Abstract
Many studies have demonstrated that premenopausal women are at increased risk for various pain disorders. Pain-sensing neurons, termed “nociceptors,” in the trigeminal ganglia (TG) and dorsal root ganglia (DRG) express receptors for inflammatory mediators and noxious physical stimuli and transmit signals for central processing of pain sensation. Estrogen receptors (ERs) are also expressed on nociceptors in the TG and DRG, and there is ample literature to suggest that activation of ERs can influence pain mechanisms. However, the mechanism for ER modulation of nociceptor activity is incompletely understood. The aim of this study was to characterize the effect of 17β-estradiol (17β-E2) on signaling of the inflammatory mediator bradykinin (BK) in primary cultures of rat sensory neurons and a behavioral model of thermal allodynia in rats. Here, we show that exposure to 17β-E2 rapidly (within 15 min) enhanced responses to BK in vitro and in vivo. The 17β-E2-mediated enhancement of BK signaling was not blocked by the transcription inhibitor anisomycin and was mediated by a membrane-associated ER. The effect of 17β-E2 to enhance BK responses required activation of β1-containing, RGD-binding integrins. These data show that 17β-E2 rapidly enhances inflammatory mediator responses both in vitro and in vivo and suggest that 17β-E2 acting at primary sensory pain neurons may participate in regulating the sensitivity of women to painful stimuli.
Introduction
Epidemiological studies have demonstrated that women are at increased risk for a variety of pain disorders, including temporomandibular joint disorder, fibromyalgia, headache, and arthritis, among others (Fillingim et al., 2009). Under controlled experimental conditions, women are generally more sensitive to pain-causing stimuli than men (Cairns and Gazerani, 2009; Fillingim et al., 2009), and this sex difference extends to many studies in animals as well (Wiesenfeld-Hallin, 2005; Cairns and Gazerani, 2009). The mechanisms for sex differences in pain responsiveness are numerous, complex, and far from understood. Although some of these differences may be accounted for by cognitive and sociocultural gender differences, there is strong evidence for significant biological differences in pain perception and processing between the sexes. It is noteworthy that many studies have identified the important role played by sex hormones, in particular estrogen (Wiesenfeld-Hallin, 2005; Craft, 2007; Cairns and Gazerani, 2009; Fillingim et al., 2009).
The actions of estrogen on nociception are also complex and multifactorial. Both pronociceptive and antinociceptive effects have been attributed to estrogen in human and animal models (Wiesenfeld-Hallin, 2005; Craft, 2007; Cairns and Gazerani, 2009; Fillingim et al., 2009). Such apparently contradicting differences in the effects of estrogen on nociception may be related to differences among pain conditions (inflammatory, neuropathic, etc.), differential actions of estrogen at multiple levels of the pain transmission/perception pathways, and time-dependent effects of estrogen, including genomic versus nongenomic (rapid) signaling.
Of the many places within the pain transmission pathway that estrogen may act, there is abundant evidence indicating that estrogen can regulate the activity of the primary sensory neurons involved in pain transmission, termed nociceptors. Estrogen receptors (ERs) are expressed in nociceptors of the trigeminal ganglia (TG) and dorsal root ganglia (DRG) (Yang et al., 1998; Bereiter et al., 2005), and treatment with 17β-estradiol (17β-E2) influences a variety of functions and cellular processes in nociceptors such as expression of trkA mRNA (Liuzzi et al., 1999), expression of calcitonin gene-related peptide mRNA and protein (Gangula et al., 2000), extracellular signal-regulated kinase activity (Liverman et al., 2009), calcium mobilization (Chaban and Micevych, 2005), and transient receptor potential cation channel V1 (TRPV1) function (Xu et al., 2008). Furthermore, local 17β-E2 injection into the temporomandibular joint reduces nociceptive behavioral responses to intrajoint administration of formalin (Fávaro-Moreira et al., 2009), suggesting estrogen's effect on nociceptors is functionally relevant.
Classic ERs are members of the nuclear receptor superfamily and are comprised of α (ERα) and β (ERβ) subtypes, which, when activated by estrogen, act as transcription factors to regulate protein synthesis in target tissues. With respect to pain, estrogen has been shown to regulate the expression of a number of proteins involved in nociception (Aloisi and Bonifazi, 2006). The effects mediated by this “genomic” pathway typically have rather long latencies for onset of action and extended duration (hours to days). However, recent evidence strongly suggests that estrogen can have rapid (within seconds) nongenomic effects that seem to be mediated by plasma membrane-associated ERs (Hammes and Levin, 2007). In DRG neurons in vitro, estrogen, acting via ERα associated with the plasma membrane, rapidly (with 5 min) reduces ATP-mediated intracellular calcium mobilization (Chaban and Micevych, 2005). Estrogen also rapidly activates extracellular signal-regulated kinase (Liverman et al., 2009) and interferes with exchange protein activated by cAMP-mediated activation of protein kinase Cε (Hucho et al., 2006). These data suggest that estrogen can exert multiple actions (genomic and nongenomic) on primary sensory neurons to regulate pain neurotransmission.
The effects of estrogen on nociception may also differ depending on the nature of the pain stimulus (Cairns and Gazerani, 2009; Fillingim et al., 2009), and both pronociceptive and antinociceptive effects have been reported for inflammatory pain (Straub, 2007). Pain induced by inflammation results from the release of a myriad of inflammatory mediators, such as bradykinin (BK), prostaglandins, cytokines, and proteases, and it is possible that the effect of estrogen could differ depending on the specific inflammatory mediator involved or biological outcome observed.
