This is the last article in a series on the use of complementary and alternative medicine (CAM) in the U.S. Part 1 discussed the widespread use of CAM in the U.S. and the need for better-informed health professionals to provide patient counseling. Part 2 reviewed government policy and regulatory and safety concerns regarding dietary supplements.
Introduction
With the increasing popularity of CAM and dietary supplements among patients in recent years, there has been a corresponding movement to incorporate these therapies into mainstream medicine.1 These efforts are now influencing both the public and private sectors to invest more deeply and broadly in the practice of integrative medicine.1 However, despite this increasing trend, information about policies and practices regarding the use of dietary supplements in health care facilities is lacking.2 Evidence that does exist shows that such policies within medical facilities are either inconsistent or nonexistent and sometimes don’t comply with accreditation recommendations.3–5 With the growing patient interest in CAM and dietary supplements, health care facilities should establish consistent, reasonable, and enforceable policies regarding their use.6 If these products are not included on the health care institution’s formulary, a carefully considered policy regarding patients’ home supply of dietary supplements should be established.
Integrative Medicine: A Growing Trend in Health Care Facilities
In response to increased consumer interest, mainstream medicine has begun to incorporate certain “alternative” therapies in the delivery of health care.6,7 This popular intermingling of conventional medicine and CAM is referred to as “integrative medicine.”7
Leading academic institutions have also incorporated CAM into medical education, clinical practice, and research.2 Conventional medical schools such as Columbia University College of Physicians and Surgeons, Harvard Medical School, and Thomas Jefferson University have established CAM or integrative medicine centers, whereas others have added this topic to their curricula.1,7 In addition, a national consortium of academic medical centers has been formed to foster the development of integrative medical practice, research, and training.1 Hospitals outside academia are also creating CAM programs, and health care providers have been expanding benefits to include alternative practices.6,7
Many cancer treatment centers also now offer CAM therapies.8 The University of Texas MD Anderson Cancer Center provides an integrative medicine approach to cancer care that incorporates a clinical program, research, and education.8 It offers the “Place … of Wellness” program, which provides more than 75 complementary therapy opportunities to help patients with the nonmedical aspects of living with cancer.8 The Memorial Sloan-Kettering Cancer Center and the Dana Farber Cancer Institute have also established integrative medicine centers.8 In 2003, the Society for Integrative Oncology was also created to provide health care professionals with a forum for the presentation, discussion, and peer review of evidence-based CAM research and treatment modalities for cancer care.8
The Need for Standardized Policies and Practices
Despite the increased acceptance of CAM and integrative medicine, these therapies are still limited in practice.7 Consequently, many institutions have not established formal policies concerning CAM or dietary supplement use.1,7 In addition, when institutions do attempt to integrate CAM and standard medical practices, challenges arise.1,5 Because of this struggle, the task of setting an institutional policy for CAM and dietary supplement use seems to elude many centers.9 In addition, when policies do exist, little is known about the content and quality of these guidelines or how they are implemented in an inpatient setting.3
Perhaps the reason for the inconsistency and confusion in hospital policies regarding CAM is that universally accepted guidelines have not been established for the use of these therapies in an institutional setting.3 However, both the Joint Commission and the American Society of Health-System Pharmacists (ASHP) have provided guidelines concerning dietary supplement use in health care facilities.3,5 Notably, the Joint Commission’s Comprehensive Accreditation Manual for Hospitals defines a medication as “any prescription medication, sample medication, herbal remedies, vitamins, nutraceuticals, over-the-counter drugs, vaccines, diagnostic and contrast agents, used on or administered to a person to diagnosis, treat, or prevent disease or other abnormal conditions.”3 Therefore, the inclusion of herbal remedies, nutraceuticals, and vitamins in this definition makes it very clear that the Joint Commission expects health care facilities to manage dietary supplements with the same diligence and care given to any prescription or nonprescription drug.3,5 A competent policy should therefore require, at a minimum, a rigorous analysis of the impact of dietary supplement use on the patient’s care and condition, a medical order, and thorough documentation of such use.3,5
However, despite guidelines established by both the Joint Commission and ASHP, policies and practices in health care facilities regarding the management of dietary supplements are still often inconsistent and wrought with confusion.3 Studies have revealed that many institutions have not yet come to a strong consensus regarding such policies.3–5 In one study that surveyed 302 pharmacy directors in acute-care facilities, only 62% had developed and implemented policies regarding dietary supplements, whereas the remaining 38% of respondents indicated that no such policy existed.4 Additional findings for this study are listed in Table 1.4
Table 1.
