INTRODUCTION
Recombinant activated protein C (APC) (Xigris, Eli Lilly) has anticoagulant, anti-inflammatory, and fibrinolytic properties.1 In November 2001, the FDA approved the use of APC in patients with severe sepsis and at a high risk of death, defined as a score of 25 or higher in the Acute Physiology and Chronic Health Evaluation (APACHE II). At the time, the FDA also requested that additional studies be conducted by the manufacturer in children and in adults with sepsis who had a lower risk of death. In the intervening years, several studies have examined the risks and benefits of APC in patients with sepsis.
This review provides an update on the use of APC since its approval. A snapshot of major studies is provided in Table 1.
Table 1.
Summary of Significant Studies of Activated Protein C (Xigris)
| Trial | No. of Patients | Year | Objective | Design | Main Outcomes | Author |
|---|---|---|---|---|---|---|
| Protein C Worldwide Evaluation in Severe Sepsis (PROWESS) | 1,690 | 2001 | All-cause mortality at 28 days | R, DB, PC, MC | Mortality: placebo, 30.8% vs. treatment, 24.7% (P = 0.005) | Bernard2 |
| Administration of Drotrecogin Alfa (Activated) in Early-Stage Severe Sepsis (ADDRESS) | 2,640 | 2005 | All-cause mortality at 28 days in patients with severe sepsis and low risk of death | R, DB, PC, MC | Mortality: placebo, 17% vs. treatment, 18.5% (P = 0.34) | Abraham5 |
| Researching Severe Sepsis and Organ Dysfunction in Children: A Global Perspective Study (RESOLVE) | 477 | 2007 | CTCOFR: mortality and safety at 28 days | R, DB, PC, MC | CTCOFR: no difference between groups (P = 0.72)
|
Nadel12 |
| Human recombinant activated protein C for severe sepsis (review) | 4,911 | 2008 | All-cause mortality at 28 days | M | Relative risk = 0.92 (95% CI, 0.72–1.18; P = 0.42) | Marti-Carvajal17 |
| Extended Evaluation of Human Recombinant Activated Protein C (ENHANCE) | 2,375 | 2005 | All-cause mortality and safety at 28 days | SA, OL |
|
Vincent19 |
| Use of drotrecogin alfa (activated) in Italian intensive-care units | 668 | 2007 | Clinical outcomes | Survey (with NPC) | Mortality: increased in surgical patients (within 7 days); odds ratio = 2.79 | Bertolini21 |
| Evaluating use of drotrecogin alfa (activated) in adult patients with severe sepsis: a Canadian multicenter observational study | 261 | 2007 | Usage patterns and clinical outcomes | Survey |
|
Kanji22 |
| Adverse outcomes in patients with severe sepsis and baseline bleeding precautions | 73 | 2009 | Outcomes in patients with baseline bleeding precautions (outlined in PROWESS) | RT |
|
Gentry23 |
| Xigris and Prophylactic Heparin Evaluation in Severe Sepsis (XPRESS) | 1,994 | 2009 | All-cause mortality at 28 days | R, DB, PC, MC |
|
Levi26 |
| Extended drotrecogin alfa (activated) infusions in prolonged septic shock | 193 | 2009 |
Primary: resolution of shock within 72 hours after initial 96 hours of therapy Secondary: all-cause mortality at 28 days |
R, DB, PC, MC |
|
Dhainaut28 |
CI = confidence interval; CNS = central nervous system; CTCOFR = composite time to complete primary organ failure resolution; DB = double-blind; M = meta-analysis; MC = multicenter; NPC = non-parallel control group; OL = open-label; PC = placebo-controlled; R = randomized; SA = single-arm; RT = retrospective.
This subgroup of patients was receiving heparin at the baseline evaluation.