To begin to understand the contradictory effects of estrogen on inflammatory pain, we assessed the ability of 17β-estradiol (17β-E2) to regulate the effects of BK in primary cultures of TG neurons in vitro and in a behavioral model of thermal allodynia in vivo. We found that 17β-E2 rapidly enhanced both BK signaling in TG neurons and BK-induced thermal allodynia, suggesting that rapid-onset actions of estrogen, acting at primary sensory nerve terminals, may contribute to enhanced pain sensitivity in females.
Materials and Methods
Materials.
Fetal bovine serum was purchased from Gemini Bioproducts (Calabasas, CA). All other tissue culture reagents were from Invitrogen (Carlsbad, CA). 17β-Estradiol-6-(O-carboxymethyl) oxime-BSA (17β-E2-BSA) was purchased from Sigma-Aldrich (St. Louis, MO). For in vitro experiments, 17β-E2-BSA was diluted in 50 mM Tris-HCl (pH 7.2) to a working stock solution of 10 μM based on BSA concentration. For in vivo experiments, 17β-E2-BSA was dissolved in saline and administered to the rat hindpaw at doses of 30 or 50 μg that correspond to approximately 100 or 150 ng of 17β-E2, respectively, based on the molecular weight of BSA. All other drugs and chemicals were purchased from Sigma-Aldrich.
Animals.
The animal study protocol was approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio and conformed to International Association for the Study of Pain and federal guidelines. Adult male and female Sprague-Dawley rats, 200 to 250 g, were purchased from Charles River Laboratories, Inc. (Wilmington, MA). Ovariectomy was performed at Charles River Laboratories, Inc. Experiments with ovariectomized (OVX) rats were performed at least 2 weeks after surgery. Animals were housed for 1 week with food and water available ad libitum before experiments.
Rat Trigeminal Ganglion Culture.
Primary cultures of rat TG cells were prepared as described previously (Patwardhan et al., 2006; Berg et al., 2007a,b). In brief, rats were sacrificed by decapitation, and TGs were rapidly removed and chilled in Hank's balanced salt solution (HBSS; Ca2+, Mg2+ free) on ice. TGs were washed with HBSS, digested with 3 mg/ml of collagenase for 30 min at 37°C, and centrifuged. The pellet was further digested with 0.1% trypsin for 15 min at 37°C, pelleted by centrifugation (5000g for 5 min), and resuspended in Dulbecco's modified Eagle's medium (high glucose) containing 100 ng/ml of nerve growth factor (Harlan, Indianapolis, IN), 10% fetal bovine serum, 1× penicillin/streptomycin, 1× l-glutamine, and the mitotic inhibitors uridine (7.5 μg/ml) and 5-fluoro-2′-deoxyuridine (17.5 mg/ml). After trituration to disrupt tissue, the cell suspension was seeded on polylysine-coated 24- or 48-well plates. Media were changed 24 and 48 h after plating and every 48 h thereafter. Nerve growth factor and serum were removed 24 h before experiments. Cells were used on the 5th or 6th day of culture.
Measurement of Inositol Phosphate Accumulation.
BK-stimulated inositol phosphate (IP) accumulation in TG cultures was measured as described previously (Patwardhan et al., 2006; Berg et al., 2007a). Cells grown in 24- or 48-well plates were labeled with 2μCi/ml [3H]myoinositol for 24 h before experiments. After labeling, cells were rinsed three times with 1 ml of HBSS that contained 20 mM HEPES and 20 mM lithium chloride (LiCl) and were preincubated in HBSS/LiCl at 37°C in room air for 15 min. BK (various concentrations) was added to a final volume of 500 μl, and cells were further incubated for 25 min. Where indicated, 17β-E2 was added during the preincubation period. The incubation was terminated by the addition of 200 μl of ice-cold formic acid, and total [3H]IPs were separated with ion-exchange chromatography and measured with liquid scintillation spectrometry. Data are expressed as accumulation of total IPs (disintegrations per minute) or a percentage of basal IP accumulation.
Behavioral Testing.
Paw withdrawal latency (PWL) to a thermal stimulus was measured with a plantar test apparatus (Hargreaves et al., 1988) by observers blinded to the treatment allocation. In brief, rats were placed in plastic boxes with a glass floor. After a 30-min habituation period, the plantar surface of the hindpaw was exposed to a beam of radiant heat through the glass floor, and the PWL was automatically determined by a photoelectric cell. The rate of increase in temperature of the glass floor was adjusted so that baseline PWL values were close to 10 ± 2 s; cutoff time was 20 s. Measurements were taken in duplicate at least 30 s apart, and the average was used for statistical analysis. BK, Gly-Arg-Gly-Asp-Ser-Pro (GRGDSP, integrin-blocking peptide), Gly-Asp-Gly-Arg-Ser-Pro (GDGRSP, inactive, reverse-sequence integrin-blocking peptide), and 17β-E2-BSA stock solutions were diluted in saline. 17β-E2 was diluted in peanut oil. All drugs were administered via intraplantar injection at a final volume of 50 μl.
To assess a role for gene transcription in the mechanism of action of 17β-E2, we administered the transcription inhibitor anisomycin (Sigma-Aldrich) via intraplantar injection, 15 min before intraplantar injection of 17β-E2. At a concentration of 10 μM, anisomycin inhibits protein synthesis by 99% (Grollman, 1967). Using a value of 1 ml as an estimate (likely an overestimate) of paw volume, we chose a dose of 5 μg/50 μl of anisomycin that would lead to a calculated concentration of anisomycin of 20 μM.
Data Analysis.
For TG cell culture experiments, concentration-response data were fit to a logistic equation using nonlinear regression analysis to provide estimates of maximal response (Rmax), potency (EC50) and slope factor (n):
where R is the measured response at a given agonist concentration (A), Rmax is the maximal response, EC50 is the concentration of agonist that produces half-maximal response, and n is the slope index. Statistical differences in concentration-response curve parameters between groups were analyzed with Student's paired t test. When only a single concentration was used, statistical significance was assessed by using one-way analysis of variance followed by Dunnet's post hoc or Student's t test (paired) using Prism software (GraphPad Software, Inc., San Diego, CA). p < 0.05 was considered statistically significant.