Policies and Practices Regarding Dietary Supplements in Responding Institutions
| % Respondents Reporting Policy or Practice | ||
|---|---|---|
| Policy or Practice | Institutions With Policy (n = 163) | Institutions Without Policy (n = 104) |
| Written order by authorized prescriber required in medical record | 89 | 93 |
| Supplement use documented in medication administration record | 75 | 81 |
| Mechanism in place for identification by health care practitioner prior to use | 75 | 68 |
| P&T committee reviews and approves supplements for inclusion in formulary | 68 | 66 |
| Supplement use documented in pharmacy database | 65 | 67 |
| Dietary supplements prohibited at institution | 30 | 9* |
| Nonformulary requests and forms submitted prior to supplement use | 29 | 28 |
| Written/signed patient consent obtained prior to supplement use | 12 | 3† |
* = significant difference (χ2 = 16.405; d.f.= 1; P < 0.0001).
χ2 = 5.866; d.f. = 1; P < 0.015.
d.f. = degree of freedom.
Originally published in Bazzie KL, et al. Am J Health Syst Pharm 2006;63(1):65–70. © 2006, American Society of Health-System Pharmacists, Inc. All rights reserved. Reprinted with permission. (R1030)4
Even among hospitals that do have written policies concerning dietary supplements, the quality of guidelines varies and some may even compromise patient safety and quality of care.3–5 In a 2008 survey of 109 National Association of Children’s Hospitals and Related Institutions (NACHRI), only 44% of these health care facilities reported having written policies that included vitamins and minerals, herbs, and other dietary supplements.3 In addition, only 46% of the facilities surveyed required potential drug–dietary supplementinteractions to be documented, and only 32% made surgical preoperative recommendations regarding dietary supplement products.3 Investigators for this study concluded that only 11% of the 109 hospitals surveyed satisfied the 10 quality indicators that they had established for policies regarding the use of dietary supplement in health care facilities.3
Key Concerns When Developing Strategies For CAM and Dietary Supplement Usage
Unfortunately, adapting current hospital policies and pharmacy practices to patients’ use of dietary supplements is complicated by a lack of FDA regulations and safety data for these products.5 Health care professionals are also often unaware and uninformed about adverse effects and drug interactions that can occur with dietary supplements.6 The lack of regulation, evidence, and knowledge of these products further emphasizes the need for pharmacists to accurately research their use in order to prevent reactions or interactions.6 Patients receiving acute care may also have differing responses to dietary supplements, depending on their underlying pathology and other drug therapies they are taking.6
Comorbidities are also common in patients, so pharmacists must actively monitor the use of these products, just as they do for prescription medications.8 Because some dietary supplements affect clotting dynamics or cause additive sedation, patients taking these products may have to follow additional precautions prior to surgery and anesthesia.6 Doctors and pharmacists also need to educate patients that even though dietary supplement therapies are often considered “natural,” they can have an impact on the chemical balance in the body, just as prescription drugs do.6 In order to educate patients, health care provider education is critical.7
One of the most significant questions regarding dietary supplement policies in health care facilities is whether to confiscate a patient’s home supply upon admission, replace it with items from an institutional formulary, or establish practices that ensure the safe use of these substances.9 Banning dietary supplement use increases the risk that patients might smuggle these agents into the hospital and hide them from clinicians and other staff workers.5 Consumers tend to be loyal to certain products and brands that are unlikely to be on a health care facility’s formulary.9 Federal law also allows patients access to dietary supplements, irrespective of medical advice.9 For these reasons, existing policies in many facilities allow patients to bring their own supply of these products from home.5 This practice makes it particularly important to identify home supply dietary supplements in order to avoid surgery-related or drug-interaction risks.3,5
Some health care facilities allow the unrestricted use of dietary supplements.5 However, hospitals that do not require dietary supplement identification, that allow patients to take these products irrespective of risk, or that do not securely store these products are not meeting Joint Commission accreditation guidelines.5 Such a policy is also contradicted by ASHP guidelines, which discourage the availability of a home supply of dietary supplements at a patient’s bedside.4 The ASHP recommends that pharmacists identify all dietary supplement products before they are used and that self-administered medications be avoided if possible.10 The ASHP also advises that self-administration of dietary supplements during a stay in the health system may increase the risk to patients and liabilities to health care professionals and institutions.10 If an institution decides to allow patients to use dietary supplements, the ASHP also recommends that a prescribed order for the specific dietary supplement be entered into the patient’s medical record and that a pharmacist review and verify the order.10
