Abstract
Alcoholism and anxiety disorders have a huge impact on society and afflict 17.6 million and 40 million people in the United States, respectively. A strong comorbidity exists between alcoholism and anxiety disorders. Indeed, alcohol withdrawal-induced anxiety is a primary contributing factor for relapse, and anxiolytics are a common adjuvant therapy prescribed for treatment-seeking alcoholics. It is thought that the use of alcohol to self-medicate and relieve anxiety contributes to the development of addiction. Treatment for anxiety disorders and alcoholism exist but are not universally effective. The delta opioid receptor (DOR) plays a role in both alcohol consumption and anxiety, making it a very interesting clinical target. Two pharmacologically distinct DORs have been described: DOR1 and DOR2. We find here that the relative specificity of DOR agonists for DOR1 or DOR2 can greatly affect the effects they exert on ethanol consumption and anxiety. The DOR1 agonist 2-methyl-4aα-(3-hydroxyphenyl)-1,2,3,4,4a,5,12,12aα-octahydro-quinolino[2,3,30g]isoquinoline (TAN-67), although not effective in decreasing anxiety-like behavior in naive mice, has anxiolytic-like properties in ethanol-withdrawn mice. In contrast, a less subtype-selective agonist, (+)-4-[(αR)-α-((2S,5R)-4-allyl-2,5-dimethyl-1-piperazinyl)-3-methoxybenzyl]-N,N-diethylbenzamide (SNC80), while also reducing anxiety-like behavior, increases ethanol consumption. In addition, we found that the conical anxiolytic diazepam [DZ; 7-chloro-1-methyl-5-phenyl-3H-1,4-benzodiazepin-2(1H)-one] is a less effective anxiolytic in ethanol-withdrawn mice than in naive mice. Together, our findings suggest that selective DOR agonists can decrease anxiety-like behavior and are more effective than diazepam at reducing ethanol consumption. We believe the dual efficacy of DOR1 agonists makes these receptors an interesting therapeutic target for treatment-seeking alcoholics.
Introduction
Currently, three drugs have been approved by the Food and Drug Administration to treat alcoholism: Revia [naltrexone (NTX); 17-(cyclopropylmethyl)-4,5α-epoxy-3,14-dihydroxymorphinan-6-one; a nonselective opioid antagonist], Campral (acamprosate; a N-methyl-d-aspartic acid receptor antagonist), and Antabuse (disulfiram; an inhibitor of acetylaldehyde dehydrogenase). Although these drugs can be efficacious in reducing ethanol consumption, all have clinical limitations and suffer from compliance issues (Pettinati et al., 2000; Buonopane and Petrakis, 2005; Anton et al., 2006; Swift, 2007; Garbutt, 2009; Mitchell et al., 2009). One of the drugs currently in clinical trials to treat alcoholism is the neurokinin (NK1) receptor antagonist (2-chloro-phenyl)-(2-(5-pyridin-4-yl-1-(3,5-bistrifluoromethyl-benzyl)-1H-(1,2,3)triazol-4-yl)pyridin-3-yl)methanone (LY686017). It is thought that the efficacy of this drug is caused, at least in part, by the reduction of anxiety and craving in anxious alcohol-dependent subjects (George et al., 2008; Heilig et al., 2009). According to the National Institute of Mental Health, approximately 40 million American adults suffer from an anxiety disorder (AD). The individual and societal cost associated with ADs is extremely high (Wittchen, 2002; Hoffman et al., 2008). It is noteworthy that people suffering from ADs are more susceptible to substance abuse (Wittchen, 2002; Hoffman et al., 2008). Selective serotonin reuptake inhibitors (SSRIs) and benzodiazepines (BZDs) are the two classes of drug most commonly used to treat ADs (Cloos and Ferreira, 2009). SSRIs are generally well tolerated; however, they take several weeks to take effect, and each SSRI is effective only in a minority of patients (Simon, 2001), who cannot be identified before initiating treatment (Tiwari et al., 2009). BZDs are commonly prescribed to control anxiety “attacks;” however, they themselves are intoxicating and habit-forming (Isbister et al., 2004; O'Brien, 2005). It is noteworthy that BZDs can increase the palatability of ethanol and increase alcohol consumption (Soderpalm and Hansen, 1998). Thus, there is a significant need for novel targets and treatments for ADs, especially for anxiety that is comorbid with alcoholism.
One target that is linked to anxiety is the delta opioid receptor (DOR). Disruption of the gene encoding DOR or its endogenous ligand produces an anxious-like phenotype in mice (Filliol et al., 2000; Ragnauth et al., 2001; Roberts et al., 2001), suggesting that DOR agonists could be anxiolytic. The DOR is a particularly interesting target for anxiety, because it has also been found to regulate ethanol consumption. In some cases mice disrupted for DOR show enhanced ethanol consumption when alcohol-naive (van Rijn and Whistler, 2009), but DOR knockout mice also show enhanced drinking and preference for alcohol once they have been drinking for some time (Roberts et al., 2001). It is noteworthy that two DOR subtypes (DOR1 and DOR2) can be distinguished in vivo (Mattia et al., 1991; Zaki et al., 1996), and recently we found that these subtypes have opposing effects with regard to ethanol intake (van Rijn and Whistler, 2009).
As mentioned above, there is significant comorbidity between anxiety and alcohol abuse, and anxiety and stress are risk factors that predispose an individual to both the primary development of alcoholism and relapse in withdrawing and abstinent patients. Individuals abstaining from ethanol may experience increased levels of anxiety, especially within the first 24 to 48 h after their last drink, a phenomenon generally known as alcohol withdrawal syndrome. This is often treated with benzodiazepines (McKeon et al., 2008). Stress-related anxiety-induced relapse can also be modeled in preclinical animal models, because rats subjected to a foot shock paradigm, for example, show increased relapse to alcohol (Lê et al., 1999; Liu and Weiss, 2003). As would be expected if activity at the DOR were anxiolytic, rats that express more functional DORs show less anxiety-like behaviors and drink less ethanol than rats with fewer functional receptors (Margolis et al., 2008), and DOR ligands reduce ethanol seeking in withdrawn rats (Ciccocioppo et al., 2002; Marinelli et al., 2009).