For behavioral experiments, time course data were analyzed with two-way analysis of variance, followed by Bonferroni's post hoc test. Statistical inference was made when p < 0.05. Data are presented as mean ± S.E.M.
Results
17β-E2 Effects on BK Signaling in TG Cultures.
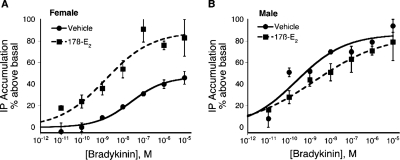
In TG cultures from OVX female rats, pretreatment with 17β-E2 rapidly and significantly increased both the potency (≈8-fold) and the efficacy (≈2-fold) of BK to stimulate the phospholipase C (PLC)-IP pathway (Fig. 1). The pEC50 values for BK were 8.00 ± 0.36 (10 nM) versus 8.87 ± 0.47 (1.3 nM) for the vehicle and 17β-E2-pretreatment groups, respectively. The maximal response (Emax) to BK was increased from 48 ± 6% (vehicle pretreatment) to 87 ± 16% above basal by 17β-E2 pretreatment. It is noteworthy that 17β-E2 did not alter the potency or maximal response of BK in TG cultures derived from male rats (pEC50: 9.64 ± 0.26 versus 8.89 ± 0.9; Emax: 86 ± 10% versus 86 ± 5% for vehicle- and 17β-E2-pretreament of male cultures, respectively). Thus, the effect of 17β-E2 on TG cultures was clearly sexually dimorphic.
Fig. 1.
17β-Estradiol rapidly enhances BK-PLC activity in TG cultures from female, but not male, rats. TG cultures (5–6 days in culture) from intact female (A) and male (B) rats were labeled with [3H]myo-inositol for 24 h. After a brief wash, cultures were treated with 17β-E2 (50 nM) or vehicle (0.1% DMSO) for 15 min (37°C) before the addition of various concentrations of BK and further incubation for 25 min (37°C). Total IP accumulation was determined as described under Materials and Methods. Data are expressed as the mean ± S.E.M. of three experiments. 17β-Estradiol pretreatment had no effect on basal IP accumulation, but significantly enhanced the maximal response to BK from 48 ± 6% above basal to 87 ± 16%.
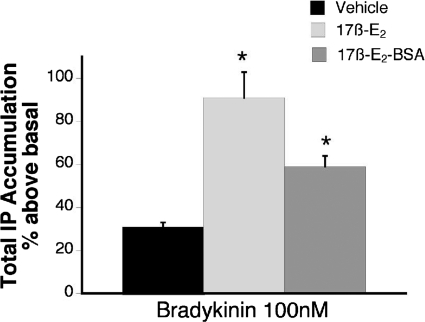
Figure 2 shows that a membrane-impermeable form of 17β-E2 (17β-E2 conjugated to BSA) also significantly enhanced the BK-PLC response. Pretreatment (15 min) with 17β-E2-BSA enhanced the response to 100 nM BK by 2-fold, whereas pretreatment with 17β-E2 increased the BK response by 3-fold.
Fig. 2.
Membrane-impermeable 17β-estradiol-BSA rapidly enhances BK-PLC activity in female TG cultures. Cultures were treated with 17β-E2 (50 nM), 17β-E2-BSA (50 nM), or vehicle (0.1% DMSO) for 15 min (37°C) before the addition of BK (100 nM) and further incubation for 25 min (37°C). Total IP accumulation was determined as described under Materials and Methods. Data are expressed as the mean ± S.E.M. of three experiments. *, p < 0.05 versus vehicle.
17β-E2 Effects on BK-Induced Thermal Allodynia.
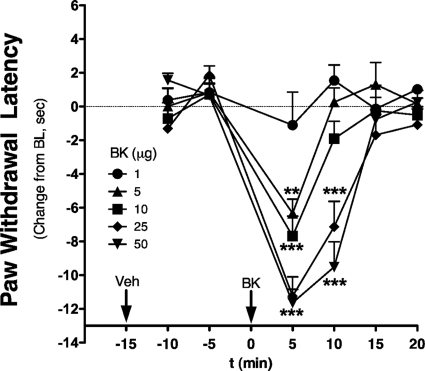
Using observers blinded to treatment allocation, the intraplantar injection of BK in the hindpaw of OVX rats produced thermal allodynia at doses above 1 μg (Fig. 3). At effective doses, the thermal allodynia was rapidly induced (within 5 min) and was transient, returning to baseline by 15 min after injection. The ED50 for BK was 2.8 μg.
Fig. 3.
BK dose-dependently induces rapid and transient thermal allodynia in OVX rats. OVX rats received an intraplantar injection of vehicle (peanut oil, 50 μl) 15 min before injection with BK (various doses, 50 μl in saline). PWL was measured in duplicate every 5 min after each injection. Data are expressed as change from individual baselines and represent mean ± S.E.M. of four to six animals per group. **, p < 0.01; ***, p < 0.001 versus 1-μg dose. Bulb intensity was adjusted for baseline PWL of 13.37 ± 0.17 s. Cutoff was 20 s.
