Abstract
To confirm seizure susceptibility (SZS) quantitative trait loci (QTLs) on chromosome (chr) 15 identified previously using C57BL/6J (B6) and DBA/2J (D2) mice and to refine their genomic map position, we studied a set of three congenic strains in which overlapping segments of chr 15 from D2 were transferred onto the B6 background. We measured thresholds for generalized electroshock seizure (GEST) and maximal electroshock seizure (MEST) in congenic strains and B6-like littermates and also tested their responses to kainic acid (KA) and pentylenetetrazol (PTZ). Results document that MEST is significantly lower in strains 15M and 15D, which harbor medial and distal (telomeric) segments of chr 15 (respectively) from D2, compared with strain 15P, which harbors the proximal (acromeric) segment of chr 15 from D2, and with control littermates. Congenic strains 15P and 15M exhibited greater KA SZS compared with strain 15D and B6-like controls. All congenic strains were similar to controls with regard to PTZ SZS. Taken together, results suggest there are multiple SZS QTLs on chr 15 and that two QTLs harbor gene variants that affect MEST and KA SZS independently. The MEST QTL is refined to a 19 Mb region flanked by rs13482630 and D15Mit159. This interval contains 350 genes, 183 of which reside in areas where the polymorphism rate between B6 and D2 is high. The KA QTL interval spans a 65 Mb region flanked by markers D15Mit13 and rs31271969. It harbors 83 genes in highly polymorphic areas, 310 genes in all. Complete dissection of these loci will lead to identification of genetic variants that influence SZS in mice and provide a better understanding of seizure biology.
Keywords: mouse, genetics, quantitative trait locus, kainic acid, pentylenetetrazole, maximal electroshock seizure threshold, generalized electroshock seizure threshold, congenic strains, epilepsy
understanding how specific genetic variation in mice is related to seizure susceptibility (SZS) provides information both on the biology of seizures and on the pathogenesis of epilepsy. Accordingly, several different lines of research have provided the impetus to focus on mouse chromosome (chr) 15 with respect to genetic influences on seizures and epilepsy. Among the genes mapped to chr 15 are jerky (27) and stargazer (19, 23), mutated alleles of which were cloned from mice characterized as having neurological syndromes that include unprovoked seizures. Analysis of such spontaneously occurring mouse mutations has provided new perspectives on the biological mechanisms underlying seizure activity (20). Moreover, extension of mouse models to clinical studies has also led to new insight into epilepsy (22). In addition to genes that were found through identification of naturally occurring mutations in rare strains of mice that have seizures, several chr 15 genes are implicated in seizure biology as a result of studying knockout mice or mice with other engineered or induced gene mutations such as Slc1a3 (25).
Previous quantitative trait locus (QTL) mapping studies of seizure-related traits in mice have also stimulated interest in chr 15. Reports from several different laboratories indicate that chr 15 QTLs are involved in mediating the difference in SZS between C57BL/6J (B6) mice, which are relatively seizure resistant, and DBA/2J (D2) mice, which are relatively seizure susceptible. Importantly, these inbred strains of mice comprise a multifactorial model of SZS (4) and thus may better represent the genetically heterogeneous architecture of common human epilepsies compared with mouse seizure models involving a mutation in a single gene. A variety of seizure paradigms have been used to study the genetic influences on SZS in the B6 and D2 inbred mouse strains and QTLs on chr 15 have been detected for susceptibility to kainic acid (KA) seizures (10), cocaine seizures (14), and electrically induced seizures (7). In all of these studies, the alleles associated with greater seizure susceptibility were derived from the D2 strain.
To confirm the existence of the chr 15 QTLs between B6 and D2 mice and to characterize resources for fine-mapping, we studied a set of congenic strains in which overlapping portions of chr 15 are introgressed from the D2 strain onto a B6 genetic background (15). In this article, we report differences in electrical seizure thresholds and KA-induced SZS between these congenic strains and control B6-like littermates. We then combine these data with analysis of DNA markers at the introgression breakpoints and haplotype block analysis of critical intervals to refine the map position of chr 15 SZS QTLs.
MATERIALS AND METHODS
Animals.
Mice used in these studies were produced and maintained at the Department of Veterans Affairs Medical Center in Coatesville, PA. Breeding pairs of mice from three chr 15 “genome-tagged” mouse strains were a gift from Dr. Richard Davis at the University of California at Los Angeles (15). The strains, 15P, 15M, and 15D, are congenic between B6 and D2 and are characterized by having restricted segments of chr 15 from the D2 strain introgressed onto the genetic background of the B6 strain. Original breeders, which were homozygous with respect to genomic regions of introgression from D2, were mated with colony B6 mice and heterozygous congenic mice were intercrossed to generate homozygous congenic mice and “wild type” B6-like littermate controls (homozygous for B6 alleles across the congenic interval) for propagation for seizure testing. All mice were maintained on a 14 h/10 h light/dark schedule and provided food and water ad libitum. The standard operating procedure in the animal facility (and for this study) involves trio breeding. Females are given their own cages where litters are delivered and nursed. At 3–4 wk of age, pups are weaned and tail-clipped to obtain DNA for genotyping purposes. Mice are group-housed by sex and are used for breeding as early as 5 wk of age or for seizure testing beginning at 8 wk of age. Only male mice were used in seizure tests. All studies were approved by the Institutional Animal Care and Use Committees governing the participating laboratories.
