC. Rabbits were infused with boluses of activated protein C (0.1, 0.2, 0.4, or 0.8 mg/kg), protein S (0.5 mg/kg), or activated protein C (0.1 or 0.01 mg/kg) plus protein S (0.5 mg/kg). APC alone produced a dose-dependent antithrombotic effect, but only the group receiving the highest dose differed significantly from controls. While a low dose of activated protein C (0.1 mg/kg) alone had no antithrombotic effect, together with protein S (0.5 mg/kg) it produced a potent response. The presented results demonstrate the in vivo significance of protein S as a cofactor to activated protein C. The data show that a potent antithrombotic effect, without hemorrhagic side effects or significant systemic anticoagulation, may be achieved by low doses of activated protein C when combined with protein S.
Full text
PDF

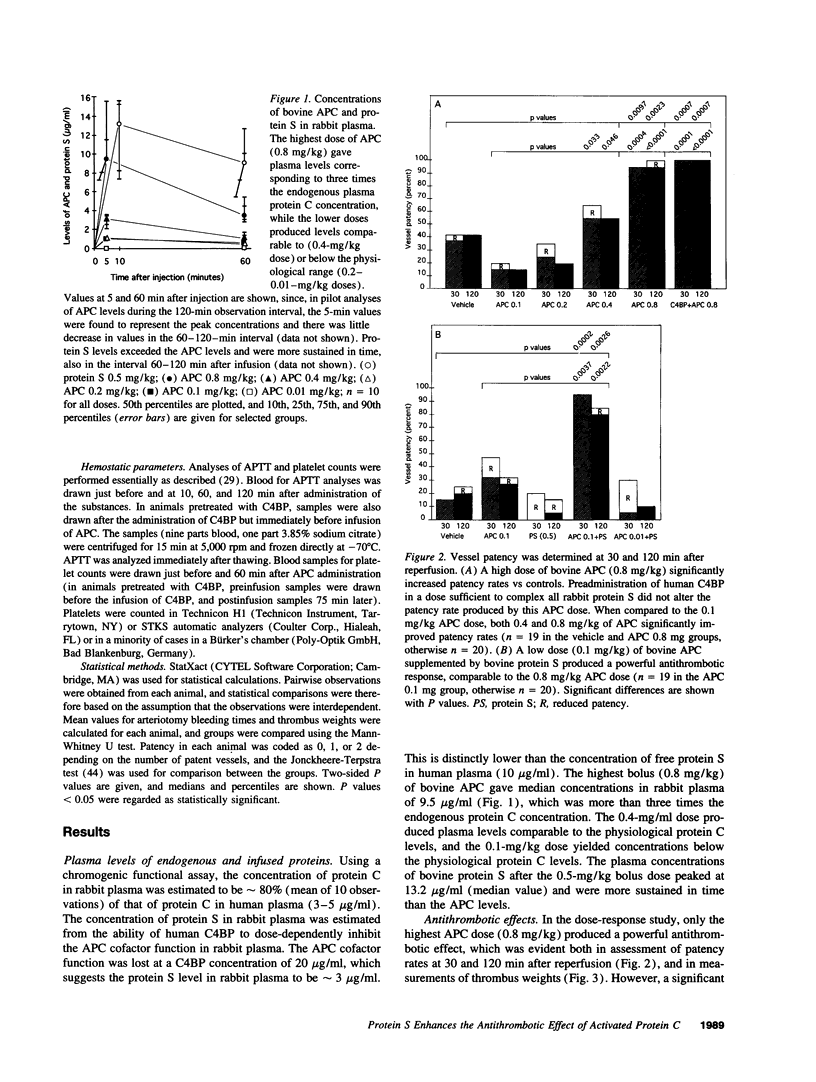
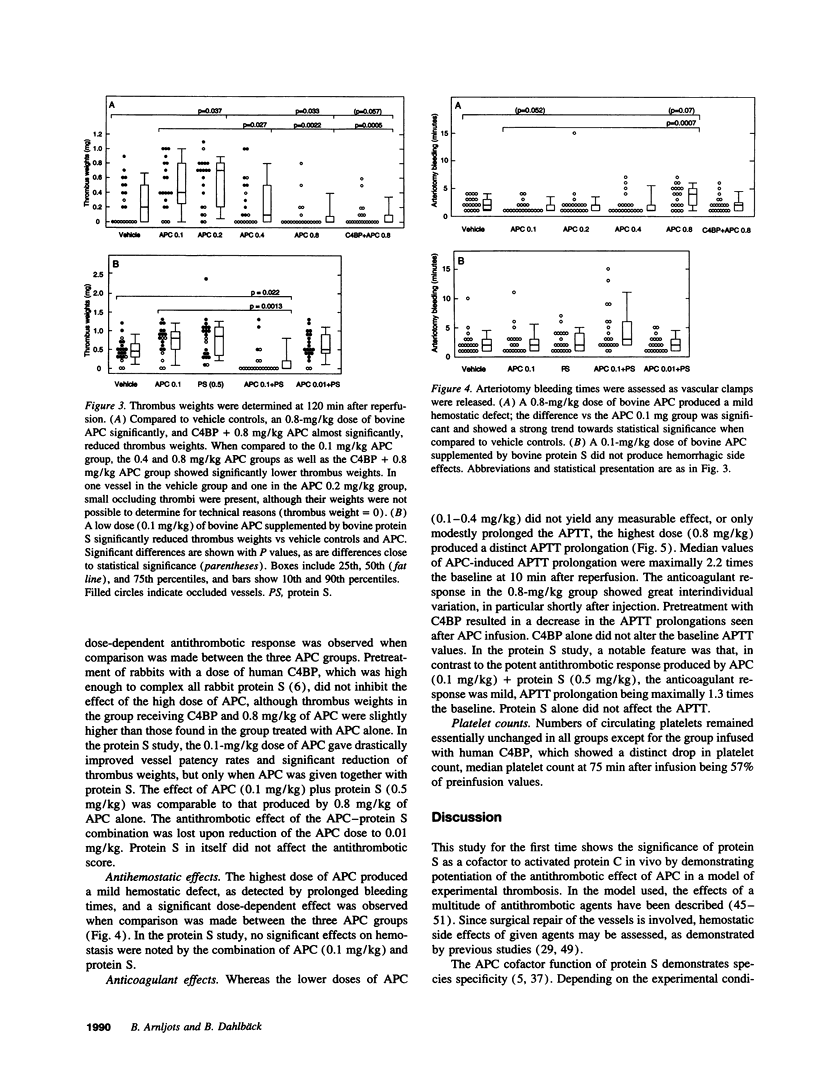
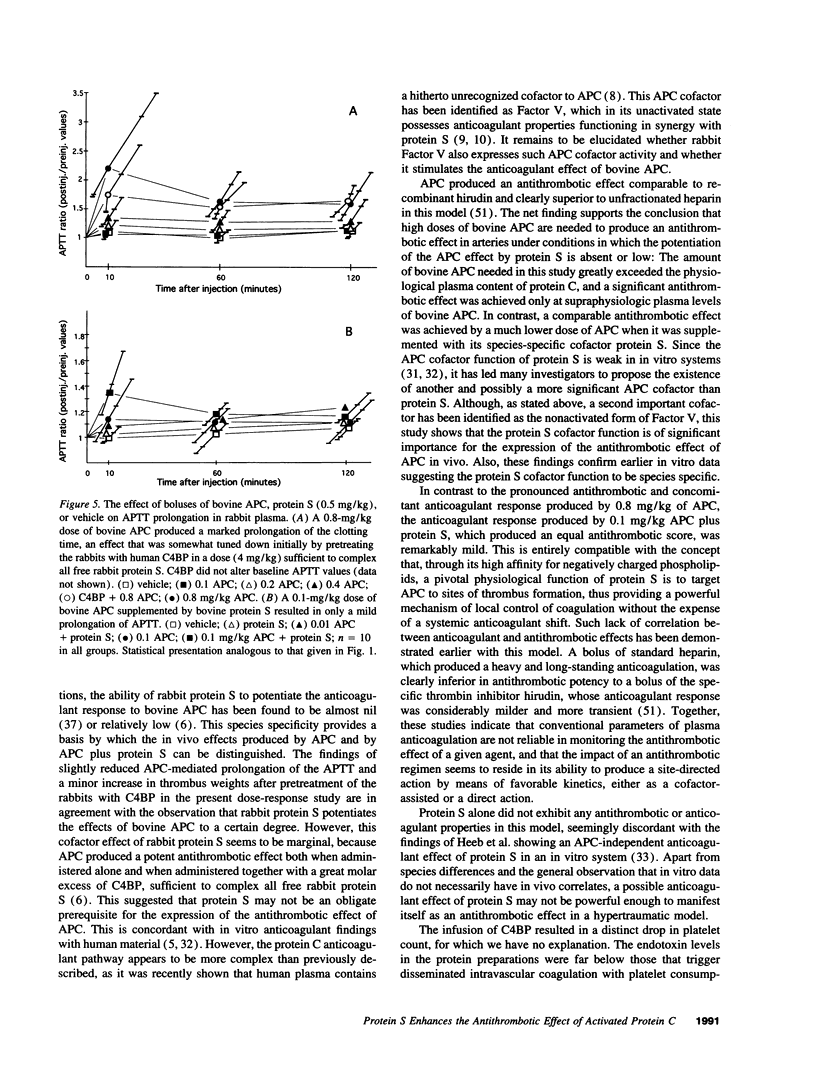


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araki H., Nishi K., Ishihara N., Okajima K. Inhibitory effects of activated protein C and heparin on thrombotic arterial occlusion in rat mesenteric arteries. Thromb Res. 1991 May 1;62(3):209–216. doi: 10.1016/0049-3848(91)90194-2. [DOI] [PubMed] [Google Scholar]
- Arnljots B., Bergqvist D., Dahlbäck B. Inhibition of microarterial thrombosis by activated protein C in a rabbit model. Thromb Haemost. 1994 Sep;72(3):415–420. [PubMed] [Google Scholar]
- Arnljots B., Bergqvist D. Inhibition of heparin-resistant microarterial thrombosis by recombinant hirudin: a specific thrombin inhibitor. Plast Reconstr Surg. 1995 Apr;95(5):894–900. [PubMed] [Google Scholar]
- Arnljots B., Dougan P., Salemark L., Bergqvist D. Effects of streptokinase and urokinase on microarterial thrombosis and haemostasis. An experimental study in rabbits. Scand J Plast Reconstr Surg Hand Surg. 1994 Mar;28(1):9–13. doi: 10.3109/02844319409015988. [DOI] [PubMed] [Google Scholar]
- Arnljots B., Dougan P., Wieslander J. B., Salemark L., Bergqvist D. Platelet accumulation and thrombus formation after microarterial injury. An experimental study in rabbits. Scand J Plast Reconstr Surg Hand Surg. 1994 Sep;28(3):167–175. doi: 10.3109/02844319409015977. [DOI] [PubMed] [Google Scholar]
- Arnljots B., Wieslander J. B., Dougan P., Salemark L. Prevention of microvascular thrombosis with low-dose tissue plasminogen activator. Plast Reconstr Surg. 1992 Aug;90(2):281–288. [PubMed] [Google Scholar]