EFFICACY
The publication of the Protein C Worldwide Evaluation in Severe Sepsis (PROWESS) trial marked a significant advance in the management of patients at a high risk of death.1,2 The study was designed to evaluate the impact of APC on mortality in patients with severe sepsis. The investigators had intended to enroll 2,280 patients, but enrollment was halted after a second interim analysis of 1,520 patients revealed a significant decrease in mortality in the APC group compared with the group receiving placebo. APC was associated with an absolute reduction in mortality of 6.1%. A subset analysis showed that the mortality benefit was limited to patients who were more severely ill (APACHE II scores of 25 or higher) in whom the use of APC was associated with an absolute reduction in mortality of 13%. Conversely, APC use in patients with APACHE II scores of below 25 had a mortality rate of 19%, which was identical to that of the placebo group. Consequently, APC was labeled for use in patients with severe sepsis and a high risk of death.
Despite the impressive findings of PROWESS, the FDA advisory committee raised significant concerns.3 Halfway through the study, the protocol was amended and the drug-manufacturing process was changed. The protocol changes were made to ensure that patients who were more likely to die within 28 days from causes other than sepsis would be excluded from the study.
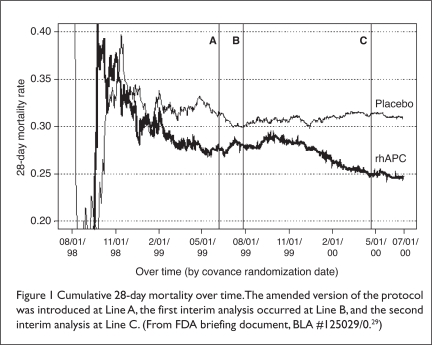
Although the intent of the amendment existed in the original protocol (i.e., to exclude patients who were likely to die from causes other than sepsis), the study’s exclusion criteria were further clarified in the amendment.4 Baseline demographics of patients in the original and amended protocols were similar. Notable differences included fewer APACHE II chronic health problems in patients in the amended protocol, more acidosis in the original group (46% vs. 26%) and higher interleukin-6 (IL-6) levels in the amended group (566 vs. 389 mcg/mL).4 Examination of mortality rates before and after the protocol amendments suggested that the changes were beneficial (Figure 1; see page 506).
Figure 1.
Cumulative 28-day mortality over time. The amended version of the protocol was introduced at Line A, the first interim analysis occurred at Line B, and the second interim analysis at Line C. (From FDA briefing document, BLA #125029/0.29)
Concerns over the study’s findings resulted in a vote of 10 to 10 by the FDA’s advisory group regarding APC approval.3 Statistical evaluation of mortality in the pre-amendment and post-amendment groups resulted in a P value of 0.08.4 Although the FDA acknowledged a potential for the amendment’s effect, data showing that patients with chronic health problems derived the most benefit from the drug (even though the protocol amendment decreased enrollment of these patients) led the FDA to conclude that the differences arose by chance. The drug was subsequently approved for clinical use.
Upon approval of APC, the FDA required an additional study—Administration of Drotrecogin Alfa (Activated) in Early Stage Severe Sepsis (ADDRESS)—to determine the drug’s efficacy in patients with severe sepsis and a low risk of death.5 The study was terminated early after 2,640 patients were enrolled because of a low likelihood of APC’s showing a beneficial effect. There was no significant difference in mortality between the APC group (18.5%) and the placebo group (17%) (P = 0.34). Within the study was a subset of 321 patients who had APACHE II scores of 25 or higher (the subgroup of patients who had benefited from APC in the PROWESS trial). Mortality rates at 28 days were 29.5% in this subgroup and 24.7% in those receiving placebo. There was no difference in mortality in patients with multiple-organ dysfunction at baseline.