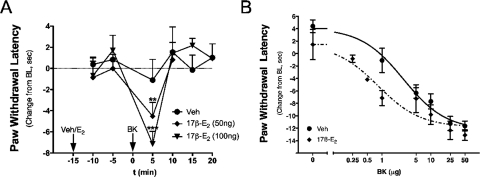
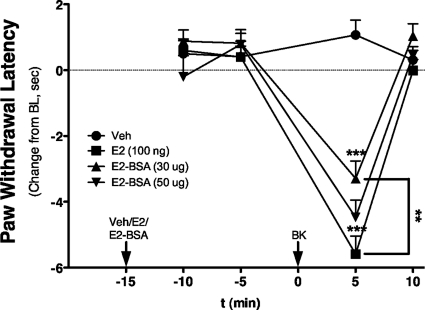
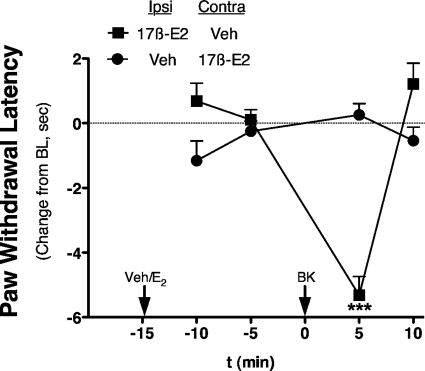
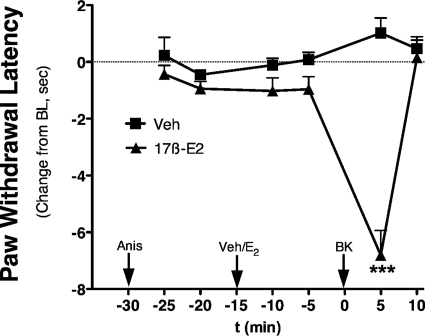
Intraplantar injection of 17β-E2 to OVX rats at doses up to 100 ng did not produce a change in PWL over the measured time period (Fig. 4). However, 15-min pretreatment with 17β-E2 rapidly sensitized responses to BK, such that an inactive dose (1 μg) now produced a significant thermal allodynia with a magnitude similar to that observed with 10 μg of BK (Fig. 3 versus 4). Moreover, the BK-sensitizing effect of 17β-E2 was dose-related, with significant differences observed with pretreatment with either 50 or 100 ng of 17β-E2 into the ipsilateral hindpaw. A dose-response analysis indicated that 17β-E2 pretreatment increased the potency of BK by ∼3-fold (ED50 2.8 μg versus 0.8 μg; Fig. 4B). Similar to the effect of 17β-E2, intraplantar injection of the membrane-impermeable 17β-E2-BSA (30 or 50 μg) did not produce a change in PWL but dose-dependently enhanced the response to BK (1 μg; Fig. 5). Injection of 17β-E2 into the contralateral hindpaw did not affect the BK response (Fig. 6), indicating that the action of 17β-E2 to enhance BK-induced thermal allodynia was peripherally mediated. The effect of 17β-E2 to enhance thermal allodynia in response to BK was not blocked by prior administration of the transcription inhibitor anisomycin into the ipsilateral hindpaw (Fig. 7).
Fig. 4.
17β-Estradiol rapidly enhances BK-induced thermal allodynia in OVX rats. A, time course of BK-induced thermal allodynia. OVX rats were injected intraplantarly with 17β-E2 (50 or 100 ng) or vehicle (peanut oil) 15 min before injection with a subthreshold dose of BK (1 μg). PWL was measured in duplicate every 5 min after each injection. Data are expressed as change from individual preinjection baselines and represent the mean ± S.E.M. of four to six animals per group. **, p < 0.01; ***, p < 0.001 versus vehicle. B, effect of estrogen on the dose-response curve for BK-induced thermal allodynia. OVX rats were injected intraplantarly with 17β-E2 (100 ng) or vehicle (peanut oil) 15 min before injection of BK (various doses, intraplantarly). PWL was measured in duplicate every 5 min after each injection. Data are expressed as change from individual preinjection baselines at the time of maximal BK effect (5 min post-BK) and represent the mean ± S.E.M. of four to six animals per group. Bulb intensity was adjusted for baseline PWL of 12.52 ± 0.22 s.
Fig. 5.
Membrane-impermeable 17β-estradiol-BSA rapidly enhances BK-induced thermal allodynia in OVX rats. OVX rats were injected intraplantarly with 17β-E2 (100 ng), 17β-E2-BSA (30 or 50 μg, equivalent to 100 or 150 ng of 17β-E2, respectively, based on BSA concentration), or vehicle (saline) 15 min before injection with a subthreshold dose of BK (1 μg). PWL was measured in duplicate every 5 min after each injection. Data are expressed as change from individual preinjection baselines and represent mean ± S.E.M. of six animals per group. ***, p < 0.001 versus vehicle; **, p < 0.01 as indicated. Bulb intensity was adjusted for baseline PWL of 10.04 ± 0.15 s.
Fig. 6.
17β-Estradiol enhancement of BK-induced thermal allodynia is peripherally restricted. OVX rats were pretreated intraplantarly (at time −15 min) with 17β-E2 (100 ng) or vehicle (peanut oil) into the ipsilateral (Ipsi) and contralateral (Contra) hindpaws (two injections), as indicated, 15 min before the injection (intraplantarly at time 0 min) of BK (1 μg in saline, subthreshold) into the ipsilateral hindpaw. Responses (PWL) of the ipsilateral hindpaw were measured in duplicate every 5 min after each injection. Data are shown as change in PWL compared with individual preinjection ipsilateral paw baseline. Each point represents the mean ± S.E.M. of six animals per group. ***, p < 0.001. Bulb intensity was adjusted for baseline PWL of 10.58 ± 0.38 s.
Fig. 7.