- Bertina R. M., Koeleman B. P., Koster T., Rosendaal F. R., Dirven R. J., de Ronde H., van der Velden P. A., Reitsma P. H. Mutation in blood coagulation factor V associated with resistance to activated protein C. Nature. 1994 May 5;369(6475):64–67. doi: 10.1038/369064a0. [DOI] [PubMed] [Google Scholar]
- Bertina R. M., van Wijngaarden A., Reinalda-Poot J., Poort S. R., Bom V. J. Determination of plasma protein S--the protein cofactor of activated protein C. Thromb Haemost. 1985 Apr 22;53(2):268–272. [PubMed] [Google Scholar]
- Bregengård C., Nordfang O., Wildgoose P., Svendsen O., Hedner U., Diness V. The effect of two-domain tissue factor pathway inhibitor on endotoxin-induced disseminated intravascular coagulation in rabbits. Blood Coagul Fibrinolysis. 1993 Oct;4(5):699–706. [PubMed] [Google Scholar]
- Broekmans A. W., Veltkamp J. J., Bertina R. M. Congenital protein C deficiency and venous thromboembolism. A study of three Dutch families. N Engl J Med. 1983 Aug 11;309(6):340–344. doi: 10.1056/NEJM198308113090604. [DOI] [PubMed] [Google Scholar]
- Comp P. C., Esmon C. T. Recurrent venous thromboembolism in patients with a partial deficiency of protein S. N Engl J Med. 1984 Dec 13;311(24):1525–1528. doi: 10.1056/NEJM198412133112401. [DOI] [PubMed] [Google Scholar]
- Dahlbäck B., Carlsson M., Svensson P. J. Familial thrombophilia due to a previously unrecognized mechanism characterized by poor anticoagulant response to activated protein C: prediction of a cofactor to activated protein C. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):1004–1008. doi: 10.1073/pnas.90.3.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlbäck B., Hildebrand B. Inherited resistance to activated protein C is corrected by anticoagulant cofactor activity found to be a property of factor V. Proc Natl Acad Sci U S A. 1994 Feb 15;91(4):1396–1400. doi: 10.1073/pnas.91.4.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlbäck B., Hildebrand B., Malm J. Characterization of functionally important domains in human vitamin K-dependent protein S using monoclonal antibodies. J Biol Chem. 1990 May 15;265(14):8127–8135. [PubMed] [Google Scholar]
- Dahlbäck B. Inhibition of protein Ca cofactor function of human and bovine protein S by C4b-binding protein. J Biol Chem. 1986 Sep 15;261(26):12022–12027. [PubMed] [Google Scholar]