When developing policies concerning dietary supplements, health care providers are therefore faced with a difficult dilemma—acquiesce to patient preferences or impose paternalistic policies that deny patient choice.9 Such decisions should involve both legal as well as clinical considerations and expertise in order to create a policy that responds to patients’ interests and that honors clinical sensibilities.9
Liability Issues for Health Care Facilities
When creating policies concerning CAM and dietary supplements, many hospitals struggle with balancing patient care and legal and ethical concerns.3 Relatively little has been scientifically proven regarding the efficacy and safety of most CAM and dietary supplement treatments, so these therapies are still considered to be nonstandard care.6 Because of this lack of efficacy and safety data, it is often not possible for health care practitioners to make informed recommendations regarding dietary supplement use.6 Therefore, the risk of unforeseen adverse effects from dietary supplement use by a patient during an admission places significant liability on clinicians and hospitals.5 Risk management, the potential for malpractice claims and awards, and the rising costs of malpractice insurance coverage must therefore be considered when dietary supplement use policies are being established.1
These liability risks also contribute to the lack of dietary supplement policies in health care facilities because rather than establish a formal protocol, many medical centers prefer to leave all decision-making to the individual clinician.9 Such a policy then presents significant liability issues for physicians, who can be held liable whether or not they have recommended that a patient use dietary supplements.6 Furthermore, even though the medical literature tends to blame dietary supplements for adverse drug interactions, it is unlikely that conventional medical products and procedures would be exempt from legal claims and awards regarding these interactions.1
As a safeguard against liability, some hospitals are also satisfied to counsel patients against dietary supplement use and then document the patient’s decision to go against medical advice in the patient’s record.9 However, even allowing the hospital nursing staff to administer potentially toxic dietary supplements of uncertain benefit may be seen as unethical and places significant liability on health care facilities and physicians.5
Yet another approach taken by some facilities to reduce potential liability is to request that patients sign a liability waiver or provide informed consent prior to permitting the use of dietary supplements.10 However, some experts have suggested that the absence of safety and efficacy data for these products prevents the valid use of informed consent.5 Patients may also refuse to sign a release and may surreptitiously use dietary supplements, which would continue to expose a health care facility to legal liability.5
Current Common Policies in Health Care Facilities
In place of a formal written policy, some health care centers have adopted other strategies regarding patient dietary supplement use.5 These strategies often resemble the established protocol for patient use of a home supply of prescription or over-the-counter medications.5
According to this informal protocol, during the intake process, a physician or nurse typically interviews the patient and identifies which conventional pharmaceuticals, dietary supplements, or other substances the individual is taking.5,9 The pharmacy staff is then informed about these substances.9 The pharmacist then researches the medical literature to identify any potential problems that may occur with the use of these medications, supplements, and other products.9 Because of a lack of efficacy and safety data regarding dietary supplements in the medical literature, this step presents a challenge and places a great deal of responsibility on the pharmacist.6 Pharmacists should prepare for this responsibility by using whatever resources are available that will help them increase their knowledge about dietary supplements.6
The physician then discusses the risks and benefits of dietary supplement use with the patient.9 The focus of this conversation generally concerns safety rather than efficacy.9 If the product is safe yet has questionable efficacy, then it’s up to the patient to decide whether it’s worth the expense.9 Even if the pharmacy staff considers a dietary supplement product to be unsafe, generally it is still the patient’s decision whether to continue to using it.9 However, the institution usually documents the patient’s decision in the chart by dictating it, writing a note, or including the information in an e-mail that is printed and included in the patient’s medical record.9
After these steps, the physician may then write an order allowing the patient to use dietary supplements from a home supply.5 The nursing staff collects the dietary supplements the patient has brought into the health care facility and sends them to the pharmacy.5 The pharmacist then attempts to verify the identity of the products.5 The pharmacist relabels the identified dietary supplements and returns them to the patient’s floor, where they are stored in the medication room or cart.5 A nurse then administers the agent or dietary supplement according to the dose and schedule ordered by the physician and documents the patient’s use in the medication administration record (MAR).5
This system works, but it is time-consuming for the health care facility staff. Furthermore, not all physicians comply with such a system.9 Some physicians simply advise patients not to take dietary supplements, whereas others bypass making any recommendation by referring patients to the pharmacy staff for information.9