Data from PROWESS suggested that APC in patients with APACHE II scores of 25 or higher would result in one life saved for approximately every eight patients treated; the FDA used this information to support the approval of APC.6 The lack of benefit of APC in ADDRESS in patients with APACHE II scores of 25 or higher raised concerns about the efficacy of APC, although the number of patients was small and the patients were considered to be at low risk for death at the time of study randomization.7 It was suggested that another study of patients with sepsis and a high risk of death be conducted to confirm the benefit of APC.8–10
Finally, a follow-up subset analysis of PROWESS and ADDRESS revealed an increased risk of death in patients with single-organ dysfunction who had recently undergone surgery (within 30 days). This discovery prompted a change in the package insert to include a warning for using APC in this patient population.11
The efficacy of APC was also evaluated in pediatric patients in a study known as RESOLVE (Researching Severe Sepsis and Organ Dysfunction in Children: A Global Perspective).12 This prospective, placebo-controlled, randomized trial enrolled children between 38 weeks and 17 years of age. After 477 subjects were enrolled, the study was terminated early because the independent monitoring committee advised that the likelihood of showing a benefit was low.13 The committee also noted an increased rate of intracranial hemorrhage in the patients receiving APC. Overall, there were 11 central nervous system (CNS) bleeding episodes in the APC group (4.6%) and five in the placebo group (2.1%) (P = 0.13). A companion editorial acknowledged the increasingly tenuous benefit of APC and reiterated the need to either definitively identify patients for whom APC was effective or pursue a definitive controlled study.14
In 2007, the European Medicines Agency stated that the risks versus the benefits of APC were unclear and called for another placebo-controlled trial to be conducted. Eli Lilly agreed to sponsor PROWESS–SHOCK. This study is currently recruiting patients with a target end date of August 2011.15 The primary objective is all-cause mortality at 28 days.
A study now under way is APROCCHS (Activated Protein C and Corticosteroids for Human Septic Shock). This placebo-controlled trial is evaluating APC with and without low-dose corticosteroids for the management of septic shock.16
A meta-analysis on the use of APC for severe sepsis, from 2008, concluded that there was no evidence to support the use of APC for managing severe sepsis or septic shock.17 The meta-analysis included four studies involving 4,911 patients (4,434 adults and 477 children).2,5,12,18 The investigators recommended a moratorium on the use of APC until additional randomized controlled trials showed a benefit of therapy.
The need for PROWESS–SHOCK and APROCCHS is a strong indicator that a state of clinical equipoise has been reached in terms of using APC for sepsis. With more than 200 centers participating in these placebo-controlled trials, it is evident that a significant segment of the critical-care community is uncertain about the role of APC. Thus, more robust evidence is needed before APC is adopted as the standard of care for sepsis.
SAFETY
The major adverse effect associated with APC is bleeding. Table 2 lists exclusion criteria used in PROWESS and in the corresponding product labeling designed to minimize the risk of bleeding during the trial. Serious bleeding was defined as any one of the following events:
intracranial hemorrhage
any life-threatening bleeding episode
any bleeding classified as serious by the investigator
any bleeding that causes a patient to require 3 units of packed red blood cells (RBCs) on two consecutive days
Table 2.
Bleeding Risk Criteria in the PROWESS Study and in the Product Label for Activated Protein C
| PROWESS Exclusion Criteria | Xigris Product Label |
|---|---|
| Active internal bleeding | Contraindication |
| Hemorrhagic stroke within 3 months | Contraindication |
| Intracranial/intraspinal surgery or severe head trauma within 2 months | Contraindication |
| Trauma with increased risk of life-threatening bleeding | Contraindication |
| Presence of an epidural catheter | Contraindication |
| Intracranial neoplasm or mass lesion or evidence of cerebral herniation | Contraindication |
| Concurrent therapeutic heparin to treat an active or thrombotic event | Precaution |
| Platelet count below 30,000/mm3 | Precaution |
| Prothrombin time–INR above 3 | Precaution |
| Gastrointestinal bleeding within 6 weeks | Precaution |
| Systemic thrombolytic therapy within 3 days | Precaution |
| Oral anticoagulants or glycoprotein IIb/IIIa inhibitors within 7 days | Precaution |
| Aspirin (> 650 mg/day) or other platelet inhibitor within 7 days | Precaution |
| Ischemic stroke within 3 months | Precaution |
| Intracranial arteriovenous malformation or aneurysm | Precaution |
| Known bleeding diathesis | Precaution |
| Chronic severe hepatic disease | Precaution |
| Any other condition in which bleeding constitutes a significant hazard or would be very difficult to manage because of its location | Precaution |
INR = International Normalized Ratio; PROWESS = Protein C Worldwide Evaluation in Severe Sepsis.