Here, we investigate the ability of DOR-selective agonists to decrease ethanol consumption and both anxiety and ethanol withdrawal-induced anxiety-like behaviors compared with the drugs currently available to treat these disorders, naltrexone [NTX; 17-(cyclopropylmethyl)-4,5α-epoxy-3,14-dihydroxymorphinan-6-one] and diazepam [DX; 7-chloro-1-methyl-5-phenyl-3H-1,4-benzodiazepin-2(1H)-one]. We found that the selective DOR agonist (+)-4-[(αR)-α-((2S,5R)-4-allyl-2,5-dimethyl-1-piperazinyl)-3-methoxybenzyl]-N,N-diethylbenzamide (SNC80) reduces anxiety-like behaviors, but also increases ethanol consumption. In comparison, the DOR1-selective agonist 2-methyl-4aα-(3-hydroxyphenyl)-1,2,3,4,4a,5,12,12aα-octahydro-quinolino[2,3,30g]isoquinoline (TAN-67), which we had previously shown to decrease ethanol intake (van Rijn and Whistler, 2009), has no effect on anxiety-like behavior in alcohol-naive mice. It is noteworthy, however, that we observe TAN-67 does reduce ethanol withdrawal-induced anxiety-like behaviors. In contrast, we found that two drugs commonly used in the treatment of alcoholism (NTX) or anxiety (diazepam) may induce anxiogenic-like behavior and have limited ability to decrease ethanol consumption, respectively. Therefore, DOR1 subtype-selective drugs may potentially have an improved ability to simultaneously reduce ethanol intake and anxiety associated with ethanol abstinence than the currently available therapeutics.
Materials and Methods
Animals and Housing
Wild-type and DOR knockout (KO) C57BL/6 mice (male, 20–25g; Taconic Farms, Germantown, NY) were housed (maximally five per cage) in ventilated Plexiglas cages at ambient temperature (21°C) in a room maintained on a 12-h light/12-h dark cycle (lights on at 8:00 AM, lights off at 8:00 PM). Food and water was provided ad libitum. The mice were given 1 week to acclimatize before the start of the experiments. All animal procedures were preapproved by the Gallo Center Institutional Animal Care and Use Committee, performed in our Association for Assessment and Accreditation of Laboratory Animal Care-certified facility, and were in accordance with National Institutes of Health Guide for the Care and Use of Laboratory Animals. Mice were not deprived of food or water at any time.
Chronic Ethanol Exposure
The limited-access two-bottle choice paradigm was performed as described previously (van Rijn and Whistler, 2009). In short, for the ethanol exposure, mice were individually housed in ventilated Plexiglas cages at ambient temperature (21°C) in a room maintained on a reversed 12-h light/12-h dark cycle (lights off at 10:00 AM, lights on at 10:00 PM). Food and water was provided ad libitum. The mice were given 1 week to acclimatize to the individual housing conditions and reverse light cycle before the start of the experiments. A two-bottle, limited-access (4 h/day) drinking paradigm was used for 5 days. During the limited drinking phase mice had access to water and either a 10% ethanol solution or a 2% sucrose solution.
Anxiety-Like Behavior Measurements
Elevated-Plus Maze.
The elevated-plus maze consisted of two closed arms and two open arms arranged perpendicular to one another. The plus maze was made of wood, painted white, and elevated 41 cm above the floor (arm length 70 cm, width 9 cm, and height 12 cm). No rim was present surrounding the open arm. During the 5-min trial, the behavior of the mouse was recorded by a camera positioned above the maze in the absence of laboratory personnel. Each mouse was placed in the center of the maze (9 × 9 cm) facing a closed arm. The surface of the maze was cleaned with disinfectant and dried before the next mouse was tested. Light intensity on the plus maze was 460 lux. The variables measured included the total number of entries into the closed and open arms and the total time spent in each region. An entry was defined as the mouse placing two paws within the boundaries of the arm. An increase in the number of entries and time spent in the open arms is indicative of an anxiolytic-like response.
Dark–Light Transition Box.
The dark–light apparatus was made up of an automated activity monitor with a dark box insert (ENV-510; Med Associates, St. Albans, VT), to create an equally spaced light and dark compartment (24 cm × 28 cm × 25 cm). The entire apparatus was positioned in a sound-attenuating chamber. The light side was illuminated to a degree of 100 lux, compared with 5 lux in the dark side. Each animal was placed facing the entrance of the dark area, and its behavior was recorded for 5 min. The dark–light transition box was cleaned with disinfectant and dried before the next mouse was tested. A photobeam-based tracking system was used to track the movement and locomotor activity of the mice within the test box and calculate the time spent in each area and the number of entries into each area. Anxiolytic-like effects were indicated by increased time spent in the illuminated compartment.
Ethanol Withdrawal-Induced Anxiety
To study ethanol withdrawal-induced anxiety-like behaviors, mice were trained in the limited-access, two-bottle choice paradigm for 5 days. On day 6 (24 h after the last access to ethanol or sucrose), anxiety levels in the withdrawn mice were tested by using the elevated-plus maze and the dark–light transition box.
Data Analysis
Baseline values for the ethanol drinking studies were determined by taking the average of the consumption over the 3 days before injection. Statistical analysis was performed with Prism software (GraphPad Software, Inc., San Diego, CA). Significance was determined by Student's t test, one-way ANOVA, or two-way ANOVA (repeated measures). A post hoc Newman-Keuls (one-way ANOVA) or Bonferroni (two-way ANOVA) test was used when a significant overall effect was found (p < 0.05).
