INTRODUCTION
According to the Centers for Disease Control and Prevention (CDC) prevalence data for 2007, diabetes is a chronic and progressive disease that affects nearly 24 million people in the U.S.1 It is projected that by the year 2030, more than 30 million people will have diabetes.2 From 90% to 95% of diagnosed cases are type-2 diabetes, which is characterized by insulin resistance and an altered secretion of pancreatic beta cells, leading to hyperglycemia.1 Diabetes is associated with various microvascular and macrovascular complications that often lead to death. In 2006, diabetes was listed as the seventh leading cause of death in the U.S. The World Health Organization (WHO) predicts that diabetes-related deaths will double between 2005 and 2030 worldwide.1,2
The United Kingdom Prospective Diabetes Study (UKPDS) established the importance of reducing glucose levels to decrease the risk of complications associated with type-2 diabetes.3 Since the publication of that report, more recent studies, such as Action to Control Cardiovascular Risk in Diabetes Trial (ACCORD) and the Veterans Affairs Diabetes Trial (VADT), have re-emphasized the importance of glycemic control and its association with vascular complications.4,5
Although numerous pharmacological agents are available for the management of the disease, type-2 diabetes is controlled in fewer than 50% of patients in the U.S.6 These statistics are alarming; more effort is needed to achieve the recommended American Diabetes Association (ADA) and American Association of Clinical Endocrinologists (AACE) glycosylated hemoglobin (HbA1c) goals of below 7% and 6.5%, respectively.7,8 Research in pharmacological management, in addition to lifestyle modifications, has flourished as a consequence of the complications associated with diabetes.
The incretin mimetic class of medications was introduced to the market in 2005 in the form of exenatide (Byetta, Amylin/Lilly), a twice-daily subcutaneous (SQ) injection.9 Incretins—glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP)—cause an increase in the amount of insulin released from pancreatic beta cells after meals.10 The incretin effect is defined as a significantly greater insulin stimulatory response after an oral glucose load, compared with an intravenous (IV) glucose infusion when plasma glucose concentrations are equivalent.11 GLP-1 levels are reduced in patients with type-2 diabetes, compared to individuals with normal glucose tolerance.11,12
Thus, product development has focused primarily on GLP-1 for type-2 diabetes. The incretin mimetic liraglutide (Victoza, Novo Nordisk) was approved in the U.S. in January 2010 as an adjunctive therapy to diet and exercise in adults with type-2 diabetes.13 Liraglutide is also approved in Europe and Japan.14
PHARMACOLOGY
Liraglutide is a long-acting human GLP-1 analogue with 97% amino acid homology to the human endogenous GLP-1. This 97% homology was achieved by substituting arginine for lysine at position 34 of endogenous GLP-1. Endogenous GLP-1 is rapidly degraded by dipeptidyl peptidase-4 (DPP-4), and its insulin effect is short-lived. Liraglutide contains a fatty acid molecule that binds to albumin and prolongs the half-life of the structure.13,15 Receptors for GLP-1 are found in pancreatic alpha and beta cells; the central and peripheral nervous systems; and the heart, lungs, and gastrointestinal (GI) tract.10 Through the messenger intracellular cyclic adenosine monophosphate (cAMP), liraglutide causes insulin to be secreted in the presence of elevated glucose levels. Because of the receptor locations, liraglutide also inhibits glucagon secretion and delays gastric emptying.
PHARMACODYNAMICS AND PHARMACOKINETICS
Liraglutide is administered subcutaneously as an isotonic solution. In pharmacokinetic studies, liraglutide exhibits its maximum concentration after 8 to 12 hours, and its half-life is 13 hours after a single injection.13,15 Endogenous GLP-1 has a half-life of approximately 2 minutes because of DDP-4 actions, but the difference in analogue structure allows for a prolonged half-life and for once-daily administration.16 The mean peak concentration (Cmax) is 35 ng/mL, and the total area under-the-curve (AUC) concentration is 960 ng • hours/mL for a single dose of 0.6 mg. Both the Cmax and AUC concentration increase proportionally as the dose increases from 0.6 to 1.8 mg.13,15 Liraglutide is greater than 98% bound to plasma albumin, and it does not have a specific organ as a major route of elimination.13
CLINICAL TRIALS17–22
Liraglutide was studied as monotherapy and in combination with other agents used to treat type-2 diabetes in six randomized, controlled phase 3 trials known as the Liraglutide Effect and Action in Diabetes (LEAD) program. The trial investigators recruited more than 3,900 patients in 40 countries. The patients had been previously treated with lifestyle interventions (diet and exercise) or lifestyle plus oral antidiabetic agents for a minimum of two months before initiation of liraglutide therapy. Table 1 presents demographic profiles of the participants.