The incidence of serious bleeding in PROWESS was 3.5% in the APC patients and 2% in the placebo group. In the APC group, 249 patients (29.3%) experienced a bleeding event during the study drug’s infusion, compared with 121 placebo patients (14.4%).
Since the approval of APC, several observational studies have been conducted, adding to the body of information regarding the risk of bleeding. ENHANCE (Extended Evaluation of Human Recombinant Activated Protein C) was an open-label study by Vincent et al., with inclusion criteria similar to those of PROWESS.19 The incidence of serious bleeding in ENHANCE was 6.5%, nearly twice the incidence of PROWESS. The incidence of intracranial hemorrhage in ENHANCE was 1.5%, compared with 0.2% in PROWESS. Results of ENHANCE led to a renewed call for another trial, led by Eichacker et al., to confirm the efficacy of APC.20
A study by Bertolini et al., conducted under the auspices of the Italian Ministry of Health, required all APC users to participate in a pharmacoeconomic surveillance program.21 The survey included 668 patients who received APC and 1,181 parallel, nonrandomized control patients who were eligible for APC but did not receive it; 324 patients who were evaluable experienced serious bleeding at an incidence of 4.6%, which was slightly higher than that seen in PROWESS. However, this variation could simply have been a reflection of differing definitions of serious bleeding.
For example, in PROWESS, one criterion for serious bleeding was the need for administration of 3 units of packed RBCs on two consecutive days. In the Italian study, by contrast, any administration of more than 2 units was considered to be an episode of serious bleeding. The crude mortality rate for the on-label use of APC was 46.4%, which was lower than the rate in the control group (54.9%; P = 0.0004). However, the two groups were not similar at baseline; control patients were older (67.8 vs. 57.9 years of age; P < 0.001), and a greater proportion of controls had septic shock (77.4% vs. 66.8% for APC; P < 0.0001). Multivariate logistic regression also showed that the use of APC in patients who underwent scheduled surgery (within seven days before or 24 hours after hospital admission) was associated with an increased risk of mortality (odds ratio [OR], 2.79; standard error, 1.31–5.97).
A Canadian survey included 261 patients, with a mortality rate of 45%.22 The higher mortality rate, compared with that of PROWESS, might be explained by a greater severity of illness in the Canadian cohort (APACHE II median, 31). Early administration of APC (before 12 hours) was associated with a lower mortality rate. Fifty-two patients (19.9%) who received APC had relative contraindications, and four had an absolute contraindication; in addition, 25 patients (9.6%) in the survey had a serious bleeding event. Logistic regression showed that having more than three failing organs (OR = 3.1; P = 0.016) or having a relative contraindication (OR = 2.7; P = 0.028) was predictive of a serious bleeding event.