Drugs
Ethanol solutions were prepared in tap water using 95% (v/v) ethanol (Gold Shield Chemical Co., Hayward, CA). TAN-67 (25 mg/kg) was purchased from Tocris Bioscience (Ellisville, MO). Sucrose, naltrexone (1 mg/kg), SNC80 (20 mg/kg), and diazepam (1 and 3 mg/kg) were purchased from Sigma-Aldrich (St. Louis, MO). All compounds were dissolved in saline. Diazepam was suspended in solution with 0.06% Tween 80. This concentration of Tween has no effect on behavior (Supplemental Fig. 1, A and B); therefore, we used saline as vehicle in all of our experiments to allow comparison across all treatment groups. All drugs were prepared immediately before injection and administered subcutaneously. All drugs were administered 30 min before the beginning of each experiment.
Results
Delta Opioid Receptors Affect Alcohol Intake and Anxiety-Like Behaviors.
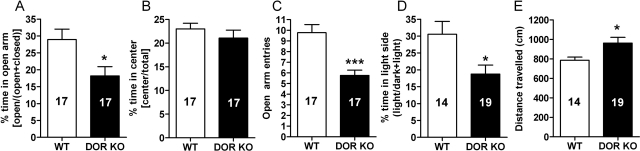
We have previously shown that C57BL/6 mice with a knockout of the DOR gene (DOR KO) consume more ethanol than wild-type mice (van Rijn and Whistler, 2009). We found that these C57BL/6 mice also show an anxiogenic phenotype, as measured by their behavior on an elevated-plus maze (Fig. 1, A–C) and dark–light transition box (Fig. 1D). The WT mice spent significantly more time in the open arm (p = 0.014) of the elevated-plus maze (Fig. 1A) and the light side (p = 0.013) of the dark–light box (Fig. 1D) compared with DOR mice. In addition, the DOR KO mice made significantly (p = 0.0001) fewer entries in the open arm of the elevated-plus maze compared with WT mice (Fig. 1B). The fewer entries in DOR KO were not caused by a generalized decreased locomotor activity because DOR KO mice were somewhat hyperlocomotive (p = 0.026) compared with WT mice (Fig. 1E). The two genotypes showed no difference in the amount of time the mice spent in the center of the elevated-plus maze (Fig. 1C).
Fig. 1.
DOR KO mice show a higher degree of anxiety-like behavior compared with WT C57BL/6 mice. A to D, anxiety-like behaviors were measured in WT and DOR KO C57BL/6 mice by using the elevated-plus maze (A–C) and dark–light transition box (D). For the elevated-plus maze relative time spent in the open arms and center was measured (A) and the number of entries into the open arm was counted (C) for 5 min. For the dark–light transition box, relative time spent in the light chambers (D) was measured for 5 min. E, locomotor activity (distance traveled) was assessed in the dark–light box. The number of animals used in each group is indicated in each bar of the histogram. *, p < 0.05; ***, p < 0.001.
DOR-Selective Ligands Can Either Increase or Decrease Ethanol Intake Depending on the Subtype Targeted.
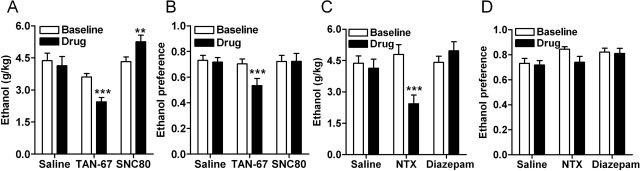
We have previously reported that agonists selective for DOR1 (TAN-67) and antagonists selective for DOR2 [naltriben; 17-(cyclopropylmethyl)-6,7-didehydro-3,14β-dihydroxy-4,5α-epoxy-6,7–2,3′-benzo furanomorphinan; NTB] decrease ethanol consumption in mice (van Rijn and Whistler, 2009 and see Fig. 2, A and B), suggesting that DOR1 and DOR2 receptors have opposing effects on ethanol consumption. Here, we found that the DOR agonist SNC80 significantly increased ethanol consumption [F(2,24) = 20.48; p < 0.0001] (Fig. 2, A and B). Thus, the pharmacological specificity of the DOR1 agonist TAN-67 was critical for its ability to reduce ethanol consumption. NTX, the current Food and Drug Administration-approved drug used in the treatment of alcoholism, can also decrease ethanol consumption in mice (van Rijn and Whistler, 2009 and see Fig. 2, C and D), but also decreased water consumption and therefore did not affect preference (Fig. 2D). In addition, the ability of NTX to antagonize all three opioid receptors (Raynor et al., 1994) may contribute to the side effects of this drug. The benzodiazepine diazepam had no significant effect on either ethanol consumption [F(2,24) = 28.22; p < 0.0001] or preference [F(2,24) = 1.78; p = 0.19], although there was a trend toward an increase in alcohol consumption (Fig. 2, C and D), consistent with previous reports that benzodiazepines can increase the palatability of ethanol (Soderpalm and Hansen, 1998). Thus, among the four drugs we tested, the DOR1-selective agonist TAN-67, the DOR-selective agonist SNC80, the opioid receptor antagonist NTX. and the benzodiazepine diazepam, only the DOR1 agonist TAN-67 reduced both ethanol consumption and preference [F(2,24) = 6.21; p = 0.0067].
Fig. 2.
DOR subtype-selective agonists have opposing actions in ethanol consumption. Wild-type C57BL/6 mice (n = 9), trained to drink in a limited-access, two-bottle choice paradigm, were injected subcutaneously with saline, 25 mg/kg of the DOR1 agonist TAN-67, or 20 mg/kg of the DOR agonist SNC80 (A and B) or 1.5 mg/kg of the nonselective opioid antagonist NTX or 1 mg/kg of the benzodiazepine diazepam (C and D). Thirty minutes after injection ethanol, water consumption were measured over a 4-h period. Ethanol preference = ethanol consumption/(ethanol consumption + water consumption). **, p < 0.01; ***, p < 0.001.
The DOR-Selective Agonist SNC80, but Not the DOR1-Selective Agonist TAN-67, Reduces Anxiety-Like Behavior in Naive Mice.