Additional Elements Suggested for Inclusion In Dietary Supplement Policies
Other emerging ad hoc approaches may also provide sensible methods for developing policies that address the widespread use of dietary supplements among patients.3,9 Areas for improvement in dietary supplement policy that have been identified include establishing a comprehensive policy that applies to all dietary supplements, enhancing methods for documenting patient use, and establishing guidelines for pre-operativeuse.3 It has also been suggested that research be conducted to precisely determine the relationship between facility policies, quality of care, and patient outcomes.3
Several features that have also been suggested for inclusion in a dietary supplement policy:
Emphasize only evidence-based use. Health care facilities may consider limiting dietary supplement use by allowing patients to only take products that have been proven safe.9 Evidence that either supports or contradicts a product’s use should also be shared with patients in order to permit them to participate in decision-making.9 Patients should be encouraged to communicate their preferences, and clinicians should be prepared to respond about whether such choices are consistent with the medical literature.9
Restrict patient use only to products that have undergone quality assurance testing. Because dietary supplements (particularly Chinese herbals) may be adulterated, quality assurance testing for these products are critical.9 Health care facilities should therefore allow patients to take only high-quality dietary supplements.9 Such products are made by manufacturers that use pharmaceutical grade-ingredients and submit to third-party quality assurance testing by the U.S. Pharmacopeia (USP).3
Limit clinicians’ scope of practice. Health care facilities might also consider limiting their providers’ scope of practice with respect to dietary supplements. Some centers ban all of their health care practitioners from recommending dietary supplements, whereas others allow pharmacists, dietitians, and nutritionists to make such recommendations because they are deemed to have relevant expertise.9
Provide adequate informational resources to health care practitioners. Even if a facility or a health care provider doesn’t recommend dietary supplements, patients may still ask questions about these products.9 For this reason, informational resources, particularly about dietary supplement safety, should be made available to clinicians within the hospital pharmacy or information center.9 Education regarding dietary supplements might also be included in the curriculum offered by health care institutions.9
Emphasize an overall health strategy to patients, and limit the use of dietary supplements to wellness care. Some health care facilities have reported concerns that patients think of dietary supplements as a “magic pill.”9 In such situations, an effort might be made to refocus the patient on an overall health strategy for symptom relief and disease cure.9 Health care institutions may also find it helpful to reduce patient reliance on dietary supplements as part of a treatment regimen and, instead, to emphasize the potential role of these products in health maintenance.9 This approach may help to obviate the common phenomenon of patients equating the use of dietary supplements with conventional medications used in the management of medical conditions.9
A Suggested ‘Model Policy’ for Health Care Facilities
Despite the lack of consensus concerning dietary supplement policies in health care institutions, it is agreed that a model policy should be reasonable, enforceable, and, ideally, based upon established pharmacy practices.5 It has therefore been suggested that such a model policy should include the following specific features:5
a physician’s order, including the name, source, dose, and schedule of the dietary supplement
the identification, labeling, and recording of the substance by the hospital pharmacy
secure storage of the product in the medication room or cart
administration by the patient or nurse, which is documented in the MAR
the reporting of any adverse events associated with dietary supplement use internally to pharmacy and risk management personnel, as well as externally to the FDA’s MedWatch program
It is also recommended that this model policy include provisions for educating health care providers so that they are prepared to make clinical decisions and discuss dietary supplement use with patients.5
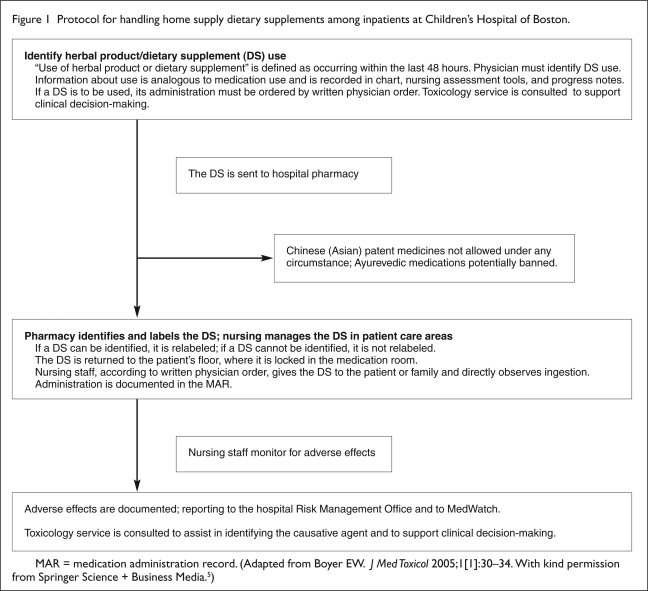
A protocol for a patient’s use of dietary supplements from a home supply that follows these recommendations was developed at Children’s Hospital in Boston (Figure 1).5 This protocol was reviewed and approved by the institution’s directors of pharmacy and nursing services, the P&T committee, and attorneys for the Risk Management Foundation of Harvard University.5 In accordance with this policy, physicians use preprinted physical and admission history forms during intake that prompt an inquiry about patients’ use of dietary supplements.5 A physician must then write an order that specifies the dose and schedule for any dietary supplements the patient will be allowed to take after hospital admission.5 Although consultation with a pharmacist is not required, clinicians are encouraged to use holistic medicine and medical toxicology services as well as online pharmacy databases.5
Figure 1.