Table 1.
Liraglutide Effect and Diabetes (LEAD) Program, Demographic Overview
| Trial | No. of Patients | Study Duration (Weeks) | Treatment Arm | Mean Diabetes Duration (Years) | Mean Baseline HbA1c (%) | Mean FPG (mg/dL) | Mean BMI |
|---|---|---|---|---|---|---|---|
| LEAD-1 | 1,041 | 26 |
Glimepiride 2–4 mg/day plus: Liraglutide 0.6 mg daily Liraglutide 1.2 mg daily Liraglutide 1.8 mg daily Placebo Rosiglitazone 4 mg daily |
7.9 | 8.4 | 176.4 | 29.9 |
| LEAD-2 | 1,091 | 26 |
Metformin 1.5–2 g/day plus: Liraglutide 0.6mg daily Liraglutide 1.2 mg daily Liraglutide 1.8 mg daily Placebo Glimepiride 4 mg daily |
7.4 | 8.4 | 180 | 31 |
| LEAD-3 | 746 | 52 | Liraglutide 1.2 mg daily Liraglutide 1.8 mg daily Glimepiride 8 mg daily |
5.4 | 8.2 | 170.5 | 33.1 |
| LEAD-4 | 533 | 26 |
Metformin 2 g/day and rosiglitazone 8 mg/day plus: Liraglutide 1.2 mg daily Liraglutide 1.8 mg daily Placebo |
9 | 8.3 | 181.8 | 33.5 |
| LEAD-5 | 581 | 26 |
Metformin 2 g/day and glimepiride 4 mg/day plus: Liraglutide 1.8 mg daily Placebo Insulin glargine |
9.4 | 8.2 | 165.6 | 30.5 |
| LEAD-6 | 464 | 26 |
Metformin 2 g/day and/or sulfonylurea daily plus: Liraglutide 1.8 mg daily Exenatide 10 mcg twice daily |
8.2 | 8.2 | 173.7 | 32.9 |
BMI = body mass index; FPG = fasting plasma glucose; HbA1c = glycosylated hemoglobin.
Glycemic Parameters
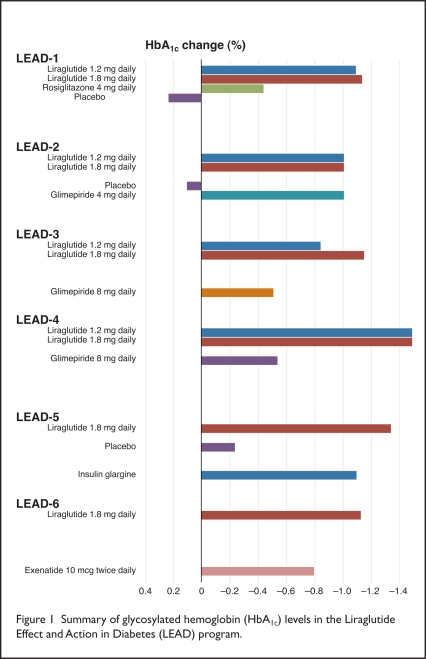
In the LEAD trials, liraglutide led to improved glycemic control as a result of the reduction in HbA1c values, compared with placebo or active comparators (Figure 1). As monotherapy, liraglutide lowered HbA1c from the mean baseline value (8.2%) by 0.84% and by 1.14% with 1.2-mg and 1.8-mg doses, respectively, compared with a 0.51% reduction with glimepiride 8 mg (Amaryl, Sanofi-Aventis).19
Figure 1.
Summary of glycosylated hemoglobin (HbA1c) levels in the Liraglutide Effect and Action in Diabetes (LEAD) program.
When added to one or two oral anti-diabetic agents, liraglutide also reduced HbA1c. In comparison with placebo, HbA1c values decreased significantly with liraglutide 1.2 mg or 1.8 mg (P < 0.001) as well as with glimepiride 4 mg when combined with metformin (Glucophage, Bristol-Myers Squibb).18 Each of these doses showed a 1.1% reduction from the mean baseline HbA1c level of 8.4%.