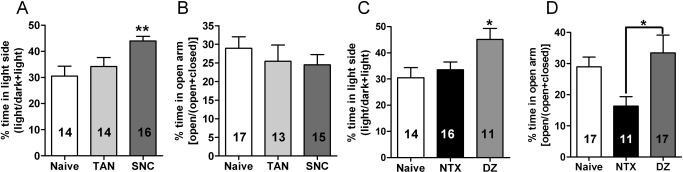
Because disruption of DORs increases anxiety-like behavior (Fig. 1), we next examined whether DOR agonists could decrease anxiety and whether this effect was subtype-specific. Both the DOR-selective agonist SNC80 [F(2,41) = 5.46; p = 0.0079] and our control anxiolytic diazepam [F(2,39); p = 0.023] produced anxiolytic-like properties in the dark–light transition box (Fig. 3, A and C), significantly increasing time spent in the light side. SNC80 and diazepam did not significantly [F(2 42) = 0.52, p = 0.60] increase the time mice spent in the open arm of the elevated-plus maze (Fig. 3D). However, this is likely because, even in the absence of drug, alcohol-naive C57BL/6 mice spent nearly 30% of their time in the open arm. Although the amount of time spent in the open arm is relatively high, this finding in not unusual for this strain of mice (Griebel et al., 2000; Lepicard et al., 2000; Carola et al., 2002; Lalonde and Strazielle, 2008). In addition, it is not uncommon for 1 mg/kg diazepam to fail to reduce anxiety in C57BL/6 mice in the elevated-plus maze paradigm (Griebel et al., 2000; Lepicard et al., 2000). It is noteworthy that we found that mice treated with NTX spent significantly [F(2,41) = 4.19; p = 0.023] less time in the open arm of the elevated-plus maze (Fig. 3D). This suggests that NTX may elicit some anxiety-like behavior even in alcohol-naive mice, which is in agreement with previous reports that have shown that in some cases NTX can increase anxiety (King et al., 1997; Maremmani et al., 1998; Kozak et al., 2007). Thus, we find the light–dark transition box seems to be the more sensitive assay for detecting anxiolytic-like effects, whereas the elevated-plus maze seems to be more sensitive for detecting anxiogenic-like effects, at least for C57BL/6 mice.
Fig. 3.
The DOR-selective agonist SNC80 decreases anxiety-like behavior in naive mice. A, naive wild-type C57BL/6 mice were injected subcutaneously with saline, 25 mg/kg of the DOR1 agonist TAN-67, or 20 mg/kg of the DOR agonist SNC80 (A and B) or 1.5 mg/kg of the nonselective opioid antagonist NTX or 1 mg/kg of the benzodiazepine diazepam (C and D). Thirty minutes after injection, anxiety-like behavior was measured by using the dark–light transition box (A and C) and the elevated-plus maze (B and D). For the dark–light transition box relative time spent in the light chambers was measured for 5 min. For the elevated-plus maze relative time spent in the open arms was measured for 5 min. The number of animals used in each group is indicated in each bar of the histogram. *, p < 0.05; **, p < 0.01.
C57BL/6 Mice Display Ethanol but Not Sucrose Withdrawal-Induced Anxiety-Like Behaviors.
One of the many problems treatment-seeking abstinent alcoholics encounter is an increased level of anxiety (McKeon et al., 2008). In rodents, forced ethanol exposure via bolus intraperitoneal injection of ethanol or use of an ethanol vapor chamber has been shown to produce anxiety-like behaviors after withdrawal (Kliethermes, 2005). Here, we developed a paradigm to measure anxiety-like behavior after withdrawal from voluntary ethanol consumption. As above, mice were given a choice of water and 10% ethanol (or 2% sucrose as a controlled “preferred” substance) for 4 h a day for 5 days. After a 5-day period both 10% ethanol and 2% sucrose were preferred over water to a similar extent (Fig. 4A). Mice were then examined for anxiety-like behavior 24 h after their last ethanol or sucrose exposure. Ethanol-withdrawn mice showed a significant reduction in time spent in the open arm of the elevated-plus maze compared with naive mice or sucrose-withdrawn mice [F(2,28) = 8.02; p = 0.0018] (Fig. 4B), indicative of increased anxiety. In the dark–light transition paradigm we observed an anxiogenic trend between the naive and ethanol-withdrawn mice. In comparison with sucrose-withdrawn mice, we found that ethanol-withdrawn mice do in fact spend significantly less time in the light side of the dark–light box [F(2,31) = 4.46; p = 0.020] (Fig. 4C).
Fig. 4.
Mice trained to voluntarily consume ethanol display ethanol withdrawal-induced anxiety-like behavior. A, C57BL/6 mice (n = 9) were trained to consume either ethanol or sucrose (see Materials and Methods). B and C, anxiety-like behavior was measured by using the elevated plus maze (B) and dark–light transition box (C). For the elevated-plus maze relative time spent in the open arms was measured for 5 min. For the dark–light transition box relative time spent in the light chambers was measured for 5 min. D, locomotor activity (distance traveled) was assessed in the dark–light box. The number of animals used in each group is indicated in each bar of the histogram. *, p < 0.05; **, p < 0.01.
The changes in anxiety-like behavior were unlikely caused by changes in general locomotion, because ethanol or sucrose withdrawal did not have a significant effect on locomotor activity [F(2,31) = 1.14; p = 0.33] (Fig. 4D).
DOR-Selective Agonists Attenuate Ethanol Withdrawal-Induced Anxiety-Like Behavior.