DNA marker genotyping.
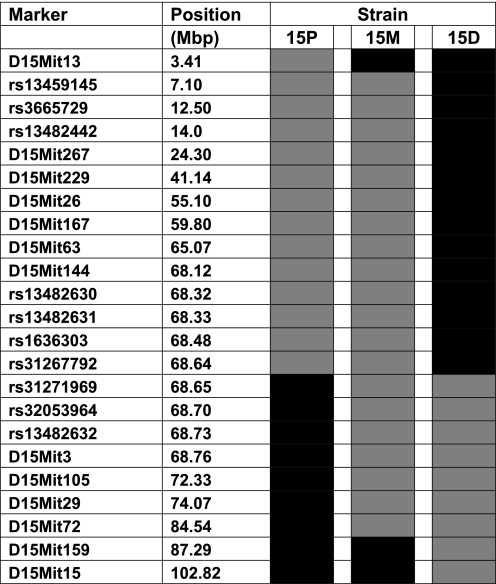
The panel of DNA markers used to characterize the three chr 15 congenic strains is shown in Fig. 1. Previously published methods were used to analyze microsatellite markers (9, 10). For fine-mapping at introgression breakpoints, we used PCR/RFLP assay methods to analyze single nucleotide polymorphism (SNP) DNA markers. SNPs were identified from publically accessible databases (e.g., http://www.informatics.jax.org/) and confirmed with B6 and D2 genomic DNA. Three SNP markers were analyzed in the interval between 3 and 24 Mbp to define the breakpoint at the 5′ (acromeric) end of the introgressed interval in strain 15M. Seven SNP markers were analyzed in the interval between 68 and 69 Mbp to define the 3′ (telomeric) introgression breakpoint in strain 15P and the 5′ (acromeric) introgression breakpoint in strain 15D. PCR primer sequences and information on strain-specific restriction enzyme patterns are available upon request. SNP genotype scoring was based on the analysis of digested fragments on a 7% nondenaturing polyacrylamide gel. Gels were stained with ethidium bromide and images captured under ultraviolet illumination. Microsatellite markers were scored as described previously (10). Haplotype blocks in critical QTL intervals were compared using a publically accessible web-based resource (http://mouse.perlegen.com/mouse/mousehap.html). The critical QTL intervals were scanned at a resolution of 1 Mbp and all genes whose starting or ending coordinates fell within nonshared (or unknown) haplotype blocks were counted.
Fig. 1.
Schematic diagram of chr 15 showing regions of D2 genomic introgression (gray blocks) onto the B6 background (black blocks) for three chr 15 congenic strains (15P, 15M, and 15D). The intersection of gray and black blocks represents “breakpoint” intervals for each strain where there is no genotype information. Marker positions are taken from the database at http://uswest.ensembl.org/Mus_musculus/Info/Index.
Seizure testing.
Electroshock seizure thresholds were determined using a constant current electroshock unit (model #7801; Ugo Basile, Varese, Italy) as described previously (5–7). Mice were tested with a single electric shock once per day beginning at age 8–9 wk. Initially, current level was set at 20 mA, and it was increased by 2 mA with each successive daily trial until a maximal seizure was observed. Other parameters of the stimulus were held constant (60 Hz, 0.4 ms pulse width, 0.2 s duration) and all shocks were delivered via ear-clip electrodes. Seizures were elicited at all current intensities utilized: lower intensities produced facial and forelimb clonus, whereas higher intensities produced generalized and maximal seizures. A generalized seizure was defined by loss of upright posture (i.e., falling over) and bilateral limb clonus. The current value at which mice first exhibit a generalized seizure is taken as the generalized electroshock seizure threshold (GEST). A maximal seizure was defined by bilateral tonic extension of hindlimbs. The current value at which mice exhibit tonic hindlimb extension is taken as the maximal electroshock seizure threshold (MEST). The sequence of responses that characterized a trial in which a maximal seizure was observed is as follows: bilateral tonic forelimb flexion, bilateral tonic hindlimb flexion, and bilateral tonic hindlimb extension. These signs are sometimes preceded or accompanied by wild running in the observation chamber. Mice were euthanized by cervical dislocation under CO2 anesthesia immediately after a trial in which a maximal seizure was elicited.
Susceptibility to KA-induced seizures was quantified using a paradigm employed previously to map KA SZS QTLs (10). Mice were tested with a single injection of KA at a dose of 25 mg/kg sc and observed for 90 min during which time behavioral seizure activity was assessed. Endpoints were established as latencies to specific stages of the KA seizure response, which comprises a specific sequence of behavioral events. The first distinct seizure response observed is myoclonus. This type of focal or partial seizure may involve a whole body jerk, facial twitching with head turning/head bobbing, or alternating rhythmic movements of fore limbs. The next clearly identifiable seizure stage is a generalized seizure that is characterized by rearing with bilateral forepaw clonus (usually involving falling over and inability to maintain upright posture) or wild running and jumping. Status epilepticus was defined as rearing and bilateral forepaw clonus occurring continuously for 10 min. The 90 min observation period was extended accordingly if a mouse exhibited behavior consistent with a classification of status epilepticus after 80 min.