When triple therapy combining liraglutide, metformin, and rosiglitazone (Avandia, GlaxoSmithKline) was used, liraglutide 1.2 mg and 1.8 mg resulted in HbA1c reductions of 1.48%, compared with reductions of 0.54% in the placebo group (P < 0.001).20
Liraglutide 1.8 mg was also compared with insulin glargine (Lantus, Sanofi-Aventis) plus a combination of metformin and glimepiride. The reduction from the mean baseline HbA1c of 8.2% was significantly reduced with liraglutide (by 1.48%) compared with insulin glargine (by 1.09%) and placebo (by 0.24%) (P < 0.0001).21
LEAD-6 was a head-to-head comparison of another incretin mimetic, exenatide (Byetta) 10 mcg twice daily, and liraglutide 1.8 mg once daily, both with the addition of metformin and/or glimepiride. The mean baseline HbA1c for this study population (8.2%) was subsequently reduced more significantly with liraglutide (1.12%) than with exenatide (0.79%) (P < 0.0001).22 In the trial’s extension period, patients using exenatide were switched to liraglutide. During those additional 14 weeks, mean HbA1c values decreased from 7.2% to 6.9% (P < 0.0001) after the switch.23
The primary endpoint for the LEAD program was a reduction in HbA1c values. The liraglutide treatment arms experienced a greater reduction in comparison to other treatment arms, indicating that a higher proportion of subjects achieved the ADA’s HbA1c goal of below 7%. With liraglutide monotherapy, 43% and 51% of patients attained the ADA’s goal with liraglutide 1.2 mg (P < 0.001) and 1.8 mg (P < 0.0001), respectively, compared with 28% in the glimepiride group.19
When liraglutide was combined with other agents in the other LEAD trials, the percentage of patients achieving therapeutic goals ranged from 35% to 54%, compared with a range of 7% to 46% in those receiving placebo and other comparators.17,18,20–22
The combination of fasting plasma glucose (FPG) and postprandial plasma glucose (PPG) contributes to overall glycemic control and plays a role in microvascular and macrovascular complications associated with diabetes.24 In the LEAD trials, liraglutide alone and in combination with oral antidiabetic drugs reduced FPG and PPG levels. There was evidence of a dose-dependent effect of liraglutide on fasting levels; the 1.8-mg dose reduced fasting levels more than the 1.2-mg dose. As monotherapy, liraglutide 1.8 mg and 1.2 mg and glimepiride 8 mg each reduced FPG levels by 26 mg/dL, 15 mg/dL, and 5 mg/dL, respectively.19 Both doses of liraglutide were significant when compared with glimepiride. When liraglutide was combined with glimepiride or metformin, or both, a similar pattern of reduction occurred and was significant (P < 0.0001) when compared with placebo. When liraglutide was compared with exenatide, FPG levels were reduced by 29 mg/dL and by 11 mg/dL, respectively.22
Liraglutide also resulted in lower PPG levels throughout the LEAD program. When liraglutide was combined with glimepiride, reductions of 45 mg/dL and 49 mg/dL were seen with liraglutide 1.2 mg and 1.8 mg, respectively. This was significantly greater than the 33-mg/dL reduction achieved with rosiglitazone/glimepiride (P < 0.05 for both doses).17 PPG levels were significantly decreased in the liraglutide and metformin groups, compared with the placebo patients (P < 0.001).18 The estimated treatment difference for exenatide was greater than that for liraglutide in self-reported PPG levels at breakfast (P < 0.0001) and at lunch (P = 0.005).22
Body Weight
Endogenous GLP-1 suppresses appetite and energy intake in both normal-weight and obese individuals.25 Weight loss and decreased food intake have also been exhibited in animal studies of liraglutide.26,27 The LEAD trials showed a decrease in mean weight ranging from 1 to 3.2 kg (2.2 to 7.04 pounds) over the course of 26 or 52 weeks with liraglutide except in the LEAD-1 study.18–22 Weight reduction was more statistically significant for liraglutide than for the comparative treatments. In LEAD-1, when glimepiride was combined with liraglutide, a reduction in body weight was observed with liraglutide 1.8 mg and placebo. Weight loss in both groups was less than 0.5 kg (1 pound).17
Astrup et al. compared daily injections of liraglutide 1.2 mg, 1.8 mg, 2.4 mg, 3 mg, or placebo with orlistat (Xenical, Roche) 120 mg, given orally three times daily for 20 weeks. Participants receiving liraglutide lost significantly more weight (4.8–7.2 kg, or 11–16 pounds), compared with those receiving placebo (2.4 kg, or 9 pounds) and orlistat (4.1 kg, or 10.5 pounds). Participants with type-1 and type-2 diabetes were excluded from this trial.28
DRUG INTERACTIONS13
One of the effects of liraglutide is delayed gastric emptying; therefore, this agent has the potential to affect the absorption of oral medications. In clinical trials, liraglutide did not affect the absorption of any tested oral drug to any clinical relevance. Caution should be exercised when liraglutide is combined with medications that need to be rapidly absorbed to be therapeutically effective.