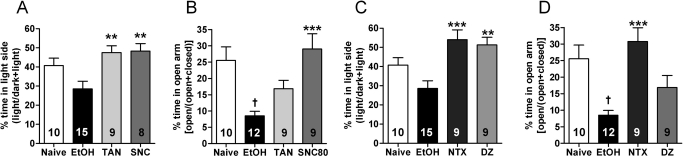
We next examined whether DOR-selective agonists could decrease ethanol withdrawal-induced anxiety-like behavior. We found that SNC80 significantly reduced withdrawal-induced anxiety-like behavior in both the dark–light transition box [F(3,38) = 5.80; p = 0.002] (Fig. 5A) and the elevated-plus maze [F(3,36) = 8.36; p = 0.0002] (Fig. 5B). It is noteworthy that although the DOR-1-selective agonist TAN-67 was ineffective at reducing anxiety-like behavior in alcohol-naive mice, it did reduce anxiety-like behavior in ethanol-withdrawn mice. Specifically, in ethanol-withdrawn mice, TAN-67 significantly increased the amount of time spent in the light side of the dark–light transition box (Fig. 5A). In addition, ethanol-withdrawn mice given TAN-67 no longer spent significantly less time in the open arm than ethanol-naive mice (Fig. 5B). Both NTX and diazepam significantly [F(3,39) = 8. 04; p = 0.0003] increased time spent in the light side of the of the dark–light transition box (Fig. 5C). However, NTX, but not diazepam, significantly [F(3,36) = 9.09; p = 0.0001] increased the time spent in the open arm of the elevated-plus maze (Fig. 5D). Benzodiazepines and ethanol both allosterically activate GABAA receptors. Thus, the reduction in efficacy of diazepam in the ethanol-withdrawn mice could reflect “cross-tolerance” at this target as a function of decreased number or function of GABAA receptors as a consequence of alcohol exposure (Sanna et al., 2003). To examine this possibility, we tested whether a higher dose could overcome the apparent tolerance. However, 3 mg/kg diazepam produced significant sedative effects, immobilizing mice (Supplemental Fig. 1C).
Fig. 5.
DOR-selective agonists can abolish ethanol withdrawal-induced anxiety. Ethanol-withdrawn (24 h after last exposure) C57BL/6 mice were injected subcutaneously with saline, 25 mg/kg of the DOR1 agonist TAN-67, and 20 mg/kg of the DOR agonist SNC80 (A and B) or 1.5 mg/kg of the nonselective opioid antagonist NTX or 1 mg/kg of the benzodiazepine diazepam (C and D). Thirty minutes after injection, anxiety-like behavior was measured by using the dark–light transition box (A and C) and the elevated-plus maze (B and D). For the dark–light transition box relative time spent in the light chambers was measured for 5 min. For the elevated-plus maze relative time spent in the open arms was measured for 5 min. The number of animals used in each group is indicated in each bar of the histogram. *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus ethanol; †, p < 0.05 versus naive.
Discussion
Here, we show that some, but not all, DOR subtype-selective ligands show dual efficacy at reducing ethanol consumption and ethanol withdrawal-induced anxiety. We and others have previously reported that DOR KO mice consume more ethanol and show increased anxiety-like behavior compared with wild-type mice (Filliol et al., 2000; Roberts et al., 2001; van Rijn and Whistler, 2009). Together, these studies suggest that DORs play a role in both anxiety and drinking behavior. Anxiety is a key risk factor for relapse in human alcoholics, which has led to the use of anxiolytics as adjunct therapy in the treatment of alcoholism. However, here we found that anxiolytics, including the benzodiazepine diazepam and the DOR-selective agonist SNC80, although reducing anxiety-like behaviors, increase rather than decrease drinking. It is noteworthy that we found that the DOR1-selective agonist TAN-67, which showed no effect on anxiety-like behaviors in alcohol-naive mice, reduced both drinking and alcohol withdrawal-induced anxiety.
We hypothesize that the diversity in the effectiveness of distinct opioid drugs for ethanol consumption and anxiety can be attributed to the existence of multiple receptor subtypes that differentially effect behavior. Two pharmacologically distinct DOR subtypes have been described in vivo. Intriguingly, ligands selective for DOR1 or DOR2 have opposing effects on alcohol consumption (van Rijn and Whistler, 2009). Thus, the existence of two DOR subtypes with opposing effects on drinking could explain why results from DOR KO mice do not always directly correlate with those seen with DOR subtype-selective ligands.
Our finding that the DOR-selective agonist SNC80 reduces the expression of anxiety-like behaviors in mice is in agreement with previously published work in rats showing SNC80 decreases anxiety-like behaviors (Saitoh et al., 2004) and that the DOR-selective antagonist naltrindole (Perrine et al., 2006) and the DOR2 subtype-selective antagonist naltriben produce an anxiogenic-like effect in the elevated-plus maze in rats (Saitoh et al., 2005). However, although it decreases anxiety, SNC80 increases alcohol consumption. Thus, it seems that decreasing anxiety per se does not lead to a decrease in ethanol consumption. It is noteworthy that we found that, unlike SNC80 (and diazepam), the DOR1 agonist TAN-67 decreased ethanol consumption. Although TAN-67 was not anxiolytic in alcohol-naive mice, it eliminated alcohol withdrawal-induced anxiety. Thus TAN-67 is distinguished from SNC80 (and diazepam) in that it reduces rather than enhances drinking and has a selective effect on alcohol withdrawal-induced anxiety. SNC80 and TAN-67 both are highly selective for DOR over the mu opioid receptor (Knapp et al., 1995, 1996). However, the dextrorotary enantiomer of TAN-67 has been shown to interact with spinal nociceptin/orphanin FQ receptor with nanomolar affinity (Kamei et al., 1999). Further studies are required to determine to what degree, if any, the racemic mixture of TAN-67 or the dextrorotary enantiomer affect ethanol consumption and anxiety through the nociceptin receptor. Our finding that TAN-67 shifts from being ineffective in reducing anxiety-like behavior in naive mice to having anxiolytic properties in ethanol-withdrawn mice corresponds to findings that DORs may be up-regulated after chronic stress and ethanol consumption (Commons, 2003; Margolis et al., 2008). Moreover, we have previously shown that removal of DORs eliminates the effects of TAN-67 on ethanol consumption entirely (van Rijn and Whistler, 2009).