Pretreatment with the transcription inhibitor anisomycin has no effect on 17β-E2-mediated enhancement of BK-stimulated thermal allodynia. OVX rats were pretreated (intraplantarly at time −30 min) with anisomycin (5 μg) before pretreatment (intraplantarly at time −15 min) with 17β-E2 (100 ng) or vehicle (peanut oil). Fifteen minutes later (at time 0 min), animals were injected intraplantarly with a subthreshold dose of BK (1 μg in saline), and PWL responses were measured in duplicate every 5 min after each injection. Data are presented as change in PWL compared with individual preinjection paw baseline. Each point represents the mean ± S.E.M. of six animals per group. ***, p < 0.001. Bulb intensity was adjusted for baseline PWL of 12.44 ± 0.49 s.
RGD-Binding Integrins Mediate the Effect of 17β-E2 on BK Responses In Vitro and In Vivo.
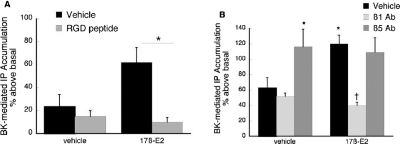
Integrins are expressed on many cell types, including afferent neurons, and the RGD subclass of these heterodimeric proteins regulates the signaling activity of several membrane-bound receptors, including opioid receptors on peptidergic TG neurons (Litvak et al., 2000; Short et al., 2000; Berg et al., 2007b). Accordingly, we evaluated whether disruption of the RGD subclass of integrins modulates the effect of 17β-E2 to enhance BK-stimulated IP accumulation in TG cultures derived from OVX rats (Fig. 8). Administration of an integrin antagonist (soluble RGD-containing peptide, GRGDSP) completely blocked the effect of 17β-E2 for increasing the BK-mediated accumulation of IP (Fig. 8A). Control studies verified that administration of GRGDSP peptide did not alter basal or BK-stimulated IP accumulation (data not shown). Furthermore, selective blockade of integrin activity with monoclonal anti-β1 subunit antibodies also completely blocked the effect of 17β-E2 (Fig. 8B). It is noteworthy that antibodies directed against integrins containing the β5 subunit enhanced BK-stimulated IP accumulation by approximately 2-fold, and the addition of 17β-E2 did not further augment the BK response. Thus, regulation of integrin activities rapidly modulates the ability of 17β-E2 to affect BK signaling in sensory neurons, with both excitatory and inhibitory outcomes mediated by distinct integrin subtypes.
Fig. 8.
17β-Estradiol enhancement of BK-PLC activity is blocked by integrin antagonists. A, effect of soluble RGD peptide. TG cultures from female rats were pretreated with Gly-Arg-Gly-Asp-Ser-Pro (soluble RGD peptide; 100 μM) or vehicle for 15 min. After pretreatment, cells were treated with 17β-E2 (50 nM) or vehicle (0.1% DMSO) for 15 min (37°C). Cultures were then exposed to BK (1 nM) for 25 min (37°C), and total IP accumulation was determined as under Materials and Methods. Data are the mean ± S.E.M. of three experiments. *, p < 0.05. B, effect of soluble anti-integrin antibodies. TG cultures from female rats were pretreated with subtype-selective antibody (1:100) or normal rat IgG for 30 min (37°C) before the addition of 17β-E2 (50 nM) or vehicle (0.1% DMSO) for 15 min (37°C). BK-stimulated (1 nM) IP accumulation was measured after 25-min incubation (37°C) as described under Materials and Methods. Data are expressed as mean ± S.E.M. of three experiments. *, p < 0.05 compared with vehicle-vehicle condition; †, p < 0.05 compared with vehicle-17β-E2.
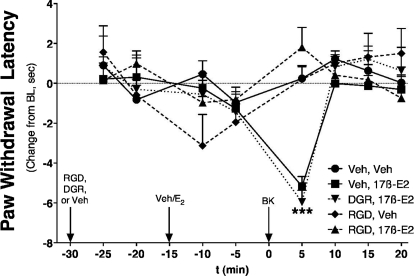
We next evaluated whether integrin mediation of 17β-E2 effects are replicated in vivo. The intraplantar injection of the soluble integrin antagonist peptide (GRGDSP, 25 μg) into the ipsilateral hindpaw completely blocked 17β-E2-mediated enhancement of BK-induced thermal allodynia in OVX rats (Fig. 9) without having an effect on its own. In contrast, the inactive, reverse sequence (GDGRSP, 25 μg) was ineffective at blocking 17β-E2-mediated enhancement of BK-induced thermal allodynia.
Fig. 9.
17β-Estradiol enhancement of BK-induced thermal allodynia is blocked by an integrin antagonist. OVX rats were pretreated intraplantarly with Gly-Arg-Gly-Asp-Ser-Pro (RGD peptide, 25 μg), reverse control sequence (DGR, 25 μg), or saline 15 min before pretreatment (intraplantarly) with 17β-E2 (100 ng) or vehicle (peanut oil). Fifteen minutes after 17β-E2/vehicle injection, animals were injected intraplantarly with BK (1 μg). PWL was measured in duplicate every 5 min after each injection. Data are expressed as change in PWL compared with individual preinjection and represent the mean ± S.E.M. of 5 to 14 animals per group. ***, p < 0.001 versus all other groups. Bulb intensity was adjusted for baseline PWL of 11.12 ± 0.27 s.
Discussion
Although a role for estrogen in gender differences in pain sensitivity has been supported by numerous studies (Wiesenfeld-Hallin, 2005; Craft, 2007; Cairns and Gazerani, 2009; Fillingim et al., 2009), typically such estrogen effects have been attributed to the classic “genomic” pathway where an intracellular ER complex acts as a transcription factor to regulate protein synthesis. Acting via this classic pathway, the effects of estrogen generally are characterized as having a long latency for onset of action and a long duration of action, which persists even after estrogen is removed from the system. Here, we show that 17β-E2 can rapidly (within minutes) increase BK signaling in primary cultures of sensory neurons in vitro and nociceptive responsiveness to BK in vivo. The rapid onset of action of 17β-E2 suggests that the effect is not mediated by the classic “genomic” pathway. Over the past several years, an emerging body of evidence indicates that estrogen can also regulate cellular activity via ERs associated with the plasma membrane (Hammes and Levin, 2007). Typically, these effects are of short-onset latency (within seconds) and do not require changes in transcription. The effectiveness of a membrane-impermeable form of 17β-E2 (17β-E2 conjugated to BSA) to enhance BK signaling in TG cultures and nociceptive responses in vivo and the lack of effect of the transcription inhibitor anisomycin in vivo also support the hypothesis that the effect of estrogen is not mediated by the classic genomic pathway.