- Dahlbäck B. Protein S and C4b-binding protein: components involved in the regulation of the protein C anticoagulant system. Thromb Haemost. 1991 Jul 12;66(1):49–61. [PubMed] [Google Scholar]
- Emekli N. B., Ulutin O. N. The protective effect of autoprothrombin II - anticoagulant on experimental DIC in animals. Haematologica. 1980 Oct;65(5):644–651. [PubMed] [Google Scholar]
- Faioni E. M., Franchi F., Asti D., Sacchi E., Bernardi F., Mannucci P. M. Resistance to activated protein C in nine thrombophilic families: interference in a protein S functional assay. Thromb Haemost. 1993 Dec 20;70(6):1067–1071. [PubMed] [Google Scholar]
- Greengard J. S., Sun X., Xu X., Fernandez J. A., Griffin J. H., Evatt B. Activated protein C resistance caused by Arg506Gln mutation in factor Va. Lancet. 1994 May 28;343(8909):1361–1362. doi: 10.1016/s0140-6736(94)92497-x. [DOI] [PubMed] [Google Scholar]
- Griffin J. H., Evatt B., Wideman C., Fernández J. A. Anticoagulant protein C pathway defective in majority of thrombophilic patients. Blood. 1993 Oct 1;82(7):1989–1993. [PubMed] [Google Scholar]
- Gruber A., Griffin J. H., Harker L. A., Hanson S. R. Inhibition of platelet-dependent thrombus formation by human activated protein C in a primate model. Blood. 1989 Feb 15;73(3):639–642. [PubMed] [Google Scholar]
- Gruber A., Hanson S. R., Kelly A. B., Yan B. S., Bang N., Griffin J. H., Harker L. A. Inhibition of thrombus formation by activated recombinant protein C in a primate model of arterial thrombosis. Circulation. 1990 Aug;82(2):578–585. doi: 10.1161/01.cir.82.2.578. [DOI] [PubMed] [Google Scholar]
- Hanson S. R., Griffin J. H., Harker L. A., Kelly A. B., Esmon C. T., Gruber A. Antithrombotic effects of thrombin-induced activation of endogenous protein C in primates. J Clin Invest. 1993 Oct;92(4):2003–2012. doi: 10.1172/JCI116795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Dahlbäck B. Molecular cloning, expression and functional characterization of rabbit anticoagulant vitamin-K-dependent protein S. Eur J Biochem. 1993 Nov 1;217(3):857–865. doi: 10.1111/j.1432-1033.1993.tb18314.x. [DOI] [PubMed] [Google Scholar]
- He X., Dahlbäck B. Rabbit plasma, unlike its human counterpart, contains no complex between protein S and C4b-binding protein. Thromb Haemost. 1994 Apr;71(4):446–451. [PubMed] [Google Scholar]
- Heeb M. J., Mesters R. M., Tans G., Rosing J., Griffin J. H. Binding of protein S to factor Va associated with inhibition of prothrombinase that is independent of activated protein C. J Biol Chem. 1993 Feb 5;268(4):2872–2877. [PubMed] [Google Scholar]