Protocol for handling home supply dietary supplements among inpatients at Children’s Hospital of Boston.
MAR = medication administration record. (Adapted from Boyer EW. J Med Toxicol 2005;1[1]:30–34. With kind permission from Springer Science + Business Media.5)
The dietary supplements in the patient’s home supply are then sent to the pharmacy, but they are relabeled only if they can be definitively identified.5 The pharmacy maintains an ongoing record of patients’ dietary supplement use.5 The dietary supplement is then locked in the medication room until the dosing schedule calls for its administration.5 The nurses then delivers the dietary supplement to the patient or caregiver for administration. After observing ingestion by the patient, the nurse records the event in the MAR.5 If any adverse events are observed, they are reported internally to hospital pharmacy and risk-management personnel and externally to MedWatch.5
Each aspect of this policy is designed to educate clinicians regarding dietary supplement use, engage patients, or adhere to directives from credentialing organizations.5 Providing educational services to physicians and encouraging discussion with patients broadens clinical knowledge about dietary supplement use.5 Requirements for physician’s orders, pharmacy and nursing documentation, and the secure storage of dietary supplements complies with Joint Commission accreditation requirements.5 The patients’ sense of participation in their own medical care is enhanced by allowing them or a caregiver to administer the dietary supplement.5 In addition, allowing self-administration may relieve the hospital staff of ethical and legal burdens regarding the administration of dietary supplements with unknown benefits and toxicities.5 This suggested model strategy, however, does not include a request of patients to provide informed consent with respect to dietary supplement use.5
P&T Committee and Formulary Considerations
After a health care facility has a dietary supplement policy in place, decisions must be made about which products, if any, to include on both inpatient and outpatient formularies and which brands to stock.9 The survey of NACHRI hospitals found that most formularies (99%) in these hospitals do include vitamins and minerals (Table 2).3 However, only 2% of these institutions’ formularies include herbs, and only 38% include other types of dietary supplements (such as probiotics or melatonin).3 The most frequently cited reasons for including dietary supplements on the inpatient formularies for these institutions were a prior review by the P&T committee, safety data, clinical evidence, availability of standardized products, physician demand, cost, and proof of quality of the product (Table 3).3
Table 2.
Formulary Availability and Policies About Dietary Supplements
| Variable | No. (%) |
|---|---|
| Written policy regarding inpatient use | |
| Herbal products | 86 (79) |
| Other dietary supplements | 74 (68) |
| Vitamins and minerals | 51 (47) |
| Other policy | 8 (7) |
| Answered yes to all three (vitamins and minerals, herbs, and other dietary supplements) | 48 (44) |
| Policy based on national guidelines about dietary supplements | 18 (17) |
| Policy on staff recommendations on use of dietary supplements* | |
| Staff may make recommendations | 70 (64) |
| Staff may not make recommendations | 18 (17) |
| Policy does not specify | 38 (35) |
| Other | 17 (16) |
| Available on inpatient formulary | |
| Vitamins and minerals | 108 (99) |
| Herbal products | 2 (2) |
| Other dietary supplements† | 41 (38) |
Not all respondents answered this question, and more than one answer was allowed.
Other dietary supplements include products such as fish oil, melatonin, chondroitin, and glucosamine.
From Gardiner P, et al. Pediatrics 2008;121(4):e775–e781.
©American Academy of Pediatrics
Table 3.
Factors Affecting Inpatient Formulary Decisions Regarding Dietary Supplements
| Variable | No. (%) |
|---|---|
| P&T committee recommendations | 104 (95) |
| Safety data | 102 (94) |
| Clinical evidence | 101 (93) |
| Standardized product available | 92 (84) |
| Physician demand | 84 (77) |
| Cost | 68 (62) |
| Proof of quality of product | 63 (58) |
| Patient demand | 55 (50) |
| Third-party reimbursement covers dietary supplements | 27 (25) |
| Other factors | 8 (7) |
Not all respondents answered this question, and more than one answer was allowed.