Finally, in a study published in 2009, Gentry et al. compared the outcomes of 73 patients receiving APC with or without bleeding risk, as set forth by PROWESS.23 Serious bleeding occurred in seven of 20 patients (35%) who had a baseline bleeding risk, compared with two of 53 patients (3.8%) who had no bleeding risks (P < 0.0001). The use of APC in patients with bleeding risks at the baseline evaluation was associated with increased mortality (65% vs. 24.5%; P = 0.0015). A multivariate analysis showed that baseline bleeding risk was the only variable associated with occurrence of serious bleeding. The authors concluded that APC in patients with severe sepsis should be avoided in those with baseline bleeding risk, as identified in PROWESS.23
A companion editorial by Sweeney et al. suggested that enough evidence was available to change the APC-labeled warnings to contraindications.24 The study’s publication prompted the FDA to state that the findings were consistent with the product’s labeling, and the agency said that it was working with Eli Lilly to accumulate data on bleeding events and mortality rates in patients who receive APC.25
Prophylactic Heparin
The Xigris and Prophylactic Heparin Evaluation in Severe Sepsis (XPRESS) trial evaluated 28-day mortality rates of 1,994 patients receiving APC in combination with heparin (or low-molecular-weight heparin) compared with patients receiving placebo.26 Patients with severe sepsis are at an increased risk for thrombosis, and prophylactic heparin is usually considered the standard of care. However, because APC has anticoagulant properties, heparin therapy might be unnecessary. Further, in vitro studies have suggested that high doses of heparin may decrease APC activity through increased hepatic clearance.27 Prophylactic heparin in PROWESS was not associated with an increased incidence of bleeding in a study by Dhainaut et al.,28 but a secondary analysis showed higher mortality rates in patients receiving prophylactic heparin than in those who did not receive it.29
Results of XPRESS revealed that the 28-day mortality rate was lower in the APC/heparin group (28.3%) than in the APC/placebo group (31.9%) (P = 0.08). In addition, patients receiving heparin at baseline had significantly lower mortality rates (26.9%) compared with the placebo patients (35.6%) (P = 0.005). During days 0 to 6, there were more bleeding events with heparin (105) than with placebo (78) (P = 0.048). However, during the 28-day study period, significantly more ischemic strokes occurred in the placebo group of patients (17 strokes) than in the heparin patients (five strokes) (P = 0.009).26 The investigators concluded that prophylactic heparin had an acceptable safety profile. For patients who are about to receive APC, discontinuing current heparin prophylaxis should be discouraged unless the risks outweigh the potential benefits.
Extended Infusions of Activated Protein C
In a multicenter, randomized, placebo-controlled trial, Dhainaut et al. evaluated the efficacy of extending APC infusions in 193 patients with prolonged septic shock for an additional 72 hours beyond the standard 96-hour infusion.30 The primary outcome was time to resolution of vasopressor-dependent shock within 72 hours; the secondary outcome included all-cause mortality at 28 days. At baseline, the extended-infusion group had significantly higher vasopressor requirements (mean norepinephrine = 1.24 vs. 1.09 mcg/kg per minute) (P = 0.034). Extended infusions did not result in a significantly decreased proportion of patients receiving vasopressors (32 patients, or 34%), compared with those receiving placebo (40 patients, or 40.4%) (P = 0.419). Similarly, there was no difference in 28-day mortality; 37 APC patients and 31 placebo patients died (P = 0.283). There was also no difference in the number of adverse drug events. Thus, extended infusions of APC did not bring about improved outcomes in terms of resolving shock, although the small sample size might have limited the ability to identify a difference.
CONCLUSION
The initial enthusiasm following the publication of the PROWESS trial has been tempered significantly, with subsequent studies showing a lack of efficacy of activated protein C (Xigris) and an increased incidence of bleeding in general clinical use. Consequently, hospitals should consider adopting the PROWESS exclusion criteria for use of the product. Results of PROWESS–SHOCK and APROCCHS should provide further evidence for defining the precise role of APC in the management of septic shock.
Footnotes
Disclosure. The author reports no financial or commercial relationships in regard to this article.
REFERENCES
- 1.Matthay MA. Severe sepsis: A new treatment with both anticoagulant and antiinflammatory properties. N Engl J Med. 2001:759–762. doi: 10.1056/NEJM200103083441009. [DOI] [PubMed] [Google Scholar]
- 2.Bernard G, Vincent JL, Laterre PF, et al. Efficacy and safety of activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 3.FDA, Center for Drug Evaluation Research 2001 Meeting Documents, March 27, 2007. Available at: www.fda.gov/ohrms/dockets/ac/cder01.htm#Anti-Infective. Accessed June 7, 2010.