17β-E2 administration to the rat hindpaw rapidly and significantly enhanced BK-induced thermal allodynia via a local mechanism of action restricted to the injected hindpaw. Given that ERs are expressed in primary sensory neurons (Yang et al., 1998; Bereiter et al., 2005) and together with the results from primary culture of sensory neurons, these data indicate that 17β-E2 acts locally, likely at membrane-associated receptors on primary sensory neuron terminals, to rapidly enhance pain sensitivity to the inflammatory mediator BK.
Several studies have found that estrogen treatment can increase responsiveness to a variety of painful stimuli (Wiesenfeld-Hallin, 2005; Craft, 2007; Cairns and Gazerani, 2009; Fillingim et al., 2009). However, decreases in pain sensation have been reported as well (Kuba et al., 2005; Multon et al., 2005). Many of these previous studies have incorporated prolonged application of estrogen and therefore the observed effects could be mediated via genomic or nongenomic pathways or both. In addition, many previous studies have used experimental designs that evaluated the integrated outcome of peripheral and central mechanisms. To the best of our knowledge, this is the first report demonstrating peripheral and rapid-onset actions of 17β-E2 to enhance nociceptive responsiveness to BK.
It has been reported that 17β-E2 delivered directly into the temporomandibular joint of anesthetized OVX rats reduced the nocifensive response (rubbing and flinching) to intrajoint injection of formalin measured over 45 min (Fávaro-Moreira et al., 2009). A nongenomic mechanism was suggested on the basis of the effectiveness of a membrane-impermeable form of 17β-E2 (conjugated to BSA). In the present study, we found that local 17β-E2 administration enhanced thermal allodynia in response to intraplantar injection of BK measured 5 min after BK. Differences in estrogen's effect on pain responses have been suggested to be caused by differences among pain conditions (Cairns and Gazerani, 2009; Fillingim et al., 2009). Formalin elicits a prolonged hyperalgesic response that has both inflammatory and neurogenic components and is caused in part by the release of multiple inflammatory mediators (BK, prostaglandins, cytokines, proteases, arachidonic acid metabolites, sympathetic amines, etc.) and direct activation of nociceptors. Central sensitization (increased sensitivity of spinal cord neurons caused by prolonged afferent input to the spinal cord) also plays a role in the formalin-induced pain response. It is possible that the more complex pain stimulus elicited by formalin is differentially influenced by 17β-E2 than that produced by BK.
The receptor signaling mechanisms that mediate the rapid-onset action of estrogen are not well defined. In some cases, these actions seem to be mediated by classic estrogen receptors (ERα or ERβ or splice variants, thereof) that are associated with the plasma membrane or are present in the cytosol (Razandi et al., 1999; Clarke et al., 2000; Watson et al., 2002). Post-translational changes (e.g., palmitoylation) have been proposed to traffic ERα and ERβ subtypes to plasma membrane microdomains enriched in cholesterol, receptors, and signaling molecules, such as caveolae (Acconcia et al., 2003; Razandi et al., 2003). Several signal transduction pathways have been implicated in rapid ER signaling, including mitogen-activated protein kinase, adenylyl cyclase, PLC, nitric oxide, and phosphatidylinositol 3-kinase (Kelly and Levin, 2001; for reviews, see Hall et al., 2001; Levin, 2002). Experiments to identify the signaling pathways that mediate estrogen's action on BK responsiveness are underway.
Here, we report that certain members of the RGD-binding family of integrins mediate the ability of 17β-E2 to enhance BK signaling. Integrins are a heterogeneous class of cell-surface proteins expressed by virtually every cell type and are best known to be involved in the regulation of several vital cell functions, including adhesion, migration, proliferation, and differentiation (van der Flier and Sonnenberg, 2001). Recent evidence indicates that integrins can also regulate signaling by seven-transmembrane spanning receptors (Litvak et al., 2000; Short et al., 2000; Berg et al., 2007b).
Eighteen α and eight β subunits have been identified, forming at least 24 different α/β integrins (van der Flier and Sonnenberg, 2001). Integrins composed of α4, α5, α8, αIIb, or αv subunits (10 of the known 24 integrins contain one of these α subunits) bind to molecules containing an RGD sequence (van der Flier and Sonnenberg, 2001), which is exposed in extracellular matrix molecules such as fibronectin and vitronectin. Upon binding to an RGD sequence immobilized within extracellular matrix molecules, integrins become activated and signal to a variety of intracellular signaling cascades, including ion channels, kinases, and associated proteins (Coppolino and Dedhar, 2000; Juliano, 2002; Martin et al., 2002). However, soluble RGD-containing peptides or soluble antibodies against integrin subunits block integrin signaling (Chavis and Westbrook, 2001). Here, we found that soluble RGD peptides completely blocked the enhancement of BK signaling in vitro and in vivo without affecting BK signaling on its own. In addition, a soluble antibody directed against the β1 integrin subunit blocked the effect of 17β-E2. These data suggest that 17β-E2 regulation of BK signaling is mediated by RGD-binding integrins that contain the β1 subunit. It is noteworthy that application of a soluble antibody against the β5 integrin subunit enhanced BK signaling in both the absence and presence of 17β-E2, and the effect on BK signaling was not further increased by 17β-E2. These data suggest that β5-containing integrins provide tonic inhibition of BK signaling that can be overcome by activation of β1-containing integrins (by 17β-E2) and underscore the complexity of integrin regulation of cellular functions.