From Gardiner P, et al. Pediatrics 2008;121(4):e775–e781.
©American Academy of Pediatrics
An effective approach taken by one managed health care organization was to form a P&T subcommittee to evaluate common dietary supplements for efficacy and safety.11 The goal was to develop a guide listing P&T committee recommendations regarding dietary supplement use.11 The subcommittee met monthly and evaluated the following criteria concerning these agents:11
proposed or common uses
evidence-based indications, if any
adverse events, drug interactions, and precautions
dosing and other recommendations for use
formulation and origin
The P&T subcommittee then assigned a recommendation of “neutral,” “use with extreme caution,” or “negative” to 85 different dietary supplements.11 The evidence-based guide for dietary supplement use that this P&T subcommittee developed was well received and met the dual goals of responding to patient demand and providing ongoing education to clinicians.11
The ASHP’s “Statement on the Use of Dietary Supplements” is also a valuable source of guidance regarding the review of dietary supplements for formulary inclusion.4 The ASHP recommends that dietary supplements undergo a formulary review process at least as rigorous as that for prescription and nonprescription drugs.10 The ASHP advises that the inclusion of any product in a health system’s formulary should be based on comparative data regarding efficacy, adverse effects, potential therapeutic advantages and disadvantages, the potential for drug–dietary supplement interactions, and cost.10 The ASHP guidelines also advise that the lack of evidence of safety and efficacy for dietary supplements and the variability in product content and quality make most of these products unsuitable for inclusion in health system formularies.10
Conclusion
With increased patient use of CAM and dietary supplements, health care facilities should establish consistent, reasonable, and enforceable policies regarding the use of these products.6 If they are not included on the formulary for a health care facility, a carefully considered policy regarding patients’ home supplies of dietary supplements needs to be established.
References
- 1.Micozzi MS. Integrative medicine in pharmacy and therapeutics. P&T. 2003;28(10):666–672. [Google Scholar]
- 2.Vohra S, Feldman K, Johnston B, et al. Integrating complementary and alternative medicine into academic medical centers: Experience and perceptions of nine leading centers in North America. BMC Health Services Res. 2005;5(78):1–7. doi: 10.1186/1472-6963-5-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gardiner P, Phillip RS, Kemper K, et al. Dietary supplements: Inpatient policies in U.S. children’s hospitals. Pediatrics. 2008;121(4):e775–e781. doi: 10.1542/peds.2007-1898. [DOI] [PubMed] [Google Scholar]
- 4.Bazzie KL, Witmer DR, Pinto B, et al. National survey of dietary supplement policies in acute care facilities. Am J Health Syst Pharm. 2006;63(1):65–70. doi: 10.2146/ajhp050106. [DOI] [PubMed] [Google Scholar]
- 5.Boyer EW. Issues in the management of dietary supplement use among hospitalized patients. J Med Toxicol. 2005;1(1):30–34. doi: 10.1007/BF03160903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen KR, Cerone P, Ruggiero R. Complementary/alternative medicine use: Responsibilities and implications for pharmacy services. P&T. 2002;27(9):440–446. [Google Scholar]
- 7.Kantor M. The role of rigorous scientific evaluation in the use and practice of complementary and alternative medicine. J Am Coll Radiol. 2009;6(4):254–262. doi: 10.1016/j.jacr.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 8.McHughes M, Timmerman B. A review of the use of CAM therapy and the sources of accurate and reliable information. Manag Care Pharm. 2005;11(8):695–703. doi: 10.18553/jmcp.2005.11.8.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen MH, Ruggie M, Micozzi MS. The Practice of Integrative Medicine: A Legal and Operational Guide. New York: Springer; 2006. p. 224. [Google Scholar]
- 10.American Society of Health-System Pharmacists ASHP statement on the use of dietary supplements. Am J Health Syst Pharm. 2004;61:1707–1711. doi: 10.1093/ajhp/61.16.1707. [DOI] [PubMed] [Google Scholar]
- 11.Dunn JD, Cannon HE, Lewis T, Shane-McWhorter L. Development of a complementary and alternative medicine (CAM) pharmacy and therapeutics (P&T) subcommittee and CAM guide for providers. J Manag Care Pharm. 2005;11(3):252–258. doi: 10.18553/jmcp.2005.11.3.252. [DOI] [PMC free article] [PubMed] [Google Scholar]