Susceptibility to pentylenetetrazol (PTZ)-induced seizures was quantified according to methods utilized previously in our laboratory for mapping PTZ SZS QTLs (8). Mice were tested with a single injection of PTZ at a dose of 80 mg/kg sc and observed for 45 min during which time behavioral seizure activity was assessed. Endpoints were established based on latencies to three specific stages of the PTZ seizure response: myoclonus (focal or partial seizure involving a whole body jerk or facial or forelimb clonus), generalized seizure (loss of upright posture, bilateral clonus), and maximal seizure (tonic hindlimb extension).
Data analysis.
Phenotype data including strain, age, sex, weight, and seizure responses (thresholds and latencies) are maintained in Excel spreadsheet format. Strain averages for MEST were compared using ANOVA with strain as the independent variable. GEST was analyzed nonparametrically with a Kruskal-Wallis ANOVA due to unequal group variance. For KA tests, latencies for first myoclonic seizure, first generalized seizure and status epilepticus were compared between strains using the Kaplan-Meier method. For PTZ tests, latencies to myoclonus and generalized seizure were analyzed by ANOVA. Post hoc analyses to examine statistical relationships between strains for SZS endpoints were conducted using the Tukey test (ANOVA) or a Log-Rank test (Kaplan-Meier) with Holm-Bonferroni correction. In addition, we compared the number of mice from each strain achieving the endpoint of status epilepticus (KA) or maximal seizure (PTZ) using the Fisher exact test. For this analysis, each congenic strain was compared individually to the B6-like control group (3 separate tests) and P values were adjusted using the Holm-Bonferroni method. Analyses were performed using the Truepistat software program (Richardson, TX) or the R software environment for statistical computing and graphics (http://www.r-project.org/).
RESULTS
Figure 1 depicts the markers on chr 15 that were used to characterize genomic regions of introgression in the three congenic strains. Inspection of marker patterns reveals the large overlapping intervals between strains 15P and 15M and between strains 15M and 15D. Surprisingly, we discovered that the distal (i.e., 3′) end of the introgressed region in strain 15P is no more than 9 Kb away from the proximal (i.e., 5′) end of the introgressed region in strain 15D (rs312267792 and rs31271969 in Fig. 1). It is possible that there is an overlap between strains 15P and 15D in the region flanked by rs31267792 and rs31271969; however, we were unable to test this possibility due to the lack of polymorphisms within the interval that distinguish B6 and D2 strains. There are no complete genes and only one expressed sequence tag mapped to the 9 Kb interval between rs31267792 and rs31271969 in Fig. 1.
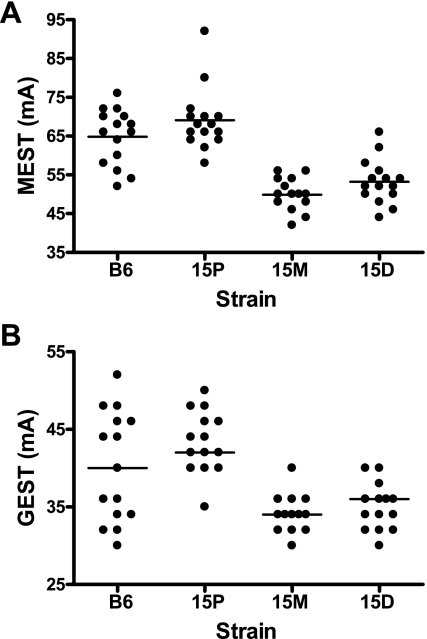
Electrical seizure thresholds for chr 15 congenic strains are shown in Fig. 2. Compared with both B6-like controls and strain 15P, mice from strains 15M and 15D have significantly lower mean MEST values (P < 0.001 for 15M vs. B6-like controls and 15P; P < 0.001 for 15D vs. B6-like controls and 15P). No significant differences are observed between strain 15P and B6-like controls or between strains 15M and 15D (Fig. 2A). Although a similar trend is observed for GEST in that 15M and 15D mice have lower mean GEST values than both 15P and B6-like control mice (Fig. 2B), unequal group variance necessitated comparison of strain medians. The differences in medians are only statistically significant for comparisons between congenic strains with strain 15D exhibiting a higher value than strains 15M and 15P (P < 0.001 for both comparisons). No significant differences were observed when congenic strains were compared with B6-like control mice (P = 0.556 for 15P; P = 0.114 for 15M; P = 0.245 for 15D).
Fig. 2.
Scatter plot of maximal electroshock seizure (MEST, A) and generalized electroshock seizure (GEST, B) values in chr 15 congenic strains and B6-like littermate controls (n = 15 per group). The solid lines show the mean MEST value. For MEST, strains 15M and 15D are significantly different than B6 controls (P < 0.001) and strain 15P (P < 0.001) (ANOVA/Tukey). For GEST, comparison of median values revealed that strain 15P was significantly different from strains 15M and 15D (P < 0.001, Kruskal-Wallis ANOVA and Holm-Bonferroni adjusted pair-wise Wilcoxon rank sum).