- Hillarp A., Dahlbäck B. Novel subunit in C4b-binding protein required for protein S binding. J Biol Chem. 1988 Sep 5;263(25):12759–12764. [PubMed] [Google Scholar]
- Koster T., Rosendaal F. R., de Ronde H., Briët E., Vandenbroucke J. P., Bertina R. M. Venous thrombosis due to poor anticoagulant response to activated protein C: Leiden Thrombophilia Study. Lancet. 1993 Dec 18;342(8886-8887):1503–1506. doi: 10.1016/s0140-6736(05)80081-9. [DOI] [PubMed] [Google Scholar]
- Marlar R. A., Neumann A. Neonatal purpura fulminans due to homozygous protein C or protein S deficiencies. Semin Thromb Hemost. 1990 Oct;16(4):299–309. doi: 10.1055/s-2007-1002683. [DOI] [PubMed] [Google Scholar]
- Regan L. M., Lamphear B. J., Huggins C. F., Walker F. J., Fay P. J. Factor IXa protects factor VIIIa from activated protein C. Factor IXa inhibits activated protein C-catalyzed cleavage of factor VIIIa at Arg562. J Biol Chem. 1994 Apr 1;269(13):9445–9452. [PubMed] [Google Scholar]
- Shen L., Dahlbäck B. Factor V and protein S as synergistic cofactors to activated protein C in degradation of factor VIIIa. J Biol Chem. 1994 Jul 22;269(29):18735–18738. [PubMed] [Google Scholar]
- Smirnov M. D., Pyzh M. V., Borovikov D. V., Atorozhilova A. N., Dobrovolsky A. B., Golubych V. L., Gratsiansky N. A. Low doses of activated protein C delay arterial thrombosis in rats. Thromb Res. 1990 Feb 15;57(4):645–650. doi: 10.1016/0049-3848(90)90082-n. [DOI] [PubMed] [Google Scholar]
- Solymoss S., Tucker M. M., Tracy P. B. Kinetics of inactivation of membrane-bound factor Va by activated protein C. Protein S modulates factor Xa protection. J Biol Chem. 1988 Oct 15;263(29):14884–14890. [PubMed] [Google Scholar]
- Stenflo J. A new vitamin K-dependent protein. Purification from bovine plasma and preliminary characterization. J Biol Chem. 1976 Jan 25;251(2):355–363. [PubMed] [Google Scholar]
- Stenflo J., Jönsson M. Protein S, a new vitamin K-dependent protein from bovine plasma. FEBS Lett. 1979 May 15;101(2):377–381. doi: 10.1016/0014-5793(79)81048-0. [DOI] [PubMed] [Google Scholar]
- Svensson P. J., Dahlbäck B. Resistance to activated protein C as a basis for venous thrombosis. N Engl J Med. 1994 Feb 24;330(8):517–522. doi: 10.1056/NEJM199402243300801. [DOI] [PubMed] [Google Scholar]