Alcohol-dependent individuals, who abstain from consuming ethanol, may experience several withdrawal symptoms, including anxiety, deliriums, and potentially life-threatening seizures 24 to 48 h after their last drink. Although these symptoms may be mild in occurrence in general, the percentage of people reporting these symptoms increases in the subpopulation of heavy drinkers undergoing detoxification procedures. Benzodiazepines are a common treatment for alcohol-withdrawal symptoms (McKeon et al., 2008). Several rodent models exist that can reproduce the ethanol withdrawal-induced anxiety observed in humans. In general, the methods used to induce ethanol dependence require forced exposure to the ethanol, such as ethanol-containing liquid diets, ethanol vapor chambers, and intraperitoneal ethanol injections (Kliethermes, 2005). The forced nature of these methods, however, does not reflect human behavior and may further complicate any extrapolation of data obtained in mice to the human situation. Another issue in these rodent models is that withdrawn animals often have a reduction in their locomotor activity, which could be mistaken as a sign of anxiety-like behavior (Kliethermes, 2005). We found that, in a model of voluntary ethanol consumption, ethanol but not sucrose withdrawal increased the expression of anxiety-like behavior. It is noteworthy that the locomotor activity of these mice was not affected. Therefore, we believe this paradigm may be a suitable model for studying ethanol withdrawal-induced anxiety-like behavior. Most importantly, using this model we found that both SNC80 and TAN-67 were effective in reducing ethanol withdrawal-induced anxiety-like behavior and were, in fact, more effective than diazepam.
The ability of TAN-67 to decrease ethanol withdrawal-induced anxiety-like behavior is surprising because TAN-67 does not affect anxiety-like behavior in naive mice. DOR expression has been shown to change under the influence of chronic stressors, such as inflammation (Cahill et al., 2003), and chronic exposure to morphine (Cahill et al., 2001), ethanol (Margolis et al., 2008), and stress (Commons, 2003). We hypothesize that drinking and/or withdrawal from ethanol may change the number of functional DORs, in particular DOR1s. An increase in DOR function could explain our finding that both TAN-67 and SNC80 become more effective in the ethanol-withdrawn mice. In particular, we would expect chronic ethanol to up-regulate the number or function of DOR1s in circuits or brain regions that control anxiety. This hypothesis is supported by recent findings that chronic ethanol exposure recruited functional DORs in the central nucleus of the amygdala (Bie et al., 2009). Intriguingly, a recent study showed that DORs are involved in the function of benzodiazepines (Primeaux et al., 2006). Thus a change in the number of functional DORs, induced by heavy ethanol consumption and stress, could thereby also possibly affect the efficacy of benzodiazepines.
In conclusion, we show that the current drugs available to combat alcoholism in the human population, although effective at controlling individual aspects of the disease, are not ideally suited to treat both consumption and withdrawal-induced anxiety. Diazepam reduces anxiety-like behavior in naive mice but increases ethanol palatability and consumption and loses efficacy as an anxiolytic after drinking. Conversely, NTX can reduce ethanol consumption, but it suffers from side effects such as dysphoria and increased pain sensitivity and may increase anxiety. Here, we show that drugs that selectively target DOR subtypes could show promise as “dual efficacy” drugs to reduce both ethanol consumption and withdrawal-induced anxiety. The choice of DOR subtype will be key to the success of this strategy because the DOR1-selective drug TAN-67 decreased both alcohol withdrawal-induced anxiety and consumption, whereas the DOR agonist SNC80 decreased anxiety but increased consumption. In short, we believe that selectively targeting DOR1s with agonist drugs could be a promising new strategy for reducing ethanol withdrawal-induced anxiety without increasing the drive for alcohol consumption.
Supplementary Material
Acknowledgments
We thank Chris Kliethermes for valuable discussion and review of the manuscript and Madeline Ferwerda for genotyping and maintenance of mouse colonies.
This work was supported by the Department of Defense [Grant DAMD62-10-5-071] (to J.L.W.), the National Institute on Alcohol Abuse and Alcoholism [Grant AA017072-01] (to J.L.W.), National Institutes of Health National Institute on Drug Abuse [Grants DA015232, DA019958] (to J.L.W.), and funds provided by the State of California for medical research on alcohol and substance abuse through the University of California, San Francisco (to J.L.W.).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.170969.
 The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
- DOR
- delta opioid receptor
- DOR1
- DOR subtype 1
- DOR2
- DOR subtype 2
- KO
- knockout
- AD
- anxiety disorder
- SSRI
- selective serotonin reuptake inhibitor
- BZD
- benzodiazepine
- ANOVA
- analysis of variance
- WT
- wild type
- TAN-67 (SB-205607)
- 2-methyl-4aα-(3-hydroxyphenyl)-1,2,3,4,4a,5,12,12aα-octahydro-quinolino[2,3,30g]isoquinoline
- SNC80
- (+)-4-[(αR)-α-((2S,5R)-4-allyl-2,5-dimethyl-1-piperazinyl)-3-methoxybenzyl]-N, N-diethylbenzamide
- DZ
- 7-chloro-1-methyl-5-phenyl-3H-1,4-benzodiazepin-2(1H)-one
- NTX
- 17-(cyclopropylmethyl)-4,5α-epoxy-3,14-dihydroxymorphinan-6-one
- NTB
- 17-(cyclopropylmethyl)-6,7-didehydro-3,14β-dihydroxy-4,5α-epoxy-6,7–2,3′-benzo furanomorphinan
- LY686017
- (2-chloro-phenyl)-(2-(5-pyridin-4-yl-1-(3,5-bistrifluoromethyl-benzyl)-1H-(1,2,3)triazol-4-yl)pyridin-3-yl)methanone.