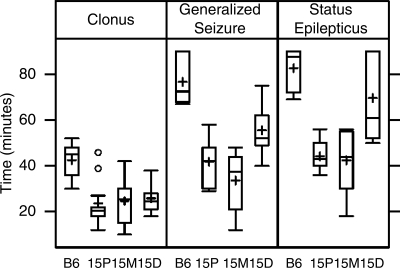
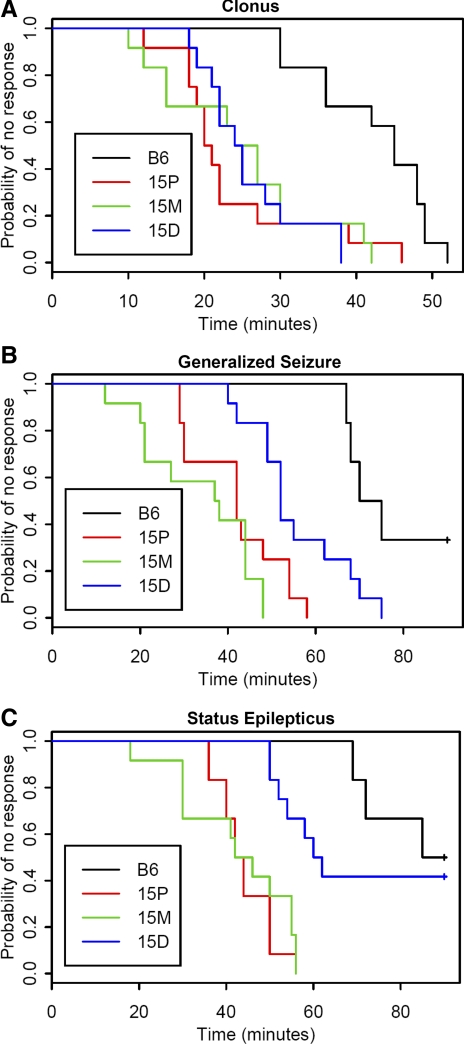
Figure 3 shows a summary of strain-specific responses to KA for the latency endpoints of clonus, generalized seizure, and status epilepticus, and Fig. 4, A–C, shows the respective Kaplan-Meier curves. For all three endpoints, a log-rank test found significant differences between the group curves (P < 0.001 for all endpoints). Holm-Bonferroni-adjusted log-rank tests between each pair of groups found significant differences when comparing congenic strains 15P and 15M to B6-like controls for all three endpoints (P < 0.001 for all such comparisons). There were no significant differences between congenic strains 15P and 15M for any endpoint (P = 1.000 for clonus, P = 0.161 for generalized seizure, P = 0.648 for status epilepticus). Mice from congenic strain 15D were significantly different than B6-like control mice for clonus and generalized seizure (P < 0.001 and P = 0.002, respectively), but not significantly different for status epilepticus (P = 0.485). Congenic strain 15D was not significantly different than either strain 15P or strain 15M for clonus (P = 1.000 for both strains) but was significantly different than both strains for generalized seizure (P = 0.024 for 15P, P < 0.001 for 15M) and for status epilepticus (P < 0.001 for 15P, P = 0.001 for 15M). We also observed that significantly more 15P and 15M mice succumbed to status epilepticus compared with B6-like controls (P = 0.041 for both comparisons, Fisher exact test with Holm-Bonferroni correction).
Fig. 3.
Box-plot of the distribution of kainic acid (KA) seizure endpoint responses in chr 15 congenic strains and B6-like littermate controls (n = 12 per group). The horizontal line inside each box represents the median response value, and the plus sign represents the mean response value. The top line and bottom line of each box represent the 75th percentile and 25th percentile, respectively. The line above extends to the largest value within 1.5 × IQR (interquartile range) of the 75th percentile, where IQR is the 75th percentile minus the 25th percentile, and the line below extends to the smallest observation within 1.5 × IQR of the 25th percentile. The 2 circles above the line on Clonus 15P are outliers that are >1.5 × IQR above the 75th percentile.
Fig. 4.
Kaplan-Meier plots for KA seizure endpoints in chr 15 congenic strains and B6-like littermate controls (n = 12 per group). Colored lines represent strain trajectories for achieving each endpoint. Statistical comparisons were conducted using Holm-Bonferroni-adjusted log-rank tests. A: clonus: all congenic strains are significantly different from B6-like controls (P < 0.001); there are no differences between any of the other groups (P = 1.000). B: generalized seizure: all congenic strains are significantly different from B6-like controls (P < 0.001 for 15M and 15P, P = 0.002 for 15D); 15D is significantly different from other congenic strains (P = 0.024 compared with 15P, P < 0.001 compared with 15M); there is no significant difference between 15M and 15P (P = 0.161). C: status epilepticus: 15P and 15M are significantly different from B6-like controls (P < 0.001 for both) and from 15D (P < 0.001 compared with 15P, P = 0.001 compared with 15M). There is no significant difference between 15D and B6-like controls (P = 0.485) or between 15P and 15M (P = 0.648).
Testing with PTZ revealed similar seizure responses for all congenic strains, and no statistically significant differences were observed when congenic strains were compared with B6-like controls. The time-courses to myoclonus and generalized seizure were not significantly different (P = 0.952 and P = 0.898, respectively, ANOVA) and there was no significant difference in the proportion of mice from each strain that exhibited a PTZ-induced maximal seizure (P = 1.000, Fisher exact). These results are summarized in Table 1.
Table 1.
PTZ-induced seizure responses in chr 15 congenic strains
| B6 | 15P | 15M | 15D | |
|---|---|---|---|---|
| Latency to myoclonus, s | 333 ± 74 | 348 ± 99 | 320 ± 99 | 326 ± 80 |
| Latency to generalized seizure, s | 438 ± 108 | 450 ± 93 | 415 ± 91 | 400 ± 102 |
| Fraction with maximal seizure | 0.33 | 0.17 | 0.33 | 0.33 |
Latency values are means ± SD (n = 6 per group). Mice were tested with 1 injection of pentylenetetrazol (PTZ, 80 mg/kg sc) and observed for 45 min. Latencies were analyzed with ANOVA, and maximal seizure responses were analyzed with Fisher's exact test. No statistically significant differences were obtained.