- 4.FDA Clinical Review, BLA#125029/0. Drotrecogin alfa (activated) [Recombinant Human Activated Protein C (rhAPC)], Xigris. Available at: www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/approvalApplications/TherapeuticBiologicApplications/UCM113441.pdf. Indianapolis: Eli Lilly; November 21, 2001. Accessed June 7, 2010.
- 5.Abraham E, Laterre P-F, Garg R, et al. Drotrecogin alfa (activated) for adults with severe sepsis and a low risk of death. N Engl J Med. 2005;353:1332–1341. doi: 10.1056/NEJMoa050935. [DOI] [PubMed] [Google Scholar]
- 6.Siegel JP. Assessing the use of activated protein C in the treatment of severe sepsis. N Engl J Med. 2002;347:1030–1034. doi: 10.1056/NEJMsb021512. [DOI] [PubMed] [Google Scholar]
- 7.Parillo JE. Severe sepsis and therapy with activated protein C. N Engl J Med. 2005;353:1398–1400. doi: 10.1056/NEJMe058160. [DOI] [PubMed] [Google Scholar]
- 8.Friedrich JO. Drotrecogin alfa (activated) in severe sepsis. N Engl J Med. 2006;354:94–95. doi: 10.1056/NEJMc052759. [DOI] [PubMed] [Google Scholar]
- 9.LaRosa SP. Drotrecogin alfa (activated) in severe sepsis. N Engl J Med. 2006;354:96. [PubMed] [Google Scholar]
- 10.Warren HS, Suffredini AF, Eichacker PQ, Munford RS. Risks and benefits of activated protein C for severe sepsis. N Engl J Med. 2002;347:1027–1030. doi: 10.1056/NEJMsb020574. [DOI] [PubMed] [Google Scholar]
- 11.Important drug warning, Indianapolis: Eli Lilly; February 4, 2005. Available at: www.fda.gov/downloads/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/UCM164904.pdf. Accessed June 8, 2010.
- 12.Nadel SK, Goldstein B, Williams MD, et al. Drotrecogin alfa (activated) in children with severe sepsis: A multicentre phase III randomized controlled trial. Lancet. 2007;369:836–843. doi: 10.1016/S0140-6736(07)60411-5. [DOI] [PubMed] [Google Scholar]
- 13.Re: Discontinuation of Study F1K-MC-EVBP, Investigation of the Efficacy and Safety of Drotrecogin Alfa (Activated) in Pediatric Severe Sepsis. Indianapolis: Eli Lilly; April 21, 2005. Available at: www.fda.gov/downloads/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/UCM164902.pdf. Accessed June 10, 2010.
- 14.Opal SM. Can we resolve the treatment of sepsis? Lancet. 2007;369:803–804. doi: 10.1016/S0140-6736(07)60383-3. [DOI] [PubMed] [Google Scholar]
- 15.Finfer S, Ranieri VM, Thompson BT, et al. Design, conduct, analysis, and reporting of a multinational placebo-controlled trial of activated protein C for septic shock. Intens Care Med. 2008;34:1935–1947. doi: 10.1007/s00134-008-1266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.University of Versailles Activated Protein C and Corticosteroids for Human Septic Shock (APROCCHS) 2008. Available at: http://clinicaltrials.gov/ct2/show/NCT00625209?term=activated+protein+c&rank=1. Accessed February 23, 2010.