References
- Anton RF, O'Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, et al. (2006) Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA 295:2003–2017 [DOI] [PubMed] [Google Scholar]
- Bie B, Zhu W, Pan ZZ. (2009) Ethanol-induced δ-opioid receptor modulation of glutamate synaptic transmission and conditioned place preference in central amygdala. Neuroscience 160:348–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonopane A, Petrakis IL. (2005) Pharmacotherapy of alcohol use disorders. Subst Use Misuse 40:2001–2020, 2043–2048 [DOI] [PubMed] [Google Scholar]
- Cahill CM, Morinville A, Hoffert C, O'Donnell D, Beaudet A. (2003) Up-regulation and trafficking of δ opioid receptor in a model of chronic inflammation: implications for pain control. Pain 101:199–208 [DOI] [PubMed] [Google Scholar]
- Cahill CM, Morinville A, Lee MC, Vincent JP, Collier B, Beaudet A. (2001) Prolonged morphine treatment targets δ opioid receptors to neuronal plasma membranes and enhances δ-mediated antinociception. J Neurosci 21:7598–7607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carola V, D'Olimpio F, Brunamonti E, Mangia F, Renzi P. (2002) Evaluation of the elevated plus-maze and open-field tests for the assessment of anxiety-related behavior in inbred mice. Behav Brain Res 134:49–57 [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Martin-Fardon R, Weiss F. (2002) Effect of selective blockade of μ(1) or δ opioid receptors on reinstatement of alcohol-seeking behavior by drug-associated stimuli in rats. Neuropsychopharmacology 27:391–399 [DOI] [PubMed] [Google Scholar]
- Cloos JM, Ferreira V. (2009) Current use of benzodiazepines in anxiety disorders. Curr Opin Psychiatry 22:90–95 [DOI] [PubMed] [Google Scholar]
- Commons KG. (2003) Translocation of presynaptic δ opioid receptors in the ventrolateral periaqueductal gray after swim stress. J Comp Neurol 464:197–207 [DOI] [PubMed] [Google Scholar]
- Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F, Befort K, Gavériaux-Ruff C, Dierich A, LeMeur M, et al. (2000) Mice deficient for δ- and μ-opioid receptors exhibit opposing alterations of emotional responses. Nat Genet 25:195–200 [DOI] [PubMed] [Google Scholar]
- Garbutt JC. (2009) The state of pharmacotherapy for the treatment of alcohol dependence. J Subst Abuse Treat 36:S15–S23; quiz S24–S25. [PubMed] [Google Scholar]
- George DT, Gilman J, Hersh J, Thorsell A, Herion D, Geyer C, Peng X, Kielbasa W, Rawlings R, Brandt JE, et al. (2008) Neurokinin 1 receptor antagonism as a possible therapy for alcoholism. Science 319:1536–1539 [DOI] [PubMed] [Google Scholar]
- Griebel G, Belzung C, Perrault G, Sanger DJ. (2000) Differences in anxiety-related behaviours and in sensitivity to diazepam in inbred and outbred strains of mice. Psychopharmacology (Berl) 148:164–170 [DOI] [PubMed] [Google Scholar]
- Heilig M, Thorsell A, Sommer WH, Hansson AC, Ramchandani VA, George DT, Hommer D, Barr CS. (2009) Translating the neuroscience of alcoholism into clinical treatments: from blocking the buzz to curing the blues. Neurosci Biobehav Rev doi:10.1016/j.neubiorev.2009.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman DL, Dukes EM, Wittchen HU. (2008) Human and economic burden of generalized anxiety disorder. Depress Anxiety 25:72–90 [DOI] [PubMed] [Google Scholar]
- Isbister GK, O'Regan L, Sibbritt D, Whyte IM. (2004) Alprazolam is relatively more toxic than other benzodiazepines in overdose. Br J Clin Pharmacol 58:88–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei J, Ohsawa M, Suzuki T, Saitoh A, Endoh T, Narita M, Tseng LF, Nagase H. (1999) The modulatory effect of (+)-TAN-67 on the antinociceptive effects of the nociceptin/orphanin FQ in mice. Eur J Pharmacol 383:241–247 [DOI] [PubMed] [Google Scholar]
- King AC, Volpicelli JR, Gunduz M, O'Brien CP, Kreek MJ. (1997) Naltrexone biotransformation and incidence of subjective side effects: a preliminary study. Alcohol Clin Exp Res 21:906–909 [PubMed] [Google Scholar]
- Kliethermes CL. (2005) Anxiety-like behaviors following chronic ethanol exposure. Neurosci Biobehav Rev 28:837–850 [DOI] [PubMed] [Google Scholar]
- Knapp RJ, Landsman R, Waite S, Malatynska E, Varga E, Haq W, Hruby VJ, Roeske WR, Nagase H, Yamamura HI. (1995) Properties of TAN-67, a nonpeptidic δ-opioid receptor agonist, at cloned human δ- and μ-opioid receptors. Eur J Pharmacol 291:129–134 [DOI] [PubMed] [Google Scholar]
- Knapp RJ, Santoro G, De Leon IA, Lee KB, Edsall SA, Waite S, Malatynska E, Varga E, Calderon SN, Rice KC, et al. (1996) Structure-activity relationships for SNC80 and related compounds at cloned human δ and μ opioid receptors. J Pharmacol Exp Ther 277:1284–1291 [PubMed] [Google Scholar]
- Kozak AT, Spates CR, McChargue DE, Bailey KC, Schneider KL, Liepman MR. (2007) Naltrexone renders one-session exposure therapy less effective: a controlled pilot study. J Anxiety Disord 21:142–152 [DOI] [PubMed] [Google Scholar]
- Lalonde R, Strazielle C. (2008) Relations between open-field, elevated plus-maze, and emergence tests as displayed by C57/BL6J and BALB/c mice. J Neurosci Methods 171:48–52 [DOI] [PubMed] [Google Scholar]
- Lê AD, Poulos CX, Harding S, Watchus J, Juzytsch W, Shaham Y. (1999) Effects of naltrexone and fluoxetine on alcohol self-administration and reinstatement of alcohol seeking induced by priming injections of alcohol and exposure to stress. Neuropsychopharmacology 21:435–444 [DOI] [PubMed] [Google Scholar]