DISCUSSION
Several independent laboratories have mapped SZS QTLs on chr 15 in B6 and D2 mice and a summary of these findings is shown in Table 2. In the present study, we used congenic strains to confirm and refine the map position of chr 15 QTLs for electroshock seizure threshold and seizure response to systemic KA injection. Determination of MEST in the three chr 15 congenic strains revealed that strains 15M and 15D have significantly lower values compared with either strain 15P or B6-like littermates (Fig. 2). These results are consistent with the position of MEST QTL Szs13 near markers D15Mit158 (80.1 Mbp) and D15Mit34 (90.5 Mbp) (7) and suggest that the gene(s) underlying Szs13 is captured in both congenic strains 15M and 15D. Characterization of the overlapping regions of introgression between congenic strains 15M and 15D allows refinement of the map position for Szs13 to the 18.65 Mb interval between rs31267792 (68.64 Mbp) and D15Mit159 (87.29 Mbp) (Fig. 1). It is of interest that a QTL for susceptibility to cocaine-induced seizures, Cosz3, has also been mapped to this region (14).
Table 2.
Chromosome 15 seizure susceptibility QTLs mapped previously in B6 and D2 mice
| Seizure Paradigm | QTL1 | Markers | Position, Mbp | Susceptibility Allele | Reference |
|---|---|---|---|---|---|
| Kainate | Szv3 | D15Nds2-D15Mit46 | 21.2–60.6 | D2 | 10 |
| MEST | D15Mit13-D15Mit229 | 3.4–41.1 | D2 | 7 | |
| MEST | Szs13 | D15Mit158-D15Mit34 | 80.0–90.5 | D2 | 7 |
| Cocaine | Cosz3 | D15Mit34-D15Mit161 | 83.58–96.84 | D2 | 14 |
QTL, quantitative trait locus; MEST, maximal electroshock seizure threshold.
As listed in the database at http://www.informatics.jax.org/.
While results of electroshock seizure testing confirm the presence of an MEST QTL on the distal aspect of chr 15, there was no evidence for a more proximally located (closer to the acromere) QTL that was mapped previously near markers D15Mit13 (3.41 Mbp) and D15Mit229 (41.14 Mbp) (7). Reasons for failure to confirm the proximal MEST QTL are unknown although there are several possibilities that may be put forth for consideration. One explanation is that the original finding was a false positive result. This seems unlikely given that the magnitude of the logarithm of the odds (LOD) score was similar to that for the distal (Szs13) QTL (LOD = 3.5 in an additive genetic model) but must be considered nonetheless since this value is just above the threshold for declaring genome-wide significance in an F2 intercross (17). Another explanation is that the QTL is real but that the gene(s) mediating the QTL effect interacts with other genetic variants unique to the D2 strain background. Such variants would not be present in the congenic strains since they have a B6 genetic background. Further work is required to investigate these and other possibilities.
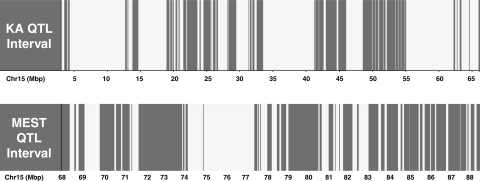
The 19 Mb interval that defines the MEST Szs13 QTL on chr 15 contains ∼350 known or predicted genes. The haplotype block structure of the genome of the laboratory mouse facilitates identification of regions that are identical by descent (IBD) between inbred strains (28), and this can assist in prioritizing genes within a QTL interval for further study. Figure 5 depicts the substantial fraction of genome that is IBD, and thus where there is a 10 times lower polymorphism rate, between the strains (28). Taking this approach reveals that of the 350 genes in the QTL interval between 68 and 88 Mbp, 183 of them reside outside regions shared by B6 and D2. However, given that there are polymorphisms between B6 and D2 strains documented across the entire interval and that new mutations are constantly occurring over evolution, some consideration must be given to all genes in the interval. With respect to the non-IBD regions, however, where there is a greater likelihood that the causative gene is located, several candidates stand out based on biological function and relationship to seizures. Most notable among all genes in the interval is Cacng2 (calcium channel, voltage-dependent, gamma subunit 2), well-known as the cause of seizures in the spontaneous mouse mutant stargazer (19). This gene falls outside IBD segments and exhibits numerous intronic SNPs between B6 and D2 strains as well as several other SNPs located in the 5′ untranslated region. Other interesting genes in the interval include Kcnj4 (inward rectifier potassium channel subfamily J member 4), Cacna1i (calcium channel, voltage-dependent, alpha 1I subunit), and Kcnk9 (potassium channel, subfamily K, member 9). The bias toward ion channel genes comes from the “channelopathy” concept of epilepsy (16), although it is clear that mutations in certain genes not directly associated with ion channel function can also cause epilepsy.
Fig. 5.
Haplotype block patterns for B6 and D2 mice in the KA (top) and MEST (bottom) quantitative trait locus (QTL) intervals on chr 15. White blocks are regions of the genome that are identical by descent between the strains. Gray blocks are regions that have higher polymorphism rate. Genomic location (in Mb) is shown on the x-axis.