- 17.Marti-Carvajal A, Salanti G, Cardona-Zonilla AF. Human Recombinant Activated Protein C for Severe Sepsis (Review) 3. Cochrane Library: John Wiley & Sons; 2008. [DOI] [PubMed] [Google Scholar]
- 18.Bernard GR, Ely EW, Wright TJ, et al. Safety and dose relationship of recombinant human activated protein C for coagulopathy in severe sepsis. Crit Care Med. 2001;29:2051–2059. doi: 10.1097/00003246-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Vincent JL, Bernard GR, Beale R, et al. Drotrecogin alfa (activated) treatment in severe sepsis from the global open-label trial ENHANCE: Further evidence for survival and safety implications for early treatment. Crit Care Med. 2005;33:2266–2277. doi: 10.1097/01.ccm.0000181729.46010.83. [DOI] [PubMed] [Google Scholar]
- 20.Eichacker PQ, Danner RL, Suffredini AF, et al. Reassessing recombinant activated protein C for sepsis: Time for a new randomized controlled trial. Crit Care Med. 2005;33:2426–2428. doi: 10.1097/01.ccm.0000183002.26587.ff. [DOI] [PubMed] [Google Scholar]
- 21.Bertolini G, Rossi C, Anghileri A, et al. Use of drotrecogin alfa (activated) in Italian intensive care units: The results of a nationwide survey. Intens Care Med. 2007;33:426–434. doi: 10.1007/s00134-007-0554-x. [DOI] [PubMed] [Google Scholar]
- 22.Kanji S, Perrault MM, Chant C, et al. Evaluating the use of drotrecogin alfa (activated) in adult severe sepsis: A Canadian observational study. Intens Care Med. 2007;33:517–523. doi: 10.1007/s00134-007-0555-9. [DOI] [PubMed] [Google Scholar]
- 23.Gentry CA, Gross KB, Sud B, Drevets DA. Adverse outcomes associated with use of drotrecogin alfa (activated) in patients with severe sepsis and baseline bleeding precautions. Crit Care Med. 2009;37:19–25. doi: 10.1097/CCM.0b013e318192843b. [DOI] [PubMed] [Google Scholar]
- 24.Sweeney DA, Natanson C, Eichacker PQ. Recombinant human activated protein C, package labeling and hemorrhage risk. Crit Care Med. 2009;37:327–329. doi: 10.1097/CCM.0b013e3181935102. [DOI] [PubMed] [Google Scholar]
- 25.Early communication about an ongoing safety review [of] Xigris (drotrecogin alfa [activated]), February 4, 2009. Available at: www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/UCM079519.htm. Accessed February 23, 2010.
- 26.Levi M, Levy M, Williams MD, et al. Prophylactic heparin in patients with severe sepsis treated with drotrecogin alfa (activated) Am J Respir Crit Care Med. 2007;176:483–490. doi: 10.1164/rccm.200612-1803OC. [DOI] [PubMed] [Google Scholar]
- 27.Friedrich U, Blom AM, Dahlback B, Villoutreix BO. Structural and energetic characteristics of the heparin-binding site in anti-thrombotic protein C. J Biol Chem. 2001;276:2422–2428. doi: 10.1074/jbc.M011567200. [DOI] [PubMed] [Google Scholar]
- 28.Dhainaut JF, Laterre PF, Janes JM, et al. Activated Protein C Worldwide Evaluation in Sepsis (PROWESS) Study Group. Drotrecogin alfa (activated) in the treatment of severe sepsis patients with multiple-organ dysfunction. Intens Care Med. 2003;29:894–903. doi: 10.1007/s00134-003-1731-1. [DOI] [PubMed] [Google Scholar]
- 29.FDA briefing document, Anti-Infective Advisory Committee. Drotrecogin alfa (activated) recombinant human activated protein C (rhAPC, Xigris). BLA #125029/0. Rockville, Md. September 12, 2001. Available at: www.fda.gov/ohrms/dockets/ac/01/briefing/3797b1_02_FDAbriefing.pdf.
- 30.Dhainaut JF, Antonelli M, Wright P, et al. Extended drotrecogin alfa (activated) treatment in patients with prolonged septic shock. Intens Care Med. 2009;35:1187–1195. doi: 10.1007/s00134-009-1436-1. [DOI] [PubMed] [Google Scholar]