- Lepicard EM, Joubert C, Hagneau I, Perez-Diaz F, Chapouthier G. (2000) Differences in anxiety-related behavior and response to diazepam in BALB/cByJ and C57BL/6J strains of mice. Pharmacol Biochem Behav 67:739–748 [DOI] [PubMed] [Google Scholar]
- Liu X, Weiss F. (2003) Stimulus conditioned to foot-shock stress reinstates alcohol-seeking behavior in an animal model of relapse. Psychopharmacology (Berl) 168:184–191 [DOI] [PubMed] [Google Scholar]
- Maremmani I, Marini G, Fornai F. (1998) Naltrexone-induced panic attacks. Am J Psychiatry 155:447. [DOI] [PubMed] [Google Scholar]
- Margolis EB, Fields HL, Hjelmstad GO, Mitchell JM. (2008) Delta-opioid receptor expression in the ventral tegmental area protects against elevated alcohol consumption. J Neurosci 28:12672–12681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli PW, Funk D, Harding S, Li Z, Juzytsch W, Lê AD. (2009) Roles of opioid receptor subtypes in mediating alcohol-seeking induced by discrete cues and context. Eur J Neurosci 30:671–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattia A, Vanderah T, Mosberg HI, Omnaas JR, Bowen WD, Porreca F. (1991) Pharmacological characterization of [D-Ala2,Leu5,Ser6]enkephalin (DALES): antinociceptive actions at the δ noncomplexed-opioid receptor. Eur J Pharmacol 192:371–375 [DOI] [PubMed] [Google Scholar]
- McKeon A, Frye MA, Delanty N. (2008) The alcohol withdrawal syndrome. J Neurol Neurosurg Psychiatry 79:854–862 [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Bergren LJ, Chen KS, Rowbotham MC, Fields HL. (2009) Naltrexone aversion and treatment efficacy are greatest in humans and rats that actively consume high levels of alcohol. Neurobiol Dis 33:72–80 [DOI] [PubMed] [Google Scholar]
- O'Brien CP. (2005) Benzodiazepine use, abuse, and dependence. J Clin Psychiatry 66 (Suppl 2):28–33 [PubMed] [Google Scholar]
- Perrine SA, Hoshaw BA, Unterwald EM. (2006) Delta opioid receptor ligands modulate anxiety-like behaviors in the rat. Br J Pharmacol 147:864–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettinati HM, Volpicelli JR, Pierce JD, Jr, O'Brien CP. (2000) Improving naltrexone response: an intervention for medical practitioners to enhance medication compliance in alcohol dependent patients. J Addict Dis 19:71–83 [DOI] [PubMed] [Google Scholar]
- Primeaux SD, Wilson SP, McDonald AJ, Mascagni F, Wilson MA. (2006) The role of δ opioid receptors in the anxiolytic actions of benzodiazepines. Pharmacol Biochem Behav 85:545–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragnauth A, Schuller A, Morgan M, Chan J, Ogawa S, Pintar J, Bodnar RJ, Pfaff DW. (2001) Female preproenkephalin-knockout mice display altered emotional responses. Proc Natl Acad Sci USA 98:1958–1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynor K, Kong H, Chen Y, Yasuda K, Yu L, Bell GI, Reisine T. (1994) Pharmacological characterization of the cloned κ-, δ-, and μ-opioid receptors. Mol Pharmacol 45:330–334 [PubMed] [Google Scholar]
- Roberts AJ, Gold LH, Polis I, McDonald JS, Filliol D, Kieffer BL, Koob GF. (2001) Increased ethanol self-administration in δ-opioid receptor knockout mice. Alcohol Clin Exp Res 25:1249–1256 [PubMed] [Google Scholar]
- Saitoh A, Kimura Y, Suzuki T, Kawai K, Nagase H, Kamei J. (2004) Potential anxiolytic and antidepressant-like activities of SNC80, a selective δ-opioid agonist, in behavioral models in rodents. J Pharmacol Sci 95:374–380 [DOI] [PubMed] [Google Scholar]
- Saitoh A, Yoshikawa Y, Onodera K, Kamei J. (2005) Role of δ-opioid receptor subtypes in anxiety-related behaviors in the elevated plus-maze in rats. Psychopharmacology (Berl) 182:327–334 [DOI] [PubMed] [Google Scholar]
- Sanna E, Mostallino MC, Busonero F, Talani G, Tranquilli S, Mameli M, Spiga S, Follesa P, Biggio G. (2003) Changes in GABA(A) receptor gene expression associated with selective alterations in receptor function and pharmacology after ethanol withdrawal. J Neurosci 23:11711–11724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon G. (2001) Choosing a first-line antidepressant: equal on average does not mean equal for everyone. JAMA 286:3003–3004 [DOI] [PubMed] [Google Scholar]
- Söderpalm AH, Hansen S. (1998) Benzodiazepines enhance the consumption and palatability of alcohol in the rat. Psychopharmacology (Berl) 137:215–222 [DOI] [PubMed] [Google Scholar]
- Swift R. (2007) Emerging approaches to managing alcohol dependence. Am J Health Syst Pharm 64:S12–S22 [DOI] [PubMed] [Google Scholar]
- Tiwari AK, Souza RP, Müller DJ. (2009) Pharmacogenetics of anxiolytic drugs. J Neural Transm 116:667–677 [DOI] [PubMed] [Google Scholar]
- van Rijn RM, Whistler JL. (2009) The δ(1) opioid receptor is a heterodimer that opposes the actions of the δ(2) receptor on alcohol intake. Biol Psychiatry 66:777–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittchen HU. (2002) Generalized anxiety disorder: prevalence, burden, and cost to society. Depress Anxiety 16:162–171 [DOI] [PubMed] [Google Scholar]
- Zaki PA, Bilsky EJ, Vanderah TW, Lai J, Evans CJ, Porreca F. (1996) Opioid receptor types and subtypes: the δ receptor as a model. Annu Rev Pharmacol Toxicol 36:379–401 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.