The method for measuring MEST used in our laboratory involves electroshock stimuli given once per day with an incremental increase in electrical current on each successive trial. It is based on classical procedures developed a number of decades ago (26) and has been instrumental in the development of anti-epilepsy drugs. The paradigm involves an initial electrical shock administered at a relatively low current intensity and a daily increase in the magnitude of current until a maximal seizure is elicited. As a result of this step-wise ramping of electrical current intensity on a day-by-day basis, various behavioral seizure responses are observable over the course of the test. One of the endpoints that may be scored reliably as current is increased is the transition from partial to generalized seizure or the threshold for generalized seizure (GEST). Determining GEST in a specific animal is only possible if the initial trial does not induce a generalized seizure. Given our standard procedure for auricular stimulation in which electroshock seizure tests start at a current level of 20 mA, it is not unusual for some mice from certain seizure-susceptible strains, like D2, to express a generalized seizure during the first trial. Such a result obviates the possibility of determining the true GEST; however, in seizure-resistant strains, like B6, GEST is considerably >20 mA and average values may be determined accurately. In this way, we were able to measure GEST in the chr 15 congenic strains and B6-like littermates and discovered a similar strain pattern to that observed for MEST (Fig. 2B). Although statistical evaluation of data did not reveal a significant difference between any of congenic strains and B6-like controls, possible differences may be obscured by a higher degree of variability in the measurement since the study was powered for the MEST endpoint. It is possible that larger group sizes would reveal a pattern of significant strain differences in GEST similar to that observed for MEST. Thus the possibility that there is a functional genetic relationship between susceptibility to a generalized seizure and susceptibility to a maximal seizure warrants further investigation.
In contrast to the electroshock seizure studies, testing with KA revealed a distinct pattern of results with mice from congenic strains 15P and 15M documented to be significantly more seizure susceptible than both B6-like littermates and mice from strain 15D (Figs. 3 and 4). Greater susceptibility compared with B6-like littermate control mice was characterized by shorter latencies to the first partial seizure (clonus), to the first generalized seizure, and to the development of status epilepticus. This finding is consistent with initial KA QTL mapping both in relation to the physical map position of Szv3 (Table 2) and phenotyping results, which showed that Szv3 influences multiple KA seizure latency endpoints (10). Increased seizure susceptibility was also reflected by the finding that a significantly greater fraction of mice from 15P and 15M achieved status epilepticus compared with B6-like controls. Although strain 15D exhibited significantly shorter latency to generalized seizure than B6-like littermates, this latency was also significantly longer than strains 15P and 15M. Latency to status epilepticus was also significantly longer in 15D compared with 15P and 15M. Differences in KA SZS phenotypes between the congenic strains suggest that multiple genetic variants mediate these effects in each strain with the combination of factors present in the region of overlap between strains 15P and 15M having the greatest influence. This region is defined by markers D15Mit13 (3.41 Mbp) and rs31271969 (68.65 Mbp). It spans ∼65 Mbp (Fig. 1) but houses only 310 genes. Moreover, when genes in shared haplotype blocks between B6 and D2 are eliminated from consideration (Fig. 5), the number drops to 83. Notable genes on this shorter list include Kcnv1 (potassium channel, subfamily V, member 1) and Adcy8 (adenylate cyclase 8).
Comparison of mouse QTL intervals with syntenic regions of the human genome has the potential to expedite the QTL-to-gene discovery process. Thus, we were interested to determine whether human epilepsy genes have been mapped to regions syntenic with MEST and KA QTLs. With respect to the MEST QTL interval between 68 and 88 Mb, the main genomic regions of synteny in humans are 8q22-24 and 22q13. The 8q22-24 region is of interest due to the presence of KCNQ3, mutations in which have been identified as one of the causes of benign familial neonatal epilepsy (2). Although mouse Kcnq3 is in a region of low polymorphism frequency between B6 and D2, a number of intronic and synonymous coding SNPs have been annotated in the gene as well as one donor splice site SNP (rs39357957). It is not known whether this latter polymorphism leads to the production of functionally different potassium channels that affect seizure susceptibility. Another gene of interest in the region is JRK, the human homolog of the mouse Jerky gene that has been reported to lead to a rare form of epilepsy when mutated (22). Several other novel loci in the 8q22-24 region have been identified for rare syndromes that include epilepsy (1, 3, 12, 24). In the 22q13–15 region, novel linkages and associations with human epilepsy include the SCA10 locus (21) as well as mutations in the genes TEF (13) and MLC1 (18). The syntenic human genomic regions to the KA SZS QTL interval between 3 and 68 Mb include 15p13–15 and 8q22-24. Although human KCNQ3 is within the syntenic region of 8q22-24, mouse Kcnq3 maps just outside this QTL interval and thus is not a candidate gene for the KA QTL. There is no evidence for epilepsy-related gene mutations on human chr 15p.
Overall, there are several conclusions that may be drawn from the results of the experiments described above. First, the data suggest that the genes underlying the previously mapped QTLs on chr 15 for differences in electrical seizure threshold between B6 and D2 mice are distinct from those underlying the KA QTL Szv3. This conclusion is based partly on the lack of confirmation of the more proximal MEST QTL and is consistent with the fact that the strains were not differentially susceptible to the GABA-A receptor antagonist PTZ. Second, since the phenotypic differences observed between these strains are robust, further refined mapping with a recombinant congenic strain approach is feasible. This idea is also supported by the haplotype structure of the two QTL intervals. Thus, we cautiously conclude that continued genetic dissection of the congenic strains described in this study may yield the identity of the chr 15 genes and their variation that influence the natural seizure susceptibility and resistance of D2 and B6 mice, respectively.