Although the risk of hypoglycemia is low when liraglutide is used as monotherapy, the risk is increased when it is combined with other antidiabetic medications. In the LEAD program, seven patients receiving liraglutide experienced hypoglycemia and needed another person to assist with treatment; however, six of the seven patients were also receiving a sulfonylurea, which might have contributed to the hypoglycemia.
ADVERSE DRUG EVENTS13,22
Gastrointestinal (GI) adverse events were the most commonly reported side effects with liraglutide. In the LEAD-1 through LEAD-5 trials, GI events were reported in 41% of liraglutide-treated patients and in 17% of the comparator-treated groups. Dose-related events were nausea, diarrhea, and vomiting (Table 2). GI tolerability improved during the course of treatment and with dose titration.
Table 2.
Gastrointestinal Adverse Events Reported in the Liraglutide Effect and Action in Diabetes (LEAD) Program*
| Liraglutide† | ComparableTreatment Arms | |
|---|---|---|
| Nausea | 18% | 4% |
| Diarrhea | 11% | 4% |
| Vomiting | 6% | 1% |
Of the liraglutide patients, 46% reported GI effects, compared with 43% of exenatide-treated patients.
CONTRAINDICATIONS, WARNINGS, AND PRECAUTIONS13,17
A boxed warning in the product labeling states that liraglutide is contraindicated in patients with a personal or family history of medullary thyroid carcinoma or in those with multiple endocrine neoplasia syndrome type-2 (MEN-2). Animal studies suggest that liraglutide can cause thyroid C-cell tumors at clinically relevant exposures, in accordance with the dose and duration of treatment. Compared with controls, rats receiving liraglutide showed a statistically significant increase in cancer. It is unknown whether liraglutide causes thyroid C-cell tumors or medullary thyroid carcinoma in humans. Patients should be counseled on the signs and symptoms of thyroid tumors (e.g., a lump in the neck, hoarseness, or difficulty swallowing or breathing).
Pancreatitis, seen with the first marketed incretin mimetic, was also one of the parameters observed with liraglutide. In clinical trials, pancreatitis was reported in seven patients treated with liraglutide and in one patient receiving a comparator agent. Five of the seven patients had acute pancreatitis, and two had the chronic form. One patient had pancreatitis with necrosis and died; however, clinical causality with liraglutide could not be established.
Another case of pancreatitis was subsequently reported in connection with liraglutide. In LEAD-1, five patients with a previous diagnosis of pancreatitis were randomly assigned to all of the treatment arms. Each of those patients completed the trial without reporting pancreatitis as an adverse event. There is no conclusive evidence establishing a risk of pancreatitis with liraglutide. If pancreatitis develops during liraglutide therapy, it is recommended that liraglutide not be restarted after the problem has been resolved.
Liraglutide-mediated secretion of insulin is glucose-dependent; therefore, the risk of hypoglycemia is low as a result of this mechanism. As mentioned earlier, when liraglutide is combined with other antidiabetic agents, the risk of hypoglycemia increases. Patients should be counseled on the signs, symptoms, and treatment of hypoglycemia when they are taking other glucose-lowering agents with liraglutide.
DOSAGE AND ADMINISTRATION13
The recommended starting dose of liraglutide is 0.6 mg subcutaneously once daily for one week to reduce any GI side effects. Because this dose is not effective for glycemic control, it is titrated to 1.2 mg. If glycemic control is not achieved, the dose is increased to 1.8 mg once daily. The SQ injection may be given in the abdomen, thigh, or upper arm without regard to meals or time of day. The injection site and timing can be changed without any dose adjustments. Dosage adjustments are not required for patients with renal or hepatic impairment.
Liraglutide causes beta cells to increase insulin production, and reducing doses of insulin secretagogues should be considered to decrease the risk of hypoglycemia.
Prior to use, liraglutide must be refrigerated. After the first use, the liraglutide pen can be stored at room temperature (15°C to 30°C) or refrigerated for 30 days.
COST29
Liraglutide is sold in a prefilled, multi-dose pen that delivers doses of 0.6 mg, 1.2 mg, or 1.8 mg. The average wholesale prices for a month’s supply of the 1.2-mg and 1.8-mg doses are approximately $289 and $433, respectively.