- Tans G., Rosing J., Thomassen M. C., Heeb M. J., Zwaal R. F., Griffin J. H. Comparison of anticoagulant and procoagulant activities of stimulated platelets and platelet-derived microparticles. Blood. 1991 Jun 15;77(12):2641–2648. [PubMed] [Google Scholar]
- Taylor F. B., Jr, Chang A., Esmon C. T., D'Angelo A., Vigano-D'Angelo S., Blick K. E. Protein C prevents the coagulopathic and lethal effects of Escherichia coli infusion in the baboon. J Clin Invest. 1987 Mar;79(3):918–925. doi: 10.1172/JCI112902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor F., Chang A., Ferrell G., Mather T., Catlett R., Blick K., Esmon C. T. C4b-binding protein exacerbates the host response to Escherichia coli. Blood. 1991 Jul 15;78(2):357–363. [PubMed] [Google Scholar]
- Voorberg J., Roelse J., Koopman R., Büller H., Berends F., ten Cate J. W., Mertens K., van Mourik J. A. Association of idiopathic venous thromboembolism with single point-mutation at Arg506 of factor V. Lancet. 1994 Jun 18;343(8912):1535–1536. doi: 10.1016/s0140-6736(94)92939-4. [DOI] [PubMed] [Google Scholar]
- Walker F. J. Protein S and the regulation of activated protein C. Semin Thromb Hemost. 1984 Apr;10(2):131–138. doi: 10.1055/s-2007-1004415. [DOI] [PubMed] [Google Scholar]
- Walker F. J. Regulation of activated protein C by protein S. The role of phospholipid in factor Va inactivation. J Biol Chem. 1981 Nov 10;256(21):11128–11131. [PubMed] [Google Scholar]
- Warr T. A., Rao L. V., Rapaport S. I. Disseminated intravascular coagulation in rabbits induced by administration of endotoxin or tissue factor: effect of anti-tissue factor antibodies and measurement of plasma extrinsic pathway inhibitor activity. Blood. 1990 Apr 1;75(7):1481–1489. [PubMed] [Google Scholar]
- Wieslander J. B., Dougan P., Stjernquist U., Mecklenburg C. V. Effect of dextran 70 and saline on thrombus formation following arteriotomy and intimectomy in small arteries. Microsurgery. 1986;7(4):168–177. doi: 10.1002/micr.1920070409. [DOI] [PubMed] [Google Scholar]
- Wieslander J. B., Dougan P. Washout of vessels with heparin does not improve patency following severe microarterial trauma: an experimental study. Ann Plast Surg. 1990 Mar;24(3):216–222. doi: 10.1097/00000637-199003000-00005. [DOI] [PubMed] [Google Scholar]
- Zöller B., Dahlbäck B. Linkage between inherited resistance to activated protein C and factor V gene mutation in venous thrombosis. Lancet. 1994 Jun 18;343(8912):1536–1538. doi: 10.1016/s0140-6736(94)92940-8. [DOI] [PubMed] [Google Scholar]