In summary, we used a set of three chr 15 congenic strains to confirm and fine-map QTLs for MEST and KA SZS in B6 and D2 mice. The MEST QTL interval has been reduced to ∼19 Mbp and harbors a number of biologically relevant quantitative trait gene candidates. In addition, we confirmed a chr 15 QTL for KA SZS that is represented by a larger, but relatively gene poor genomic interval of ∼65 Mbp that offers a more restricted set of candidate genes. MEST has been established as an endpoint with greatest relevance to generalized epilepsy, whereas KA treatment is better established as a model of partial epilepsy (11). Epidemiological studies in humans suggest potential overlap of susceptibility factors for generalized and partial epilepsy (29), and thus we hypothesize that there are some QTLs in common between models involving MEST and KA in B6 and D2 mice. Results of the present study suggest that loci on chr 15 are not shared between these two seizure models. Ultimately, it is hoped that identification of the gene variants underlying these QTLs will lead to better understanding of the biological basis of seizures and epilepsy as well as new targets for the development of antiepilepsy medications.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant NS-40554 (T. N. Ferraro), The Center for Neurobiology and Behavior in the Department of Psychiatry at the University of Pennsylvania, and the Research Service at the Department of Veterans Affairs Medical Center, Coatesville, PA.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The content of this manuscript does not represent the official views of the U.S. Government.
REFERENCES
- 1.Bugiani M, Gyftodimou Y, Tsimpouka P, Lamantea E, Katzaki E, d'Adamo P, Nakou S, Georgoudi N, Grigoriadou M, Tsina E, Kabolis N, Milani D, Pandelia E, Kokotas H, Gasparini P, Giannoulia-Karantana A, Renieri A, Zeviani M, Petersen MB. Cohen syndrome resulting from a novel large intragenic COH1 deletion segregating in an isolated Greek island population. Am J Med Genet 146A: 2221– 2226, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Charlier C, Singh NA, Ryan SG, Lewis TB, Reus BE, Leach RJ, Leppert M. A pore mutation in a novel KQT-like potassium channel gene in an idiopathic epilepsy family. Nat Genet 18: 53– 55, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Deglincerti A, De Giorgio R, Cefle K, Devoto M, Pippucci T, Castegnaro G, Panza E, Barbara G, Cogliandro RF, Mungan Z, Palanduz S, Corinaldesi R, Romeo G, Seri M, Stanghellini V. A novel locus for syndromic chronic idiopathic intestinal pseudo-obstruction maps to chromosome 8q23-q24. Eur J Hum Genet 15: 889– 897, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Ferraro TN. Sixty years in the making: a multifactorial mouse model of seizure susceptibility. In: Encyclopedia of Basic Epilepsy Research (vol. 1), edited by Schwartzkroin PA.Oxford: Academic Press, 2009, p. 374–381 [Google Scholar]
- 5.Ferraro TN, Golden GT, Dahl JP, Smith GG, Schwebel CL, Macdonald R, Lohoff FW, Berrettini WH, Buono RJ. Analysis of a quantitative trait locus for seizure susceptibility in mice using bacterial artificial chromosome-mediated gene transfer. Epilepsia 48: 1667– 1677, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Ferraro TN, Golden GT, Smith GG, Martin JF, Lohoff FW, Gieringer TA, Zamboni D, Schwebel CL, Press DM, Kratzer SO, Zhao H, Berrettini WH, Buono RJ. Fine mapping of a seizure susceptibility locus on mouse Chromosome 1: nomination of Kcnj10 as a causative gene. Mamm Genome 15: 239– 251, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Ferraro TN, Golden GT, Smith GG, Longman RL, Snyder RL, DeMuth D, Szpilzak I, Mulholland N, Eng E, Lohoff FW, Buono RJ, Berrettini WH. Quantitative genetic study of maximal electroshock seizure threshold in mice: evidence for a major seizure susceptibility locus on distal chromosome 1. Genomics 75: 35– 42, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Ferraro TN, Golden GT, Smith GG, St Jean P, Schork NJ, Mulholland N, Ballas C, Schill J, Buono RJ, Berrettini WH. Mapping loci for pentylenetetrazol-induced seizure susceptibility in mice. J Neurosci 19: 6733– 6739, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferraro TN, Schill JF, Ballas C, Mulholland N, Golden GT, Smith GG, Buono RJ, Berrettini WH. Genotyping microsatellite polymorphisms by agarose gel electrophoresis with ethidium bromide staining: application to quantitative trait loci analysis of seizure susceptibility in mice. Psychiatr Genet 8: 227– 233, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Ferraro TN, Golden GT, Smith GG, Schork NJ, St Jean P, Ballas C, Choi H, Berrettini WH. Mapping murine loci for seizure response to kainic acid. Mamm Genome 8: 200– 208, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Fisher RS. Animal models of the epilepsies. Brain Res Brain Res Rev 14: 245– 278, 1989 [DOI] [PubMed] [Google Scholar]