P&T COMMITTEE CONSIDERATIONS30,31
Liraglutide is not considered a first-line therapy for the treatment of type-2 diabetes. Guidelines from the ADA/European Association for the Study of Diabetes (EASD) and AACE guidelines were published before the FDA’s approval of liraglutide. In the 2009 ADA/EASD consensus statement, the GLP-1 agonist exenatide is considered a tier 2 therapy in combination with the use of metformin and lifestyle modifications. Tier 2 therapies are considered less well validated in the treatment guidelines. The AACE treatment algorithm considers GLP-1 use for HbA1c levels above 7.5% and in combination with another treatment modality. For use in dual therapy, the AACE recommends GLP-1, a DPP-4 inhibitor or an insulin secretagogue (a sulfonylurea or a meglitinide), in that order. Although sulfonylureas are more cost-effective than the other therapeutic classes, they are a less desirable treatment choice because of the risk of hypoglycemia, weight gain, and a lack of improved glycemic control for an extended period of time. In contrast, DPP-4 inhibitors and GLP-1 receptor agonists exert their actions in a glucose-dependent manner without weight gain or the need for frequent dosage adjustments.
In a head-to-head study of liraglutide and exenatide, weight loss was comparable with both agents; however, liraglutide was better tolerated and produced greater reductions in levels of HbA1c.22,23 Both drugs are similar in cost per month, but liraglutide’s effectiveness in glycemic reduction and its flexibility of once-daily administration without regard to meals make it the better treatment option.
CONCLUSION
Liraglutide (Victoza) is the second agent in the incretin mimetic class of drugs approved for patients with type-2 diabetes and is the first incretin mimetic with a pharmacokinetic profile for once-daily administration. Thus far, studies have shown that liraglutide significantly reduces fasting and postprandial glucose concentrations and HbA1c levels; it is also associated with a low risk of hypoglycemia and weight gain, which may often be observed with other agents. Liraglutide is currently being evaluated for its effects on beta-cell function and cardiovascular profiles.
Footnotes
Disclosure: The authors report no financial or commercial relationships in regard to this article.
REFERENCES
- 1.Centers for Disease Control and Prevention(CDC) National Diabetes Fact Sheet: General information and national estimates on diabetes in the United States, 2007. Available at: www.cdc.gov/diabetes/pubs/pdf/ndfs_2007.pdf. Accessed March 18, 2010. [Google Scholar]
- 2.Wild S, Roglic G, Green A, et al. Global prevalence of diabetes, estimates for the year 2000, and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 3.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes. Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 4.Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes.Action to Control Cardiovascular Risk in Diabetes [ACCORD] Study Group. N Engl J Med. 2008;353:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duckworth W, Abraira C, Moritz T, et al. the VADT Investigators Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 6.Resnick HE, Foster GL, Bardsley J, Ratner RE. Achievement of American Diabetes Association clinical practice recommendations among U.S. adults with diabetes, 1999–2002: The National Health and Nutrition Examination Survey. Diabetes Care. 2006;29:531–537. doi: 10.2337/diacare.29.03.06.dc05-1254. [DOI] [PubMed] [Google Scholar]
- 7.America Diabetes Association Position statement: Standards of Medical Care in Diabetes—2010. Diabetes Care. 2010;33(Suppl):S11–S61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Association of Clinical Endocrinologists Medical guidelines for the management of diabetes mellitus. Endocr Pract. 2007;13(Supp1):S3–S66. doi: 10.4158/EP.13.S1.1. [DOI] [PubMed] [Google Scholar]
- 9.Amylin and Lilly announce FDA approval of Byetta (Exenatide) injection, April 29, 2005. Available at: www2.prnewswire.com. Accessed May 3, 2010.
- 10.Drucker DJ, Nauck M. The incretin system: Glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 11.Toft-Nielsen MB, Damhotl MB, Madsbad S, et al. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab. 2001;86:3717–3723. doi: 10.1210/jcem.86.8.7750. [DOI] [PubMed] [Google Scholar]
- 12.Nauck M, Stockman F, Ebert R, Creutzfeldt W. Reduced incretin effect in type 2 (non–insulin-dependent) diabetes. Diabetologia. 1986;29:46–54. doi: 10.1007/BF02427280. [DOI] [PubMed] [Google Scholar]
- 13.Victoza (liraglutide), product information . Princeton, N.J: Novo Nordisk Inc.; Jan, 2010. [Google Scholar]
- 14.Novo Nordisk receives U.S. approval for Victoza (liraglutide) for the treatment of type 2 diabetes, January 26, 2010. Available at: www.novonordisk.com/press/sea/sea.asp?sShowNewsItemGUID=7e58c0fd-2e96-4188-b201-a691ea2b4471&sShowLanguageCode=en-GB. Accessed March 10, 2010.