The mechanism by which RGD-binding integrins participate in the regulation of BK signaling by 17β-E2 is unknown. Integrins regulate a variety of intracellular protein kinase signaling cascades (Coppolino and Dedhar, 2000; Juliano, 2002; Martin et al., 2002) that, via cross-talk mechanisms, may regulate signaling or trafficking of the BK receptor system. Alternatively, integrins promote powerful changes in the organization of the cytoskeleton and can regulate the constituents of caveolin-containing membrane microdomains (Salanueva et al., 2007), which spatially restrict the distribution of, and signaling by, a variety of receptor systems (Allen et al., 2007). Receptor-mediated signaling can be either enhanced or decreased by trafficking of receptors and signaling molecule partners (e.g., G proteins) between membrane compartments (Allen et al., 2007). Consequently, by regulating the signaling molecule composition of membrane microdomains, integrins could regulate BK signaling. Although the present studies do not distinguish among these mechanisms, they provide strong documentation for a rapid estrogen-mediated regulation of BK signaling and function that critically involves mediation by the RGD class of integrins.
In summary, 17β-E2 rapidly enhanced BK signaling in cultures of rat primary sensory neurons and enhanced BK-induced thermal allodynia in rats. The action of 17β-E2 was probably nongenomic, mediated by cell membrane-associated ERs located on peripheral primary sensory nerve terminals, and required β1-containing integrins. Collectively, these results indicate that 17β-E2 acting on primary sensory neurons may participate in the enhanced sensitivity of women to painful stimuli.
Acknowledgments
We thank Teresa Sanchez, Michelle Silva, Mona Bains, and Rachel Gambulos for excellent technical assistance.
This work was supported by the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grants R01-NS055835, R01-NS061884, R01-NS058655], the National Institute of Dental and Craniofacial Research [COSTAR Training Grant T32DE14318] (to M.P.R.), the Texas Advanced Research Program [Grant 003659-0023], and The Oral and Maxillofacial Surgery Foundation.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.167445.
- TG
- trigeminal ganglia
- DRG
- dorsal root ganglia
- BK
- bradykinin
- 17β-E2
- 17β-estradiol
- PWL
- paw withdrawal latency
- ER
- estrogen receptor
- OVX
- ovariectomized
- DMSO
- dimethyl sulfoxide
- BSA
- bovine serum albumin
- HBSS
- Hank's balanced salt solution
- IP
- inositol phosphate
- PLC
- phospholipase C.
References
- Acconcia F, Bocedi A, Ascenzi P, Marino M. (2003) Does palmitoylation target estrogen receptors to plasma membrane caveolae? IUBMB Life 55:33–35 [DOI] [PubMed] [Google Scholar]
- Allen JA, Halverson-Tamboli RA, Rasenick MM. (2007) Lipid raft microdomains and neurotransmitter signalling. Nat Rev Neurosci 8:128–140 [DOI] [PubMed] [Google Scholar]
- Aloisi AM, Bonifazi M. (2006) Sex hormones, central nervous system and pain. Horm Behav 50:1–7 [DOI] [PubMed] [Google Scholar]
- Bereiter DA, Cioffi JL, Bereiter DF. (2005) Oestrogen receptor-immunoreactive neurons in the trigeminal sensory system of male and cycling female rats. Arch Oral Biol 50:971–979 [DOI] [PubMed] [Google Scholar]
- Berg KA, Patwardhan AM, Sanchez TA, Silva YM, Hargreaves KM, Clarke WP. (2007a) Rapid modulation of μ-opioid receptor signaling in primary sensory neurons. J Pharmacol Exp Ther 321:839–847 [DOI] [PubMed] [Google Scholar]
- Berg KA, Zardeneta G, Hargreaves KM, Clarke WP, Milam SB. (2007b) Integrins regulate opioid receptor signaling in trigeminal ganglion neurons. Neuroscience 144:889–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns BE, Gazerani P. (2009) Sex-related differences in pain. Maturitas 63:292–296 [DOI] [PubMed] [Google Scholar]
- Chaban VV, Micevych PE. (2005) Estrogen receptor-α mediates estradiol attenuation of ATP-induced Ca2+ signaling in mouse dorsal root ganglion neurons. J Neurosci Res 81:31–37 [DOI] [PubMed] [Google Scholar]
- Chavis P, Westbrook G. (2001) Integrins mediate functional pre- and postsynaptic maturation at a hippocampal synapse. Nature 411:317–321 [DOI] [PubMed] [Google Scholar]
- Clarke CH, Norfleet AM, Clarke MS, Watson CS, Cunningham KA, Thomas ML. (2000) Perimembrane localization of the estrogen receptor α protein in neuronal processes of cultured hippocampal neurons. Neuroendocrinology 71:34–42 [DOI] [PubMed] [Google Scholar]
- Coppolino MG, Dedhar S. (2000) Bi-directional signal transduction by integrin receptors. Int J Biochem Cell Biol 32:171–188 [DOI] [PubMed] [Google Scholar]
- Craft RM. (2007) Modulation of pain by estrogens. Pain 132 (Suppl 1):S3–S12 [DOI] [PubMed] [Google Scholar]
- Fávaro-Moreira NC, Torres-Chávez KE, Fischer L, Tambeli CH. (2009) Peripheral estradiol induces temporomandibular joint antinociception in rats by activating the nitric oxide/cyclic guanosine monophosphate signaling pathway. Neuroscience 164:724–732 [DOI] [PubMed] [Google Scholar]
- Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL., 3rd (2009) Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain 10:447–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangula PR, Lanlua P, Wimalawansa S, Supowit S, DiPette D, Yallampalli C. (2000) Regulation of calcitonin gene-related peptide expression in dorsal root ganglia of rats by female sex steroid hormones. Biol Reprod 62:1033–1039 [DOI] [PubMed] [Google Scholar]
- Grollman AP. (1967) Inhibitors of protein biosynthesis. II. Mode of action of anisomycin. J Biol Chem 242:3226–3233 [PubMed] [Google Scholar]
- Hall JM, Couse JF, Korach KS. (2001) The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem 276:36869–36872 [DOI] [PubMed] [Google Scholar]
- Hammes SR, Levin ER. (2007) Extranuclear steroid receptors: nature and actions. Endocr Rev 28:726–741 [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. (1988) A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32:77–88 [DOI] [PubMed] [Google Scholar]
- Hucho TB, Dina OA, Kuhn J, Levine JD. (2006) Estrogen controls PKCε-dependent mechanical hyperalgesia through direct action on nociceptive neurons. Eur J Neurosci 24:527–534 [DOI] [PubMed] [Google Scholar]
- Juliano RL. (2002) Signal transduction by cell adhesion receptors and the cytoskeleton: functions of integrins, cadherins, selectins, and immunoglobulin-superfamily members. Annu Rev Pharmacol Toxicol 42:283–323 [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Levin ER. (2001) Rapid actions of plasma membrane estrogen receptors. Trends Endocrinol Metab 12:152–156 [DOI] [PubMed] [Google Scholar]
- Kuba T, Kemen LM, Quinones-Jenab V. (2005) Estradiol administration mediates the inflammatory response to formalin in female rats. Brain Res 1047:119–122 [DOI] [PubMed] [Google Scholar]
- Levin ER. (2002) Cellular functions of plasma membrane estrogen receptors. Steroids 67:471–475 [DOI] [PubMed] [Google Scholar]
- Litvak V, Tian D, Shaul YD, Lev S. (2000) Targeting of PYK2 to focal adhesions as a cellular mechanism for convergence between integrins and G protein-coupled receptor signaling cascades. J Biol Chem 275:32736–32746 [DOI] [PubMed] [Google Scholar]
- Liuzzi FJ, Scoville SA, Bufton SM. (1999) Effects of short-term estrogen replacement on trkA mRNA levels in axotomized dorsal root ganglion neurons. Exp Neurol 159:433–440 [DOI] [PubMed] [Google Scholar]
- Liverman CS, Brown JW, Sandhir R, Klein RM, McCarson K, Berman NE. (2009) Oestrogen increases nociception through ERK activation in the trigeminal ganglion: evidence for a peripheral mechanism of allodynia. Cephalalgia 29:520–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin KH, Slack JK, Boerner SA, Martin CC, Parsons JT. (2002) Integrin connections map: to infinity and beyond. Science 296:1652–1653 [DOI] [PubMed] [Google Scholar]
- Multon S, Pardutz A, Mosen J, Hua MT, Defays C, Honda S, Harada N, Bohotin C, Franzen R, Schoenen J. (2005) Lack of estrogen increases pain in the trigeminal formalin model: a behavioural and immunocytochemical study of transgenic ArKO mice. Pain 114:257–265 [DOI] [PubMed] [Google Scholar]
- Patwardhan AM, Diogenes A, Berg KA, Fehrenbacher JC, Clarke WP, Akopian AN, Hargreaves KM. (2006) PAR-2 agonists activate trigeminal nociceptors and induce functional competence in the Δ opioid receptor. Pain 125:114–124 [DOI] [PubMed] [Google Scholar]
- Razandi M, Alton G, Pedram A, Ghonshani S, Webb P, Levin ER. (2003) Identification of a structural determinant necessary for the localization and function of estrogen receptor α at the plasma membrane. Mol Cell Biol 23:1633–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Greene GL, Levin ER. (1999) Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERα and ERβ expressed in Chinese hamster ovary cells. Mol Endocrinol 13:307–319 [DOI] [PubMed] [Google Scholar]
- Salanueva IJ, Cerezo A, Guadamillas MC, del Pozo MA. (2007) Integrin regulation of caveolin function. J Cell Mol Med 11:969–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short SM, Boyer JL, Juliano RL. (2000) Integrins regulate the linkage between upstream and downstream events in G protein-coupled receptor signaling to mitogen-activated protein kinase. J Biol Chem 275:12970–12977 [DOI] [PubMed] [Google Scholar]
- Straub RH. (2007) The complex role of estrogens in inflammation. Endocr Rev 28:521–574 [DOI] [PubMed] [Google Scholar]
- van der Flier A, Sonnenberg A. (2001) Function and interactions of integrins. Cell Tissue Res 305:285–298 [DOI] [PubMed] [Google Scholar]
- Watson CS, Campbell CH, Gametchu B. (2002) The dynamic and elusive membrane estrogen receptor-α. Steroids 67:429–437 [DOI] [PubMed] [Google Scholar]
- Wiesenfeld-Hallin Z. (2005) Sex differences in pain perception. Gend Med 2:137–145 [DOI] [PubMed] [Google Scholar]
- Xu S, Cheng Y, Keast JR, Osborne PB. (2008) 17β-estradiol activates estrogen receptor β-signalling and inhibits transient receptor potential vanilloid receptor 1 activation by capsaicin in adult rat nociceptor neurons. Endocrinology 149:5540–5548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Ozawa H, Lu H, Yuri K, Hayashi S, Nihonyanagi K, Kawata M. (1998) Immunocytochemical analysis of sex differences in calcitonin gene-related peptide in the rat dorsal root ganglion, with special reference to estrogen and its receptor. Brain Res 791:35–42 [DOI] [PubMed] [Google Scholar]