- 12.Fong GCY, Shah PU, Gee MN, Serratosa JM, Castroviejo IP, Khan S, Ravat SH, Mani J, Huang Y, Zhao HZ, Medina MT, Treiman LJ, Pineda G, Delgado-Escueta AV. Childhood absence epilepsy with tonic-clonic seizures and electroencephalogram 3–4-Hz spike and multispike-slow wave complexes: linkage to chromosome 8q24. Am J Hum Genet 63: 1117– 1129, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gachon F, Fonjallaz P, Damiola F, Gos P, Kodama T, Zakany J, Duboule D, Petit B, Tafti M, Schibler U. The loss of circadian PAR bZip transcription factors results in epilepsy. Genes Dev 18: 1397– 1412, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hain HS, Crabbe JC, Bergeson SE, Belknap JK. Cocaine-induced seizure thresholds: quantitative trait loci detection and mapping in two populations derived from the C57BL/6 and DBA/2 mouse strains. J Pharmacol Exp Ther 293: 180– 187, 2000 [PubMed] [Google Scholar]
- 15.Iakoubova OA, Olsson CL, Dains KM, Ross DA, Andalibi A, Lau K, Choi J, Kalcheva I, Cunanan M, Louie J, Nimon V, Machrus M, Bentley LG, Beauheim C, Silvey S, Cavalcoli J, Lusis AJ, West DB. Genome-tagged mice (GTM): two sets of genome-wide congenic strains. Genomics 74: 89– 104, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Kullmann DM. Neurological channelopathies. Annu Rev Neurosci 33: 151– 172, 2010. [DOI] [PubMed] [Google Scholar]
- 17.Lander E, Kruglyak L. Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results. Nat Genet 11: 241– 247, 1995 [DOI] [PubMed] [Google Scholar]
- 18.Leegwater PAJ, Yuan BQ, van der Steen J, Mulders J, Konst AAM, Ilja Boor PK, Mejaski-Bosnjak V, van der Maarel SM, Frants RR, Oudejans CBM, Schutgens RBH, Pronk JC, van der Knapp MS. Mutations of MLC1 (KIAA0027), encoding a putative membrane protein, cause megalencephalic leukoencephalopathy with subcortical cysts. Am J Hum Genet 68: 831– 838, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Letts VA, Valenzuela A, Kirley JP, Sweet HO, Davisson MT, Frankel WN. Genetic and physical maps of the stargazer locus on mouse chromosome 15. Genomics 43: 62– 68, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Liu W, Seto J, Donovan G, Toth M. Jerky, a protein deficient in a mouse epilepsy model, is associated with translationally inactive mRNA in neurons. J Neurosci 22: 176– 182, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsuura T, Achari M, Khajavi M, Bachinski LL, Zoghbi HY, Ashizawa T. Mapping of the gene for a novel spinocerebellar ataxia with pure cerebellar signs and epilepsy. Ann Neurol 45: 407– 411, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Moore T, Hecquet S, McLellann A, Ville D, Grid D, Picard F, Moulard B, Asherson P, Makoff AJ, McCormick D, Nashef L, Froguel P, Arzimanoglou A, LeGuern E, Bailleul B. Polymorphism analysis of JRK/JH8, the human homologue of mouse jerky, and description of a rare mutation in a case of CAE evolving to JME. Epilepsy Res 46: 157– 167, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Noebels JL, Qiao X, Bronson RT, Spencer C, Davisson MT. Stargazer: a new neurological mutant on chromosome 15 in the mouse with prolonged cortical seizures. Epilepsy Res 7: 129– 135, 1990 [DOI] [PubMed] [Google Scholar]
- 24.Shimizu A, Asakawa S, Sasaki T, Yamazaki S, Yamagata H, Kudoh J, Minoshima S, Kondo I, Shimizu N. A novel giant gene CSMD3 encoding a protein with CUB and sushi multiple domains: a candidate gene for benign adult familial myoclonic epilepsy on human chromosome 8q23.3-q241. Biochem Biophys Res Commun 309: 143– 154, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Stoffel W, Korner R, Wachtmann D, Keller BU. Functional analysis of glutamate transporters in excitatory synaptic transmission of GLAST1 and GLAST1/EAAC1 deficient mice. Brain Res Mol Brain Res 128: 170– 181, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Swinyard EA. Electrically induced convulsions. In: Experimental Models of Epilepsy, edited by Purpura DP, Penry JK, Woodbury DM, Tower DB, Walter RB. New York: Raven, p. 433– 458 [Google Scholar]
- 27.Toth M, Grimsby J, Buzsaki G, Donovan GP. Epileptic seizures caused by inactivation of a novel gene, jerky, related to centromere binding protein-B in transgenic mice. Nat Genet 11: 71– 75, 1995 [DOI] [PubMed] [Google Scholar]
- 28.Wiltshire T, Pletcher MT, Batalov S, Barnes SW, Tarantino LM, Cooke MP, Wu H, Smylie K, Santrosyan A, Copeland NG, Jenkins NA, Kalush F, Mural RJ, Glynne RJ, Kay SA, Adams MD, Fletcher CF. Genome-wide single-nucleotide polymorphism analysis defines haplotype patterns in mouse. Proc Natl Acad Sci USA 100: 3380– 3385, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winawer MR, Marini C, Grinton BE, Rabinowitz D, Berkovic SF, Scheffer IE, Ottman R. Familial clustering of seizure types within the idiopathic generalized epilepsies. Neurology 65: 523– 528, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]