- 15.Agerso H, Jensen LB, Elbrond B, et al. The pharmacokinetics, pharmacodynamics, safety, and tolerability of NN2211, a new long-acting GLP-1 derivative in healthy men. Diabetologia. 2002;45:195–202. doi: 10.1007/s00125-001-0719-z. [DOI] [PubMed] [Google Scholar]
- 16.Russell-Jones D. Molecular, pharmacological, and clinical aspects of liraglutide, a once-daily human GLP-1 analogue. Mol Cell Endocrinol. 2009;297:137–140. doi: 10.1016/j.mce.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 17.Marre M, Shaw J, Brandle M, et al. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks, produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU) Diabet Med. 2009;26:268–278. doi: 10.1111/j.1464-5491.2009.02666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nauck M, Frid A, Hermansen K, et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin in type 2 diabetes: The LEAD (Liraglutide Effect and Action in Diabetes)-2 study. Diabetes Care. 2009;32:84–90. doi: 10.2337/dc08-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 MONO): Randomized, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373:473–481. doi: 10.1016/S0140-6736(08)61246-5. [DOI] [PubMed] [Google Scholar]
- 20.Zinman B, Gerich J, Buse JB, et al. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met + TZD) Diabetes Care. 2009;32:1224–1230. doi: 10.2337/dc08-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russell-Jones D, Vaag A, Schmitz O, et al. Liraglutide vs. insulin glargine and placebo in combination with metformin and sulphonylurea therapy in type 2 diabetes mellitus (LEAD-5 met + SU): A randomised controlled trial. Diabetologia. 2009;52:2046–2055. doi: 10.1007/s00125-009-1472-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buse JB, Rosenstock J, Sesti G, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: A 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6) Lancet. 2009;374:39–47. doi: 10.1016/S0140-6736(09)60659-0. [DOI] [PubMed] [Google Scholar]
- 23.Buse JB, Sesti G, Schmidt W, et al. Switching to once-daily liraglutide from twice-daily exenatide further improves glycemic control in patients with type 2 diabetes using oral agents. Diabetes Care. 2010;33:1300–1303. doi: 10.2337/dc09-2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: Variations with increasing levels of HbA(1c) Diabetes Care. 2003;26:881–885. doi: 10.2337/diacare.26.3.881. [DOI] [PubMed] [Google Scholar]
- 25.Flint A, Raben A, Astrup A, Holst JJ. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest. 1998;101:515–520. doi: 10.1172/JCI990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raun K, Von-Voss P, Gotfredsen CF, et al. Liraglutide, a long-acting glucagon-like peptide-1 analog, reduces body weight and food intake in obese candy-fed rats, whereas a dipeptidyl peptidase–IV inhibitor, vildagliptin, does not. Diabetes. 2007;56:8–15. doi: 10.2337/db06-0565. [DOI] [PubMed] [Google Scholar]
- 27.Larsen PJ, Fledelius C, Knudsen LB, Tang-Christensen M. Systemic administration of the long-acting GLP-1 derivative NN2211 induces lasting and reversible weight loss in both normal and obese rats. Diabetes. 2001;50:2530–2539. doi: 10.2337/diabetes.50.11.2530. [DOI] [PubMed] [Google Scholar]
- 28.Astrup A, Rossner S, Van Gaal L, et al. Effects of liraglutide in the treatment of obesity: A randomised, double-blind, placebo-controlled study. Lancet. 2009;374:1606–1616. doi: 10.1016/S0140-6736(09)61375-1. [DOI] [PubMed] [Google Scholar]
- 29.McKesson Corp., San Francisco. Available at www.mckesson.com. Accessed June 12, 2010.
- 30.Nathan DM, Buse JB, Davidson MD, et al. Medical management of hyperglycemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy—a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by the American Association of Clinical Endocrinologist/American College of Endocrinology consensus panel on type 2 diabetes mellitus: An algorithm for glycemic control. Endocrinol Pract. 2009;15:540–559. doi: 10.4158/EP.15.6.540. [DOI] [PubMed] [Google Scholar]